Abstract
Adenocalymma bracteatum is a shrub of dense foliage and yellow flowers, easily found on grasslands areas in Central Brazil. The aim of this study was to determine the reproductive biology and the flower visitors of A. bracteatum in a pasture area nearby Ivinhema city, MS (Brazil). The flowering peak occurs in winter. The flower reflects ultraviolet light. Anthesis begins at 6:30h, and pollen and nectar are the resources to visitors. We captured 1,038 floral visitors. The bees Apis mellifera (L.), Trigona sp., Trigona spinipes (Fabricius), (Hymenoptera: Apoidea) and the ant Cephalotes sp. (Hymenoptera: Formicidae) were the main visitors. The reproductive tests indicate that A. bracteatum is self compatible, justifying its expansion in altered environments; however, the largest reproductive success was dependant on cross-pollination and self-pollination, evidencing the pollinators importance. Adenocalymma bracteatum presents melittophilous syndrome and bumblebees were the main pollinators in the area. The correlations observed between the climatic variables and the main pollinator species were low or medium.
Floral trait; pillager; pollinator; bee
ECOLOGY, BEHAVIOR AND BIONOMICS
Pollination of Adenocalymma bracteatum (Bignoniaceae): floral biology and visitors
Stela Almeida-SoaresI; Leandro P PolattoI; João C S DutraII; Helena M Torezan-SilingardiIII
IPPG em Entomologia e Conservação da Biodiversidade, Univ Federal da Grande Dourados, CP 351, 79804-907 Dourados, MS, Brasil; stelasoares@ufpr.br, lppolatto@gmail.com
IIPPG em Ciências Biológicas (Zoologia), Univ Estadual Paulista, Campus de Rio Claro, CP 199, 13506-900 Rio Claro, SP, Brasil; jcstanzani@uol.com.br
IIIInstituto de Biologia, Univ Federal de Uberlândia, CP 593, 38400-902 Uberlândia, MG, Brasil; torezan@inbio.ufu.br
ABSTRACT
Adenocalymma bracteatum is a shrub of dense foliage and yellow flowers, easily found on grasslands areas in Central Brazil. The aim of this study was to determine the reproductive biology and the flower visitors of A. bracteatum in a pasture area nearby Ivinhema city, MS (Brazil). The flowering peak occurs in winter. The flower reflects ultraviolet light. Anthesis begins at 6:30h, and pollen and nectar are the resources to visitors. We captured 1,038 floral visitors. The bees Apis mellifera (L.), Trigona sp., Trigona spinipes (Fabricius), (Hymenoptera: Apoidea) and the ant Cephalotes sp. (Hymenoptera: Formicidae) were the main visitors. The reproductive tests indicate that A. bracteatum is self compatible, justifying its expansion in altered environments; however, the largest reproductive success was dependant on cross-pollination and self-pollination, evidencing the pollinators importance. Adenocalymma bracteatum presents melittophilous syndrome and bumblebees were the main pollinators in the area. The correlations observed between the climatic variables and the main pollinator species were low or medium.
Key words: Floral trait, pillager, pollinator, bee
The interactions between animals and plants are influenced by several community-specific biotic and abiotic factors. The study of such characteristics, joined to the species phenology, is crucial to understand the co-evolutionary and ecological processes observed between plants and pollinators (Pellmyr & Thompson 1996, Thompson 1999, 2005). Although widely spread and diversified, the relations between plants and pollinators are seldom studied regarding the impact of seasonal changes and species phenology on ecological interactions (Marques & Oliveira 2004, Torezan-Silingardi & Oliveira 2004, Silva & Torezan-Silingardi 2009). Therefore, the study of the behaviour of floral visitors is an important tool to identify patterns of ecological interactions between visitors and flowers, commonly known as "pollination ecology" (Quesada et al 2008). Most of such relations are mutualistic, whereby plant species offer floral resources and obtain pollination benefits. Fewer relations are unilateral, where plants can be used as substrates or for other purposes such as for prey capture, mating, and resting (Gonçalves-Alvim & Macedo 1998, Gottsberger & Silberbauer-Gottsberger 2006). Other relations involve the pillage of plant resources by floral visitors, i.e. the use of resources by illegitimate visitation incapable of promote pollination (Inouye 1980).
Pollination ecology approaches several processes that associate behavioral interactions among floral visitors and the morphological and physiological traits of the flower (Proctor et al 1996, Waser & Ollerton 2006, Mitchell et al 2009, Del-Claro & Torezan-Silingardi 2009). Vitalli-Veiga & Machado (1994) argued that from the animal perspective, pollination is a by-product of the collection of a useful resource provided by a plant, usually nectar or pollen. For plants, pollination is a tool for maximizing gene flux. Therefore, insect-plant trophic relations during the pollination process are crucial for the nourishment and survival of flower visitors (Gonçalves-Alvim & Macedo 1998, Gottsberger & Silberbauer-Gottsberger 2006, Nascimento & Del-Claro 2007). Pollination studies enhance our understanding of such interactions and provide feedback information for the appropriate management and preservation of tropical environments. When the species being studied belong to important families characterized by numerous species, high abundance of individuals, and wider geographic distribution, the information obtained is still more relevant. This is the case of the Bignoniaceae family (Udulutsch et al 2004, Kinoshita et al 2006), with about 800 species and the predominance of lianas belonging to tribe Bignoniae in the Neotropical region (Gentry 1974a, 1980).
With the aim of increasing the knowledge about the importance of ecological relations on pollination, the objective of this study was to identify the floral visitors of Adenocalymma bracteatum (tribe Bignoniae), to describe the behaviour of the most frequent and the potential effective pollinators, and to characterize the floral and reproductive biology of the relation between environmental factors and the visiting species.
Material and Methods
Field research was conducted on a roadside approximately three kilometers north of urban Ivinhema city, state of Mato Grosso do Sul (22º18'S, 53º48'W), Brazil. This is a transition area in terms of floristic composition, containing grasses, shrubs and creepers (in pastures), and a secondary forest in intermediate stage of regeneration according to the criteria proposed by Budowski (1965, 1970), where A. bracteatum is one of the predominant plant species in both environments, with aggregate distribution and occurrence in dense groups.
