Abstract
Small ruminant lentiviruses isolated from peripheral blood leukocytes and target organs can be propagated in vitro in fibroblasts derived from goat synovial membrane cells. These cells are obtained from tissues collected from embryos or fetuses and are necessary for the establishment of the fibroblast primary culture. A new alternative type of host cells, derived from goat umbilical cord, was isolated and characterized phenotypically with its main purpose being to obtain cell monolayers that could be used for the diagnosis and isolation of small ruminant lentiviruses in cell culture. To accomplish this goal, cells were isolated from umbilical cords; characterized phenotypically by flow cytometry analysis; differentiate into osteogenic, chondrogenic and adipogenic lineage; and submitted to viral challenge. The proliferation of goat umbilical cord cells was fast and cell monolayers formed after 15 days. These cells exhibited morphology, immunophenotype, growth characteristics, and lineage differentiation potential similar to mesenchymal stem cells of other origins. The goat umbilical cord derived cells stained positive for vimentin and CD90, but negative for cytokeratin, CD34 and CD105 markers. Syncytia and cell lysis were observed in cell monolayers infected by CAEV-Cork and MVV-K1514, showing that the cells are permissive to small ruminant lentivirus infection in vitro. These data demonstrate the proliferative competence of cells derived from goat umbilical cords and provide a sound basis for future research to standardize this cell lineage.
Keywords:
CAEV; MVV; Cell culture; MSC; Flow cytometry
Introduction
The small ruminant lentivirus (SRLV) comprises two types of viruses, the Caprine Arthritis Encephalitis virus (CAEV) and the Maedi Visna virus (MVV); both of which are widely distributed throughout the world. CAEV and MVV share genetic similarities, molecular mechanisms of replication, morphology and similar biological interactions in their hosts. These lentiviruses cause persistent infections, including encephalitis, arthritis, progressive pneumonia, and mastitis in goats and sheep. Like other lentiviruses, CAEV and MVV infect cells of the monocyte/macrophage lineage and dendritic cells.11 Narayan O, Clements JE. Biology and pathogenesis of lentiviruses. J Gen Virol. 1989;70:1617-1639.,22 Ryan S, Tiley L, McConnell I, Blacklaws B. Infection of dendritic cells by the Maedi-Visna lentivirus. J Virol. 2000;74:10096-10103.
CAEV and MVV can replicate in primary fibroblasts derived from synovial membranes or from choroid plexus cultures,33 Chebloune Y, Sheffer D, Karr BM, Stephens E, Narayan O. Restrictive type of replication of ovine/caprine lentiviruses in ovine fibroblast cell cultures. Virology. 1996;222:21-30.,44 Sihvonen L, Veijalainen P. Kinetics of Maedi virus production in sheep choroid plexus cells. Vet Microbiol. 1981;6:1-8. and can also replicate in the immortalized TIGEF cell line (T Immortalized Goat Embryonic Fibroblast).55 Teixeira MFS, Veronique L, Msebli-Lakahl L, Chettab A, Chebloune Y, Mornex JF. Imortalization of caprine fibroblasts permissive for replication of small ruminant lentiviroses. Am J Vet Res. 1997;58:579-584. These cells are obtained from the tissues collected from embryos or fetuses and are necessary for the establishment of the fibroblast primary culture.
An alternative method would involve using cells derived from goat umbilical cords, which is an invaluable tool that does not use embryos or fetuses. Generally, the umbilical cord would be discarded after calving. However, it is an excellent source material, rich in cells that are able to originate several cell types. The umbilical cord is a well-known source of mesenchymal stem cells (MSCs), which have been isolated and characterized in umbilical cord samples from canine,66 Lee KS, Nah JJ, Lee BC, et al. Maintenance and characterization of multipotent mesenchymal stem cells isolated from canine umbilical cord matrix by collagenase digestion. Res Vet Sci. 2013;94:144-151. equine77 Reed SA, Johnson SE. Refinement of culture conditions for maintenance of undifferentiated equine umbilical cord blood stem cells. J Equine Vet Sci. 2012;32:360-366. and ovine88 Fadel L, Viana BR, Feitosa MLT, et al. Protocols for obtainment and isolation of two mesenchymal stem cell sources in sheep. Acta Cir Brasil. 2011;26:267-273. species. MSCs are a multipotent adult stem cell.99 Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147.
Undifferentiated MSCs exhibit fibroblast-like morphology and are characterized phenotypically by the expression of surface markers; however, the characterization of these cells has not yet been fully defined. There are several positive markers described, and there is a consensus that undifferentiated MSCs should be positive for CD29, CD44, CD90, CD105, CD73 and negative for CD34, CD45, CD14 and CD3.1010 Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301.,1111 Rebelatto CK, Aguiar AM, Moretão MP, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood). 2008;233:901-913. MSCs are multipotent stromal cells capable of differentiating to mesenchymal lineages, including tissues such as adipose tissue, bone, cartilage and muscle.99 Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147.,1212 Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;31:595-596.
