Abstract
The aim of this study was to determine whether an edge effect could be observed in the structure and composition of phlebotomine assemblages in five forest fragments on São Luís Island. The study also investigated whether there were any differences in species along the forest edge-to-interior gradient and in species richness and abundance between the fragments studied. To capture the insects a transect was defined in each fragment, and eight light traps were set up at 15 m intervals from the edge. Phlebotomines were found in all fragments, and a total of 2972 specimens (1188 males and 1784 females) belonging to 24 species were collected. Of these, the most abundant was Lutzomyia antunesi (Coutinho), followed by Brumptomyia avellari (Costa Lima), L. infraspinosa (Mangabeira), L. flaviscutellata (Mangabeira), L. claustrei Abonnenc, Léger & Fauran, L. wellcomei (Fraiha, Shaw & Lainson), L. sordellii (Shannon & Del Ponte) and L. paraensis (Costa Lima). No significant differences were observed in the number of individuals or species along the edge-to-interior gradient. However, a higher distribution of some species in certain regions of the forest could be observed graphically. There was no correlation between fragment size and the number of species or individuals.
Edge effect; gradient biodiversity; sandfly
PUBLIC HEALTH
The effect of fragmentation on phlebotomine communities (Diptera: Psychodidae) in areas of ombrophilous forest in São Luís, state of Maranhão, Brazil
PCB AzevedoI; GN LopesI; RS FontelesI; GC VasconcelosI; JLP MoraesII; JMM RebêloII
IDepto de Biologia
IILab de Entomologia e Vetores, Depto de Patologia. Univ Federal do Maranhão, São Luís, MA, Brasil
Correspondence Correspondence: José M M Rebêlo Depto de Patologia Univ Federal do Maranhão Praça Madre Deus, 02, 65025-560 São Luís, MA, Brasil macariorebelo@uol.com.br
ABSTRACT
The aim of this study was to determine whether an edge effect could be observed in the structure and composition of phlebotomine assemblages in five forest fragments on São Luís Island. The study also investigated whether there were any differences in species along the forest edge-to-interior gradient and in species richness and abundance between the fragments studied. To capture the insects a transect was defined in each fragment, and eight light traps were set up at 15 m intervals from the edge. Phlebotomines were found in all fragments, and a total of 2972 specimens (1188 males and 1784 females) belonging to 24 species were collected. Of these, the most abundant was Lutzomyia antunesi (Coutinho), followed by Brumptomyia avellari (Costa Lima), L. infraspinosa (Mangabeira), L. flaviscutellata (Mangabeira), L. claustrei Abonnenc, Léger & Fauran, L. wellcomei (Fraiha, Shaw & Lainson), L. sordellii (Shannon & Del Ponte) and L. paraensis (Costa Lima). No significant differences were observed in the number of individuals or species along the edge-to-interior gradient. However, a higher distribution of some species in certain regions of the forest could be observed graphically. There was no correlation between fragment size and the number of species or individuals.
Keywords: Edge effect, gradient biodiversity, sandfly
Introduction
Continuous growth in human activities has led to a drastic reduction in natural areas as a result of the transformation of continuous habitats into different-sized fragments located within a nonforest matrix and subject to different degrees of isolation and disturbance. Such growth constitutes one of the main threats to biodiversity faced worldwide (Corlett 2000, Tabarelli et al 2004).
One consequence of this fragmentation is the edge effect in forest remnants. The edge effect modifies the forest structure, microclimate and species composition (Murcia 1995) as a result of new events in the evolutionary history of natural plant and animal populations. Consequently, demographic parameters such as mortality and birth rates for each species are affected differently, and ecosystem structure and dynamics are in turn affected (Viana & Pinheiro 1998). The structural and functional characteristics around the matrix also play an important role in determining both the extent of the changes induced at the edge and which species are present (Laurance 1991, Gascon et al 1999, Mesquita et al 1999).
