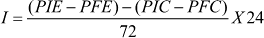Abstracts
The objective of this work was to compare the influence of dietary protein on performance and feeding behavior of immature males and females of Ceratitis capitata (Wiedemann). The protein source was beer yeast at 6.5 and 1.5 g.100 ml-1. The following parameters were evaluated: percentage of emergence, total life cycle, adult size, diet consumption, feeding preference and discrimination threshold for yeast. Immature adults showed similar protein requirements regardless of sex. Both males and females showed similar feeding behavior, preferring to feed on the diet with higher protein content. The discrimination threshold for levedure in both sexes was 0.4 g.100 ml-1. We concluded that immature males of C. capitata show similar protein requirements as the immature females.
Size sexual dimorphism; fruit fly; feeding behavior
O objetivo deste trabalho foi comparar a influência da proteína na performance e no comportamento alimentar de fêmeas e machos imaturos de Ceratitis capitata (Wiedemann). A fonte de proteína utilizada foi a levedura de cerveja nas concentrações de 6,5 g e 1,5 g por 100ml de água. Foi avaliada a influência da proteína nos seguintes parâmetros: porcentagem de emergência, duração do ciclo de vida, tamanho do adulto, consumo de dietas, seleção de dietas e limiar de discriminação para a levedura. Os resultados indicaram que na fase imatura machos e fêmeas apresentam exigência protéica similar para performance adequada. Ambos sexos apresentaram comportamento alimentar semelhante, escolhendo sempre dietas contendo maior quantidade de proteína. O limiar de discriminação para a levedura nos imaturos de ambos os sexos foi 0,4 g/100 ml de água. Com base nos resultados aqui apresentados, é possível inferir que os machos de C. capitata apresentam exigência protéica similar à das fêmeas em sua fase imatura.
Dimorfismo sexual de tamanho; mosca-das-frutas; comportamento alimentar
ECOLOGY, BEHAVIOR AND BIONOMY
Influence of protein on feeding behavior of Ceratitis capitata Wiedemann (Diptera: Tephritidae): comparison between immature males and females
Influência da proteína no comportamento de alimentação de Ceratitis capitata Wiedemann (Diptera: Tephritidae): comparação entre fêmeas e machos imaturos
Maria do C. Plácido-SilvaI; Fernando S. ZucolotoII; Iara S. Joachim-BravoI
IDepto. Biologia Geral, Instituto de Biologia Universidade Federal da Bahia. Rua Barão do Geremoabo, s/n Campus Universitário de Ondina, 40170-290, Salvador, BA
IIDepto. Biologia, Faculdade de Filosofia Ciências e Letras de Ribeirão Preto USP. Av. Bandeirantes, 3900 Monte Alegre, 14.040-901, Ribeirão Preto, SP
ABSTRACT
The objective of this work was to compare the influence of dietary protein on performance and feeding behavior of immature males and females of Ceratitis capitata (Wiedemann). The protein source was beer yeast at 6.5 and 1.5 g.100 ml-1. The following parameters were evaluated: percentage of emergence, total life cycle, adult size, diet consumption, feeding preference and discrimination threshold for yeast. Immature adults showed similar protein requirements regardless of sex. Both males and females showed similar feeding behavior, preferring to feed on the diet with higher protein content. The discrimination threshold for levedure in both sexes was 0.4 g.100 ml-1. We concluded that immature males of C. capitata show similar protein requirements as the immature females.
Key words: Size sexual dimorphism, fruit fly, feeding behavior
RESUMO
O objetivo deste trabalho foi comparar a influência da proteína na performance e no comportamento alimentar de fêmeas e machos imaturos de Ceratitis capitata (Wiedemann). A fonte de proteína utilizada foi a levedura de cerveja nas concentrações de 6,5 g e 1,5 g por 100ml de água. Foi avaliada a influência da proteína nos seguintes parâmetros: porcentagem de emergência, duração do ciclo de vida, tamanho do adulto, consumo de dietas, seleção de dietas e limiar de discriminação para a levedura. Os resultados indicaram que na fase imatura machos e fêmeas apresentam exigência protéica similar para performance adequada. Ambos sexos apresentaram comportamento alimentar semelhante, escolhendo sempre dietas contendo maior quantidade de proteína. O limiar de discriminação para a levedura nos imaturos de ambos os sexos foi 0,4 g/100 ml de água. Com base nos resultados aqui apresentados, é possível inferir que os machos de C. capitata apresentam exigência protéica similar à das fêmeas em sua fase imatura.
