ABSTRACT
Early diagnosis of tuberculosis is of major clinical importance. Among 4733 clinical specimens collected from 3363 patients and subjected to Ziehl-Neelsen microscopy, 4109 were inoculated onto Löwenstein-Jensen slants and 3139 in Bactec/9000MB. Polymerase Chain Reaction (PCR) was performed in 3139 specimens, whereas, a genotypic assay was directly applied in 93 Mycobacterium tuberculosis complex PCR-positive for isoniazid and rifampicin resistance detection specimens (GenoType MTBDRplus). Recovered M. tuberculosis isolates (64) as well as, 21 more sent from Regional Hospitals were tested for antimycobacterial resistance with a phenotypic (manual MGIT-SIRE) and a genotypic assay (GenoType MTBDRplus). PCR in the clinical specimens showed excellent specificity (97.4%) and accuracy (96.8%), good sensitivity (70.4%), but low positive predictive value (40.3%). MGIT-SIRE performed to M. tuberculosis did not confer a reliable result in 16 isolates. Of the remaining 69 isolates, 15 were resistant to streptomycin, seven to isoniazid, seven to ethambutol and five to rifampicin. GenoType MTBDRplus correctly detected isoniazid (seven) and rifampicin-resistant M. tuberculosis strains (five), showing an excellent performance overall (100%). Susceptibility results by the molecular assay applied directly to clinical specimens were identical to those obtained from recovered isolates of the corresponding patients. Combining molecular and conventional methods greatly contribute to early diagnosis and accurate susceptibility testing of tuberculosis.
Keywords:
Mycobacterium tuberculosis; PCR; GenoType MTBDRplus
Introduction
Despite the declining incidence of tuberculosis in Europe, it still remains an important health issue.11 European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2014. Sweden: Stockholm; 2014.,22 Papaventsis D, Nikolaou S, Karabela S, et al. Tuberculosis in Greece: bacteriologically confirmed cases and anti-tuberculosis drug resistance, 1995-2009. Euro Surveill. 2010;15. The notification rate of tuberculosis steadily decreases in Greece during the last decade, however, there are certain concerns since the incidence is significantly high mainly due to underreporting.11 European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2014. Sweden: Stockholm; 2014.
2 Papaventsis D, Nikolaou S, Karabela S, et al. Tuberculosis in Greece: bacteriologically confirmed cases and anti-tuberculosis drug resistance, 1995-2009. Euro Surveill. 2010;15.-33 Jelastopulu E, Alexopoulos EC, Venieri D, et al. Substantial underreporting of tuberculosis in West Greece: implications for local and national surveillance. Euro Surveill. 2009;14:.
The prompt diagnosis of patients with active disease is challenging, even in developed countries with sufficient resources.44 Smith A, Miller RF, Story A, Booth HL. A&E department: a missed opportunity for diagnosis of TB?. Thorax. 2006;61:364-365. Tuberculosis diagnosis is mainly based on culture-based techniques characterized by a long turn-around time.55 Drobniewski F, Nikolayevskyy V, Maxeiner H, et al. Rapid diagnostics of tuberculosis and drug resistance in the industrialized world: clinical and public health benefits and barriers to implementation. BMC Med. 2013;11:190. Today, in order to reduce the time of diagnosis, molecular assays combined with conventional methods are used, either directly in clinical specimens or in isolates recovered from cultures. Therefore, since acid-fast bacilli smears lack sensitivity and Mycobacterium tuberculosis (MTB) treatment is accompanied by complications, PCR based methods have been used for its identification directly in clinical specimens in order to rapidly aid clinicians.55 Drobniewski F, Nikolayevskyy V, Maxeiner H, et al. Rapid diagnostics of tuberculosis and drug resistance in the industrialized world: clinical and public health benefits and barriers to implementation. BMC Med. 2013;11:190.,66 Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2013;167:603-662. Nowadays, clinical specimens originating from patients with suspicion of tuberculosis must be subjected to smear microscopy, culture, and MTB-PCR assays.55 Drobniewski F, Nikolayevskyy V, Maxeiner H, et al. Rapid diagnostics of tuberculosis and drug resistance in the industrialized world: clinical and public health benefits and barriers to implementation. BMC Med. 2013;11:190. The delay in microbiologic confirmation of the disease combined with underreporting may provoke a delay in public health measures to identify and cure infected patients and households.