One group that has been neglected in studies on this subject are the blood-feeding insects, which can be important disease vectors (De Luca et al 2003), particularly the phlebotomines (Diptera: Psychodidae), implicated in the transmission of leishmaniasis (Martins et al 2004). Phlebotomines are holometabolous (Killick-Kendrick 1999) that live in a moist substrate and feed on organic material (Ferro et al 1997). However, adults feeding habits will depend on their sex. Although both males and females feed on plant sap, only females develop blood-feeding habits (Chaniotis 1974), attacking a wide range of vertebrate species.
As a result of the changes brought about by forest fragmentation, the amount of resources available for adult phlebotomines and/or their larvae may be affected (De Luca et al 2003), leading to changes in vector species communities, with consequences for the parasitic cycle of Leishmania, the causative agent of leishmaniasis. This process can also favor the selection of species that are able to colonize the peridomestic environment and areas that have been modified, bringing the vector and human population closer together (Azevedo et al 1990).
Unfortunately, there are very few surveys of phlebotomine faunas in natural systems despite recognition of the importance of these studies for conservation projects, for increasing our knowledge of areas where these insects can be found and for minimizing human contact with them. This study therefore aimed to investigate whether there is any variation in the structure and composition of phlebotomine communities on the forest edge-to-interior gradient and to determine species richness and abundance in the various fragments studied.
Material and Methods
Collection area
The study was carried out in a forest on the Merck farm, in the southeastern area of São Luís Island (2°38'60"S and 44º8'53"W), in the São José de Ribamar municipality, state of Maranhão, Brazil.
The vegetation in the area consists of ombrophilous forest comprising terra firme forest and seasonally flooded forest, which can be classified as Dense Ombrophilous Lowland Forest and Dense Alluvial Ombrophilous Forest, respectively (IBGE 1992). The vegetation also includes swamp, restinga (sandy coastal strips and their characteristic vegetation) and a 20 year-old secondary forest. The climate is moist, mesothermal and tropical, with two well-defined seasons: the rainy season, from January to June, during which an average of 94% of the total annual rainfall is concentrated, and the dry season, from July to December, when only 6% of the rainfall occurs. Total annual rainfall is high, around 1,900 mm. Temperatures are high throughout the year (average temperature is 26°C) with little variation.
Sampling
Five fragments of terra firme forest of different sizes were sampled. In three of these fragments 1 (F1) (51.73 ha), 2 (F2) (110.71 ha) and 3 (F3) (76.81 ha), the matrix consisted of roads and a secondary forest, and in the other two, fragments 4 (F4 - 52.12 ha) and 5 (F5 - 56.17 ha), the matrix was a swamp (Fig 1).
In each fragment, insects were captured in a 105 m long transect defined from the edge of the fragment to the interior of the forest. Eight Hoover Pugedo light traps were installed along each of these transects at 15 m intervals from the edge (i.e., at 0, 15, 30, 45, 60, 75, 90 and 105 m). The traps were installed at 6 pm and collected at 6 am the following morning once a month for four months (July to October), giving a total sampling effort of 96 h/fragment/month.
The collected phlebotomines were cleared in 10% potassium hydroxide, acetic acid, distilled water and lactophenol, and mounted in Berlese fluid. They were later identified according to Young & Duncan (1994).
Statistical analysis
Diversity in the area was analyzed using the Shannon index, and species similarity among the fragments was measured with the Morisita index.
The relationship between the two types of matrix and the species abundance of the specimens collected in the fragments bordering the matrices was analyzed using one-way ANOVA. Spearman's correlation coefficient was used to determine whether there was a relationship between fragment size and the number of species and individuals found in a given fragment.
The Kruskal-Wallis non parametric test was used to investigate whether species abundance varied along the forest edge-to-interior gradient. Together with this, dispersion graphs and mean abundance values were used to relate abundance to distance from the edge for each species.