Palavras-chave: Dimorfismo sexual de tamanho, mosca-das-frutas, comportamento alimentar
Food consumption and utilization by animal species is crucial to provide satisfactory growth, development and reproduction. Thus, the amount consumed and the nutritional quality of food by an insect during its immature stages play a role in its immature development as well as in its adult phase (Slansky & Rodriguez 1987).
Among nutrients naturally used by insects, proteins play an important role in metabolic processes. They are the elements that most influence insect growth and fecundity (Hagen et al. 1984). Several studies try to establish a relationship between protein consumption and female reproductive maturity in several species (Robacker 1991, Cangussu & Zucoloto 1993, Ashworth & Wall 1995). Nevertheless, there are few records about the protein requirements and the influence of protein on male performance.
Several studies demonstrated that protein consumption are directly related to fitness of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), a world pest of fruits. Lack of proteinaceous food during the immature stages interferes in life cycle duration, emergence, adult size and egg production during the pre-oviposition period (Zucoloto 1988). During the adult stage, the shortage of protein affects the production of eggs specially after the pre-oviposition period, i.e., after the fifth day after emergence (Cangussu & Zucoloto 1992, 1995). Notwithstanding, most studies focus on female performance. Only recently the influence of nutrients on male performance has been investigated in detail (Kaspi et al. 2002, Rodriguero et al. 2002).
The objective of the present work is to compare the influence of protein in the development and feeding behavior of males and females of C. capitata, during the immature phase. The protein requirements of males were investigated, so as to answer a polemic question: as males do not require protein for egg production and also taking into account that females show larger body size at emergence, do males have the same protein requirements as females? The protein needs of males and females were evaluated through the following parameters: percent of emergence, life cycle duration (eclosion-emergence) and adult size. The consumption and selection for proteinaceous diets was also analysed, as was the larval discrimination ability for yeast.
Material and Methods
The medfly population used in this study was obtained from a laboratory colony maintained at the Laboratório de Entomologia from the Centro Nacional de Pesquisa de Mandioca e Fruticultura - CNPMF/EMBRAPA - Cruz das Almas - BA. The colony was maintained for approximately 15 years and routinely received wild material collected from infested fruits of tropical almond (Teminalia catappa L.). During the present work, ten collections were made in Itaparica and Salvador, and varying numbers of adults were introduced to the laboratory strain.
Population maintenance in the Laboratório de Ecologia Nutricional de Insetos, from the Departamento de Biologia Geral of the Instituto de Biologia da Universidade Federal da Bahia, followed the methodology described by Zucoloto (1987).
Adults received water and artificial diet daily (Zucoloto et al. 1979). Adult food was composed of: 6.5 g of beer yeast (Mãe-Terra), 11 g of sugar (União), 2 g of agar (Isofar), 1 g of citric acid (Vetec), 1 g of Nipagin (Isofar) and 100 ml of distilled water.
Beer yeast used for medfly lab rearing was chosen as the protein source for the test. Two diets containing different protein contents were used. The first consisted of a suitable protein content, as described above (denominated as Diet 6.5 g). The second contained only the minimum necessary as to fulfill protein requirements to larval development (1.5 g yeast/ 100 ml water) (Zucoloto et al. 1979), denominated Diet 1.5 g.
To evaluate the protein requirements of immature males and females, 25 newly emerged larvae were placed on small pieces (1.0 g) of the diet to be tested. Daily, new pieces were added to each dish until larvae completed their development. Larvae were maintained in BODs at 26ºC and 12 replicates were ran for each diet. The following parameters were compared for males and females: life cycle duration (egg hatch adult emergence), percent of eclosion, adult size. Adult size was estimated through the distance between R4+5 and Cu - M of the left wing (Zucoloto 1987) in 15 newly emerged pairs of each diet type. Adults were taken randomly from the 12 replicates. These parameters are suitable to the study of the effect of food on fruit fly larvae (Zucoloto 1988).
In order to evaluate if the food intake of immature males and females is equivalent during a determined period, an estimate of consumption was taken for 72h, according to the methodology standardized by Joachim-Bravo & Zucoloto (1998): a 1g-piece of diet 6.5 g was placed on a petri dish (90x16 mm) internally covered with humid filter paper. A 2-d old larva was placed on the piece of diet. 72h later, the larva was removed and the remaining diet weighed. The larva was reared individually in suitable diet until adult emergence, when fly was sexed. For each three experimental dishes, one check dish was used, with 1 g of diet without larvae. This check was used to calculate water loss due to evaporation along the experiment. This procedure was repeated until 20 males and 20 females were obtained.
