Abstract
In this work, four isolates of endophytic fungi (Alternaria alternata, Colletotrichum gloesporioides, Glomerella cingulata and Nigrospora sphaerica), deposited in the culture collection ‘University Recife Mycologia’ (URM) at the Universidade Federal de Pernambuco, were characterized for the genes ITS 1 and 4 (region 5.8 S) and evaluated for taxol production.
Keywords
Paclitaxel; Taxol route genes; ITS; Colletotrichum gloesporioides
Endophytic microorganisms are those that live inside plants, inhabiting the aerial parts, such as the leaves and stems, without causing any damage to their hosts, unlike pathogens.11 Santos C, Paterson RR, Venâncio A, Lima N. Filamentous fungal characterizations by matrizassisted laser desorption/ionization time-of-flight mass spectrometry. J Appl Microbiol. 2010;108(2):375-385.,22 Souza IFAC, Napoleão TH, Sena KXRF, Paiva PMG, Araújo JM, Coelho LCBB. Endophytic microorganisms in leaves of Moringa oleifera collected in three localities at Pernambuco State, Northeastern Brazil. Br Microbiol Res J. 2016;13(5):1-7. However, an endophytic microorganism may become pathogenic, if there is an imbalance between their virulence and the plants defense.33 Hardoim PR, Overbeek LSV, Berg G, et al. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev. 2015;79(3):293-320. Endophytic fungi have biotechnological relevance for many applications, such as for use in bioremediation processes44 Khan AR, Ullah I, Waqas M, et al. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotoxicol Environ Saf. 2017;136:180-188. and the production of compounds with antimicrobial, antioxidant or antitumor activities.55 Wibowo M, Prachyawarakorn V, Aree T, Mahidol C, RuchirawaT S, Kittakoop P. Cytotoxic sesquiterpenes from the endophytic fungus Pseudolagarobasidium acaciicola. Phytochemistry. 2016;122:126-138.–77 Siridechakorn I, Yue Z, Mittraphab Y, Lei X, Pudhom K. Identification of spirobisnaphthalene derivatives with anti-tumor activities from the endophytic fungus Rhytidhysteron rufulum AS21B. Bioorg Med Chem. 2017;25(11):2878-2882. However, endophytic fungi have still been poorly explored industrially.88 Kusari S, Singh S, Jayabaskaran C. Rethinking production of Taxol® (paclitaxel) using endophyte biotechnology. Trends Biotechnol. 2014;32(6):304-311.
According to the World Health Organization (WHO), 8.8 million deaths occurred in 2015, due to cancer, and it is estimated that 12.6 million deaths will occur per year by 2030.99 World Health Organization. Projections of Mortality and Causes of Death, 2015 and 2030; 2017. Taxol (paclitaxel) is a potent drug used in the treatment of some neoplasms, as both a first – and second – line of treatment.1010 Khanna C, Rosenberg M, Vail DM. A Review of paclitaxel and novel formulations including those Suitable for use in dogs. J Vet Intern Med. 2015;29:1006-1012. It acts by inhibiting cell replication through binding to the beta-tubulin subunit of microtubules and induces apoptosis by inactivating the apoptosis inhibitory protein Bcl-2.1111 Ferlini C, Cicchillitti L, Raspaglio G, et al. Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Exp Therap Mol Targets Chem Biol. 2009;69(17), http://dx.doi.org/10.1158/0008-5472.CAN-09-0540.
http://dx.doi.org/10.1158/0008-5472.CAN-...
This compound is naturally produced by the bark of yew plants (Taxus species). However, new methods to obtain it are being explored, because its extraction or semi-synthesis of sufficient quantities to supply the current demand would imply devastation, as the removal of the bark results in the death of the Tree.1212 Meštrović T. Paclitaxel Production; 2014. Retrieved from NewsMedical: http://www.newsmedical.net/health/Paclitaxel-Production.aspx.
http://www.newsmedical.net/health/Paclit...
,1313 Wani MC, Horwit SB. Nature as a remarkable chemist: a personal story of the discovery and development of taxol. Anticancer Drugs. 2014;25(5):482-487.
