Abstracts
The spittlebug, Deois flavopicta Stål, is the main pest in cultivated pastures of the "Cerrados" (savanna) in the central region of Brazil. The insect has three discrete generations during the rainy season (September-April) and a synchronized population of diapausing eggs during the dry season (May-August). Experiments in cultivated pastures showed that nonspecific predators were able to affect significantly the mortality rates of diapausing eggs and nymphs of D. flavopicta. Predation was a density independent mortality factor that reduced diapausing eggs by approximately 60% and nymphs by 20% to 47%. The nymph mortality rate due to predation did not differ during the first and the second generations of the year. For the third generation however, the mortality rate was lower, related to a greater degree of nymphal aggregation in spatial refuges. Our direct observations indicate that among the types of predators in pastures, ants potentially make the greatest contribution to mortality, although their impact needs experimental verification. High rates of mortality of eggs and nymphs of D. flavopicta exposed to predators indicate that predation can be an important factor determining the size of adult populations. Therefore, management practices that disturb the predator community may increase the population densities of the spittlebug.
Insecta; insect community; population dynamics; survival curve
A cigarrinha-das-pastagens Deois flavopicta Stål é a principal praga de pastagens cultivadas nos Cerrados do Brasil Central. O inseto apresenta distribuição sazonal com três gerações anuais discretas durante a estação chuvosa (setembro-abril) e população sincronizada de ovos diapáusicos durante a estação seca (maio-agosto). Experimentos em pastagens cultivadas mostraram que predadores não-específicos foram capazes de alterar significativamente as taxas de mortalidade de ovos diapáusicos e ninfas de D. flavopicta. Predação foi um fator de mortalidade independente da densidade, reduzindo os ovos diapáusicos em aproximadamente 60% e ninfas de 20% a 47%. A taxa de mortalidade das ninfas resultante da predação não variou significativamente entre a primeira e a segunda geração anual. No entanto, a mortalidade de ninfas na terceira geração foi significativamente mais baixa, fato relacionado ao maior grau de agregação das ninfas em refúgios espaciais. Observações diretas de interações predador-presa nas pastagens sugerem que, potencialmente, as formigas representam o grupo que mais contribui para a mortalidade observada, embora esse impacto deva ainda ser verificado experimentalmente. Taxas de mortalidade mais altas em ovos e ninfas de D. flavopicta expostos aos predadores indicam que a predação pode ser um fator importante na determinação da população de adultos. Desta forma, práticas de manejo das pastagens que perturbam a comunidade de predadores podem resultar em densidades mais altas das cigarrinhas.
Insecta; comunidade de insetos; dinâmica populacional; curva de sobrevivência
BIOLOGICAL CONTROL
Predation as a Mortality Factor in Populations of the Spittlebug, Deois flavopicta Stål (Homoptera: Cercopidae)
EDISON R. SUJII1, MARIA A. GARCIA2, ELIANA M.G. FONTES1 AND ROBERT J. O'NEIL3
1 Embrapa/Cenargen. Controle Biológico, C. postal 2372, 70849-970 Brasilia, DF
2 Depto. Zoologia, UNICAMP IB, C. postal 6109, 13083-970, Campinas, SP
3 Dept. Entomology, Purdue University, West Lafayette, 47907-1158 IN, USA
A Predação Como Fator de Mortalidade em Populações da Cigarrinha-das-Pastagens, Deois flavopicta Stål (Homoptera: Cercopidae)
RESUMO - A cigarrinha-das-pastagens Deois flavopicta Stål é a principal praga de pastagens cultivadas nos Cerrados do Brasil Central. O inseto apresenta distribuição sazonal com três gerações anuais discretas durante a estação chuvosa (setembro-abril) e população sincronizada de ovos diapáusicos durante a estação seca (maio-agosto). Experimentos em pastagens cultivadas mostraram que predadores não-específicos foram capazes de alterar significativamente as taxas de mortalidade de ovos diapáusicos e ninfas de D. flavopicta. Predação foi um fator de mortalidade independente da densidade, reduzindo os ovos diapáusicos em aproximadamente 60% e ninfas de 20% a 47%. A taxa de mortalidade das ninfas resultante da predação não variou significativamente entre a primeira e a segunda geração anual. No entanto, a mortalidade de ninfas na terceira geração foi significativamente mais baixa, fato relacionado ao maior grau de agregação das ninfas em refúgios espaciais. Observações diretas de interações predador-presa nas pastagens sugerem que, potencialmente, as formigas representam o grupo que mais contribui para a mortalidade observada, embora esse impacto deva ainda ser verificado experimentalmente. Taxas de mortalidade mais altas em ovos e ninfas de D. flavopicta expostos aos predadores indicam que a predação pode ser um fator importante na determinação da população de adultos. Desta forma, práticas de manejo das pastagens que perturbam a comunidade de predadores podem resultar em densidades mais altas das cigarrinhas.