Results
Richness and abundance
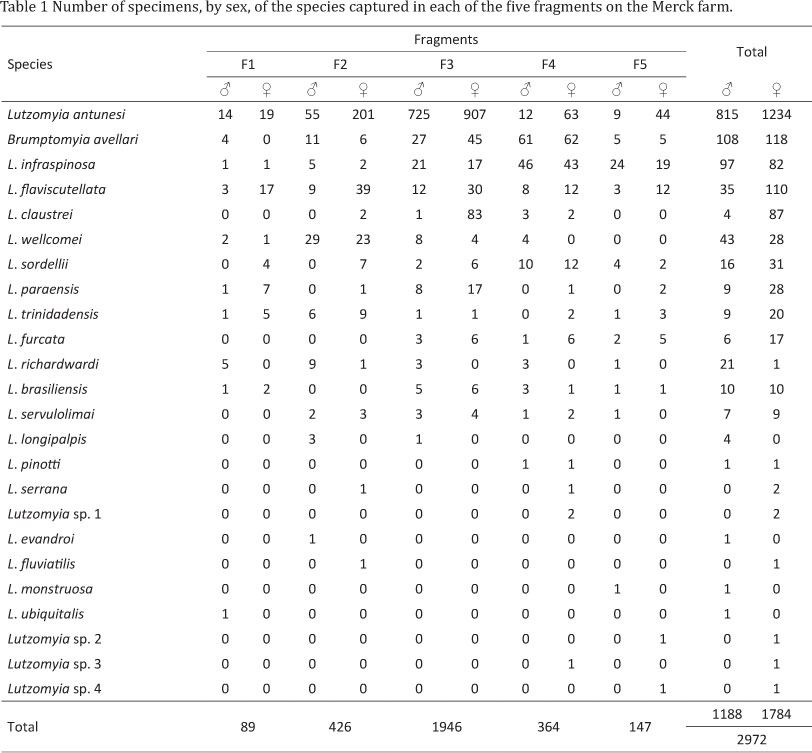
A total of 2,972 phlebotomine specimens (1,188 males and 1,784 females) belonging to 24 species and four unknown species, which have not yet been taxonomically defined, were analyzed (Table 1). Lutzomyia antunesi (Coutinho), accounted for 68.9% of all specimens sampled, while Brumptomyia avellari (Costa Lima), Lutzomyia infraspinosa (Mangabeira), L. flaviscutellata (Mangabeira), L. claustrei (Abonnenc et al), L. wellcomei (Fraiha et al), L. sordellii (Shannon & Del Ponte) and L. paraensis (Costa Lima) accounted for 26.7% of the sampled specimens. The remaining species together accounted for 4.4%.
Phlebotomines were found in all fragments (F), but species richness was greatest in F4 (F1 = 11; F2 = 14; F3 = 14; F4 = 17; F5 = 13) (Table 1), while F3 had the greatest species diversity (H') (F1 = 1.907; F2 = 1.447; F3 = 1.952; F4 = 1.813; F5 = 1.803). F1 was the most homogeneous (P) (F1 = 0.6118; F2 = 0.2834; F3 = 0.5033; F4 = 0.3605; F5 = 0.4336).
The species with the widest distribution and which were found in all the fragments were: B. avellari, L. antunesi, L. flaviscutellata, L. richardwardi, L. sordellii, L. trinidadensis, L. paraensis and L. infraspinosa. In contrast, L. monstruosa, L. ubiquitalis, L. evandroi, L. fluviatilis, L. pinottii, Lutzomyia sp. 1, Lutzomyia sp. 2, Lutzomyia sp. 3 and Lutzomyia sp. 4 each appeared in only a single fragment.