The aims of the experiments in this study were to isolate, phenotypically characterize and investigate the differentiation potentials of cells from goat umbilical cords (cGUCs), with the main purpose being to obtain cell monolayers for use in the diagnosis of small ruminant lentiviruses via isolation in cell culture.
Materials and methods
Samples
All experiments were approved by the Ethics Committee for Animal Use at the State University of Ceará, protocol number 127.769.79-0. For this study, mongrel pregnant goats were used, aged between two and three years and free of infection by SRLV.
Three umbilical cord samples were collected during calving. After birth, the goat umbilical cords were clamped at both ends, i.e., next to the fetus and the goat, and then cut and packed in the transport medium. The solutions used during transport were low glucose DMEM (Dulbecco's Modified Eagle Medium) (Gibco®, Grand Island, NY, USA), MEM-E (Minimum Essential Medium with Earle's salt and l-glutamine) (Gibco®, Grand Island, NY, USA), PBS (phosphate buffered saline) and saline solution 0.9% (SS), supplemented with 300 U/mL penicillin, 300 µg/mL streptomycin, 0.5 mg/mL amphotericin and 10% FBS. The samples were transported within 2 h to the laboratory, packed in an isothermal box with ice.
Isolation and culture of cGUCs
The tissue samples were processed in a laminar flow cabinet. The umbilical cord was washed with PBS to remove blood. The amniotic epithelium surrounding the umbilical cord was dissociated using sterile scissors and forceps. Tissue samples of umbilical cord matrix were cut into pieces of 0.5-1 cm (explants) and were plated in A25 cell culture flasks and 6-well culture plates using DMEM-low glucose or MEM-E supplemented with 300 U/mL penicillin, 300 µg/mL streptomycin, 0.5 mg/mL amphotericin and 10% FBS and cultured at 37 °C in a CO2 incubator (5% CO2).
The cell cultures were observed in an inverted microscope every day, until proliferation of the first cells from the explants had occurred. The media was changed after 5 days to avoid any mechanical stress, and thereafter, it was replenished every third day. At 80% confluence, cells were trypsinized (0.25% Trypsin-EDTA, Gibco®, Grand Island, NY, USA) and reseeded in new cell culture flasks.
Virus infectivity assay
The cells from the confluent monolayer after the third passage were infected with either the CAEV-Cork or MVV-K1514 strains, provided by Dr. Roberto Soares de Castro (Federal Rural University of Pernambuco - UFRPE). These strains originated from the Institut National de la Recherche Agronomique, Lyon-France. The virus infectivity assay was performed in duplicate for each of three separate experiments. The cells were incubated with viral suspension 105 TCID50 at a MOI of 1 for 1 h at 37 °C in a 5% CO2 incubator. Thereafter, the supernatant was discarded and the growth medium was added (DMEM supplemented with 300 U/mL penicillin, 300 µg/mL streptomycin, 0.5 mg/mL amphotericin and 2% FBS).
The cell monolayers were observed in an inverted microscope every day until the cytopathic effect caused by the virus was evident, proving that the cells were permissive for infection by SRLV.
FACS analysis
FACS analysis was performed to investigate the expression of the surface markers CD90, CD105, CD34, and the intracellular markers vimentin and cytokeratin in cGUCs after the third passage. The cells were harvested and aliquoted at a density of 106 cells/mL for each marker assay. First, these cells were incubated with antibodies against surface markers [mouse anti-Human CD90 FITC, CD105 APC and CD34 APC (BD Biosciences®, USA)] for 30 min at 4 °C in the dark. Then, the cells were permeabilized with fixation/permeabilization solution (BD Cytofix/Cytoperm Fixation/Permeabilization Kit, BD Biosciences®, USA) for 20 min at 4 °C in the dark. Permeabilized cells were incubated with antibodies against intracellular markers [mouse anti-Human vimentin PE and cytokeratin PE (BD Biosciences®, USA)] for 30 min at 4 °C in the dark. Thereafter, the cells were washed twice with BD perm/wash buffer (BD Biosciences®, USA), fixed with formaldehyde buffer (PBS with 1% formaldehyde) and analyzed using a flow cytometer (FACS Calibur BD, BD Biosciences®). A gate was defined and the minimum number of events constituting was set to 10,000. The negative control was processed in a similar manner but without antibodies. The data obtained was analyzed using Cell Quest Pro software and plotted as a histogram.