The flowering and fructification phenology of A. bracteatum was determined, and characterized by the following phenophases: floral buds, opened flowers, small and green fruits, developed fruits, completely open fruits, and seed fall (Fournier 1974, Polatto & Alves Jr 2009). Observations were conducted at fixed intervals of ten days during one year, to obtain data for a phenological species calendar.
Flowers were observed from bud formation to petal and sepal fall. Stigma receptivity was identified as opening of stigmatic lobes and stigma moisturizing aspect. Odoriferous cells (named osmophores) were detected following the method by Vogel (1962) with neutral red. Odour was analysed after storing the flowers in sealed plastic bags for 1h to concentrate the odoriferous substance. Ultra-violet absorption and reflexion spots were detected with ferric chloride solution in 1% sulphuric ether in aqueous solution (Vogel 1983).
The reproductive efficiency was assessed by isolating flower buds (n = 150) in impermeable paper bags to inhibit their contact with flower visitors. Immediately after anthesis, some flowers (n = 30) were hand pollinated with their own pollen and others (n = 30) with pollen from other flowers in the same plant to test autogamy and geitonogamy, respectively. Some (n = 30) isolated flowers were emasculated to observe the occurrence of agamospermy. Crossed pollination was tested by transferring pollen from bagged flowers to another bagged flowers of different plants (n = 30). Whole flowers (n = 30) were kept in the bags to observe spontaneous selfpollination. Buds (n = 30) were marked for fruit production in natural conditions (control). Flower development was observed up to fruit formation. The flowers and fruits produced by inflorescence (n = 30 plants) were counted and the relations between them were established to lighten the energetic input required for A. bracteatum reproduction.
The reproductive system of A. bracteatum was also analysed according to the following indexes: spontaneous self-pollination index (% of fruits produced by spontaneous self-pollination / % of fruits produced by hand selfpollination), self-incompatibility index (% of fruits produced by manual self-pollination / % of fruits formed by crossed pollination), and the reproductive efficiency index (% of fruits formed in natural conditions / % of fruits produced by crossed pollination) (Torezan-Silingardi & Oliveira 2004). These were used to assess the dependence of biotic agents as pollinators, the need of genetic flux, and the efficiency of pollinators, respectively.
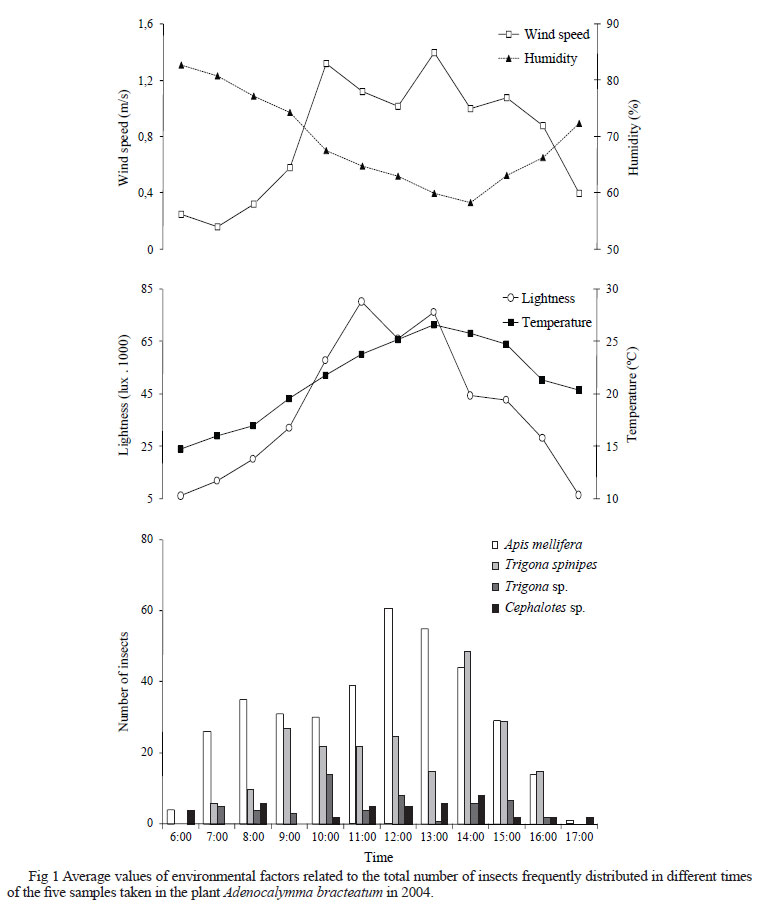
Five insect collections were conducted in each plant. The insects were captured using sweep nets and tweezers, every hour during the foraging period of flower visitors, from 6:00h to 18:00h. Environmental data on temperature, luminosity, wind speed, and relative air humidity were recorded at the beginning of each collection period. Insects were placed into separate vials containing Dietrich solution and then transferred to 70% ethanol for further identification (Borror & Delong 1969, Michener 2000). Specimens were dry-mounted and deposited in the insect collection at the Universidade Estadual de Mato Grosso do Sul (UEMS), Teaching Unity of Ivinhema, Brazil.
The behaviour of the most frequent visitors and potential pollinators was observed, described, and classified following terminology parameters defined by Inouye (1980). Data from the most frequent visitors were correlated with environmental factors using the Spearman correlation coefficient at 5% significance level.
Results and Discussion
Phenological characteristics.Adenocalymma bracteatum had only one flowering period during the 12 months of the study, from February through June with the peak in may (Table 1), coinciding with the dry and rainy periods. In a semideciduous forest area in Campinas, São Paulo state, the same species had two flowering periods in 12 months, coinciding with the driest periods (Yanagizawa & Maimoni-Rodella 2007).
The flowering pattern of this species was classified as cornucopia due to the production of several flowers per day for several weeks and high intra- and inter-population synchrony, according to the patterns proposed by Gentry (1974a,b) and Newstrom et al (1994). The daily production of several flowers per plant of A. bracteatum can provide an important attractive element to long-distance pollinators (Kill & Costa 2003).
