Abstract
Triplectides itatiaia sp. nov. is described from specimens collected on the Itatiaia massif, Mantiqueira mountain range, Rio de Janeiro state, Brazil. The new species can be distinguished by the presence of hind wing fork I petiolate, the long dorsal excision of segment X and the flat, apically rounded mesal lobes. Female and immature stages are unknown. A key to the Brazilian species in the genus is provided.
Triplectides itatiaia; new species; Triplectidini; Neotropical Region; Atlantic Forest; identification key
SYSTEMATICS, MORPHOLOGY AND PHYSIOLOGY
A new long-horned Caddisfly in the genus Triplectides Kolenati (Trichoptera: Leptoceridae) from the Itatiaia massif, southeastern Brazil
Leandro L Dumas; Jorge L Nessimian
Depto de Zoologia, Instituto de Biologia, Univ Federal do Rio de Janeiro, CP 68044, Cidade Universitária, 21944-970 Rio de Janeiro, RJ, Brazil; dumas_bioufrj@yahoo.com.br; nessimia@acd.ufrj.br
ABSTRACT
Triplectides itatiaia sp. nov. is described from specimens collected on the Itatiaia massif, Mantiqueira mountain range, Rio de Janeiro state, Brazil. The new species can be distinguished by the presence of hind wing fork I petiolate, the long dorsal excision of segment X and the flat, apically rounded mesal lobes. Female and immature stages are unknown. A key to the Brazilian species in the genus is provided.
Key words:Triplectides itatiaia, new species, Triplectidini, Neotropical Region, Atlantic Forest, identification key
The long-horned caddisfly genus Triplectides Kolenati contains about 70 described species worldwide, making it the largest in the subfamily Triplectidinae (Holzenthal 1988, Malm & Johanson 2008). The genus shows a primarily southern hemisphere distribution, being most diverse in the Australian and Neotropical regions. Mosely (1936) provided the first comprehensive revision of the genus. More recently, Morse & Neboiss (1982) reviewed the Australian species, while Holzenthal (1988) reviewed the Neotropical ones. In the Neotropics there are 13 described species (Flint et al 1999) which are distributed from southern Chile and Argentina, through most of tropical South America, Central America to southern Mexico (Holzenthal 1988). Only five species of Triplectides were recorded from Brazil: T. egleri Sattler, T. gracilis (Burmeister), T. misionensis Holzenthal, T. neotropicus Holzenthal, and T. ultimus Holzenthal (Paprocki et al 2004). In the present work, we describe and illustrate the male of a new species collected on the Itatiaia massif, Mantiqueira mountain range, Rio de Janeiro state.
Material and Methods
The material was collected with Pennsylvania light traps and preserved in 80% ethanol. To observe genital structures, the abdomen was removed and cleared in 10% KOH. The illustrations were made under a stereomicroscope equipped with a camera lucida. The terminology used in the description follows that of Holzenthal (1988). The type specimens are deposited in the Coleção Entomológica Professor José Alfredo Pinheiro Dutra, Departamento de Zoologia, Universidade Federal do Rio de Janeiro, Brazil (DZRJ).
Triplectides itatiaia sp. nov. (Figs 1-7)
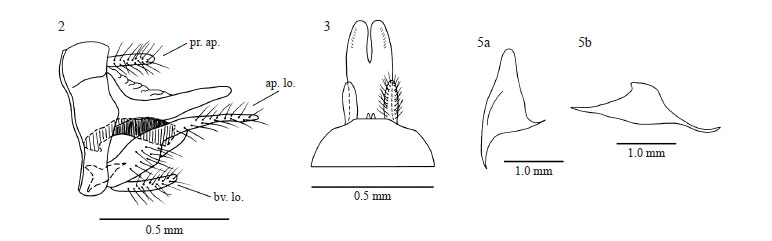
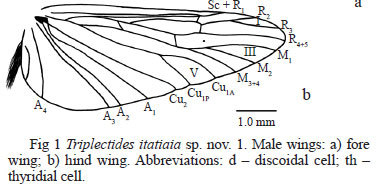
Triplectides itatiaia sp. nov. is similar to T. gracilis (Burmeister), being sympatric in the Mantiqueira mountain range of southeastern Brazil. They can be separated by wing venation, mainly by hind wing fork I being clearly petiolate in the former species (Fig 1b). In T. gracilis, the hind wing fork I is sessile or has a short petiole. In addition, in the new species the mesal lobes of the inferior appendages are apically flattened (Figs 5a, b), while in T. gracilis they are digitate, but never flattened (Figs 7a, b). Triplectides itatiaia sp. nov. is also similar to T. flintorum Holzenthal from Central America. These species are distinguished by the preanal appendages (Figs 2, 3), which are longer and more apically pointed in T. flintorum, and by tergum X that is less protruded and more rounded in the new species (Figs 2, 3). Furthermore, Holzenthal (1988) pointed out the presence of a long, thin, sclerotized strip along the dorsal midline of the phallic apparatus in T. flintorum. This structure was not found in the new species.
Triplectides ultimus Holzenthal is also sympatric with the species here described; both are endemic to the Itatiaia massif. However, these species are easily distinguishable by the shape of mesal lobe of the inferior appendages and the long, bifurcate membranous process of the tergum IX present in T. ultimus (see figs 30b, c in Holzenthal 1988).