Food consumption was calculated as follows:
being: I = ingestion; PIE = initial weight of experimental diet; PFE = final weight of experimental diet; PIC = initial weight of check diet (without larva); PFC = final weigh of check diet. With this formula, the amount consumed by the larva along 24h is inferred.
The experiment for diet selection was conducted to check if males prefer diets with lower protein content than females. It is known that, in general, larvae choose diets with higher nutritional value (Zucoloto 1987). Nevertheless, this experiment intended to check if this discrimination ability is higher in females than in males. That probably would reflect a higher protein need of females. This test followed the methodology described by Zucoloto (1987): two 0.4-g pieces of diet (6.5 g or 1.5 g diet) were placed in petri dishes (90 x16 mm) in opposite positions and equidistant from the center of the dish. Twenty newly-hatched larvae were placed in the center of each dish and 24h later, when larvae had already made their choice for the piece of diet, the different pieces of diet were separated and individually placed in dishes containing the suitable diet. Larvae were counted 48h after diet separation. These were reared with suitable diet up to emergence, when individuals were sexed. This experiment was replicated six times.
To verify whether females show better discrimination abilities to detect protein than males, the test for threshold discrimination was conducted. The procedure was similar to that formerly described (diet selection) and modified in terms of diet composition and time for larval choice. In this way, selection was for diets containing water, agar and different tested concentrations of yeast, as well as diets containing only water and agar. Three hours later, diets were separated and larvae choosing each diet were reared separately with a suitable concentration of protein until adult emergence. At emergence, adults were sexed. The initial yeast concentration was 1.0 g/100 ml of water, which was decreased at 0.2 g/100 ml intervals until larvae were not able to discriminate diets with different yeast content. At this concentration, the test formerly described was run. Each choice test was conducted six times.
Data were analyzed using Mann-Whitney at 5% of significance (Siegel 1956).
Results
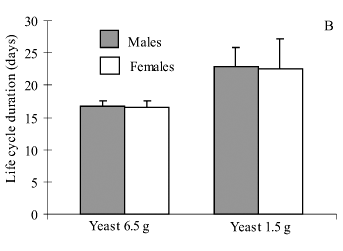
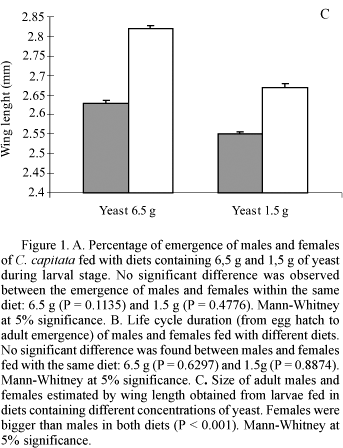
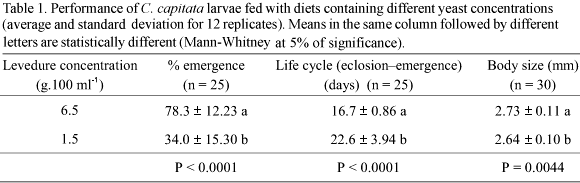
Larvae reared with the 6.5 g diet showed higher percentage of emergence, shorter life cycle (hatch-emergence) and had longer wings, when compared to larvae fed with the 1.5 g diet (Table 1). When males were compared to females in a same diet, similar results were obtained for percentage of emergence (Fig. 1A) and life cycle duration (Fig. 1B). Regarding body size, females were always bigger than males when fed on the same diet (Fig. 1C).
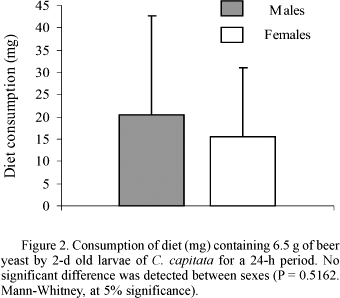
In the diet consumption experiment, males and females consumed similar amounts of diet (Fig. 2). Also, high standard deviations in relation to mean were observed, regardless of sex, showing a high degree of variability in this behavior.
To check for the existence of a relationship between diet consumption and life cycle duration, the correlation test of Spearman was conducted. Results were not significant for males (P = 0.8971) and females (P = 0.0507).