Floral characteristics. Adenocalymma bracteatum individuals produced bright yellow flowers with gamopetalous corolla in throat format and with five lobes free in the extremities, very similar to the yellow 'ipê` Tabebuia crysotricha, another Bignoniaceae species common in Brazil. The flowers were hermaphrodite with four free stamens and one staminoid in the basal part of the corolla. The four anthers were enclosed and dehiscence occurred by longitudinal slits. The gynaecium consisted of a superior ovary, a short style, and a bilabial stigma that opened when receptive and closed when touched, usually at the time pollination or senescence occurred, as usual for the Bignoniaceae species (Joly 1991).
The delicate corolla with membranous texture and enclosed anthers, in addition to other structural (zygomorphic symmetry, landing platform, and throat-format corolla) and functional traits presented by A. bracteatum, pointed to the melitophilous syndrome, being mainly adapted to pollination by large bees (sensu Faegri & van der Pijl 1979), whose bodies are more than 14 mm long and thorax is 6 mm wide, following Roubik (1989). Due to such symmetry, pollinating bees usually approached flowers from only one direction, the front side, landed in the landing platform, and penetrated the corolla to collect nectar. The flower longitudinal slits provided a morphological adaptation to increase the adjustment of the corolla to the bodies of large bees, thus ensuring the contact between pollinator and flower reproductive parts. The corolla dorsi-ventral constriction is very helpful to flower pollination because it exerts pressure on their reproductive parts during the visitation by large bees (Dutra & Machado 2001).
The development of the flowers from an inflorescence of A. bracteatum occurred as a sequence from the base to the apex in the inflorescential axis, so a single inflorescence could have flower buds, open flowers, flowers in senescence, and occasionally also one or more fruits. Anthesis started at 6 am, following a diurnal pattern. The receptive stigma could be identified early, either by its moist surface or the open stigma lobes. Pollen release occurred simultaneously to anther dehiscence, from zero to 60 min after anthesis. The throat-like floral morphology and the spatial arrangement of anthers and stigma on the upper part of the corolla opening could enhance nototribic fixation of the pollen on large bee bodies (i.e., pollen deposition on the upper thorax of bees, usually between the wings). They could also permit the transference of pollen grains to the stigma of other flowers, thus enhancing plant xenogamic reproduction. Four to five days later, the corolla was released from the calyx and occasionally remained attached to the style for a few hours. The closure of stigmatic lobes could prevent the loss of pollen grains. The sensitive stigmas are often found in plants with tubular corollas that produce fruits able to store several seeds (Bertin 1982), such as A. bracteatum. The flowers did not have osmophores and did not exhale odour. The absorption and reflection of ultra-violet rays were observed in the corolla, the anthers, and the stigma. Petal pigments are known to reflect ultraviolet rays and therefore guide visitors to the floral resources (Kay 1987, Harborne 1993).
Reproduction system. Adenocalymma bracteatum produced an average of 42 floral buds per inflorescence (n = 42) that bloomed gradually. The plant had a high abortion rate from the bloom phase up to the ripefruit phase, consequently only 1.6% of the buds reached fructification (mean of 0.6 fruit per inflorescence). Mitchell (1994) observed that the production of several flowers can deplete plant resources and proportionally decrease fruit production. The large number of flower abortions observed in A. bracteatum can be considered a common event in plants (Casper & Wiens 1981, Bawa & Webb 1984).
The highest fructification was obtained by manual cross pollination and can be due either to the low pollination efficacy in the region or the high levels of damage caused by floral pillagers and herbivores, which do not damage bagged flowers. Whereas, the fructification produced after natural pollination was directly affected by these factors. Several other species of Bignoniaceae also had low rates of natural fructification (Stephenson 1979, Bertin 1982, Bittencourt et al 2003, Yanagizawa & Maimoni-Rodella 2007). The absence of fruit formation in emasculated flowers, the low fructification rate obtained by spontaneous self-pollination, and the low index value of spontaneous self-pollination ( ISS < 0.25) indicates the high importance of pollinators services for this species reproduction.
Our results are similar to those obtained in a previous study on crossed and natural pollination, and emasculation for the same species (Yanagizawa & Maimoni-Rodella 2007). However, differently from our study, those authors could not reach fructification in their tests on manual and spontaneous pollination. The self-incompatibility index value (ISI = 0.93) obtained for A. bracteatum in our study classifies the species as highly self-compatible (sensu Bullock 1985), as previously found for another Bignoniaceae, Astianthus viminalis (ISI = 0.94) by Bullock (1985). The A. bracteatum self-incompatibility results obtained in our study places the species in a small group within the family, because a recent survey with 37 species of Bignoniaceae revealed that 86% of them were self-incompatible (Bittencourt et al 2003). Several authors have reported different levels of self-incompatibility within the family (Bawa 1974, Stephenson & Thomas 1977, Correia et al 2005, Yanagizawa & Maimoni-Rodella 2007). The values reported are usually lower than those reported in our study. The reproductive efficacy obtained (0.33) suggests that flower visitors were able to promote pollination in A. bracteatum, although it could be greater if the effective pollinators foraged the flowers more frequently and the floral pillagers and floral herbivores were absent (Table 2).
Flower visitors. Insect collection in A. bracteatum resulted in 1,038 individuals belonging to seven orders: Hymenoptera (81%), Thysanoptera (6.8%), Diptera (6%), Coleoptera (3%), Lepidoptera (2%), Hemiptera (1%), and Orthoptera (0.2%). The most frequent species within the order Hymenoptera were the bees Apis mellifera (L.) (36%) (Hymenoptera: Apidae), Trigona spinipes (Fabricius) (22%), Trigona sp. (5%) and the ant Cephalotes sp. (4%) (Formicidae). No aggression among floral visitors was observed even when there were few flowers. Apis mellifera and T. spinipes were often found visiting the same flower, where one collected pollen, while the other obtained nectar.