Another issue of increasing importance worldwide is the resistance of M. tuberculosis to first-line drugs.11 European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2014. Sweden: Stockholm; 2014.,22 Papaventsis D, Nikolaou S, Karabela S, et al. Tuberculosis in Greece: bacteriologically confirmed cases and anti-tuberculosis drug resistance, 1995-2009. Euro Surveill. 2010;15. This mainly arises from non-adherence to antimycobacterial treatment regimens, or the migration flow from regions with high rates of multi-drug resistant tuberculosis (MDR-TB).11 European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2014. Sweden: Stockholm; 2014.,22 Papaventsis D, Nikolaou S, Karabela S, et al. Tuberculosis in Greece: bacteriologically confirmed cases and anti-tuberculosis drug resistance, 1995-2009. Euro Surveill. 2010;15.,77 Rovina N, Karabela S, Constantoulakis P, et al. MIRU-VNTR typing of drug-resistant tuberculosis isolates in Greece. Ther Adv Respir Dis. 2011;5:229-236. Strains exhibiting resistance to both isoniazid (INH) and rifampicin (RMP) are characterized as MDR-TB.11 European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2014. Sweden: Stockholm; 2014. Identification of resistance to first-line drugs is important to be posed as soon as possible since infection by such strains needs prolonged treatment and displays lower cure rates.66 Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2013;167:603-662. Nowadays, in addition to traditional methodology, DNA strip assays that can reliably detect the most common mutations associated with rpoB (RMP resistance) and katG/inhA (INH resistance) genes, such as GenoType MTBDRplus, can be applied in the Diagnostic Laboratory either at the DNA extracts of isolated strains, or directly to the clinical specimen.55 Drobniewski F, Nikolayevskyy V, Maxeiner H, et al. Rapid diagnostics of tuberculosis and drug resistance in the industrialized world: clinical and public health benefits and barriers to implementation. BMC Med. 2013;11:190.,88 Bwanga F, Hoffner S, Haile M, Joloba ML. Direct susceptibility testing for multi drug resistant tuberculosis: a meta-analysis. BMC Infect Dis. 2009;9:67.
The aim of the present study was to evaluate the combination of molecular assays and conventional methods for the diagnosis of tuberculosis and characterization of antimicrobial resistance among M. tuberculosis isolates.
Materials and methods
Study population/sample collection
A total of 4733 clinical specimens were collected from 3363 patients attending the University General Hospital of Patras (UGHP) and the Regional General Hospital of Pirgos (RGHP), both located in Southwestern Greece, during a three-year period (January 2009 to December 2011). From many patients more than one sample was collected based on clinical grounds. The study was approved by the Ethics Committee of the UGHP.
The clinical specimens included: sputa (n = 1233), sputa after bronchoscopy (SAB, n = 360), bronchial lavages (BL, n = 1087), gastric aspirates (n = 117), urine (n = 490), pericardial (n = 19), peritoneal (n = 10), cerebrospinal (CSF, n = 346), synovial (n = 20), ascitic (n = 201) and pleural fluids (n = 688), as well as, tissue specimens (n = 162). Samples were assigned into two groups: pulmonary specimens (n = 2680) and extrapulmonary specimens (n = 2053).
Direct detection of M. tuberculosis complex in clinical specimens
Ziehl-Neelsen (ZN) staining was performed to all clinical specimens (4733). In addition, molecular methods were performed among 3139 clinical specimens, according to manufacturer's instructions. Testing was performed whenever requested by the clinicians. COBAS AMPLICOR MTB PCR (Roche Diagnostics, Rotkreuz, Switzerland) was used in 814 specimens obtained during January to September of 2009, whereas, COBAS TaqMan MTB (Roche Diagnostics) was applied afterwards in 2325 specimens, thereafter (October 2009-December 2011).