Adipogenic differentiation
For adipogenic differentiation, trypsinized cells (1 × 104 cells/cm2) were seeded into a 24-well plate and incubated with DMEM supplemented with 300 U/mL penicillin, 300 µg/mL streptomycin, 0.5 mg/mL amphotericin and 10% FBS. After 24 h, the medium was discarded, and the cells were incubated with StemPro® Adipogenesis Differentiation Basal Medium (Gibco®, Grand Island, NY, USA). The differentiation medium was replaced every 3-4 days. After 14 days of culture, the cells were fixed with 10% formalin at room temperature, washed with PBS, and stained with 0.5% Oil-Red O (Sigma-Aldrich®, St. Louis, MO, USA) for 15 min and Mayer's Hematoxylin (Sigma-Aldrich®, St. Louis, MO, USA) for 5 min to visualize lipid droplets.
Chondrogenic differentiation
Chondrogenic differentiation was induced in micromass cultures by seeding 5 µL droplets of cell solution (1.6 × 107 viable cells/mL) in the center of a 24-well plate. The cells were incubated with DMEM supplemented with 300 U/mL penicillin, 300 µg/mL streptomycin, 0.5 mg/mL amphotericin and 10% FBS for 2 h. Afterward, the cells were fed with a specific chondrogenesis differentiation medium (StemPro®, Gibco®, Grand Island, NY, USA). The medium was replaced every 2-3 days. After 14 days, chondrogenic differentiation was confirmed by staining for Alcian Blue (Sigma-Aldrich®, St. Louis, MO, USA).
Osteogenic differentiation
To induce osteogenic differentiation, the cells from the confluent monolayer of the third passage (5 × 103 cells/cm2) were seeded into a 24-well plate and incubated with DMEM supplemented with 300 U/mL penicillin, 300 µg/mL streptomycin, 0.5 mg/mL amphotericin and 10% FBS. After 24 h, the medium was discarded, and cells were cultured in StemPro® Osteocyte Differentiation Basal Medium (StemPro®, Gibco®, Grand Island, NY, USA). The medium was replaced every 2-3 days. After 14 days of culture, the osteogenic differentiation of stem cells was confirmed by Alizarin Red S staining (Sigma-Aldrich®, St. Louis, MO, USA).
Results
Growth and culture characteristics
Goat umbilical cord cells started to migrate out of explants after 3-6 days of culture, and the cells gradually grew into small colonies. In the samples transported in DMEM and MEM-E, cell proliferation started 3 days after beginning the incubation, whereas samples transported in PBS started proliferating after 5 days and the samples packed in SS after 6 days. These data demonstrated that DMEM and MEM-E enable better preservation of the samples and, consequently, enable better acquisition of cells.
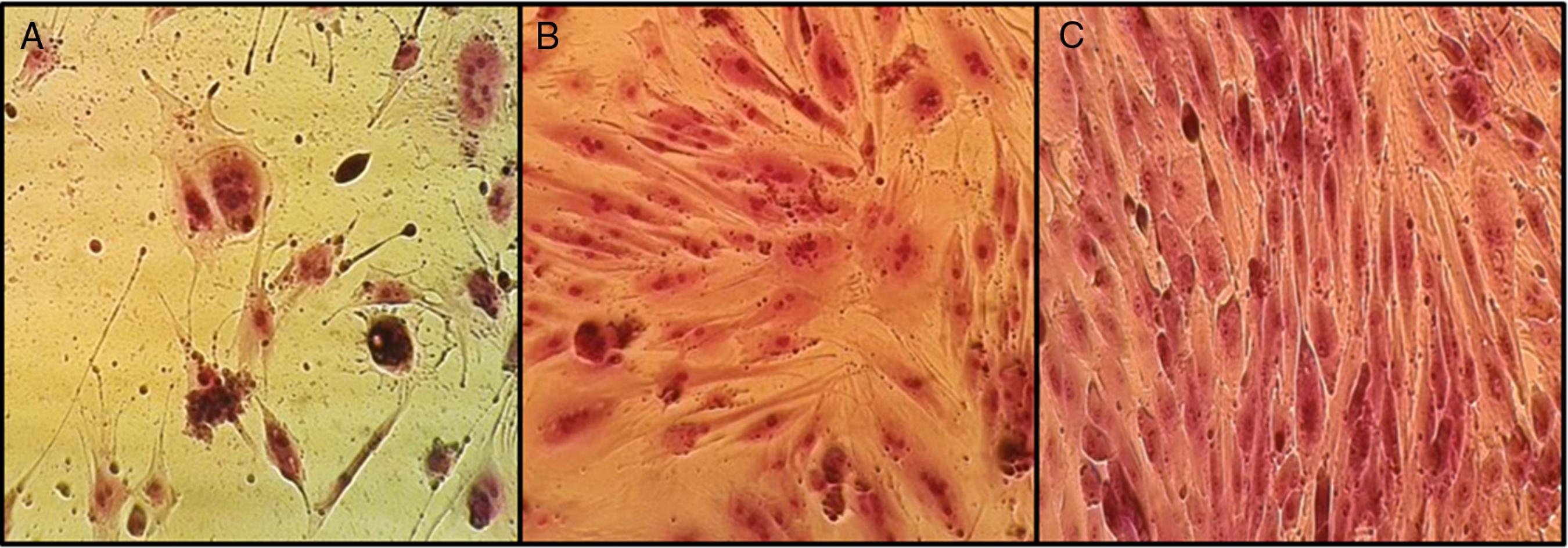
During the initial days of incubation, cells displayed a non-fibroblastic, rounded, epithelial cell-like phenotype (Fig. 1A). Later, cells attained a typical fibroblast-like spindle-shaped structure (Fig. 1B). As growth continued, adjacent colonies interconnected with each other, and a confluent monolayer was obtained (Fig. 1C). Cell monolayers derived from goat umbilical cords were used in viral challenge assays, for immunotyping by flow cytometry, and in mesodermal lineage differentiation assays.