PALAVRAS-CHAVE: Insecta, comunidade de insetos, dinâmica populacional, curva de sobrevivência
ABSTRACT - The spittlebug, Deois flavopicta Stål, is the main pest in cultivated pastures of the "Cerrados" (savanna) in the central region of Brazil. The insect has three discrete generations during the rainy season (September-April) and a synchronized population of diapausing eggs during the dry season (May-August). Experiments in cultivated pastures showed that nonspecific predators were able to affect significantly the mortality rates of diapausing eggs and nymphs of D. flavopicta. Predation was a density independent mortality factor that reduced diapausing eggs by approximately 60% and nymphs by 20% to 47%. The nymph mortality rate due to predation did not differ during the first and the second generations of the year. For the third generation however, the mortality rate was lower, related to a greater degree of nymphal aggregation in spatial refuges. Our direct observations indicate that among the types of predators in pastures, ants potentially make the greatest contribution to mortality, although their impact needs experimental verification. High rates of mortality of eggs and nymphs of D. flavopicta exposed to predators indicate that predation can be an important factor determining the size of adult populations. Therefore, management practices that disturb the predator community may increase the population densities of the spittlebug.
KEY WORDS: Insecta, insect community, population dynamics, survival curve
Since 1960, several grass species of the genus Brachiaria (Poaceae) have been introduced from Africa and planted in extensive areas, from the Central to Northern regions of Brazil (Seiffert 1980). The colonization of new pastures with few natural enemies, along with a phenology that is synchronized with the seasonal change in physical factors and the life cycle of the host plant, has favored frequent spittlebug (Homoptera: Cercopidae) outbreaks in several regions of Brazil (Cosenza & Naves 1980). Spittlebug population levels are controlled by weather variations, intrinsic regulators of the population and by the growth pattern of the host plant (Menezes et al. 1983). While resistant to drought, introduced Brachiaria species did not possess resistance to spittlebugs (Nilakhe 1987, Cosenza et al. 1989).
Among spittlebugs, Deois flavopicta (Stal) is the main species in pasture areas of Central Brazil in the "Cerrados" region. Each year this species has three discrete generations, during the rainy season, and it aestivates during the dry season as diapausing eggs (Fontes et al.1995). Low temperatures during the early part of the diapause period accelerate diapause development, whereas contact with liquid water determines the timing of post-diapause hatching (Pires et al. 2000). With the start of the rainy season, synchronized emergence of first instars occurs after the natural termination of diapause during the dry season (Sujii et al.1995).
Various species of insects, spiders, nematodes, and birds are cited as natural enemies of spittlebug (Villacorta 1980; Barbosa et al. 1984; Carneiro 1988; Hewitt & Nilakhe 1986; Bueno 1987; Marques 1988; Pires et al. 1993). Among them, parasitoids of D. flavopicta are few, but include the egg parasitoid Anagrus sp. that attacks ca. 2% of the eggs (Pires et al. 1993). Entomopathogens include Metarhizium anisopliae (Metsch.) Sorokin, Beauveria bassiana (Bals.) Vuilllemin, and Paecylomyces lilacinus (Thom.) Samson (Faria & Tigano 1996), but our field experience and knowledge of this insect indicate that their impact on spittlebug populations is minimal. Predators such as ants (Hymenoptera: Formicidae) (Hewitt & Nilakhe 1986) and Salpingogaster nigra Schiner (Diptera: Syrphidae) (Marques 1988) are mentioned, for some regions, as the main possible causes of mortality that are able to significantly alter the density of spittlebug populations. However, the impact of predators on the regulation of D. flavopicta population has not been experimentally investigated.