Taxol can also be produced by some fungi such as Colletotrichum gloeosporioides, Glomerella cingulata, Nigrospora sphaerica, Pestalotiopsis guepinii, Alternaria alternata, and Fusarium solani.1414 Gond SK, Kharwar RN, White JF. Will fungi be the new source of the blockbuster drug taxol? Fung Biol Rev. 2014;28:77-84. Genetic screening for taxol production is currently used to identify microorganisms that produce it, and three key genes are usually investigated: ts (encoding taxadiene synthase), dbat (encoding 10-deacetylbaccatin III-10-O-acetyltransferase), and bapt (encoding C-13 phenylpropanoyl side chain-CoA acyltransferase).1515 Xiong Z, Yang Y, Zhão N, Wang Y. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus × media. BMC Microbiol. 2013, http://dx.doi.org/10.1186/1471-2180-13-71.
http://dx.doi.org/10.1186/1471-2180-13-7...
Due to the great importance of taxol in anti-cancer therapy, there is a constant search for new production methods. In this work, four isolates of endophytic fungi, deposited in a culture collection from the Universidade Federal de Pernambuco, were evaluated for their ability to produce taxol through a search of the genes involved in the taxol metabolic pathway. Next, the presence of taxol in the cell mass and metabolic liquid of fungal cultures was investigated by LC/MS. In addition, the isolates were molecularly characterized for the ITS 1 and 4 genes (region 5.8 S).
The study was performed with four isolates of endophytic fungi that, belong to different genera and were, previously deposited in the culture collection ‘University Recife Mycologia (URM)’ of the Departamento de Micologia at the Universidade Federal de Pernambuco. The place of collection and the plant from which they were isolated are described in Table 1. All of the samples were stored lyophilized and/or in mineral oil.
The isolates were reactivated in Potato Dextrose Agar (PDA) medium. The standard cetyl trimethylammonium bromide (CTAB) method was used for the extraction of total DNA. The genes ITS 1 and 4 (ITS1-5.8S-ITS2 rDNA) were amplified using the oligonucleotide primers ITS1 and ITS4. The rDNA gene sequences of the samples were compared with others deposited in the NCBI Genbank database using the BLAST tool (http://www.ncbi.nlm.nih.gov/BLAST/). The quality of the sequencing was analyzed using the Pregap4 4.0 and Gap4 4.0 programs of the Staden package. Screening of the genes in the taxol etabolic pathway was performed using three conserved sequences of the main genes involved in taxol biosynthesis: ts, dbat and bapt (Table 2).
To obtain the pre-inoculum, fungi were cultivated in Petri dishes in PDA medium for 7 days at 28 °C. Next, blocks (6 mm diameter) were made, and six of them were transferred to 2-L Erlenmeyers flasks, with one quarter of their capacity filled with Potato Dextrose medium (24 g/L), supplemented with chloramphenicol (0.1 g/L), phenylalanine (0.04 g/L), magnesium sulfate heptahydrate (2 g/L), and ammonium sulphate (12 g/L). Three flasks were prepared for each fungus. The fungi were cultivated at 28 °C under agitation (160 rpm) and every 7 days an erlenmeyer of each species was used for extraction.
The cell mass was treated with ethyl acetate in order to extract intracellular secondary metabolites. For this, 10 mL of the solvent was added to 1 g of the wet weight of each cell mass, and the mixture was subjected to agitation (180 rpm) for 20 min. For extraction of the compounds present in the metabolic liquid, ethyl acetate was added to the liquid at a ratio of 2:1 (v/v) and subjected to stirring at 180 rpm for 30 min.
To verify taxane production, the extracts were evaluated by thin layer chromatography (TLC) in a saturated chamber, using 60 F254 silica gel plates with 0.25 mm thickness (Macherey-Nagel, Germany). The standard, paclitaxel (Cayman Chemical, MI, USA), was dissolved in methanol, and the Liebermann-Burchard reagent, anisaldehyde-H2SO4, and sulfuric vanillin were used as chemical developers. Chloroform–acetonitrile (7:3 v/v) was used as the elution system.