Isolation of M. tuberculosis strains
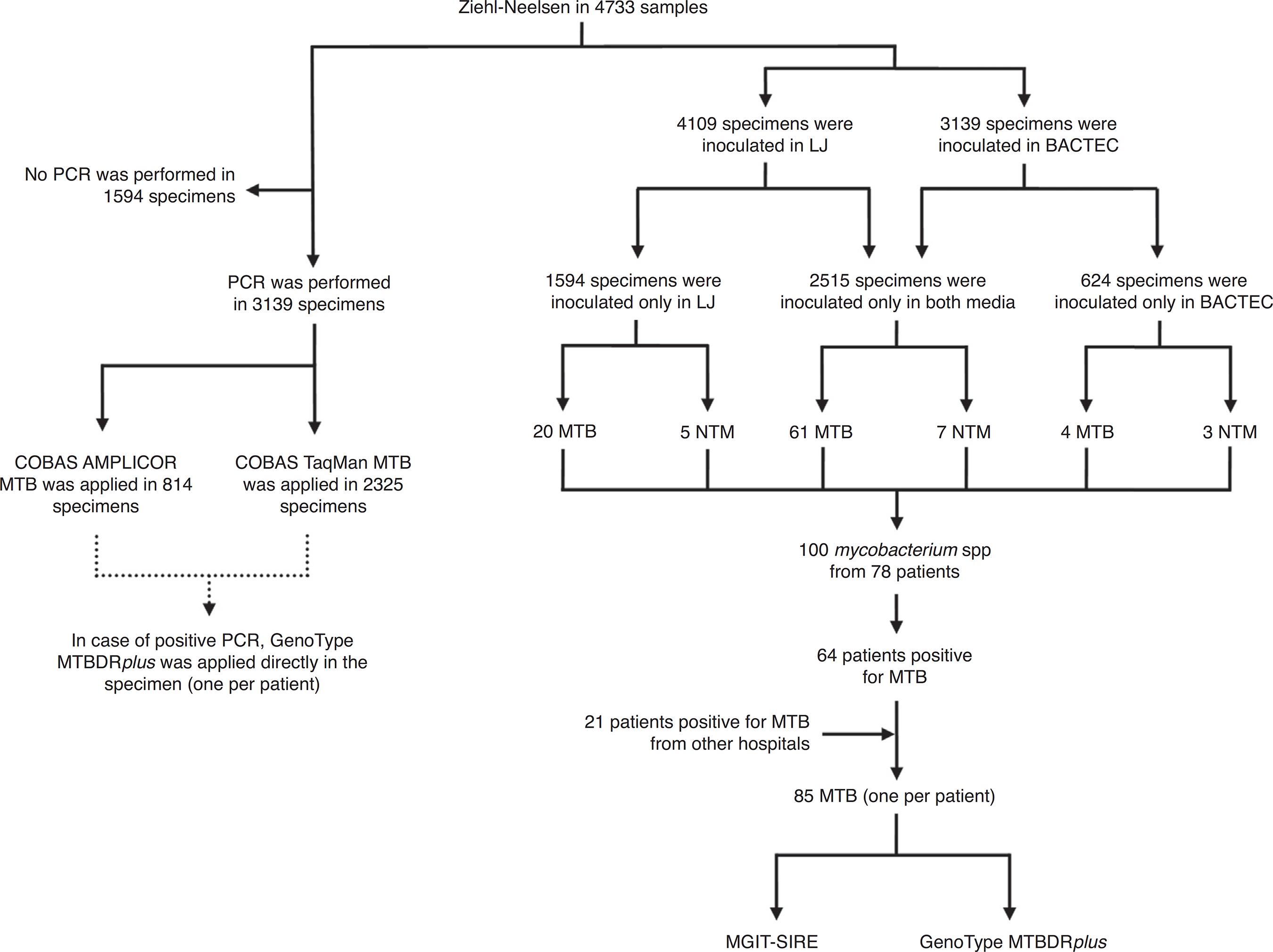
Specimens collected from non-sterile sites were concentrated and decontaminated by the N-acetyl-cystein (NALC)-NaOH method (Becton Dickinson, Le Pont-De-Claix, France). Fig. 1 shows the processing of clinical specimens. Inoculation of 4109 specimens onto Löwenstein-Jensen slants (LJ, bioMérieux, Marcy l’Etoile, France) and 3139 into Bactec/9000MB culture vials (Becton Dickinson) was carried out. Into both media, 2515 specimens were inoculated. Acid-fast bacteria recovered from solid and liquid culture media were identified to species level by a reverse line blot hybridization (RLBH) assay (GenoType MycobacteriumCM/AS; Hain Lifescience GmbH, Nehren, Germany).