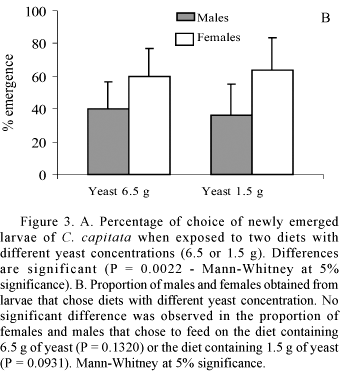
The diet selection experiment showed that immature individuals prefer to feed on diet with higher protein content (6.5 g diet) rather than on the 1.5 g diet (Fig. 3A). Males and females obtained from each diet emerged at equal proportions, indicating that there is no difference attributable to sex (Fig 3B).
In the experiment for discrimination threshold for the yeast, larvae chose to feed on diets containing yeast with at least 0.4 g/ 100 ml (Table 2). At 0.3 and 0.2 g/100 ml, larvae did not discriminate between diets with or without yeast (Table 2). No significant difference was found between males and females regarding the threshold for yeast discrimination at 0.4 g/100 ml or 0.3 g/100 ml, suggesting similar discrimination abilities in males and females (Fig. 4). Thus, the threshold for yeast discrimination for both sexes was considered 0.4 g/100 ml.
Discussion
The hypothesis that females would require more protein than males is not supported by our results. Thus, what would explain the similar protein requirements of males and females of C. capitata?
A possible explanation would be that, during the larval stage, all organs are being formed in holometabolous insects, thus needs for plastic materials would be high for individuals of both sexes (Ferro & Zucoloto 1992). Proteins are so important for immature stages as they are for adults of several insect species. During the larval stage, the ingestion of proteinaceous food is important for growth, survival, storage of nutritive material for the pupal stage and for utilization during the adult stage, mainly for egg production in some insect species (Chan et al. 1990). Regarding males, protein are essential for the formation of sexual glands, pheromones and growth, aspects that, according to some authors, could influence the sexual performance of males (Slansky & Scriber 1985, Blay & Yuval 1997). Several hypotheses could explain the observation that males become sexually more active due to the ingestion of suitable amounts of protein. Some of them are that sperm production would occur with higher intensity or earlier, pheromone production would be increased and mating behavior improved (Slansky & Scriber 1985, Warburg & Yuval 1997, Rodriguero et al. 2002). Several studies involving the ingestion of protein have been conducted for C. capitata, showing that nutrients affect its performance. Shortage of protein during the immature stage of this species leads to a decrease in the percentage of emergence, increase in development time and reduction in adult female size (Cangussu & Zucoloto 1997).
In the present work, both males and females were equally affected when they were fed on diet with low yeast concentration. The same was observed for adult size. Notwithstanding, females were also bigger than males in diets with 6.5 g and 1.5 g. This difference in body size of males and females size sexual dimorphism is common among almost all taxa and generally it is assumed as an adaptive trait (Preziosi & Fairbairn 2000, Badyaev 2002). In insects, this dimorphism generally occurs with bigger females (Biedermann 2002). Several authors attribute larger female size to its ability to produce and store eggs (Zamudio 1998, Villagra et al. 2001). On the other hand, smaller male size can be explained as a result of selection pressure for higher mobility (Zamudio 1998) and/or resource allocation to gonads and consequent trade-off for body size (Yasuda & Dixon 2002).
Regarding physiological factors involved in maintenance of the sexual dimorphism in C. capitata, the present work did not reveal differences between sexes. Females and males showed the same development time, ingested the same amount of diet, showed the same discrimination ability and threshold for discriminating yeast.
In relation to development time, some works with other insects demonstrated sexual dimorphism for size associated to protandry, what would contribute to smaller male size (Fischer & Fiedler 2001, Ernsting & Isaaks 2002, De-Block & Stoks 2003). In C. capitata, this trend is not observed. Thus, other factors should contribute to the difference in body size observed in males and females of this species.
As females emerge with larger body size and accumulate eggs, it would be expected that they also required higher amounts of protein and consumed higher amounts of food than males. Nevertheless, results obtained in the present study do not support this hypothesis either. For some insects the higher consumption of food by females contributes to their large size. In Adalia bipunctata (L.) (Coleoptera: Coccinelidae), for instance, males and females take the same time to accomplish their development, but females ingest more food during the 4th larval instar and grow bigger than males (Yasuda & Dixon 2002).
Data presented in this work showed also a high degree of variability in food consumption (high standard deviations) regardless of sex. This observation demands further investigation as, for instance, a correlation between the amount of food ingested by larvae and life cycle duration (hatch-emergence) was not observed. It is possible that the fact of this population being hybrid may have led to this high variability.