High performance liquid chromatography (HPLC) was performed using a Prominence LC-20AT liquid chromatograph (Shimadzu, Kyoto, Japan), consisting of an LC-20AT quaternary pump, DGU-20As degasser, CTO-20AC column oven, SPDM-20A diode array detector (DAD), SIL-20A autoinjector and CBM-20A communication module, controlled by the LcSolution software. The extracts were passed through 0.45 µm membrane filters (Supelco, Sigma–Aldrich, MO, USA). A flow rate of 1.0 mL/min was employed. The solvents used were methanol, acetonitrile (Merck, Darmstadt, Germany), and ultrapure water at a ratio of 25:35:40 (v/v/v). The taxol content was estimated using a standard curve (Y = 5189.7X + 1083.3), with a limit of detection of 0.0473 µg/mL and a limit of quantitation of 0.1435 µg/mL.
Liquid chromatography coupled with mass spectrometry (LC/MS) was performed with an LC/MS ACQUITY UPLC H-Class-SQ Detector 2 column (Waters), with a dimension of 2.1 × 100 mm, pore size 130 Å, and particle size 1.7 µm. The mobile phase was acetonitrile/MilliQ water/methanol at flow rate of 0.610 mL/min.
In the present work, we evaluated four isolates from this collection, obtained from different plants collected in different regions of Pernambuco, in regard to their ability to produce taxol.
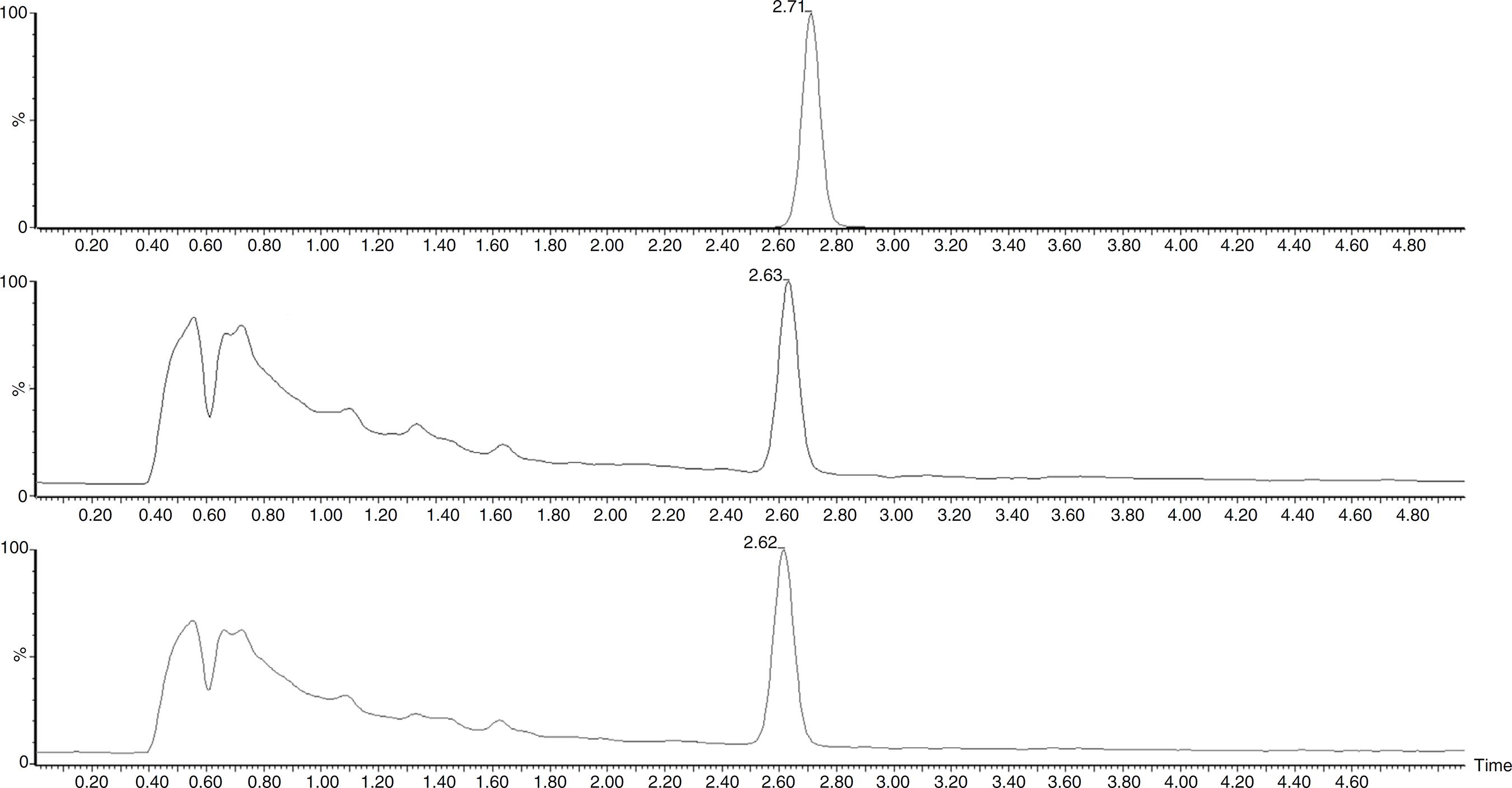
The isolates were previously identified by Stackebrant and Goebel.1616 Stackebrandt E, Goebel BM. Taxonomic note: a place for DNA–DNA reassociation and 16s rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44(4):846-849. In the present work, we performed a molecular characterization of the isolates based on the ITS genes. For all the isolates, the sequences of the ITS rDNA genes obtained, showed a homology of greater than or equal to 99% (Table 3) with the previously determined species, except for N. sphaerica. All four isolates had the ts and dbat genes, and only two presented the bapt gene. Extracts from the cell mass and metabolic liquid were then evaluated by TLC, but taxol was not identified in any sample. This result may be associated with interfering substances present in the samples. On the other hand, HPLC analysis showed that the extracts from the metabolic liquid