Clinical specimens’ flowchart. ZN, Ziehl-Neelsen; LJ, Löwenstein-Jensen; MTB, M. tuberculosis; NTM, non-tuberculous mycobacteria; MGIT-SIRE, Mycobacteria Growth Indicator Tube for susceptibility testing.
Susceptibility testing
Molecular based method
In case of a positive PCR result for the presence of M. tuberculosis complex in the clinical specimens, GenoType MTBDRplus (Hain Lifescience GmbH) was directly applied in the DNA extract, in order to rapidly detect the presence of mutations conferring resistance to INH and RMP.
Culture based method
Sixty four MTB (one per patient) isolated from 3363 patients, and another 21 isolates obtained from patients hospitalized in other Regional Hospitals of Southwestern Greece that were sent to our Microbiology Laboratory, were tested for susceptibility to antimycobacterial agents. Susceptibility testing was performed by a phenotypic method (manual Mycobacteria Growth Indicator Tube, MGIT-SIRE; Becton Dickinson) for streptomycin, INH, RMP and ethambutol (concentrations tested: 0.1 mg/L, 1.0 mg/L, 1.0 mg/L and 5.0 mg/L, respectively). In order to identify the most common mutations conferring resistance to INH and RMP the GenoType MTBDRplus (Hain Lifescience GmbH) was applied according to the instructions of the manufacturer.
Statistical analysis
SPSS version 19.0 (SPSS, Chicago, IL) software was used for data analysis. Specificity, sensitivity, positive and negative predictive values (PPV, NPV) were calculated in order to assess the diagnostic performance of ZN and PCR, as well as, the performance of GenoType MTBDRplus to INH and RMP resistance. The gold standard comparator for M. tuberculosis detection was positivity of liquid and/or LJ cultures, whereas, for INH and RMP resistance it was the manual MGIT-SIRE testing. The accuracy of aforementioned methods was investigated using receiver operating characteristic (ROC) analysis.
Results
From a total of 4733 clinical specimens, 100 specimens collected from 78 patients (2.1%) were culture-positive for mycobacteria by either method. Ninety-three (2.3%) out of 4109 specimens inoculated onto LJ were positive, whereas, 75 out of 3139 (2.4%) were positive from Bactec vials. ZN was positive in 72 (1.5%) samples, 64 of which were pulmonary (2.4% vs 0.4%, p < 0.001). Even though ZN showed high specificity, NPV, and accuracy for pulmonary and extrapulmonary specimens, its sensitivity and PPV were low (Table 1). BL and SAB samples showed higher PPV as compared to sputa. Samples from which a "non-tuberculous mycobacteria" (NTM) were isolated did not demonstrate a positive ZN stain.
Performance of Ziehl-Neelsen in the identification of culture-positive clinical specimens for mycobacteria.
In total, 78 patients had positive culture by either media: 64 M. tuberculosis, three M. kansasii, three M. fortuitum, two M. simiae and one of each M. avium, M. malmoense and M. gordonae. It was not possible to identify strains to species level from three patients, thus, they were classified as NTM (non-tuberculous mycobacteria).
From the 3139 clinical specimens tested by culture and PCR (COBAS AMPLICOR MTB PCR or COBAS TaqMan MTB), results were valid in 2958 specimens. Of these, 71 specimens (2.4%) were culture-positive and 124 (4.2%) PCR-positive for M. tuberculosis complex (Table 2). On the other hand, invalid results were found in 181 specimens (5.8%) (negative PCR result for the internal control), of which 125 were pulmonary (125/2122, 5.9%) and 56 extrapulmonary (56/1017, 5.5%). PCR showed high specificity, NPV, and accuracy, good sensitivity and poor PPV, especially for extrapulmonary fluids (gastric aspirates, urine, pericardial, peritoneal, CSF, synovial, ascitic, and pleural), as shown in Table 2. No statistically significant difference in the performance of COBAS AMPLICOR MTB PCR as compared to COBAS TaqMan MTB was observed. From the 85 total M. tuberculosis (64 being ours plus 21 from other sites, Fig. 1), phenotypic susceptibility testing by manual MGIT-SIRE did not confer a reliable result in 16 isolates (18.8%), due to medium contamination by other bacteria. From the remaining 69 isolates, 15 (21.7%) were resistant to streptomycin, seven (10.1%) to INH, seven (10.1%) to ethambutol and five (7.2%) to RMP.
Performance of PCR in the identification of culture-positive clinical specimens for M. tuberculosis (excluding 181 samples for which PCR result was invalid).