Fructification depends on several factors, including quality and amount of pollen grains produced per flower, nectar concentration and amount, flower number and distribution, number of competing insects, simultaneous flowerings, distance from flowering to colony (Vitalli-Veiga et al 1999, Silva & Torezan-Silingardi 2008), inadequate pollination (Torezan-Silingardi & Del-Claro 1998), lack of nutritional resources in the plant (Schemske & Horvitz 1988), strong wind and rain, chance, or excessive bud production by the mother-plant to ensure that enough flowers remain for fructification after herbivore activity (Strauss et al 1996, Strauss 1997). All these factors can vary, and so can the attractiveness of any flowering.
The relationships of environmental variables on A. bracteatum and the four most frequent insect species are shown in Fig 1. The degree of association between the most frequent visitors and environmental factors revealed that the visits of A. mellifera had a positive correlation with luminosity (0.811) - Spearman correlation coefficient and temperature (0.692), and a negative correlation with humidity (-0.664). Apis mellifera were very active from 12:00h to 13:00h, (Online Supplementary Material 2 ), when temperature was around 23ºC and luminosity was 80,260 lux, wind speed was 1 m/s, and relative air humidity was 64%. Early in the morning, this bee either landed on pre-anthesis buds and opened them with fast movement of its mandibles and forelegs, or landed on the opened flowers and entered the corolla to extract almost all the nectar at the base of the corolla tube. Then, the bee went to other flowers in the same or other inflorescences of the same plant species. Apis mellifera was considered an occasional pollinator because it seldom touched the flower reproductive organs during foraging activity. This bee had a similar behavior while collecting pollen from shrubs of Myrtaceae in the cerrado of São Paulo state. Honeybees would arrive early to the flowers and force the petals to open to have access to the floral resource, visiting several flowers in the same plant before moving on to a different shrub (Torezan-Silingardi & Del-Claro 1998).

Trigona spinipes was positively correlated to luminosity (0.640) and temperature (0.650), although negatively correlated to humidity (-0.682). The activity of this bee was more intense from 14:00h to 15:00h, at 25ºC, wind speed of 1 m/s, air relative humidity of 63%, and luminosity of 66,200 lux. This bee species behaved as a nectar pillager, according to the classification by Inouye (1980). Trigona spinipes grasped the floral tube of A. bracteatum with their legs and began to cut the wall with their mandibles, making round openings of different sizes that led to the nectariferous chamber, and further introduced their mouth pieces into the openings and sucked the nectar, behaving as described by Polatto et al (2007).
Trigona sp. no had correlation with environmental factors and hour of capture. The greatest activity was from 10:00h to 11:00h at 21ºC, luminosity of 57,840 lux, wind speed of 1 m/s, and air relative humidity of 65%. Trigona sp. behaved similarly to T. spinipes, considered a nectar pillager, but visits were shorter and involved fewer individuals (Online Supplementary Material).
Based on our data, we suggest two hypotheses about the importance of insects that pillage nectar on A. bracteatum. The first one is that T. spinipes and Trigona sp. can be beneficial to fruit production. This would happen as the effective pollinators would probably be forced to move from flower to flower more often and to fly longer distances between plants due to the decreased amount of nectar in the flower, as already observed by others in different systems (Silberbauer-Gottsberger & Gottsberger 1988, Cushman & Beattie 1991, Maloof 2001). The second hypothesis is that the pillage has potential negative effects on plant reproduction, what seems to be more applicable to our study. The negative effects of pillage have been shown in previous research due to the damage to the reproductive tissues (Traveset et al 1998, Polatto & Alves Jr 2008), the lower attractiveness of pillaged flowers to efficient pollinators (Roubik 1982, Irwin & Brody 1998, Traveset et al 1998, Cotton 2001), the resource investment to replace the removed nectar (Pyke 1991, Navarro 1999), and also the aggressive interference of pillagers on pollinators (Roubik 1982).
Cephalotes sp. had no correlation with environmental factors and hour of collection. As reported by Polatto et al (2007), we also found this ant sucking nectar from flowers and buds, and walking on peduncles and branches. However, we have no direct evidence that these ants act as plant pollinators. Dutra & Machado (2001) suggested the existence of a symbiotic behaviour between ants and plants, where one provides food and the other keeps herbivores away (see examples in Del-Claro 2004 and Korndörfer & Del-Claro 2006).
Due to its large size and foraging behaviour on flowers of A. bracteatum, Eulaema nigrita (Lepeletier) was considered an effective pollinator because it touched the stigma on every visit, as it collected pollen grains by vibration and promoted flower pollination. Torezan-Silingardi & Del Claro (1998) observed that flowers of Campomanesia pubescens (Myrtaceae) intensely visited by A. mellifera were avoided by the main native pollinator, E. nigrita. The same authors found that fructification of the studied species was threatened by the intense activity of A. mellifera, which collected the pollen but did not transfer it to the stigma. Adversely, E. nigrita behaved as pillager of the Bignoniaceae Arrabidaea conjugata in the 'restingas` of Maricá, state of Rio de Janeiro, where Euglossa cordata (Latreille), Centris analis (Fabricius) and Centris tarsata (Smith) were considered effective pollinators (Correia et al 2005). From a total of 15 species of floral visitors found in A. bracteatum in a mesophyllous forest in Campinas, state of São Paulo, only the bees Epicharis rustica (Olivier) and Eulaema sp. were considered effective pollinators (Yanagizawa & Maimoni-Rodella 2007).
The large bees with hairy thorax Bombus sp. and Xylocopa sp. remained in hover flight and facing the corolla for a few minutes, and then landed on the landing platform and walked toward the corolla base with the tongue stretched out on the longitudinal slit. The bees then entered the flower, touched its reproductive structures and pressed their thorax against the flower reproductive organs to adjust their bodies to the corolla morphology. Since these were legitimate visits, these bees can be considered as effective pollinators. Another trait favouring efficient pollination by many large pollinators is their potential mobility among plants (Polatto & Alves Jr 2008).
Our observations of A. bracteatum suggest that the bees Trigona spinipes and Trigona sp. are nectar pillagers and A. mellifera may be a sporadic pollinator. Eulaema nigrita, Bombus sp. and Xylocopa sp. are the main pollinators and act during the mornings. The correlations observed between the climatic variables and the main pollinator species were low or medium.
Acknowledgments
The authors are indebted to Universidade Estadual de Mato Grosso do Sul, for the financial and structural support granting of scholarships to S A Soares and L P Polatto and also to Fapemig for a PosDoc grant to H M Torezan-Silingardi.