produced by C. gloeosporioides (14th and 21st days) exhibited a peak with a retention time close to that of paclitaxel, and the UV spectrum was also similar to this standard, confirming taxol production. The standard paclitaxel showed a retention time of 2.71, min and the sample presented a peak at 2.62 ± 0.02 min (Fig. 1). In addition, the molecular mass detected for the compound, produced by C. gloeosporioides, was 852.32 g/mol, which is close to the molecular mass of taxol (853.906 g/mol). The taxol content was estimated at 5.24 µg/mL in the sample collected on the 14th day and 4.4 µg/mL in the sample from the 21st day.
LC/MS profile of standard paclitaxel (A) and metabolic liquid of Colletotrichum gloeosporioides collected after 14 (B) and 21 (C) days.
The search for new strategies to obtain taxol is of utmost importance, as about 10,000 kg of yew leaves and bark are needed to isolate 1 kg of this substance.1212 Meštrović T. Paclitaxel Production; 2014. Retrieved from NewsMedical: http://www.newsmedical.net/health/Paclitaxel-Production.aspx.
http://www.newsmedical.net/health/Paclit...
In addition, the semi-synthesis methods consume a large amount of trees and are not enough to meet the demand.88 Kusari S, Singh S, Jayabaskaran C. Rethinking production of Taxol® (paclitaxel) using endophyte biotechnology. Trends Biotechnol. 2014;32(6):304-311. Alternative strategies, such as the optimization of Taxus cell cultures and production using microbial sources, have gained increasing attention. The URM culture collection possesses a large collection of endophytic fungi that have still not been screened for their biotechnological potential. This is the first report of an evaluation of the biotechnological potential of some of the isolates in the collection for taxol production.
In addition, molecular analysis was performed to confirm the identity of the isolates. According to Nilsson et al.,1717 Nilsson RH, Tedersoo L, Lindah BD, et al. Towards standardization of the description and publication of next-generation sequencing datasets of fungal communities. New Phytologist. 2008, http://dx.doi.org/10.1111/j.1469-8137.2011.03755.x.
http://dx.doi.org/10.1111/j.1469-8137.20...