The GenoType MTBDRplus method applied to all 85 isolates correlated very well with the conventional susceptibility method. Based on the mutations found, seven isolates were identified as resistant to INH (8.2%) and five to RMP (5.9%). The most common mutation patterns identified by this method are depicted in Table 3. Only one multi-drug resistant isolate was detected by phenotypic and/or genotypic methodologies. Three isolates (one MDR and two INH-resistant) exhibited a subpopulation of susceptible cells (additional hybridization with the wild type probe) originating from previously treated patients. The performance of GenoType MTBDRplus as compared to MGIT-SIRE among the 69 isolates for which both methods conferred a result, excellent (100%) sensitivity, specificity, PPV, NPV and accuracy were recorded for the identification of resistance to INH and RMP.
In addition, the application of GenoType MTBDRplus method directly to 93 PCR-positive clinical specimens from different patients (48 culture-negative specimens included), showed excellent performance for 72 samples with an OD >0.350 by COBAS AMPLICOR MTB PCR or a threshold cycle Ct ≤36 by COBAS TaqMan MTB. No differences were detected among the susceptibility result of GenoType MTBDRplus when applied directly to specimens or the isolate recovered from the same patient (45 patients).
Discussion
Despite the declining tuberculosis incidence in Europe, delay in the diagnosis by traditional culture-based methods has as a consequence serious adverse effects on treatment, and an increase of M. tuberculosis resistance rates to primary anti-tuberculous agents. Since sensitivity and PPV of ZN are poor, the application of new molecular methods is necessary for rapid, direct and reliable diagnosis of tuberculosis, as well as, identification of resistance.11 European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2014. Sweden: Stockholm; 2014.,66 Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2013;167:603-662.
PCR (COBAS AMPLICOR MTB PCR or COBAS TaqMan MTB) showed high specificity, NPV and accuracy for the detection of M. tuberculosis complex from both pulmonary and extrapumonary samples. PCR's sensitivity was higher when applied in pulmonary samples, as compared to extrapulmonary ones (71.4% vs 62.5%), probably due to the fact that the two PCR kits used in this study were designed for pulmonary samples only, as published.99 Bloemberg GV, Voit A, Ritter C, Deggim V, Bottger EC. Evaluation of Cobas TaqMan MTB for direct detection of the Mycobacterium tuberculosis complex in comparison with Cobas Amplicor MTB. J Clin Microbiol. 2013;51:2112-2117.
10 Cho WH, Won EJ, Choi HJ, et al. Comparison of AdvanSure TB/NTM PCR and COBAS TaqMan MTB PCR for detection of Mycobacterium tuberculosis complex in routine clinical practice. Ann Lab Med. 2015;35:356-361.-1111 Tortoli E, Urbano P, Marcelli F, Simonetti TM, Cirillo DM. Is real-time PCR better than conventional PCR for Mycobacterium tuberculosis complex detection in clinical samples?. J Clin Microbiol. 2012;50:2810-2813. The overall reported sensitivity of PCR differed widely in previous publications (61.3-96.1%).1010 Cho WH, Won EJ, Choi HJ, et al. Comparison of AdvanSure TB/NTM PCR and COBAS TaqMan MTB PCR for detection of Mycobacterium tuberculosis complex in routine clinical practice. Ann Lab Med. 2015;35:356-361.
11 Tortoli E, Urbano P, Marcelli F, Simonetti TM, Cirillo DM. Is real-time PCR better than conventional PCR for Mycobacterium tuberculosis complex detection in clinical samples?. J Clin Microbiol. 2012;50:2810-2813.
12 Fegou E, Jelastopulu E, Sevdali M, Anastassiou ED, Dimitracopoulos G, Spiliopoulou I. Sensitivity of the Cobas Amplicor system for detection of Mycobacterium tuberculosis in respiratory and extrapulmonary specimens. Clin Microbiol Infect. 2005;11:593-596.
13 Wang SX, Tay L. Evaluation of three nucleic acid amplification methods for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 1999;37:1932-1934.-1414 Yang YC, Lu PL, Huang SC, Jenh YS, Jou R, Chang TC. Evaluation of the Cobas TaqMan MTB test for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011;49:797-801. In our study, the overall PCR sensitivity was 70.4%, since only 72 (1.5%) specimens were smear-positive. The main advantage of PCR is speed, and, when combined with GenoType MTBDRplus assay directly in the PCR-positive specimen can minimize the time delay from specimen collection to effective treatment administration. This can also facilitate public health authorities to trace household contacts, especially in the case of resistant tuberculosis and stop further transmission.