References
Bawa K S (1974) Breeding systems of tree species of a lowland tropical community. Evolution 28: 85-92.
Bawa K S, Webb C J (1984) Flower, fruit and seed abortion in tropical forest trees: implications for the evolution of paternal and maternal reproductive patterns. Am J Bot 71: 736-751.
Bertin R I (1982) Floral biology, hummingbird pollination and fruit production of Trumpet Creeper (Campsis radicans, Bignoniaceae). Am J Bot 69: 122-124.
Bittencourt Jr N S, Gibbs P E, Semir J (2003) Histological study of post-pollination events in Spathodeacampanulata Beauv. (Bignoniaceae), a species with lateacting self-incompatibility. Ann Bot 91: 827-834.
Borror D J, Delong D M (1969) Introdução ao estudo dos insetos. São Paulo, Edgars Blucher, 653p.
Budowski G (1965) Distribuiton of tropical American forest species in a light of sucessional process. Turrialba 15: 40-42.
Budowski G (1970) The distinction between old secondary and climax species in tropical central American lowland forests. Trop Ecol 11: 44-48.
Bullock S H (1985) Breeding systems in the flora of a tropical deciduous forest in Mexico. Biotropica 17: 287-301.
Casper B B, Wiens D (1981) Fixed rates of random ovule abortion in Cryptantha flava (Boraginaceae) and its possible relation to seed dispersal. Ecology 62: 866-869.
Correia M C R, Pinheiro M C B, Lima H A (2005) Biologia floral e polinização de Arrabidaea conjugata (Vell.) Mart. (Bignoniaceae). Acta Bot Bras 19: 501-510.
Cotton P A (2001). The behavior and interactions of birds visiting Erythrina fusca flowers in the Colombian Amazon. Biotropica 33: 662-669.
Cushman J H, Beattie A J (1991) Mutualisms: assessing the benefits to hosts and visitors. Trends. Ecol Evol 6: 191-195.
Del-Claro K (2004) Multitrophic relationships, conditional mutualisms, and the study of interaction biodiversity in tropical savannas. Neotrop Entomol 33: 665-672.
Del-Claro K, Torezan-Silingardi H M (2009) Insect-plant interactions: new pathways to a better comprehension of ecological communities in neotropical savannas. Neotrop Entomol 38: 159-164.
Dutra J C S, Machado V L L (2001). Entomofauna visitante de Stenolobium stans (Juss.) Seem (Bignoniaceae), durante seu período de floração. Neotrop Entomol 30: 43-53.
Faegri K, Van der Pijl L (1979). The principles of pollination ecology. 3. ed. London, Perganon Press, 244p.
Fournier L A (1974). Un método cuantitativo para la medición decaracterísticas fenológicas en árboles. Turrialba 24: 422-423.
Gentry A H (1974a) Coevolutionary patters in central american Bignoniaceae. Ann Mo Bot Gard 61: 728-759.
Gentry A H (1974b) Flowering phenology and diversity in tropical Bignoniaceae. Biotropica 6: 64-68.
Gentry A H (1980) Bignoniaceae - Part I (Crescentieae and Tourrettieae). Flora Neotrop 25: 1-130.
Gonçalves-Alvim S J, Macedo C F (1998) Insetos visitantes de capítulos de Heterocondylus alatus (Asteraceae) no Parque Nacional da Serra da Canastra, MG. Braz J Ecol 2: 102-107.
Gottsberger G, Silberbauer-Gottsberger I (2006) Life in the Cerrado: a South American tropical seasonal ecosystem. Vol.II. Pollination and seed dispersal. Reta Verlag, Uml, 383p.
Harborne J B (1993) Introduction to ecological biochemistry. 4. ed. London, Academic Press, 332p.
Inouye D W (1980) The terminology of floral larceny. Ecology 61: 1251-1253.
Irwin R E, Brody A K (1998) Nectar robbing in Ipomopsis agregata: effects on pollinator behavior and plant fitness. Oecologia 116: 519-527.
Joly A B (1991) Botânica: introdução à taxonomia vegetal. São Paulo, Nacional, 777p.
Kay Q O N (1987) Ultraviolet patterning and ultraviolet-absorbing pigments in flowers of the Leguminosae, p.317-354. In Stirton C H (ed) Advances in legume systematics Part 3, Royal Botanic Gardens, Kew, 480p.
Kill L H P, Costa J G (2003) Biologia floral e sistema de reprodução de Annona squamosa L. (Annonaceae) na região de Petrolina-PE. Cienc Rur 33: 851-856.
Kinoshita L S, Torres R B, Forni-Martins E R, Spinelli T, Ahn Y J, Constâncio S S (2006) Composição florística e síndromes de polinização e de dispersão da mata do Sítio São Francisco, Campinas, SP, Brasil. Acta Bot Bras 20: 313-327.
Korndörfer A P, Del-Claro K (2006) Ant defense versus induced defense in Lafoensia pacari (Lythraceae), a myrmecophilous tree of the Brazilian cerrado. Biotropica 38: 786-788.
Maloof J E (2001) The effects of a bumble bee nectar robber on plant reproductive success and pollinator behavior. Am J Bot 88: 1960-1965.
Marques M C M, Oliveira P E A M (2004) Fenologia de espécies do dossel e do sub-bosque de duas florestas de restinga na Ilha do Mel, sul do Brasil. Rev Bras Bot 27: 713-723.
Michener C D (2000) The bees of the world. Baltimore & London, Maryland, The Johns Hopkins University Press, 913p.
Mitchell R J (1994) Effects of floral traits, pollinator visitation, and plant size on Ipomopsis aggregata fruit production. Am Nat 5: 143-150.
Mitchell R J, Irwin R E, Flanagan R J, Karron J D (2009) Ecology and evolution of plant-pollinator interactions. Ann Bot 103: 1355-1363.
Nascimento E A, Del-Claro K (2007) Floral visitors of Chamaecrista debilis (Vogel) Irwin & Barneby (Fabaceae-Caesalpinoidea) at cerrado of Estação Ecológica de Jataí, São Paulo State, Brazil. Neotrop Entomol 36: 619-624.