Outbreaks of D. flavopicta, common in Central Brazil, cause severe damage to pastures and cattle production. Population-regulating factors are being explored in search for environmentally safe methods for managing this pest. In this paper, we investigated the role of predators in the regulation of D. flavopicta populations. This knowledge will support new proposals for management of pastures to favor natural biological control and reduce the occurrence of economically damaging populations of spittlebugs.
Material and Methods
Experimental Plots. This study was conducted in three experimental plots located at Embrapa/Cenargen, Embrapa/Cerrados, and Granja do Torto, in Brasilia, DF, Brazil. At Embrapa/Cenargen, the experiments were run in a 0.8 ha of pasture cultivated with Brachiaria decumbens Stapf (Poaceae). At Embrapa/Cerrados and Granja do Torto the experiments were conducted in pastures of ca. 3 ha, cultivated with Brachiaria ruziziensis Stapf.
Predation of Diapausing Eggs During the Dry Season. Beginning June 1996, groups of diapausing eggs were placed in 15 randomly selected sites within the pasture. To mark the position of the eggs, allow minimal alteration of soil permeability characteristics, and allow access to natural enemies, a 10 cm x 10 cm piece of organdy screen was placed over the soil and groups of diapausing eggs were then placed over these screens. Both screens and eggs were then covered with approximately 1 cm of soil. To test for effects of density on egg removal rates by predators, five groups of 100 eggs and 10 groups of 50 eggs were used. Screens were gathered three months later and the eggs that remained on the screens were collected by filtering, flotation in a saturated NaCl solution and observation under a stereomicroscope (see Sujii 1994). The proportions of missing eggs from the two different density conditions were compared with a "t" test (Wilkinson 1990).
Immature Mortality. Nymphal mortality in pastures was evaluated during the second generation of each year from 1994 to 1997 and during the three generations of 1995/96 (October to May) using a Modified Mark Release and Recapture - MMRR method. This method consisted of finding, marking position, and releasing all nymphs in 10 randomly selected 0.5 m x 0.5 m plots. Nymphs were marked and followed until adult emergence. A small numbered wooden stake indicated the position of the nymphs in each plot. The developmental stage of nymphs was determined by morphological characteristics, and data related with 3rd and 4th instars were joined because of the difficulty to separate them (Menezes et al. 1983, Sujii 1994). The position and development of the nymphs were recorded every one to three days.
During 1995/96 (three generations) and 1997 (second generation), nymphal cohorts protected against predators were followed through the MMRR method, as previously described. Nymphal development was measured inside 10 randomly located plots protected by a cage measuring 0.5 m x 0.5 m and 0.7 m high. The cages were wooden framed and covered with organdy screen panels to exclude predators. This set of cohorts was used as control for estimating predation in unprotected individuals.
Survival curves from first instar to teneral adults were calculated and compared graphically for each generation. Comparisons of mortality rates among different generations and years were done using Analysis of Variance followed by Tukey's Test (Wilkinson 1990). The effect of density on mortality rates in protected and unprotected quadrats was evaluated through Pearson's Correlation Coefficient (Wilkinson 1990). The pattern of nymphal aggregation was calculated through the Morisita's index of dispersion (Krebs 1989). This index was calculated using the density of nymphs in each unprotected quadrat for the set of quadrats in each generation. This index was correlated with the correspondent mortality to test if predation levels could be related to spatial distribution of nymphs.
Direct Observation and Collection of Predators. To be efficient in performing direct observations of predation, it was necessary to establish the diurnal pattern (day vs. night) of predator activity. To do so, we determined when nymphs disappeared by following a group of D. flavopicta (all instars) using the MMRR method. Observations were made twice a day at 12h intervals, early in the morning (7:00 am) and in the evening (7:00 pm), for 10 days. Rates of disappearance of nymphs, inferred as predator attack, during each period (night and day) were compared with the chi-square test.