As larger female size apparently has no relationship to higher food intake, it is suggested that females show a higher efficiency in food utilization than males. In Bombyx mori (L.), males and females do not differ in relation to food consumption or assimilation, but females show a higher efficiency in food conversion (Chandrakala et al. 1999). Further experiments will demonstrate if this is the case with C. capitata.
Data regarding tests for food selection showed that immature males and females choose the diet with higher yeast concentration. It was expected that females showed a higher sensibility to higher yeast concentration than males. Nevertheless, this was not the case. Results suggest that males and females show similar choice behavior, preferring to feed on the more suitable diet with respect to protein content. Our data corroborate those from other authors that show that C. capitata can discriminate diets with higher nutritional value (Zucoloto 1987, 1991; Canato & Zucoloto 1992).
The discrimination threshold for yeast 0.4 g/100 ml water was the same for males and females. Again, it is proposed that protein is an item as important for males and females during the larval stage, so as individuals of both sexes perceive the presence of yeast at the same amount. The threshold of 0.4 g/100 ml does not agree with that obtained by Zucoloto (1987). According to this author, the discrimination threshold for yeast was 0.1 g/100 ml. A major difference between this and the work performed by Zucoloto (1987) is that the second worked with a laboratory colony, selected along several years to feed on artificial diet. For immature stages, proteins would be the most important nutrients, what agree with the fact that larvae discriminate yeast at 0.1 g/100 ml (Zucoloto 1987). It is possible also that, in the present study, the amount or protein present in the yeast was lower than in the protein used by Zucoloto (1987).
The present study showed that immature males and females of an heterozygous population of C. capitata reared under laboratory condition show the same protein requirements and the same feeding behavior in relation to protein, regardless of body size being different at emergence. A difference in food assimilation/ conversion may explain the size difference between sexes, as no differences in consumption or performance were observed. Further studies should be conducted with laboratory strains and results should be compared with wild strains.
Acknowledgments
Thanks are due to CNPq for financial support (grant 475050/2001-0).
Literature Cited
Received 02/VIII/04. Accepted 21/II/05.
- Ashworth, J.R. & R. Wall. 1995. Effects of ovarian development and protein deprivation on the activity and locomotor responses of the blowfly, Lucilia sericata, to liver odour. Physiol. Entomol. 20: 281-285.
- Badyaev, A.V. 2002. Growing apart: An ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol. Evol. 17: 369-378.
- Biedermann, R. 2002. Mating success in the spittlebug Cercopis sanguinolenta (Scopoli, 1763) (Homoptera, Cercopidae): The role of body size and mobility. J. Ethol. 20: 13-18.
- Blay, S. & B. Yuval. 1997. Nutritional correlates of reproductive success of male Mediterranean fruit flies (Diptera: Tephritidae). Anim. Behav. 54: 59-66.
- Canato, C.M. & F.S. Zucoloto. 1992. Influência da concentração de nutrientes no valor nutritivo e seleção de dietas em larvas de Ceratitis capitata (Diptera, Tephritidae). An. Soc. Entomol. Brasil 22: 471-476.
- Cangussu, J.A. & F.S. Zucoloto. 1992. Nutritional value and selection of different diets by adult Ceratitis capitata fruit flies. J. Insect Physiol. 38: 485-491.
- Cangussu, J.A. & F.S. Zucoloto. 1993. Influence of partial malnutrition on egg production by Ceratitis capitata (Diptera, Tephritidae). Rev. Bras. Biol. 53: 155-158.
- Cangussu, J.A. & F.S. Zucoloto. 1995. Self-selection and perception threshold in adult females of Ceratitis capitata J. Insect Physiol. 41: 223-227.
- Cangussu, J.A. & F.S. Zucoloto. 1997. Effect of protein sources on fecundity, food acceptance and sexual choice by Ceratitis capitata (Diptera, Tephritidae). Rev. Bras. Biol. 5: 611-618.
- Chan, H.T., J.D. Hansen & S.Y.T. Tam. 1990. Larval diets from different protein sources for Mediterranean fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 83: 1954-1958.
- Chandrakala, M.V., R. & S.K. Rao. 1999. Food and dietary water utilization in male and female silkworms (Bombyx mori L.) of a sex-limited bivoltine race. Indian J. Seric. 38: 131-134.