Relationship between forest edge and interior
The Kruskal-Wallis test showed that there was no significant difference in species abundance along the forest edge-to-interior gradient. However, a tendency can be seen from the dispersion graph and the mean abundance values for each species at each collection point (Fig 2): the density of B. avellari, L. flaviscutellata and L. Claustrei increased from the edge to the interior, while the opposite pattern was observed for L. infraspinosa, L. furcata, L. antunesi and L. richardwardi, which were more frequent at points on the very edge. Nevertheless, no regular pattern was observed along the gradient for L. sordellii, L. wellcomei, L. brasiliensis, L. trinidadensis, L. paraensis and L. servulolimai. The remaining species were not analyzed graphically because very small numbers were collected (almost always only a single individual), making it impossible to associate them with any degree of certainty with a particular region of the forest.
ANOVA failed to show any significant interaction between the matrix (swamp or roads and forest) and species abundance (P = 0.765). The graph of the Morisita indices showed the existence of three groups: the first, made up of fragment 1 alone; the second, of fragments 4 and 5; and the last, of fragments 2 and 3 (Fig 3).
The Spearman coefficient failed to show the existence of a direct relationship between fragment size and number of species (r = 0.36; P = 0.55) or between fragment size and number of individuals (r = 0.8; P = 0.10).
Discussion
The number of species found in this study was much higher than those observed in other forest fragments on São Luís Island (Rebêlo et al 1999, Marinho et al 2008). In addition, species not previously described in forests in this region were found: L.brasiliensis, L. monstruosa, L. ubiquitalis and L. pinottii, as well as species not yet identified (Lutzomyia sp. 1, Lutzomyia sp. 2, Lutzomyia sp. 3 and Lutzomyia sp. 4). Yet, the report of L. longipalpis, L. wellcomei and L. flaviscutellata on such areas is important as these species are known to be vectors of visceral, cutaneous and diffuse cutaneous leishmaniasis agents, respectively (Lainson 1985).
In the present study, species richness varied little among fragments of the same matrix, although there were some differences in species composition between them; indeed, some species only occurred in a single fragment, with very low density. According to Martin & Rebêlo (2006), this low frequency may be associated with various factors, such as the absence of a source of blood meals and shelters in the vicinity of the collection points and the small area of influence of the light traps (5 m) (Dye et al 1991). Nonetheless some species exist naturally in very low densities, and their populations can therefore suffer considerable reductions in size in small fragments, making them more vulnerable to local extinction.
If the extinction happens, an imbalance in the community may occur, favoring other species, which may then start to occupy the empty niche, resulting in a population explosion (as may have been the case with L. antunesi). Another consequence could be the migration of species from the forested areas to the peridomestic environment, and as some of these species could be parasite vectors, this would favor outbreaks of leishmaniasis.
Lutzomyia servulolimai, L. trinidadensis, L. richardwardi, L. furcata, and L. brasiliensis are still considered low-density species despite being present in greater abundance than the species previously mentioned (between 16 and 29 individuals), corroborating the results reported by Marinho et al (2008) (with the exception of L. furcata and L. brasiliensis, which were not found). Even though present in low frequency, these species were found in both fragments bordered by terra firme vegetation and fragments bordered by swamp, indicating that despite their low densities, they are well distributed in the studied forest. In the case of L. richardwardi and L. furcata, a tendency of association between abundance and points closest to the edge could be observed.
No significant relationship between abundance and distance from the edge of the fragments was found for the most abundant species. However, a greater association with some regions of the fragments could be inferred. One such case was L. antunesi, which accounted for almost 69% of the sample. It should be stressed that 71% of this total was captured in only one collection, at collection points 15 m and 30 m from the edge, possibly indicating that the traps were located near a shelter for the species. Because of this, these data were considered outliers and excluded from the data analysis. Nonetheless, a greater association between this species and the region near the edge can still be observed.
In addition to L. antunesi, other species, such as L. infraspinosa, B. avellari, L. flaviscutellata, L. claustrei, L. wellcomei, L. sordellii and L. paraensis were also well represented; L. infraspinosa was observed more often at the beginning of the transects, and B. avellari, L. flaviscutellata and L. claustrei at the ends. The other species did not display a regular pattern, and their abundance was higher in some places along the transects sampled and lower in others.