Navarro L (1999) Pollination ecology and effect of nectar removal in Macleania bullatu (Ericaceae). Biotropica 31: 618-625.
Newstrom L E, Frankie G W, Baker H G (1994) A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La Selva. Biotropica 26: 141-159.
Pellmyr O, Thompson J N (1996) Sources of variation in pollinator contribution within a guild: the effects of plant and pollinator factors. Oecologia 107: 595-604.
Polatto L P, Dutra J C S, Alves Jr V V (2007) Biologia reprodutiva de Pyrostegia venusta (Ker-Gawl) Miers (Bignoniaceae) e comportamento de forrageamento dos visitantes florais predominantes. Rev Biol Neotrop 4: 46-57.
Polatto L P, Alves Jr V V (2008) Utilização dos recursos florais pelos visitantes em Sparattosperma leucanthum (Vell.) K. Schum. (Bignoniaceae). Neotrop Entomol 37: 389-398.
Polatto L P, Alves Jr V V (2009) Sistema reprodutivo de Sparattosperma leucanthum (Vell.) K. Schum. (Bignoniaceae). R Árvore 33: 289-296.
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Oregon, Harper Collins Publishers, 479 p.
Pyke G H (1991) What does it cost a plant to produce floral nectar? Nature 350: 58-59.
Quesada M, Rosas F, Herrerias-Diego Y, Aguliar R, Lobo J A, Sanchez-Montoya G (2008) Evolutinary ecology of pollination and reprodutionof tropical plants. In International Commision on Tropical Biology and Natural Resources (eds) Del-Claro K, Oliveira P S, Rico-Gray V, Ramirez A, Barbosa A A A, Bonet A, Scarano F R, Consol F L I, Morales Garzon F J, Nakajima J N, Costello J A, Sampaio M V, Quesada M, Morris M R, Rios M P, Ramirez N, Marcal Jr O, Macedo R H F, Marquis R J, Martins R P, Rodrigues S C, Luttge U]. In Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford, UK, [http://www.eolss.net] [Retrieved July 17, 2008]
Roubik D W (1982) The ecological impact of nectar-robbing bees and pollinating hummingbirds on a tropical shrub. Ecology 63: 354-360.
Roubik D W (1989) Ecology and natural history of tropical bees. New York, Cambridge University Press, 514p.
Schemske W, Horvitz C C (1988) Plant-animal interactions and fruit production in a Neotropical herb: a path analysis. Ecology 69: 1128-1137.
Silberbauer-Gottsberger I, Gottsberger I (1988) A polinização de plantas dos cerrados. Rev Bras Biol 48: 651-663.
Silva C I, Torezan-Silingardi H M (2009) Reprodution biology of tropical plants. In International Commission on Tropical Biology and Natural Resources (eds) Del-Claro K, Oliveira P S, Rico-Gray V, Ramirez A, Barbosa AAA, Bonet A, Scarano F R, Consol F L I, Morales Garzon F J, Nakajima J N, Costello J A, Sampaio M V, Quesada M, Morris M R, Rios M P, Ramirez N, Marcal Jr O, Macedo R H F, Marquis R J, Martins R P, Rodrigues S C, Luttge U] . In Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford, UK, [http://www.eolss.net] [Retrieved July 17, 2008]
Stephenson A G (1979) An evolutionary examination of the floral display of Catalpa speciosa (Bignoniaceae). Evolution 33: 1200-1209.
Stephenson A G, Thomas W W (1977) Diurnal and nocturnal pollination of Catalpa speciosa (Bignoniaceae). Syst Bot 2: 191-198.
Strauss S Y (1997) Floral characters link herbivores, pollinators, and plant fitness. Ecology 78: 1640-1645.
Strauss S Y, Conner J K, Rush S (1996) Foliar herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. Am Nat 147: 1098-1107.
Thompson J N (1999) The evolution of species interactions. Science 284: 2116-2118.
Thompson J N (2005) The geographic mosaic of coevolution. London, University of Chicago, 427p.
Torezan-Silingardi H M, Del-Claro K (1998) Behavior of visitors and reproductiove biology of Camponesia pubescens (Myrtaceae) in cerrado vegetation. Cienc Cult 50: 281-284.
Torezan-Silingardi H M, Oliveira P E A M (2004) Phenology and reproductive ecology of Myrcia rostrata and M. tomentosa (Myrtaceae) in central Brazil. Phyton Horn Austria 44: 23-43.
Traveset A, Willson M F, Sabag G (1998) Effect of nectarrobbing birds on fruit set of Fuchsia mageIlanica: a disrupted mutualism. Funct Ecol 12: 459-464.
Udulutsch R G, Assis M A, Picchi D G (2004) Florística de trepadeiras numa floresta estacional semidecídua, Rio Claro-Araras, estado de São Paulo, Brasil. Rev Bras Bot 27: 125-134.
Vitalli-Veiga M J, Dutra J C S, Machado V L L (1999) Visitantes florais de Lagerstroemia speciosa Pers. (Lythraceae). Rev Bras Zool 16: 397-407.
Vitalli-Veiga M J, Machado V L L (1994) Visitantes florais de Murraya exotica L. (Rutaceae). Semina 15: 153-169.
Vogel S (1962) A possible role of the boundary layer in insect flight. Nature 193: 1201-1202.
Vogel S (1983) Cophysiology of zoophilic pollination, p.560-612. In Lange O L, Nobel B S, Osmond C B, Ziegler H (eds) Physiological plant ecology. Berlin, Springer, 799p.
Waser N M, Ollerton J (2006) Plant-pollinator interactions: from specialization to generalization. Chicago, The University of Chicago Press, 445p.
Yanagizawa YA N P, Maimoni-Rodella R C S (2007) Floral visitors and reproductive strategies in five melittophilous species of Bignoniaceae in southeastern Brazil. Braz Arch Biol Technol 6: 1043-1050.
Received 22/VII/09.
Accepted 27/I/10.
Edited by Kleber Del Claro - UFU
Click to enlarge

Click to enlarge
- Bawa K S (1974) Breeding systems of tree species of a lowland tropical community. Evolution 28: 85-92.