- De-Block, M. & R. Stoks. 2003. Adaptive sex-specific life history plasticity to temperature and photoperiod in a damselfly. J. Evol. Biol. 16: 986-995.
- Ernsting, G. & J.A. Isaaks. 2002. Gamete production and sexual size dimorphism in an insect (Orchesella cincta) with indeterminate growth. Ecol. Entomol. 27: 145-151.
- Ferro, M.I.T. & F.S. Zucoloto. 1992. Efeitos da quantidade de aminoácidos na performance de larvas de Ceratitis capitata (Diptera, Tephritidae). Naturalia 17: 111-117.
- Fischer, K. & K. Fiedler. 2001. Sexual differences in life-history traits in the butterfly Lycaena tityrus: A comparison between direct and diapause development. Entomol. Exp. Appl. 100: 325-330.
- Hagen, K.S., R.H. Dadd & J. Reese. 1984. The food of insects. Ecol. Entomol. [s. v.]: 79-112.
- Joachim-Bravo, I.S. & F.S. Zucoloto. 1998. Performance and feeding behavior of Ceratitis capitata: Comparison of a wild population and a laboratory population. Entomol. Exp. Appl. 87: 67-72.
- Kaspi, R., S. Mossinson, T. Drezner, B. Kamensky & B. Yuval. 2002. Effects of larval diet on development rates and reproductive maturation of male and female Mediterranean fruit flies. Physiol. Entomol. 27: 29-38.
- Preziosi, R.F. & D.J. Fairbairn. 2000. Lifetime selection on adult body size and components of body size in a waterstrider: Opposing selection and maintenance of sexual size dimorphism. Evolution 54: 558-566.
- Robacker, D.C. 1991. Specific hunger in Anastrepha ludens: Effects on attractiveness of proteinaceous and fruit-derived lures. Environ. Entomol. 20: 1680-1686.
- Rodriguero, M.S., J.C. Vilardi, M.T. Vera, J.P. Cayol & E. Rial. 2002. Morphometric traits and sexual selection in medfly (Diptera: Tephritidae) under field cage conditions. Fla. Entomol. 85: 143-149.
- Siegel, S. 1956. Nonparametric statistics for the behavioral sciences. New York, MacGraw-Hill, 340p.
- Slansky Jr., F. & J.G. Rodriguez. 1987. Nutritional ecology of insects, mites, spiders and related invertebrates, New York, Wiley,1016p.
- Slansky Jr., F. & M.R. Scriber. 1985. Food consumption and utilization, p.88-151. In Comprehensive insect physiology, biochemistry and pharmacology, vol. 9. Ed. Kerkut, S. A. and Gilbert, J. F. Oxford, Pergamon Press, 743p.
- Villagra, C.A., C.A. Villalobos, D.H. Tapia & A.K. Rodriguez. 2001. Sexual dimorphism and behaviour in the water strider Gerris chilensis (Berg) (Hemiptera: Gerridae). Rev. Chil. Entomol. 28: 87-93.
- Warburg, M.S. & B. Yuval. 1997. Effects of energetic reserves on behavioral patterns of Mediterranean fruit flies. Oecologia 112: 314-319.
- Yasuda, H. & A.F.G. Dixon. 2002. Sexual size dimorphism in the two spot ladybird beetle Adalia bipunctata: Developmental mechanism and its consequences for mating. Ecol. Entomol. 27: 493-498.
- Zamudio, K. 1998. The evolution of female-biased sexual size dimorphism: A population-level comparative study in horned lizards (Phrynosoma). Evolution 52: 1821-1833.
- Zucoloto, F.S. 1987. Feeding habits of Ceratitis capitata: Can larvae recognize a nutritional effective diet? J. Insect Physiol. 33: 349-353.
- Zucoloto, F.S. 1988. Qualitative and quantitative competition for food in Ceratitis capitata Rev. Bras. Biol. 48: 523-526.
- Zucoloto, F.S. 1991. Effects of flavour and nutritional value on diet selection by Ceratitis capitata larvae (Diptera: Tephritidae). J. Insect Physiol. 37: 21-25.
- Zucoloto, F.S., S. Puschel & C.M. Message. 1979. Valor nutritivo de algumas dietas artificiais para Anastrepha obliqua (Diptera: Tephritidae). Bol. Zool. Univ. S. Paulo 4: 75-80.
Publication Dates
-
Publication in this collection
26 Sept 2005 -
Date of issue
Aug 2005
History
-
Accepted
21 Feb 2005 -
Received
02 Aug 2004