a margin of 2% is acceptable for intraspecific divergence in the ITS region sequences. The ts gene has been proposed as a primary screening method to identify taxol-producing fungi whereas dbat has been reported to be more diagnostic as a molecular marker.1818 Flores-Bustamante ZR, Rivera-Orduña FN, Martínez-Cárdenas A, Flores-Cotera LB. Microbial paclitaxel: advances and perspectives. J Antibiot. 2010;63:460-467. However, it is common to only detect one or two of the genes associated with taxol production in microorganisms. For example, endophytic fungi from different genera, isolated from Salacia oblonga bark, were screened for the presence of two genes; seven had the dbat gene, and one contained bapt.1919 Roopa G, Madhusudhan MC, Sunil KCR, et al. Identification of Taxol-producing endophytic fungi isolated from Salacia oblonga through genomic mining approach. J Genet Eng Biotechnol. 2015;13:119-127. Despite the detection of the genes, the effective production of the compound must also be confirmed. According to Xiong et al.,1515 Xiong Z, Yang Y, Zhão N, Wang Y. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus × media. BMC Microbiol. 2013, http://dx.doi.org/10.1186/1471-2180-13-71.
http://dx.doi.org/10.1186/1471-2180-13-7...
the ts and dbat genes are essential for taxol biosynthesis, but are not diagnostic, because the bpat gene encodes the enzyme required to convert the precursor baccatin III to taxol. In the present study, all of the isolates presented ts and dbat. However, our data showed that only the C. gloeosporioides isolate is a promising producer of paclitaxel, although it was negative for the bpat gene.
The other isolates may also be able to produce taxol, but under conditions different from those used here. It has been reported that stress conditions can be favorable for the production of this compound. Somjaipeng et al.2020 Somjaipeng S, Medina A, Magan N. Environmental stress and elicitors enhance taxol production by endophytic strains of Paraconiothyrium variabile and Epicoccum nigrum. Enzyme Microb Technol. 2016;90:69-75. reported that stress, associated with environmental factors (water activity or pH), and the presence of elicitors (ammonium acetate, jasmonic acid, phenyl-alanine, salicylic acid, serine, silver nitrate and sodium acetate) induced the production of taxol by the endophytic fungi Paraconiothyrium variabile and Epicoccum nigrum, isolated from Taxus baccata. In addition, the production of metabolites by endophytes may depend on others factors, such as and multipartite interactions with host plants, as well as the selection pressures of biotic (such as pathogens and predators) and abiotic (such as precursors of metabolites and environmental conditions) factors.88 Kusari S, Singh S, Jayabaskaran C. Rethinking production of Taxol® (paclitaxel) using endophyte biotechnology. Trends Biotechnol. 2014;32(6):304-311. Many studies have been conducted searching for taxol-producing microorganisms among the endophytes found in Taxus plants.1515 Xiong Z, Yang Y, Zhão N, Wang Y. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus × media. BMC Microbiol. 2013, http://dx.doi.org/10.1186/1471-2180-13-71.
http://dx.doi.org/10.1186/1471-2180-13-7...
,1919 Roopa G, Madhusudhan MC, Sunil KCR, et al. Identification of Taxol-producing endophytic fungi isolated from Salacia oblonga through genomic mining approach. J Genet Eng Biotechnol. 2015;13:119-127.,2020 Somjaipeng S, Medina A, Magan N. Environmental stress and elicitors enhance taxol production by endophytic strains of Paraconiothyrium variabile and Epicoccum nigrum. Enzyme Microb Technol. 2016;90:69-75. In this study, the isolate from endophytic C. gloeosporioides possessed two genes involved in taxol biosynthesis, and the presence of this compound was confirmed in the metabolic liquid it produced. This study shows the biotechnological potential of an isolate stored at the URM collection as a producer of a substance with high pharmacological relevance. However, future studies using polymer nanoparticles may facilitate the industrial use of taxol in antitumor activities.
Acknowledgments
The authors express their gratitude to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for fellowships (PMGP, THN and MTSC) as well as to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We are also grateful for the Plataforma de Sequenciamento (LABCEN-CB) of the Universidade Federal de Pernambuco.
References
-
1Santos C, Paterson RR, Venâncio A, Lima N. Filamentous fungal characterizations by matrizassisted laser desorption/ionization time-of-flight mass spectrometry. J Appl Microbiol 2010;108(2):375-385.