Direct observation of predators was made for ca. 120 min. per sampling along eight transects of 0.5 m x 20 m randomly marked in a pasture of B. ruziziensis at Granja do Torto. During observations, prey and predators seen inside transects were recorded. Predators were followed for five minutes. When an attack on a spittlebug occurred, both prey and predator were counted and collected. Unknown insects and predators that were already attacking prey were also collected for identification. Insect samples were sent to Universidade Estadual de Campinas, Comissão Executiva de Pesquisa da Lavoura Cacaueira, and Universidade de Brasilia for identification. Data were plotted in order to describe the insect community present in the pastures.
Results
Predator Effect on Survival of Diapausing Eggs. During the sampling collection at the end of dry season, no disturbance or water run off was observed in the soil over the diapausing eggs. This observation suggests that missing eggs were mainly caused by action of predators. The percentages of diapausing eggs missing from groups of 50 and 100 eggs per sample were respectively 60.0 ± 26.15% and 62.8 ± 19.61% (mean ± standard deviation), and did not statistically differ (t-test, t = 0.21; d.f. = 14; P = 0.84). However, the average numbers of missing eggs from plots with 100 eggs (62.8 ± 19.61%) was higher than from plots with 50 eggs (30.0 ± 12.40%), revealing that the number of attacks per plot increased with prey density.
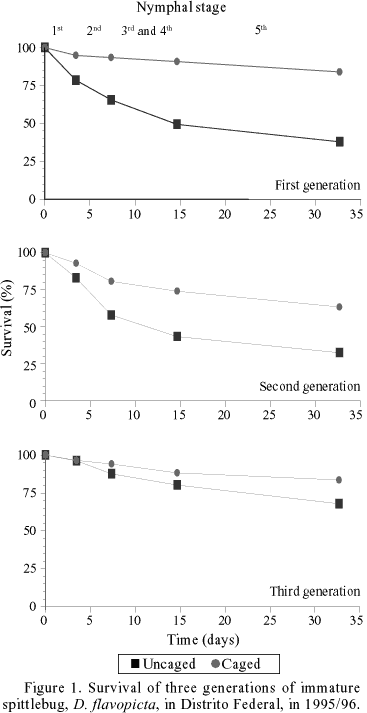
Nymph Survival. For the different generations of 1996, survival curves of uncaged nymphs, mainly first and second generations, had similar general shapes, with mortality occurring early. In contrast, nymphs protected with anti-predator cages had relatively constant rates of mortality and higher overall survivorship rates (Fig. 1). In 1995/96, the good distribution and high levels of precipitation during the period of October-May appear to have favored nymphal survival and produced high spittlebug densities. The average densities of nymphs during the three population peaks were 40, 60 and 20 individuals/m², reaching numbers up to 200 individuals/m² in some plots during the 2nd population peak. Survival curves of the 2nd generation from different years (1994-97) showed similar shapes and rates (Fig. 2).
Nymphs survival rates were higher in the quadrats protected by cages in comparison to the quadrats without cages (Table 1). A total of 1,562 nymphs was observed, 888 in cages and 674 in quadrats without cages, during 1995/96. The densities varied between 3 to 108 nymphs/quadrat (equivalent to 12 to 432 individuals/m²). Of these, 928 individuals reached the adult stage (664 from cages and 264 from quadrats without cages). Average survival rates in the protected quadrats did not differ during 1995/96 and 1997 generations, and were higher than those in the quadrats without cages, in the first and second generations (See ANOVA, Table 1).