Primary culture of cGUCs: morphological features and cell monolayers formed. (A) Epithelial cell-like phenotype. (B) Typical fibroblastoid shape. (C) cGUCs exhibiting a large, flattened fibroblast-like morphology after the third passage.
The growth media used in this study (DMEM or MEM-E supplemented with 10% FBS) were highly suitable to sustaining the cells, resulting in high rates of growth and expansion of the cell stock. After 15 days, cells had grown to 80% confluence, at which time the first passage was performed. In later passages, cultured cells reached 80-90% confluence faster, in approximately 3-4 days.
Cytopathicity of SRLV in cGUCs
The cGUCs are highly permissive to SRLV infection in vitro. The cytopathic effects appeared 7 days after inoculation. Classical giant multinucleated cells (syncytia) were observed in the monolayers infected with CAEV-Cork virus (Fig. 2B). The cells infected with MVV-K1514 virus underwent syncytia formation and cell lysis. The cytopathic effects progressed, resulting in the progressive destruction of the cell monolayer (Fig. 2A). The negative control for the viral infectivity assay is shown in Fig. 2C.
Cytopathic effects caused by MVV and CAEV. (A) Cell lysis and syncytia caused by MVV. (B) Giant multinucleated cells caused by CAEV. (C) Negative control for the viral infectivity assay.
Immunophenotypic characterization by flow cytometry
In vitro cultured, third-passage cGUCs were analyzed by FACS. FACS analysis revealed that these cells were positive for the expression of vimentin (91.6%) and CD90 (23%) but were negative when staining for CD105 (3.66%), CD34 (2.95%) and cytokeratin (1.67%) (Fig. 3).
FACS analysis of Vimentin and CD90, in vitro culture, third passage cGUCs. (A) Vimentin PE. (B) CD90 FITC. Calibrated histogram representing the number of events on the Y-axis and fluorescent intensity on the X-axis. The red area histogram indicate negative control.
Mesodermal lineage differentiation
Adipogenic differentiation of cGUCs was confirmed by Oil Red-O staining. After incubating these cells with adipogenic-inducing medium for 14 days, small lipid droplets were present in the cytoplasm (Fig. 4A).
Mesodermal lineage differentiation. Adipogenic differentiation (A). Chondrogenic differentiation (B). Osteogenic differentiation (C).
The chondrogenic potential of cGUCs was evaluated by in vitro micromass culture of these cells in a specific differentiation medium. After 14 days of incubation, the accumulation of sulfated proteoglycans was visualized by Alcian Blue staining (Fig. 4B).
In the osteogenic differentiation assay, the cells proliferated and reached complete confluence after 8-10 days of incubation with differentiation medium. The cellular aggregates were then observed and were characterized by calcium deposits, which were demonstrated by positive Alizarin Red staining (Fig. 4C).
Discussion
The umbilical cord is composed of several different components (amniotic membrane, umbilical cord matrix, umbilical cord vein, and umbilical cord blood) that can be used as sources of MSCs.66 Lee KS, Nah JJ, Lee BC, et al. Maintenance and characterization of multipotent mesenchymal stem cells isolated from canine umbilical cord matrix by collagenase digestion. Res Vet Sci. 2013;94:144-151. In this study, cGUCs were isolated from the umbilical cord matrix and were cultured in DMEM or MEM supplemented with 10% FBS. These conditions were found to be very well suited for the maintenance of the cultured cells, and contributed to quick amplification of the cell stock. Several culture media have been tested for the maintenance of MSCs, such as MEM, DMEM, RPMI-1640 and Basal Medium Eagle (BME), supplemented with FBS, generally at a concentration of 10-20%. The choice of the culture medium is important for the successful growth of MSCs during in vitro cell culture.1313 Tapp H, Hanley EN, Patt JC, Gruber HE. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med. 2009;234:1-9.