- Bawa K S, Webb C J (1984) Flower, fruit and seed abortion in tropical forest trees: implications for the evolution of paternal and maternal reproductive patterns. Am J Bot 71: 736-751.
- Bertin R I (1982) Floral biology, hummingbird pollination and fruit production of Trumpet Creeper (Campsis radicans, Bignoniaceae). Am J Bot 69: 122-124.
- Bittencourt Jr N S, Gibbs P E, Semir J (2003) Histological study of post-pollination events in Spathodeacampanulata Beauv. (Bignoniaceae), a species with lateacting self-incompatibility. Ann Bot 91: 827-834.
- Borror D J, Delong D M (1969) Introdução ao estudo dos insetos. São Paulo, Edgars Blucher, 653p.
- Budowski G (1965) Distribuiton of tropical American forest species in a light of sucessional process. Turrialba 15: 40-42.
- Budowski G (1970) The distinction between old secondary and climax species in tropical central American lowland forests. Trop Ecol 11: 44-48.
- Bullock S H (1985) Breeding systems in the flora of a tropical deciduous forest in Mexico. Biotropica 17: 287-301.
- Casper B B, Wiens D (1981) Fixed rates of random ovule abortion in Cryptantha flava (Boraginaceae) and its possible relation to seed dispersal. Ecology 62: 866-869.
- Correia M C R, Pinheiro M C B, Lima H A (2005) Biologia floral e polinização de Arrabidaea conjugata (Vell.) Mart. (Bignoniaceae). Acta Bot Bras 19: 501-510.
- Cotton P A (2001). The behavior and interactions of birds visiting Erythrina fusca flowers in the Colombian Amazon. Biotropica 33: 662-669.
- Cushman J H, Beattie A J (1991) Mutualisms: assessing the benefits to hosts and visitors. Trends. Ecol Evol 6: 191-195.
- Del-Claro K (2004) Multitrophic relationships, conditional mutualisms, and the study of interaction biodiversity in tropical savannas. Neotrop Entomol 33: 665-672.
- Del-Claro K, Torezan-Silingardi H M (2009) Insect-plant interactions: new pathways to a better comprehension of ecological communities in neotropical savannas. Neotrop Entomol 38: 159-164.
- Dutra J C S, Machado V L L (2001). Entomofauna visitante de Stenolobium stans (Juss.) Seem (Bignoniaceae), durante seu período de floração. Neotrop Entomol 30: 43-53.
- Faegri K, Van der Pijl L (1979). The principles of pollination ecology. 3. ed. London, Perganon Press, 244p.
- Fournier L A (1974). Un método cuantitativo para la medición decaracterísticas fenológicas en árboles. Turrialba 24: 422-423.
- Gentry A H (1974a) Coevolutionary patters in central american Bignoniaceae. Ann Mo Bot Gard 61: 728-759.
- Gentry A H (1974b) Flowering phenology and diversity in tropical Bignoniaceae. Biotropica 6: 64-68.
- Gentry A H (1980) Bignoniaceae - Part I (Crescentieae and Tourrettieae). Flora Neotrop 25: 1-130.
- Gonçalves-Alvim S J, Macedo C F (1998) Insetos visitantes de capítulos de Heterocondylus alatus (Asteraceae) no Parque Nacional da Serra da Canastra, MG. Braz J Ecol 2: 102-107.
- Gottsberger G, Silberbauer-Gottsberger I (2006) Life in the Cerrado: a South American tropical seasonal ecosystem. Vol.II. Pollination and seed dispersal. Reta Verlag, Uml, 383p.
- Harborne J B (1993) Introduction to ecological biochemistry. 4. ed. London, Academic Press, 332p.
- Inouye D W (1980) The terminology of floral larceny. Ecology 61: 1251-1253.
- Irwin R E, Brody A K (1998) Nectar robbing in Ipomopsis agregata: effects on pollinator behavior and plant fitness. Oecologia 116: 519-527.
- Joly A B (1991) Botânica: introdução à taxonomia vegetal. São Paulo, Nacional, 777p.
- Kay Q O N (1987) Ultraviolet patterning and ultraviolet-absorbing pigments in flowers of the Leguminosae, p.317-354. In Stirton C H (ed) Advances in legume systematics Part 3, Royal Botanic Gardens, Kew, 480p.
- Kill L H P, Costa J G (2003) Biologia floral e sistema de reprodução de Annona squamosa L. (Annonaceae) na região de Petrolina-PE. Cienc Rur 33: 851-856.
- Kinoshita L S, Torres R B, Forni-Martins E R, Spinelli T, Ahn Y J, Constâncio S S (2006) Composição florística e síndromes de polinização e de dispersão da mata do Sítio São Francisco, Campinas, SP, Brasil. Acta Bot Bras 20: 313-327.
- Korndörfer A P, Del-Claro K (2006) Ant defense versus induced defense in Lafoensia pacari (Lythraceae), a myrmecophilous tree of the Brazilian cerrado. Biotropica 38: 786-788.
- Maloof J E (2001) The effects of a bumble bee nectar robber on plant reproductive success and pollinator behavior. Am J Bot 88: 1960-1965.
- Marques M C M, Oliveira P E A M (2004) Fenologia de espécies do dossel e do sub-bosque de duas florestas de restinga na Ilha do Mel, sul do Brasil. Rev Bras Bot 27: 713-723.
- Michener C D (2000) The bees of the world. Baltimore & London, Maryland, The Johns Hopkins University Press, 913p.
- Mitchell R J (1994) Effects of floral traits, pollinator visitation, and plant size on Ipomopsis aggregata fruit production. Am Nat 5: 143-150.
- Mitchell R J, Irwin R E, Flanagan R J, Karron J D (2009) Ecology and evolution of plant-pollinator interactions. Ann Bot 103: 1355-1363.
- Nascimento E A, Del-Claro K (2007) Floral visitors of Chamaecrista debilis (Vogel) Irwin & Barneby (Fabaceae-Caesalpinoidea) at cerrado of Estação Ecológica de Jataí, São Paulo State, Brazil. Neotrop Entomol 36: 619-624.