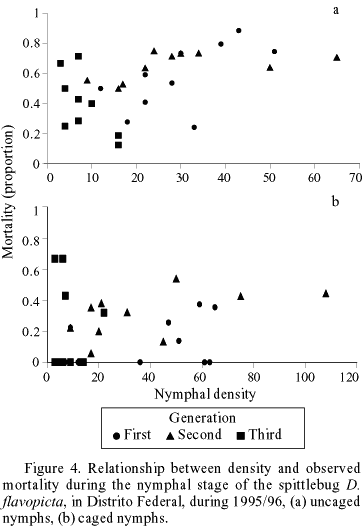
For 1995/1996, mortality rates remained constant across the instars during the three annual generations for uncaged populations (Fig. 3). In the caged treatments, no significant correlation between density and proportion of nymphal mortality was found within cages (r = 0.305, n = 30, P = 0.108) (Fig. 4b). A significant correlation was observed between nymphal densities and mortality rate, for uncaged treatments (Pearson's coefficient, r = 0.525 n = 30, P = 0.003) (Fig. 4a). However, there was no significant correlation between nymphal density and mortality rates within non-caged treatments when each generation is analyzed independently (Table 2). A negative correlation was found between the Morisita's index and the mortality rates by predation in different generations (Pearson's coefficient, r = -0.91, n = 4, P = 0.09; Fig. 5).
Direct Observations in the Insect Communities in Pastures. Observation of 62 nymphs showed that they predominantly (62%) disappear during the day. Based on this preliminary observation, eight surveys of the macrofauna (insects and spiders >  2mm) in pastures were done in different hours of the day. One was done at night (7:00 to 9:00 pm) and another at the transition of sundown to the night (6:00 to 8:00 pm). All others were distributed in order to cover the whole photophase period.
2mm) in pastures were done in different hours of the day. One was done at night (7:00 to 9:00 pm) and another at the transition of sundown to the night (6:00 to 8:00 pm). All others were distributed in order to cover the whole photophase period.
The spittlebug, D. flavopicta, was the most abundant insect amongst species belonging to 15 taxonomic groups of herbivores and detritivores observed in the study area. That species represented 95% of observed individuals and was present in all surveys (Fig. 6). The second most common insects were Acrididae and Cicadellidae (ca. 3%). Spiders and ants were the most abundant groups of predators. Small ants such as Pheidole, Solenopsis, Conomyrma and Mycocepurus were underestimated because of their recruiting behavior (Fig. 7).
Of the predators observed attacking or feeding upon spittlebugs, ants were the most abundant group (Table 3). Small ants (2-5 mm), like those belonging to the genera Pheidole and Mycocepurus, were observed attacking the early instars, while larger ants (10-14 mm) such as Pachycondyla obscuricornis Emery and Ectatomma brunneum Smith were observed preying upon 3rd5th instars. In spite of an abundance of nymphs, spiders were found attacking only adults of D. flavopicta. This result is consistent with spiders using movement as a stimulus for recognizing prey.
Discussion
Our results indicate that predation is a significant factor determining D. flavopicta's population density. They do so by significantly altering the population density of D. flavopicta immature stages, e.g. diapausing eggs and nymphs. The mortality rates of diapausing eggs were not related to population density, and did not vary significantly among years in the same pasture. However, the number of eggs presumably attacked by predators increased in relation to density. The response is consistent with a type 1 functional response, with an undefined plateau (Holling 1959 apud Hassel 1978). Our field observations showed the presence of small harvesting ants that could play an important role in the mortality of diapausing eggs. Ants of the genera Solenopsis, Pheidole, Conomyrma and Cyphomyrmex have been reported to prey on spittlebug eggs and nymphs (Hewitt & Nilakhe 1986). The high mortality rate of D. flavopicta eggs, presumably by predators (ca. 60%), is similar to that observed for eggs of Zulia entreriana Berg. (Homoptera: Cercopidae) in pastures of B. decumbens in the State of Mato Grosso, Brazil (Hewitt 1986).
Mortality rates were higher in nymphal populations exposed to predators than in protected ones; this result confirms the importance of predators as a mortality factor for D. flavopicta nymphs. In spite of this, no preference for nymphs of D. flavopicta was noted among the predators. In caged cohorts, nymphs mortality rates were similar for all generations of the same year. However, in cohorts exposed to predators, mortality was significantly lower in the third and last generations of the year. We suspect that the predators undergo behavioral changes in response to seasonal alterations; e.g. the reduction of precipitation and day length with the progression of the wet season could be one explanation for this observation. Ants, including some species observed in our study, were reported to be more abundant in the wet season in tropical areas (Levings 1983). A shift in prey, due to the seasonal change in D. flavopicta nymphs abundance is another possible cause of reduction in the mortality rate (Fontes et al. 1995, Sujii et al. 1995, Begon et al. 1996).