During the initial days of incubation, the population of cells was heterogeneous, including both epithelial-like and fibroblast-like cells. However, after passaging, the epithelioid cell population disappeared from the culture and most of the cells exhibited a fibroblast-like appearance. These data are consistent with the observations of Pratheesh et al. 1414 Pratheesh MD, Gade NE, Katiyar AN, et al. Isolation, culture and characterization of caprine mesenchymal stem cells derived from amniotic fluid. Res Vet Sci. 2013;94:313-319. who observed similar morphological features in caprine mesenchymal stem cells derived from amniotic fluid (cAF-MSCs) and also with Ren et al.1515 Ren Y, Wu H, Zhou X, et al. Isolation, expansion, and differentiation of goat adipose-derived stem cells. Res Vet Sci. 2012;93:404-411. in their study with goat adipose-derived stem cells (ADSCs). In addition, the fibroblast-like cells derived from goat umbilical cord matrix are similar to MSCs in their general features.
The aim of the present study was to investigate the capacity of cells from goat umbilical cord matrix to generate cell monolayers that could be used for viral diagnosis in cell culture assays. It was demonstrated that these cells are highly permissive to SRLV infection in vitro. Syncytia and cell lysis were observed after 7 days of inoculation with the CAEV-Cork strain, and similar results were observed with the MVV-K1514 strain. These results resemble the effects observed after infection of synovial membrane cells, which are the cells traditionally used for this purpose.
High amounts of small ruminant lentivirus can be produced in vitro using fibro-epithelial synovial membrane cells or choroid plexus cells from goats or sheep.1616 Sigurdsson B, Thormar H, Pálsson P. Cultivation of visna virus in tissue culture. Archiv für die gesamte Virusforschung. 1960;10:368-381.,1717 Crawford TB, Adams S, Sande RD, Gorhan JR, Henson JB. The connective tissue component of the caprine arthritis - encephalitis syndrome. Am J Pathol. 1980;100:443-454. However, the disadvantage is that these types of cells can only be obtained from embryos or fetuses, whereas goat umbilical cords offer a great alternative for the generation of fibroblasts because the umbilical cord would be discarded after calving and thus represents a highly convenient source material for generation of cells.
Other cell types permissive for small ruminant lentivirus infection are microglia1818 Adebayo IA, Olaleye OD, Awoniyi TA. Affinity (tropism) of caprine arthritis encephalitis virus for brain cells. Afr J Med Med Sci. 2008;39:89-93.; dendritic cells22 Ryan S, Tiley L, McConnell I, Blacklaws B. Infection of dendritic cells by the Maedi-Visna lentivirus. J Virol. 2000;74:10096-10103.; epithelial cells from the lung1919 Carrozza ML, Mazzei M, Bandecchi P, Arispici M, Tolari F. In situ PCR-associated immunohistochemistry identifies cell types harbouring the Maedi-Visna virus genome in tissue sections of sheep infected naturally. J Virol Methods. 2003;107:121-127.; mammary gland2020 Bolea R, Monleon E, Carrasco L, et al. Maedi-Visna virus infection of ovine mammary epithelial cells. Vet Res. 2006;37:133-144.; third eye lid2121 Capucchio MT, Sanna E, Sanna MP, Farigu S, Minelli R, Guarda F. Maedi-Visna virus detection in ovine third eyelids. J Comp Pathol. 2003;129:37-43.; kidney2222 Angelopoulou K, Brellou GD, Vlemmas I. Detection of Maedi-Visna virus in the kidneys of naturally infected sheep. J Comp Pathol. 2006;134:329-335.; uterus and epididymis2323 Ali Al Ahmad MZ, Dubreil L, Chatagnon G, et al. Goat uterine epithelial cells are susceptible to infection with caprine arthritis encephalitis virus (CAEV) in vivo. Vet Res. 2012;43:5.,2424 Lamara A, Fieni F, Chatagnon G, Larrat M, Dubreil L, Chebloune Y. Caprine arthritis encephalitis virus (CAEV) replicates productively in cultured epididymal cells from goats. Comp Immunol Microbiol Infect Dis. 2013;36:397-404. and endothelium; smooth myocytes1919 Carrozza ML, Mazzei M, Bandecchi P, Arispici M, Tolari F. In situ PCR-associated immunohistochemistry identifies cell types harbouring the Maedi-Visna virus genome in tissue sections of sheep infected naturally. J Virol Methods. 2003;107:121-127.,2525 Leroux C, Cordier G, Mercier I, et al. Ovine aortic smooth muscle cells allow the replication of Visna-Maedi virus in vitro. Arch Virol. 1995;140:1-11.; granulosa cells2626 Lamara A, Fieni F, Mselli-Lakhal L, Tainturier D, Chebloune Y. Efficient replication of caprine arthritis-encephalitis virus in goat granulosa cells. Virus Res. 2001;79:165-172.; and parenchyma cells from the liver and heart.2727 Brellou GD, Angelopoulou K, Poutahidis T, Vlemmas I. Detection of Maedi-Visna Virus in the liver and heart of naturally infected sheep. J Comp Pathol. 2007;136:27-35.