It is interesting to note that B. avellari and L. infraspinosa, considered accidental species in other studies carried out on São Luís Island and in Buriticupu (in the Maranhão region of Amazonia) (Rebêlo et al 1999, Marinho et al 2008), were abundant in the present study. This finding reinforces the notion that the structure of communities can vary according to geographical area; indeed, in riparian and mesophytic forest in Urbano Santos, an area of cerrado (savanna-like grasslands) in the northeast of the state, L. infraspinosa was considered dominant, while B. avellari was not found (Martins 2004).
Three groups were found to exist among the fragments (F1; F2 and F3; and F4 and F5), coinciding with the inter-fragment matrix (swamp area; roads and secondary forest) and the proximity of the fragments to each other, which may allow vertebrates used as a source for their blood meals to transit between them. The movement of these vertebrate hosts among the fragments would bring specimens with them from one ecotope to another, which, in the absence of adverse conditions, would be colonized by these phlebotomine species.
This may explain the absence of a correlation between fragment size and the number of species and individuals found in the fragment. In addition, there may be a relationship between vertebrates and the tendency in the distribution of phlebotomine species along the edge-to-interior gradient. An example of this are phlebotomine species that are parasites of rodents. According to Malcolm (1997), these rodents can be found in greater numbers at the edges, where they can find food in either the modified habitat or the forest, thereby affecting the distribution of species that feed on their blood and use their nests for shelter.
In spite of this, the statistical analysis failed to show an edge effect on the Fazenda Merck. This may be because this area has been under regeneration for around 20 years. According to Primack & Rodrigues (2001), forests that have undergone fragmentation recently are more strongly influenced by the edge effect because of ecological changes associated with the abrupt artificial edges of the forest remnants. Based on this, the study area could be considered to be in a more advanced stage of ecological succession. Primack & Rodrigues also suggest that secondary forests surrounding fragments help to protect them from external conditions and minimize the impact of fragmentation, in contrast to what happens when matrices consist of pastures or other open areas.
Given the great diversity of phlebotomines found in this area, it is important that environmental preservation programs be implemented. As well as helping to preserve other species of flora and fauna, such programs are fundamental to avoid alterations in the structure of communities and the consequent migration and adaptation of phlebotomine species to other areas, where they can act as vectors of infection.
Received 12 August 2009 and accepted 11 November 2010
Edited by Eunice Galati - FSP/USP
- Azevedo ACR, Rangel EF, Costa ME, David J, Vasconcelos AW, Lopes UG (1990) Natural infection of Lutzomyia (Nyssomyia) whitmani (Antunes & Coutinho, 1939) by Leishmania of the braziliensis complex in Baturité, Ceará State, northeast Brazil. Mem Inst Oswaldo Cruz 85: 251.
- Chaniotis BN (1974) Sugar-feeding behaviour of Lutzomyia trapidoi (Diptera: Psychodidae) under experimental conditions. J Med Entomol 11: 73-79.
- Corlett RT (2000) Environmental heterogeneity and species survival in degraded tropical landscapes, p 333-355. In Hutchings MJ, John EA, Stewart AJA (eds) The ecological consequences of environmental heterogeneity. Londres, British Ecological Society, 425p.
- De Luca AS, Vasconcelos HL, Barrett TV (2003) Distribution of sandflies (Diptera: Phlebotominae) in forest remnants and adjacent matrix habitats in Brazilian Amazonia. Braz J Biol 63: 401-410.
- Dye C, Davies CR, Lainson R (1991) Communication among phlebotomine sandflies: afield study of domesticated Lutzomyia longipalpis populations in Amazonian Brazil. Anim Behav 42: 183-192.
- Ferro C, Pardo R, Torres M, Morrison AC (1997) Larval microhabitats of Lutzomyia longipalpis (Diptera: Psychodidae) in an endemic focus of visceral leishmaniasis in Colombia. J Med Entomol 34: 719-728.