Based on our overall data, we suggest that predation of nymphs could be acting in a density-dependent mode (as proposed by Murray 1982), and that it could influence spittlebug population levels. However, when population densities in each quadrat are analyzed independently, there is no significant correlation between nymph density and mortality rates. There was also small variation and a negative correlation between the aggregation pattern of nymphs, measured through the Morisita's index (Sujii 1994), and the average mortality caused by predators among generations. These results indicate that low densities and high aggregation allow sub-populations of nymphs to find refuges from predators. High nymph densities resulted in a reduction in the average distance among individual clumps and increased probability of being found by predators. Such a situation would produce higher and less variable rates of mortality (Begon & Mortimer 1986).
The relatively constant rate of mortality among the years and within the rainy season verified in the present work is consistent with the idea that there are no specialized predators in population levels capable of regulating D. flavopicta populations. Nevertheless, the exposure of eggs and nymphs to general predators resulted in significantly higher mortality rates than occurred in the absence of predators. This predation of nymphs is expected to reduce the abundance of adults, and therefore, cultural practices and pasture management should be planned to optimize the impact of predatory action. In contrast, incorrect management of pastures may disrupt predator communities and result in high densities of D. flavopicta and, possibly, other pasture spittlebugs.
Acknowledgments
We thank João Sávio O. Paes, Cristiane Avelar, Shirley Silva, and Andrea Serafini for their help with samplings and experiments, Paulo Oliveira, Inara Leal and Marcio Pie from UNICAMP, Jacques Delabie from CEPEC/CEPLAC, and Dr. Helena Castanheira from UnB, for identifying the ants. Embrapa and Conselho Nacional de Pesquisa (CNPq) provided financial support (141.803/95-9). This study was part of a PhD Dissertation at the Universidade Estadual de Campinas, UNICAMP, Departamento de Zoologia.
Literature Cited
Received 1/11/01. Accepted 5/10/02.
- Barbosa, F.R., W.M. Moreira & C. Czepack. 1984.Beauveria bassiana (Bals.) Vuill: Promissor agente de controle biológico para a cigarrinha-das-pastagens Deois flavopicta (Stal, 1854). EMGOPA, Goiânia, 17p. (EMGOPADDI. Boletim de pesquisa, 2).
- Begon, M., J.L. Harper & C.R. Townsend. 1996. Ecology: individuals, populations and communities. 3rd ed. Blackwell Science, Oxford, 1068p.
- Begon, M. & M. Mortimer 1986. Population ecology: a unified study of animals and plants. 2nd ed., Blackwell, Oxford, 220p.
- Bueno, V.H.P. 1987. Aspectos biológicos e ritmo diário das atividades de Porasilus barbiellinii predador da cigarrinha-das-pastagens. Pesq. Agropec. Bras. 22: 903-915.
- Carneiro, M.F. 1988. Eficiência de diferentes cepas de Metarhizium anisopliae no controle de Deois flavopicta Pesq. Agropec. Bras. 23: 685689.
- Cosenza, G.W. & M.A. Naves. 1980. O controle da cigarrinha das pastagens. EMBRAPA/CPAC, Brasilia, 4 p. (EMBRAPA/CPAC, Comunicado Técnico, 6).
- Cosenza, G.W., R.P. de Andrade, D.T. Gomes & C.M.C. da Rocha. 1989. Resistência de gramíneas forrageiras à cigarrinha-das-pastagens. Pesq. Agropec. Bras. 24: 961968.
- Faria, M.R. & M.S. Tigano. 1996. Coleção de fungos entomopatogênicos do Cenargen. Embrapa, Serviço de Produção e Informação, Brasília. 76p.