- Navarro L (1999) Pollination ecology and effect of nectar removal in Macleania bullatu (Ericaceae). Biotropica 31: 618-625.
- Newstrom L E, Frankie G W, Baker H G (1994) A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La Selva. Biotropica 26: 141-159.
- Pellmyr O, Thompson J N (1996) Sources of variation in pollinator contribution within a guild: the effects of plant and pollinator factors. Oecologia 107: 595-604.
- Polatto L P, Dutra J C S, Alves Jr V V (2007) Biologia reprodutiva de Pyrostegia venusta (Ker-Gawl) Miers (Bignoniaceae) e comportamento de forrageamento dos visitantes florais predominantes. Rev Biol Neotrop 4: 46-57.
- Polatto L P, Alves Jr V V (2008) Utilização dos recursos florais pelos visitantes em Sparattosperma leucanthum (Vell.) K. Schum. (Bignoniaceae). Neotrop Entomol 37: 389-398.
- Polatto L P, Alves Jr V V (2009) Sistema reprodutivo de Sparattosperma leucanthum (Vell.) K. Schum. (Bignoniaceae). R Árvore 33: 289-296.
- Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Oregon, Harper Collins Publishers, 479 p.
- Pyke G H (1991) What does it cost a plant to produce floral nectar? Nature 350: 58-59.
- Quesada M, Rosas F, Herrerias-Diego Y, Aguliar R, Lobo J A, Sanchez-Montoya G (2008) Evolutinary ecology of pollination and reprodutionof tropical plants. In International Commision on Tropical Biology and Natural Resources (eds) Del-Claro K, Oliveira P S, Rico-Gray V, Ramirez A, Barbosa A A A, Bonet A, Scarano F R, Consol F L I, Morales Garzon F J, Nakajima J N, Costello J A, Sampaio M V, Quesada M, Morris M R, Rios M P, Ramirez N, Marcal Jr O, Macedo R H F, Marquis R J, Martins R P, Rodrigues S C, Luttge U]. In Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford, UK, [http://www.eolss.net] [Retrieved July 17, 2008]
- Roubik D W (1982) The ecological impact of nectar-robbing bees and pollinating hummingbirds on a tropical shrub. Ecology 63: 354-360.
- Roubik D W (1989) Ecology and natural history of tropical bees. New York, Cambridge University Press, 514p.
- Schemske W, Horvitz C C (1988) Plant-animal interactions and fruit production in a Neotropical herb: a path analysis. Ecology 69: 1128-1137.
- Silberbauer-Gottsberger I, Gottsberger I (1988) A polinização de plantas dos cerrados. Rev Bras Biol 48: 651-663.
- Silva C I, Torezan-Silingardi H M (2009) Reprodution biology of tropical plants. In International Commission on Tropical Biology and Natural Resources (eds) Del-Claro K, Oliveira P S, Rico-Gray V, Ramirez A, Barbosa AAA, Bonet A, Scarano F R, Consol F L I, Morales Garzon F J, Nakajima J N, Costello J A, Sampaio M V, Quesada M, Morris M R, Rios M P, Ramirez N, Marcal Jr O, Macedo R H F, Marquis R J, Martins R P, Rodrigues S C, Luttge U]
- In Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford, UK, [http://www.eolss.net] [Retrieved July 17, 2008]
- Stephenson A G (1979) An evolutionary examination of the floral display of Catalpa speciosa (Bignoniaceae). Evolution 33: 1200-1209.
- Stephenson A G, Thomas W W (1977) Diurnal and nocturnal pollination of Catalpa speciosa (Bignoniaceae). Syst Bot 2: 191-198.
- Strauss S Y (1997) Floral characters link herbivores, pollinators, and plant fitness. Ecology 78: 1640-1645.
- Strauss S Y, Conner J K, Rush S (1996) Foliar herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. Am Nat 147: 1098-1107.
- Thompson J N (1999) The evolution of species interactions. Science 284: 2116-2118.
- Thompson J N (2005) The geographic mosaic of coevolution. London, University of Chicago, 427p.
- Torezan-Silingardi H M, Del-Claro K (1998) Behavior of visitors and reproductiove biology of Camponesia pubescens (Myrtaceae) in cerrado vegetation. Cienc Cult 50: 281-284.
- Torezan-Silingardi H M, Oliveira P E A M (2004) Phenology and reproductive ecology of Myrcia rostrata and M. tomentosa (Myrtaceae) in central Brazil. Phyton Horn Austria 44: 23-43.
- Traveset A, Willson M F, Sabag G (1998) Effect of nectarrobbing birds on fruit set of Fuchsia mageIlanica: a disrupted mutualism. Funct Ecol 12: 459-464.
- Udulutsch R G, Assis M A, Picchi D G (2004) Florística de trepadeiras numa floresta estacional semidecídua, Rio Claro-Araras, estado de São Paulo, Brasil. Rev Bras Bot 27: 125-134.
- Vitalli-Veiga M J, Dutra J C S, Machado V L L (1999) Visitantes florais de Lagerstroemia speciosa Pers. (Lythraceae). Rev Bras Zool 16: 397-407.
- Vitalli-Veiga M J, Machado V L L (1994) Visitantes florais de Murraya exotica L. (Rutaceae). Semina 15: 153-169.
- Vogel S (1962) A possible role of the boundary layer in insect flight. Nature 193: 1201-1202.
- Vogel S (1983) Cophysiology of zoophilic pollination, p.560-612. In Lange O L, Nobel B S, Osmond C B, Ziegler H (eds) Physiological plant ecology. Berlin, Springer, 799p.
- Waser N M, Ollerton J (2006) Plant-pollinator interactions: from specialization to generalization. Chicago, The University of Chicago Press, 445p.
- Yanagizawa YA N P, Maimoni-Rodella R C S (2007) Floral visitors and reproductive strategies in five melittophilous species of Bignoniaceae in southeastern Brazil. Braz Arch Biol Technol 6: 1043-1050.
Publication Dates
-
Publication in this collection
17 Jan 2011 -
Date of issue
Dec 2010
History
-
Accepted
27 Jan 2010 -
Received
22 July 2009