The phenotypic characterization of cGUCs was performed by flow cytometry. The general strategy for identifying in vitro cultivated mesenchymal stem cells was executed as per the ISCT recommendation (International Society for Cytotherapy), which is to analyze the expression of cell-surface markers such as CD-73, CD-44, CD-90 and CD-105.2828 Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;4:315-317.
29 Donzelli E, Salvade‘ A, Mimo P, et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch Oral Biol. 2007;52:64-73.
30 Yu Y, Yao AH, Chen N, et al. Mesenchymal stem cells over-expressing hepatocyte growth factor improve small-for-size liver grafts regeneration. Mol Ther. 2007;15:1382-1389.-3131 De Macedo Braga LM, Lacchini S, Schaan BD, et al. In situ delivery of bone marrow cells and mesenchymal stem cells improves cardiovascular function in hypertensive rats submitted to myocardial infarction. J Biomed Sci. 2008;15:365-374. It was demonstrated that the cGUCs are positive for vimentin and CD90, whereas they were negative for cytokeratin, CD105 and CD34. These results can be compared with those of other studies. Ren et al. 1515 Ren Y, Wu H, Zhou X, et al. Isolation, expansion, and differentiation of goat adipose-derived stem cells. Res Vet Sci. 2012;93:404-411. described MSCs from goat adipose that stained positively for vimentin, CD49d and CD13 and negatively for CD34 and CD106. Caprine mesenchymal stem cells derived from amniotic fluid were positive for CD90, CD105, CD73, and negative for CD34.1414 Pratheesh MD, Gade NE, Katiyar AN, et al. Isolation, culture and characterization of caprine mesenchymal stem cells derived from amniotic fluid. Res Vet Sci. 2013;94:313-319.
Vimentin is present in normal and pathological mesenchymal tissues and is an important marker of the mesoderm. Positive vimentin staining verified that the cGUCs were derived from mesodermal stem cells.3232 Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402-1416. Cytokeratin-negative staining demonstrated the predominance of fibroblast-like cells and is consistent with the observation that the epithelial-like cells disappeared from culture and could no longer be found from third passage onward. Additionally, most of the cells exhibited a fibroblast-like appearance. CD34 is a surface marker of hematopoietic stem cells (HSCs) and is expressed in lymph nodes, bone marrow HSCs, and various endothelial cells. CD34-negative staining demonstrated that cGUCs were not derived from circulating stem cells.99 Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147.,3333 Peng H, Huard J. Muscle-derived stem cells for musculoskeletal tissue regeneration and repair. Transpl Immunol. 2004;12:311-319.
The cGUCs from umbilical cord matrix are negative for CD105. This marker is usually expressed in mesenchymal stem cells; however, there is not yet a definitive marker for MSCs. There are a large number of positive markers described, but each group of investigators uses different markers, none of which are specific or used singly. Perhaps the differences between studies may be attributed to variations in culture methods or the stage of cell differentiation.3434 Bydlowski SP, Debes AA, Maselli LMF, Janz FLL. Características biológicas das células-tronco mesenquimais. Rev Bras Hematol Hemoter. 2009;31:25-35.
In this study, FACS analysis revealed low levels of CD90 marker expression (23%) in comparison to Vimentin (91.6%). CD90 is known to be a negative regulator of hematopoietic proliferation.3535 Mayani H, Lansdrop PM. Thy-1 expression is linked to functional properties of primitive hematopoietic progenitor cells from human umbilical cord blood. Blood. 1994;83:2410-2417. Its expression, in association with CD34-negative staining, confirms that there was no evidence of hematopoietic precursors in this culture. The expression levels of surface markers are also different in other studies. Chang et al.3636 Chang Y-J, Tseng C-P, Hsu L-F, Hsieh T-B, Hwang S-M. Characterization of two populations of mesenchymal progenitor cells in umbilical cord blood. Cell Biol Int. 2006;30:495-499. isolated cells from umbilical cord blood (UCB) and observed the following two different morphologic phenotypes: flattened fibroblastic clones and spindle-shaped fibroblastic clones. CD90 was expressed differently by these two cell populations. Spindle-shaped, clonogenic MPCs expressed a high level of CD90, while flattened, clonogenic MSCs did not express CD90. These data might explain the inconsistent results regarding CD90 expression in UCB-derived MSCs in various reports.3737 Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242.