- Fontes, E.M.G., C.S. Pires & E.R. Sujii. 1995. Mixed risk-spreading strategies and the population dynamics of a Brazilian pasture pest, Deois flavopicta (Homoptera: Cercopidae). J. Econ. Entomol. 88: 1256-1262.
- Hassel, M.P. 1978. The dynamics of arthropod predator-prey systems. Princeton, New Jersey, Princeton University Press. 237p. (Monographs in Population Biology, 13).
- Hewitt, G.B. 1986. Enviromental factors affecting spittlebug egg survival during the dry season in Central Brazil. Pesq. Agropec. Bras. 21: 1237-1243.
- Hewitt, G.B. & S.S. Nilakhe. 1986. Environmental factors affecting the survival of eggs and early instar nymphs of spittlebugs Zulia entreriana and Deois flavopicta during the rainy season in central Brazil. An. Soc. Entomol. Brasil 15: 6176.
- Krebs, C.J.1989. Ecological methodology. Harper Collins, New York, 654p.
- Levings, S. C.1983. Seasonal, annual, and among-site variation in the ground ant community of a deciduous tropical forest: some cases of patchy species distributions. Ecol. Monogr. 53: 435-455.
- Marques, I.M.R. 1988. Distribuição de Salpingogaster nigra Schiner, 1868 (Diptera: Syrphidae) predador específico de ninfas de cigarrinhas da raiz (Homoptera: Cercopidae) em algumas regiões do Brasil. An. Soc. Entomol. Brasil 17: 67-74.
- Menezes M. de, M.K. El Khadi, J.M. Pereira & M.A.M. Ruiz. 1983. Bases para o controle integrado das cigarrinhas-das-pastagens na região sudeste da Bahia. CEPLAC-CEPEC, Ilhéus, 33p.
- Murray Jr., B.G. 1982. On the meaning of density dependence. Oecologia 53: 370-373.
- Nilakhe, S.S. 1987. Evaluation of grasses for resistance to spittlebug. Pesq. Agropec. Bras. 22: 767-783.
- Pires C.S.S., E.M.G. Fontes, E.R. Sujii, H.M.C. Fernandes & D.F. Gomes. 1993. Ocorrência de Anagrus sp. (Homoptera: Mymaridae) parasitando ovos de Deois flavopicta (Homoptera: Cercopidae) em pastagens do Brasil Central. An. Soc. Entomol. Brasil 22: 411-413.
- Pires, C.S.S., E.R. Sujii, E.M.G. Fontes, C.A. Tauber & M.J. Tauber. 2000. Dry-season dormancy in eggs of Deois flavopicta (Homoptera: Cercopidae): roles of temperature and moisture in nature. Environ. Entomol. 29: 714-720.
- Seiffert, N.F. 1980. Gramíneas forrageiras do gênero Brachiaria Embrapa CNPGC, Campo Grande, 83p. (Embrapa CNPGC. Circular Técnica, 1).
- Sujii, E.R. 1994. Padrão de distribuição das populações anuais e modelo fenológico para o manejo da cigarrinha-das-pastagens, Deois flavopicta (Homoptera: Cercopidae). Dissertação de mestrado, UNICAMP, Campinas, SP, 77p.
- Sujii, E.R., M.A. Garcia, E.M.G. Fontes & V. Carvalho. 1995. Efeito da temperatura e umidade sobre o término da diapausa de ovos e densidade populacional da cigarrinha-das-pastagens, Deois flavopicta (Stal) (Homoptera: Cercopidae). An. Soc. Entomol. Brasil 24: 465-478.
- Villacorta, A. 1980. Susceptibilidade de ninfas de Deois flavopicta (Stal, 1854) (Homoptera: Cercopidae) a diferentes isolamentos de Metarhizium anisopliae (Metsch) Sorokin. An. Soc. Entomol. Brasil 9: 3338.
- Wilkinson, L. 1990. SYSTAT: The System for statistics: Statistics. Evanson, Illinois, SYSTAT Inc. 676p.
Publication Dates
-
Publication in this collection
20 Feb 2003 -
Date of issue
Oct 2002
History
-
Accepted
05 Oct 2002 -
Received
01 Nov 2001