The main drawbacks of PCR for the detection of M. tuberculosis complex are inhibition and presence of false positive results. Inhibition was observed in 5.8% of samples, none of which was culture positive. The percentage is slightly higher than previously reported (1.3-4.9%).99 Bloemberg GV, Voit A, Ritter C, Deggim V, Bottger EC. Evaluation of Cobas TaqMan MTB for direct detection of the Mycobacterium tuberculosis complex in comparison with Cobas Amplicor MTB. J Clin Microbiol. 2013;51:2112-2117.,1010 Cho WH, Won EJ, Choi HJ, et al. Comparison of AdvanSure TB/NTM PCR and COBAS TaqMan MTB PCR for detection of Mycobacterium tuberculosis complex in routine clinical practice. Ann Lab Med. 2015;35:356-361.,1212 Fegou E, Jelastopulu E, Sevdali M, Anastassiou ED, Dimitracopoulos G, Spiliopoulou I. Sensitivity of the Cobas Amplicor system for detection of Mycobacterium tuberculosis in respiratory and extrapulmonary specimens. Clin Microbiol Infect. 2005;11:593-596.,1515 Huh HJ, Kwon HJ, Ki CS, Lee NY. Comparison of the genedia MTB detection kit and the cobas TaqMan MTB assay for detection of Mycobacterium tuberculosis in respiratory specimens. J Clin Microbiol. 2015;53:1012101-1012104.,1616 Kim JH, Kim YJ, Ki CS, Kim JY, Lee NY. Evaluation of Cobas TaqMan MTB PCR for detection of Mycobacterium tuberculosis. J Clin Microbiol. 2011;49:173-176. The relatively high percentage (2.5%) of PCR false positive results constitutes another controversial fact. There are two different etiologies for such an observation. Firstly, the burden of M. tuberculosis in the sample may be too low to yield a positive culture, and secondly, PCR remains positive for at least four months due to the presence of non-viable M. tuberculosis, as is the case after initiation of therapy.1717 Cohen RA, Muzaffar S, Schwartz D, et al. Diagnosis of pulmonary tuberculosis using PCR assays on sputum collected within 24 hours of hospital admission. Am J Respir Crit Care Med. 1998;157:156-161.,1818 Panaiotov S, Amicosante M. Dynamics of the laboratory results in patients with pulmonary tuberculosis. Diagn Microbiol Infect Dis. 2010;67:327-332. The latter is true for our Institution, since it is not clear among clinicians that only smear and culture, and not PCR, should be ordered in case of follow-up to assess treatment efficacy and eradication of mycobacteria.
Combining the manual MGIT-SIRE and GenoType MTBDRplus results, resistance rates of INH (8.2%) and RMP (5.9%) are comparable to those previously reported from the Greek National Reference Laboratory for Mycobacteria.22 Papaventsis D, Nikolaou S, Karabela S, et al. Tuberculosis in Greece: bacteriologically confirmed cases and anti-tuberculosis drug resistance, 1995-2009. Euro Surveill. 2010;15. On the contrary, an extremely high rate of resistance to streptomycin (21.7%) was observed, which is similar to that previously reported from our Institution.1919 Fegou E, Jelastopulu E, Nicolaou S, et al. Comparison of the manual Mycobacteria Growth Indicator tube and the Etest with the method of proportion for susceptibility testing of Mycobacterium tuberculosis. Chemotherapy. 2006;52:174-177. A possible explanation is that MGIT-SIRE shows the highest discrepancy in identifying streptomycin resistance as compared to the performance of several other methods.2020 Banu S, Rahman SM, Khan MS, et al. Discordance across several methods for drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates in a single laboratory. J Clin Microbiol. 2014;52:156-163. In a previous study from our Institution (2001-3), an equally high rate of streptomycin resistance was identified by the method of proportion we used.1919 Fegou E, Jelastopulu E, Nicolaou S, et al. Comparison of the manual Mycobacteria Growth Indicator tube and the Etest with the method of proportion for susceptibility testing of Mycobacterium tuberculosis. Chemotherapy. 2006;52:174-177. Another plausible explanation is dissemination of resistant lineages in Southwestern Greece.