38 Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7:581-588.-3939 Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625-634.
The differentiation potential of cGUCs was investigated. These cells showed a capacity to differentiate into adipocytes, chondrocytes or osteoblasts when incubated with the appropriate differentiation medium. Pratheesh et al.1414 Pratheesh MD, Gade NE, Katiyar AN, et al. Isolation, culture and characterization of caprine mesenchymal stem cells derived from amniotic fluid. Res Vet Sci. 2013;94:313-319. demonstrated that MSCs from goat amniotic fluid, such as their marrow counterparts, were able to differentiate into adipose cells in addition to differentiating into osteogenic and chondrogenic lineages. Goat adipose-derived stem cells showed the same differentiation potential.1515 Ren Y, Wu H, Zhou X, et al. Isolation, expansion, and differentiation of goat adipose-derived stem cells. Res Vet Sci. 2012;93:404-411. Goat-derived multipotent MSCs have been established from bone marrow and successfully induced to differentiate into osteogenic and adipogenic lineages under specific culture conditions.4040 Zhang Y, Fan Y, Wang Z, et al. Isolation, characterization, and gene modification of dairy goat mesenchymal stem cells from bone marrow. In Vitro Cell Dev Biol Anim. 2012;48:418-425.
The present study establishes that goat umbilical cord cells are permissive for small ruminant lentivirus infection in vitro, can be successfully used in cell culture diagnosis, and represent a new alternative source of host cells for virus isolation. These cells exhibit a fibroblast morphology, and immunophenotype, growth characteristics and mesodermal lineage differentiation potential that are all similar to MSCs. These cGUCs also express vimentin and CD90 but are negative for CD34. These data demonstrate the proliferative capacity of cells from goat umbilical cords, and they establish a sound basis for future research aimed at standardizing the cell lineage and using these cells for the diagnosis of several diseases.
Acknowledgements
This work was supported by the National Council of Scientific and Technological Development (CNPq), grant numbers 487425/2012-0 and 308995/2013-9, and by Personnel Improvement Coordination higher level (CAPES), which provided a PhD scholarship. The authors are thankful to the Post Graduate Program in Veterinary Science (PPGCV) of the State University of Ceará for supporting the implementation of the experiments; to the Federal University of Ceará, particularly to Lilia Maria Carneiro Câmara, PhD, for unconditional support in performing the FACS analysis; and to Marcos Fábio Gadelha Rocha, PhD, and Júlio Sidrim, PhD (Post Graduate Medical Microbiology - UFC). The authors also express their gratitude to owners of the farms that allowed the use of their animals for this study.
References
-
1Narayan O, Clements JE. Biology and pathogenesis of lentiviruses. J Gen Virol 1989;70:1617-1639.
-
2Ryan S, Tiley L, McConnell I, Blacklaws B. Infection of dendritic cells by the Maedi-Visna lentivirus. J Virol 2000;74:10096-10103.
-
3Chebloune Y, Sheffer D, Karr BM, Stephens E, Narayan O. Restrictive type of replication of ovine/caprine lentiviruses in ovine fibroblast cell cultures. Virology 1996;222:21-30.
-
4Sihvonen L, Veijalainen P. Kinetics of Maedi virus production in sheep choroid plexus cells. Vet Microbiol 1981;6:1-8.
-
5Teixeira MFS, Veronique L, Msebli-Lakahl L, Chettab A, Chebloune Y, Mornex JF. Imortalization of caprine fibroblasts permissive for replication of small ruminant lentiviroses. Am J Vet Res 1997;58:579-584.
-
6Lee KS, Nah JJ, Lee BC, et al. Maintenance and characterization of multipotent mesenchymal stem cells isolated from canine umbilical cord matrix by collagenase digestion. Res Vet Sci. 2013;94:144-151.
-
7Reed SA, Johnson SE. Refinement of culture conditions for maintenance of undifferentiated equine umbilical cord blood stem cells. J Equine Vet Sci. 2012;32:360-366.
-
8Fadel L, Viana BR, Feitosa MLT, et al. Protocols for obtainment and isolation of two mesenchymal stem cell sources in sheep. Acta Cir Brasil. 2011;26:267-273.
-
9Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-147.
-
10Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294-1301.
-
11Rebelatto CK, Aguiar AM, Moretão MP, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233:901-913.
-
12Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 2010;31:595-596.
-
13Tapp H, Hanley EN, Patt JC, Gruber HE. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med 2009;234:1-9.
-
14Pratheesh MD, Gade NE, Katiyar AN, et al. Isolation, culture and characterization of caprine mesenchymal stem cells derived from amniotic fluid. Res Vet Sci 2013;94:313-319.