-
2Souza IFAC, Napoleão TH, Sena KXRF, Paiva PMG, Araújo JM, Coelho LCBB. Endophytic microorganisms in leaves of Moringa oleifera collected in three localities at Pernambuco State, Northeastern Brazil. Br Microbiol Res J 2016;13(5):1-7.
-
3Hardoim PR, Overbeek LSV, Berg G, et al. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 2015;79(3):293-320.
-
4Khan AR, Ullah I, Waqas M, et al. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotoxicol Environ Saf 2017;136:180-188.
-
5Wibowo M, Prachyawarakorn V, Aree T, Mahidol C, RuchirawaT S, Kittakoop P. Cytotoxic sesquiterpenes from the endophytic fungus Pseudolagarobasidium acaciicola Phytochemistry 2016;122:126-138.
-
6Dzoyem JP, Melong R, Tsamo AT, et al. Cytotoxicity, antioxidant and antibacterial activity of four compounds produced by an endophytic fungus Epicoccum nigrum associated with Entada abyssinica Rev Bras Farmacogn 2017;27:251-253.
-
7Siridechakorn I, Yue Z, Mittraphab Y, Lei X, Pudhom K. Identification of spirobisnaphthalene derivatives with anti-tumor activities from the endophytic fungus Rhytidhysteron rufulum AS21B. Bioorg Med Chem 2017;25(11):2878-2882.
-
8Kusari S, Singh S, Jayabaskaran C. Rethinking production of Taxol® (paclitaxel) using endophyte biotechnology. Trends Biotechnol 2014;32(6):304-311.
-
9World Health Organization. Projections of Mortality and Causes of Death, 2015 and 2030; 2017.
-
10Khanna C, Rosenberg M, Vail DM. A Review of paclitaxel and novel formulations including those Suitable for use in dogs. J Vet Intern Med 2015;29:1006-1012.
-
11Ferlini C, Cicchillitti L, Raspaglio G, et al. Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Exp Therap Mol Targets Chem Biol 2009;69(17), http://dx.doi.org/10.1158/0008-5472.CAN-09-0540
» http://dx.doi.org/10.1158/0008-5472.CAN-09-0540 -
12Meštrović T. Paclitaxel Production; 2014. Retrieved from NewsMedical: http://www.newsmedical.net/health/Paclitaxel-Production.aspx
» http://www.newsmedical.net/health/Paclitaxel-Production.aspx -
13Wani MC, Horwit SB. Nature as a remarkable chemist: a personal story of the discovery and development of taxol. Anticancer Drugs 2014;25(5):482-487.
-
14Gond SK, Kharwar RN, White JF. Will fungi be the new source of the blockbuster drug taxol? Fung Biol Rev 2014;28:77-84.
-
15Xiong Z, Yang Y, Zhão N, Wang Y. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus × media. BMC Microbiol 2013, http://dx.doi.org/10.1186/1471-2180-13-71
» http://dx.doi.org/10.1186/1471-2180-13-71 -
16Stackebrandt E, Goebel BM. Taxonomic note: a place for DNA–DNA reassociation and 16s rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol 1994;44(4):846-849.
-
17Nilsson RH, Tedersoo L, Lindah BD, et al. Towards standardization of the description and publication of next-generation sequencing datasets of fungal communities. New Phytologist 2008, http://dx.doi.org/10.1111/j.1469-8137.2011.03755.x
» http://dx.doi.org/10.1111/j.1469-8137.2011.03755.x -
18Flores-Bustamante ZR, Rivera-Orduña FN, Martínez-Cárdenas A, Flores-Cotera LB. Microbial paclitaxel: advances and perspectives. J Antibiot 2010;63:460-467.
-
19Roopa G, Madhusudhan MC, Sunil KCR, et al. Identification of Taxol-producing endophytic fungi isolated from Salacia oblonga through genomic mining approach. J Genet Eng Biotechnol 2015;13:119-127.
-
20Somjaipeng S, Medina A, Magan N. Environmental stress and elicitors enhance taxol production by endophytic strains of Paraconiothyrium variabile and Epicoccum nigrum Enzyme Microb Technol 2016;90:69-75.
Edited by
Publication Dates
-
Publication in this collection
Nov 2018
History
-
Received
5 Mar 2018 -
Accepted
6 June 2018 -
Published
11 Aug 2018