Description. Adult male (Figs 1-6): Forewing length: 10 mm (n = 2). Head mostly brown, with antenna and labial palp stramineous, maxillary palp dark brown; thorax reddish brown, legs stramineous. Fore wings light brown, with several small light maculae.
Head: Frontal setal wart present between the antennal base; one pair of small antennal setal warts present; one pair of large posterior setal warts present behind eyes; one pair of long and slender setal warts on postgenal area present. Maxillary palp long, with segment IV about 1/4 the lenght of segment V.
Thorax (Fig 1): Fore wings with dorsal dark setae along costal and subcostal area, extending from base to near apex; forks I and V present in male; discoidal cell apically large; s slightly sinuous; r-m slightly shorter than s, almost touching. Hind wings broad, with forks I, III and V present; fork I with distinct petiole (Fig 1). Spur formula 2-2-4.
Male genitalia (Figs 2-7): Segment IX, in lateral view, with anterior margin slightly concave, enlarged dorsally, posterior margin almost straight, slightly protruded medially (Fig 2); tergum IX with posterior margin almost rounded, slightly protruded, with two small membranous digitate median processes (Fig 3). Preanal appendages short, digitate, bearing long setae (Figs 2, 3). Segment X in lateral aspect about two times the length of preanal appendages, wide at base, tapering from base to midlength, rounded at apex (Fig 2); dorsally with slight basal constriction, almost parallel side, with an apicomesal excision extending anteriorly to less than half the segment length (Fig 3); apical portion of segment X bearing very short, dorsal setae. Inferior appendages long, bearing long setae (Figs 2, 4); first article wide at base, constricted at 1/3 article length, narrow apical portion, with rounded apex; apicodorsal lobes long, extending beyond second article (Fig 2); basoventral lobes digitate, bearing stout setae; mesal lobes subequal in length to basoventral lobes; in lateral view, large at base, tapering apically, flattened at apex (Fig 5b); in ventral view, rounded apically (Figs 4, 5a); second article short, with sharp apex, directed mesally (Fig 4). Phallic apparatus simple, tubular, with phallotremal sclerite small, rod-like, poorly developed (Fig 6). Female and larvae: Unknown.
Holotyte male: BRAZIL, Rio de Janeiro, Itatiaia: Parque Nacional do Itatiaia, Rio Tapera, 22º26'59.64''S 44º36'19.39''W, 794 m, 13.iv.2007, J L Nessimian, L L Dumas, A P M dos Santos & N Ferreira Jr leg. (DZRJ 1654).
Paratype male: BRAZIL, Rio de Janeiro, Itatiaia: Parque Nacional do Itatiaia, Rio Campo Belo, Cachoeira Véu da Noiva track, 22º25'42.03''S 44º37'11.19''W, 982 m, 16.iv.2007, J L Nessimian, A P M dos Santos, N Ferreira Jr & L L Dumas leg. (DZRJ 1655).
Etimology. The specific epithet, itatiaia, refers to Parque Nacional do Itatiaia, the national park where the type specimens were collected. Itatiaya, from the Tupi language, means pointed rock, in reference to Pico das Agulhas Negras, the highest peak of the Itatiaia massif.
Distribution. Southeastern Brazil (Rio de Janeiro state).
Key to the Known Brazilian Species of Triplectides Kolenati (modified from Holzenthal 1988)
Acknowledgments
We thank Dr Nelson Ferreira Jr and Allan Paulo Moreira dos Santos for aid in collecting specimens. The Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA), the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) and Parque Nacional do Itatiaia are thanked for issuing collecting permits (IBAMA 14591-2). We are grateful to Dr Adolfo R Calor and the anonymous referees for improving the manuscript. This study was partially funded by CNPq, FAPERJ, and CAPES.
Received 23/VII/09.
Accepted 01/XII/09.
Edited by Marcelo Duarte - USP/MZ
- Flint Jr O S, Holzenthal R W, Harris S C (1999) Catalog of the Neotropical caddisflies (Insecta: Trichoptera). Ohio Biological Survey, Colombus, Ohio, 239p.
- Holzenthal R W (1988) Systematics of Neotropical Triplectides (Trichoptera: Leptoceridae). Ann Entomol Soc Am 81: 187-208.
- Malm T, Johanson K A (2008) Description of eleven new Triplectides species (Trichoptera: Leptoceridae) from New Caledonia. Zootaxa 1816: 1-34.
- Morse J C, Neboiss A (1982) Triplectides of Australia (Insecta: Trichoptera: Leptoceridae). Mem Nat Mus Victoria 43: 61-98.
- Mosely M E (1936) A revision of the Triplectidinae, a subfamily of the Leptoceridae (Trichoptera). Trans Royal Entomol Soc London 85: 91-129.
- Paprocki H, Holzenthal R W, Blahnik R J (2004) Checklist of the Trichoptera (Insecta) of Brazil I. Biota Neotrop 4: 1-22.
Publication Dates
-
Publication in this collection
17 Jan 2011 -
Date of issue
Dec 2010
History
-
Received
23 July 2009 -
Accepted
01 Dec 2009