- Gascon C, Lovejoy TE, Bierregaard Jr RO, Malcolm JR, Stouffer PC, Vasconcelos HL, Laurance WF, Zimmerman B, Tocher M, Borges S (1999) Matrix habitat and species richness in tropical forest remnants. Biol Conserv 91: 223-230.
- IBGE (1992) Manual técnico da vegetação brasileira. Série Manuais Técnicos em Geociências, n. 1. Rio de Janeiro, Departamento de Recursos Naturais e Estudos Ambientais - IBGE, 92p.
- Killick-Kendrick R (1999) The biology and control of phlebotomine sand flies. Clin Dermatol 17: 279-289.
- Lainson R (1985) Our present knowledge of the ecology and control of leishmaniasis in the Amazon Region of Brazil. Rev Soc Bras Med Trop 18: 47-56.
- Laurance WF (1991) Ecological correlates of extinction proneness in Australian tropical rainforest mammals. Conserv Biol 5: 79-89.
- Malcolm JR (1997) Biomass and diversity of small mammals in forest fragments, p.207-221. In Laurance WF, Bierregaard Jr RO (eds) Tropical forest remnants: ecology, management, and conservation of fragmented communities. Chicago, University of Chicago Press, 609p.
- Marinho RM, Fonteles RS, Vasconcelos GC, Azevedo PCB, Moraes JLP, Rebêlo JMM (2008) Flebotomíneos (Diptera, Psychodidae) em reservas florestais da área metropolitana de São Luís, Maranhão, Brasil. Rev Bras Entomol 52: 112-116.
- Martin AMC, Rebêlo JMM (2006) Dinâmica espaço-temporal de flebotomíneos (Diptera, Psychodidae) do município de Santa Quitéria, área de cerrado do estado do Maranhão, Brasil. Iheringia Ser Zool 96: 273-384.
- Martins FC (2004) Estrutura da vegetação e sua influência na distribuição de flebotomíneos (Diptera, Psychodidae) em um fragmento de mata ciliar do município de Urbano Santos, Maranhão. UFMA, dissertação de mestrado, 49p.
- Martins LM, Rebêlo JMM, dos Santos MCFV, Costa JML, da Silva AR (2004) Ecoepidemiologia da leishmaniose tegumentar no Município de Buriticupu, Amazônia do Maranhão, Brasil, 1996 a 1998. Cad Saúde Pública 20: 753-743.
- Mesquita RCG, Delamonica P, Laurance WF (1999) Effect of surrounding vegetation on edge-related tree mortality in Amazonian forest fragments. Biol Conserv 91: 129-134.
- Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10: 58-62.
- Primack RB, Rodrigues E (2001) Ameaças à diversidade biológica, p.69-128. In Primack RB, Rodrigues E Biologia da conservação. Brasil, Editora Planta, 327p.
- Rebêlo JMM, de Araújo JAC, Carvalho ML, Barros VLL, Silva FS, de Oliveira ST (1999) Flebótomos (Diptera, Phlebotominae) da Ilha de São Luis, zona do Golfão Maranhense, Brasil. Rev Soc Bras Med Trop 32: 247-253.
- Tabarelli M, Silva JMC, Gascon C (2004) Forest fragmentation, synergisms and the impoverishment of Neotropical forests. Biodivers Conserv 13: 1419- 1425.
- Viana VM, Pinheiro LAFV (1998) Conservação da biodiversidade em fragmentos florestais. ESALQ/USP. Série Técnica IPEF 12: 25-42.
- Young DG, Duncan MA (1994) Guide to identification and geographic distribution of Lutzomyia sandflies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). American Entomological Institute, Associated Publishers, Gainesville, Fl, 881p.
Correspondence:
Publication Dates
-
Publication in this collection
09 May 2011 -
Date of issue
Apr 2011
History
-
Received
12 Oct 2009 -
Accepted
11 Nov 2010