-
15Ren Y, Wu H, Zhou X, et al. Isolation, expansion, and differentiation of goat adipose-derived stem cells. Res Vet Sci. 2012;93:404-411.
-
16Sigurdsson B, Thormar H, Pálsson P. Cultivation of visna virus in tissue culture. Archiv für die gesamte Virusforschung 1960;10:368-381.
-
17Crawford TB, Adams S, Sande RD, Gorhan JR, Henson JB. The connective tissue component of the caprine arthritis - encephalitis syndrome. Am J Pathol. 1980;100:443-454.
-
18Adebayo IA, Olaleye OD, Awoniyi TA. Affinity (tropism) of caprine arthritis encephalitis virus for brain cells. Afr J Med Med Sci 2008;39:89-93.
-
19Carrozza ML, Mazzei M, Bandecchi P, Arispici M, Tolari F. In situ PCR-associated immunohistochemistry identifies cell types harbouring the Maedi-Visna virus genome in tissue sections of sheep infected naturally. J Virol Methods. 2003;107:121-127.
-
20Bolea R, Monleon E, Carrasco L, et al. Maedi-Visna virus infection of ovine mammary epithelial cells. Vet Res 2006;37:133-144.
-
21Capucchio MT, Sanna E, Sanna MP, Farigu S, Minelli R, Guarda F. Maedi-Visna virus detection in ovine third eyelids. J Comp Pathol 2003;129:37-43.
-
22Angelopoulou K, Brellou GD, Vlemmas I. Detection of Maedi-Visna virus in the kidneys of naturally infected sheep. J Comp Pathol 2006;134:329-335.
-
23Ali Al Ahmad MZ, Dubreil L, Chatagnon G, et al. Goat uterine epithelial cells are susceptible to infection with caprine arthritis encephalitis virus (CAEV) in vivo. Vet Res. 2012;43:5.
-
24Lamara A, Fieni F, Chatagnon G, Larrat M, Dubreil L, Chebloune Y. Caprine arthritis encephalitis virus (CAEV) replicates productively in cultured epididymal cells from goats. Comp Immunol Microbiol Infect Dis 2013;36:397-404.
-
25Leroux C, Cordier G, Mercier I, et al. Ovine aortic smooth muscle cells allow the replication of Visna-Maedi virus in vitro. Arch Virol 1995;140:1-11.
-
26Lamara A, Fieni F, Mselli-Lakhal L, Tainturier D, Chebloune Y. Efficient replication of caprine arthritis-encephalitis virus in goat granulosa cells. Virus Res. 2001;79:165-172.
-
27Brellou GD, Angelopoulou K, Poutahidis T, Vlemmas I. Detection of Maedi-Visna Virus in the liver and heart of naturally infected sheep. J Comp Pathol. 2007;136:27-35.
-
28Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006;4:315-317.
-
29Donzelli E, Salvade‘ A, Mimo P, et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch Oral Biol. 2007;52:64-73.
-
30Yu Y, Yao AH, Chen N, et al. Mesenchymal stem cells over-expressing hepatocyte growth factor improve small-for-size liver grafts regeneration. Mol Ther 2007;15:1382-1389.
-
31De Macedo Braga LM, Lacchini S, Schaan BD, et al. In situ delivery of bone marrow cells and mesenchymal stem cells improves cardiovascular function in hypertensive rats submitted to myocardial infarction. J Biomed Sci 2008;15:365-374.
-
32Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005;33:1402-1416.
-
33Peng H, Huard J. Muscle-derived stem cells for musculoskeletal tissue regeneration and repair. Transpl Immunol 2004;12:311-319.
-
34Bydlowski SP, Debes AA, Maselli LMF, Janz FLL. Características biológicas das células-tronco mesenquimais. Rev Bras Hematol Hemoter. 2009;31:25-35.
-
35Mayani H, Lansdrop PM. Thy-1 expression is linked to functional properties of primitive hematopoietic progenitor cells from human umbilical cord blood. Blood 1994;83:2410-2417.
-
36Chang Y-J, Tseng C-P, Hsu L-F, Hsieh T-B, Hwang S-M. Characterization of two populations of mesenchymal progenitor cells in umbilical cord blood. Cell Biol Int 2006;30:495-499.
-
37Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 2000;109:235-242.
-
38Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant 2001;7:581-588.
-
39Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004;22:625-634.
-
40Zhang Y, Fan Y, Wang Z, et al. Isolation, characterization, and gene modification of dairy goat mesenchymal stem cells from bone marrow. In Vitro Cell Dev Biol Anim 2012;48:418-425.
Publication Dates
-
Publication in this collection
Jan-Mar 2017
History
-
Received
6 Jan 2016 -
Accepted
16 Aug 2016