GenoType MTBDRplus showed excellent sensitivity, specificity, PPV, NPV and accuracy for the detection of INH and RMP resistance among M. tuberculosis isolates, as compared to MGIT-SIRE. This method is also useful in cases of LJ slants and Bactec vial contamination where MGIT-SIRE cannot provide a result. In our study, contamination by other bacterial species was observed in 18.8% of the total samples. As shown in a metanalysis,88 Bwanga F, Hoffner S, Haile M, Joloba ML. Direct susceptibility testing for multi drug resistant tuberculosis: a meta-analysis. BMC Infect Dis. 2009;9:67. sensitivity of GenoType MTBDRplus was high for INH (95.9%) and RMP (98.9%) resistance detection. Among the five isolates that were resistant to RMP various mutations of the rpoB gene were detected. The types of mutations were similar to those found in a previous study from Greek patients affecting most commonly codons 531 and 526.2121 Matsiota-Bernard P, Vrioni G, Marinis E. Characterization of rpoB mutations in rifampin-resistant clinical Mycobacterium tuberculosis isolates from Greece. J Clin Microbiol. 1998;36:20-23. Among the seven INH-resistant isolates, all demonstrated the same mutation in the promoter region of inhA gene (A-16G). On the contrary, a different inhA mutation (C-15T) was detected in six among 19 INH-resistant M. tuberculosis isolates from the island of Crete, Greece, while, the remaining had a mutation in katG gene leading to different prevalent clones in different geographical locations.2222 Gitti Z, Mantadakis E, Maraki S, Samonis G. GenoType(R) MTBDRplus compared with conventional drug-susceptibility testing of Mycobacterium tuberculosis in a low-resistance locale. Future Microbiol. 2011;6:357-362. The application of GenoType MTBDRplus additionally enabled the detection of a mixed population of susceptible and resistant cells to INH or RMP within the same sample. All three samples that exhibited heteroresistance originated from previously treated patients, which can explain the presence of mixed population. Apparently, the burden of the resistant population specifies the phenotypic susceptibility pattern of the isolate, which was always resistant in our study. If it was the other way around, further studies, like typing by mycobacterial interspersed repetitive unit-variable number of tandem repeats typing, should be necessary. Moreover, the excellent performance of GenoType MTBDRplus directly in the clinical specimen when the Ct ≤36 indicates that when the initial bacterial load of the sample is high enough, antimicrobial resistance for INH and RMP can be identified directly from the clinical specimen, during the early steps of laboratory diagnosis.
Despite the high accuracy of GenoType MTBDRplus in detecting M. tuberculosis resistance to INH and RMP, as well as, the contribution in identifying the occurrence of certain mutations directly from the PCR-positive specimen, this method cannot completely replace traditional phenotypic methods such as MGIT since it detects specific mutations.
Molecular methods represent a major contribution to the rapid diagnosis (COBAS AMPLICOR MTB PCR and COBAS TaqMan MTB) or susceptibility testing (GenoType MTBDRplus) of tuberculosis, since they can provide a rapid and reliable result to clinicians, while awaiting the result of traditional cultures (LJ and Bactec/9000MB) or susceptibility (MGIT-SIRE) methods. Conventional culture and susceptibility testing remain the gold standards for tuberculosis diagnosis, conferring the advantage of isolation of strains for further epidemiologic and resistance traits analyses. However, the combination of molecular and conventional methods contributes to the early tuberculosis diagnosis and drug resistance detection.
-
1
Current address: Department of Internal Medicine, Hôpital du Jura, 2800 Delémont, Switzerland.
Acknowledgements
This study was supported by funds of the Department of Microbiology, University of Patras, Patras, Greece. We thank Prof. Loukia Zerva for critically reviewing this paper.
References
-
1European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2014 Sweden: Stockholm; 2014.
-
2Papaventsis D, Nikolaou S, Karabela S, et al. Tuberculosis in Greece: bacteriologically confirmed cases and anti-tuberculosis drug resistance, 1995-2009. Euro Surveill 2010;15.
-
3Jelastopulu E, Alexopoulos EC, Venieri D, et al. Substantial underreporting of tuberculosis in West Greece: implications for local and national surveillance. Euro Surveill 2009;14:.
-
4Smith A, Miller RF, Story A, Booth HL. A&E department: a missed opportunity for diagnosis of TB?. Thorax 2006;61:364-365.
-
5Drobniewski F, Nikolayevskyy V, Maxeiner H, et al. Rapid diagnostics of tuberculosis and drug resistance in the industrialized world: clinical and public health benefits and barriers to implementation. BMC Med. 2013;11:190.
-
6Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2013;167:603-662.
-
7Rovina N, Karabela S, Constantoulakis P, et al. MIRU-VNTR typing of drug-resistant tuberculosis isolates in Greece. Ther Adv Respir Dis. 2011;5:229-236.
-
8Bwanga F, Hoffner S, Haile M, Joloba ML. Direct susceptibility testing for multi drug resistant tuberculosis: a meta-analysis. BMC Infect Dis. 2009;9:67.
-
9Bloemberg GV, Voit A, Ritter C, Deggim V, Bottger EC. Evaluation of Cobas TaqMan MTB for direct detection of the Mycobacterium tuberculosis complex in comparison with Cobas Amplicor MTB. J Clin Microbiol. 2013;51:2112-2117.
-
10Cho WH, Won EJ, Choi HJ, et al. Comparison of AdvanSure TB/NTM PCR and COBAS TaqMan MTB PCR for detection of Mycobacterium tuberculosis complex in routine clinical practice. Ann Lab Med. 2015;35:356-361.
-
11Tortoli E, Urbano P, Marcelli F, Simonetti TM, Cirillo DM. Is real-time PCR better than conventional PCR for Mycobacterium tuberculosis complex detection in clinical samples?. J Clin Microbiol 2012;50:2810-2813.
-
12Fegou E, Jelastopulu E, Sevdali M, Anastassiou ED, Dimitracopoulos G, Spiliopoulou I. Sensitivity of the Cobas Amplicor system for detection of Mycobacterium tuberculosis in respiratory and extrapulmonary specimens. Clin Microbiol Infect 2005;11:593-596.
-
13Wang SX, Tay L. Evaluation of three nucleic acid amplification methods for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol 1999;37:1932-1934.
-
14Yang YC, Lu PL, Huang SC, Jenh YS, Jou R, Chang TC. Evaluation of the Cobas TaqMan MTB test for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011;49:797-801.
-
15Huh HJ, Kwon HJ, Ki CS, Lee NY. Comparison of the genedia MTB detection kit and the cobas TaqMan MTB assay for detection of Mycobacterium tuberculosis in respiratory specimens. J Clin Microbiol. 2015;53:1012101-1012104.
-
16Kim JH, Kim YJ, Ki CS, Kim JY, Lee NY. Evaluation of Cobas TaqMan MTB PCR for detection of Mycobacterium tuberculosis. J Clin Microbiol. 2011;49:173-176.
-
17Cohen RA, Muzaffar S, Schwartz D, et al. Diagnosis of pulmonary tuberculosis using PCR assays on sputum collected within 24 hours of hospital admission. Am J Respir Crit Care Med 1998;157:156-161.
-
18Panaiotov S, Amicosante M. Dynamics of the laboratory results in patients with pulmonary tuberculosis. Diagn Microbiol Infect Dis. 2010;67:327-332.
-
19Fegou E, Jelastopulu E, Nicolaou S, et al. Comparison of the manual Mycobacteria Growth Indicator tube and the Etest with the method of proportion for susceptibility testing of Mycobacterium tuberculosis. Chemotherapy 2006;52:174-177.
-
20Banu S, Rahman SM, Khan MS, et al. Discordance across several methods for drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates in a single laboratory. J Clin Microbiol 2014;52:156-163.
-
21Matsiota-Bernard P, Vrioni G, Marinis E. Characterization of rpoB mutations in rifampin-resistant clinical Mycobacterium tuberculosis isolates from Greece. J Clin Microbiol. 1998;36:20-23.
-
22Gitti Z, Mantadakis E, Maraki S, Samonis G. GenoType(R) MTBDRplus compared with conventional drug-susceptibility testing of Mycobacterium tuberculosis in a low-resistance locale. Future Microbiol. 2011;6:357-362.
Publication Dates
-
Publication in this collection
Oct-Dec 2017
History
-
Received
31 July 2016 -
Accepted
27 Apr 2017