Entomopathogenic fungus; formulation; microbial control
BIOLOGICAL CONTROL
Cage and field assessments of Beauveria bassiana-based Mycoinsecticides for Myzus persicae Sulzer (Hemiptera: Aphididae) control in cabbage
M Michereff FilhoI; SOD OliveiraII; RS de LizI; M FariaIII
IEMBRAPA Hortaliças, Brasília, DF, Brasil
IIDepto de Biologia Animal, Univ Federal de Viçosa, Viçosa, MG, Brasil
IIIEMBRAPA Recursos Genéticos e Biotecnologia, Brasília, DF, Brasil
Correspondence Correspondence Marcos Faria EMBRAPA Recursos Genéticos e Biotecnologia CP 02372, 70770-900. Brasília, DF, Brasil faria@cenargen.embrapa.br
ABSTRACT
The efficiency of formulated Beauveria bassiana-based mycoinsecticides to control Myzus persicae (Sulzer) in cabbage was assessed under field conditions. Aqueous conidial suspensions (0.01% Tween 80 + 0.01% v/v Agral) of three fungal isolates were sprayed twice at different dates, each with 2.0 x 109 viable conidia per potted plant using screened cages. The number of nymphs and adults of M. persicae per leaf was significantly reduced in plots treated with isolates CG 864 and PL 63, with control efficiency ranging from 57% to 60%. Further field trials using screened cages with isolate CG 864 formulated as oil dispersion reduced the aphid population by 85-87% as compared to the control, whereas a 71% reduction was seen in plants treated with the aqueous conidial suspension 20 days following the first spray. The last experiment was conducted in a commercial cabbage field (without cages), in which the fungus was applied at three different dates, each with an equivalent of 1.0 x 1013 viable conidia/ha. The reduction in the number of aphids per leaf was more evident between four and five weeks following the first spray, resulting in 76-83% and 57-65% control efficiency for oil dispersions and unformulated conidia, respectively. However, with the exception of imidacloprid-treated plants, rapid aphid re-infestation was observed in all treatments. In this study, the stand-alone use of mycoinsecticides for aphid control was not a satisfactory strategy, although utilization of B. bassiana in IPM strategies remains a field to be explored.
Keywords: Entomopathogenic fungus, formulation, microbial control
Introduction
The aphid Myzus persicae (Sulzer) is a key pest of crucifers, particularly cauliflower, broccoli and cabbage in tropical regions, reducing stand and vigor of young plants, ultimately affecting product quality (Harrewijn & Minks 1989, Harrington & van Emden 2007). The indiscriminate use of pesticides for M. persicae control has lead to serious problems, such as the appearance of resistant populations, resurgence of secondary pests due to the elimination of natural enemies, intoxication of farm workers, environmental damage and risks to consumers' health (Foster et al 2000, Harrington & van Emden 2007). In organic farming, pest problems may become even more critical, since certified farmers are not allowed to use chemical pesticides. In these situations, alternative measures for prevention and control of pests are not always available or may not confer satisfactory results.
Entomopathogenic fungi are among the most used biological control agents in Brazil, and represent a viable alternative for management of many sucking insects, especially when pesticides are not allowed, as in organic cropping. Several mycoinsecticides have been used commercially in other countries to control aphids, based on formulations of Beauveria bassiana, Conidiobolus thromboides, Isaria fumosorosea, Lecanicillium longisporum, Lecanicillium muscarium and Metarhizium anisopliae (Faria & Wraight 2007).
In Brazil, some mycoinsecticides are commercialized for aphid control, including a product based on Lecanicillium sp., two based on M. anisopliae, as well as a mixture containing B. bassiana and M. anisopliae (Michereff Filho et al 2009). According to these authors, three products are formulated as oil dispersions, composed of conidia mixed with an emulsifiable oil, whereas one of them is a technical product (unformulated), sold as a colonized substrate.
Despite extensive work under laboratory conditions, publications on the efficiency of mycoinsecticides toward aphid control are rare under tropical field conditions. Therefore, the main objective of this study was to assess the effect of B. bassiana-based oil dispersions on M. persicae under cage and field conditions.
Material and Methods
Cultivation and infestation of host plants
Seedlings of cabbage, cv. Matsukase, were produced in 128-cell styrofoam trays filled with substrate for vegetables (Plantmax HT; Eucatex, Paulinia, Brazil), and subsequently transplanted at 35 days post-emergence to plastic pots (5 L capacity) or directly to the field. All plants were maintained under controlled conditions at 25 ± 2ºC, 70 ± 10% RH, and 12:12h light:dark photoperiod. In order to get age-standardized aphids for experiments, vigorous wingless adults taken from the colonies were transferred to leaves of potted plants. Adults were allowed to freely reproduce for ca. 48h, when 30-40 nymphs per leaf were present. Artificial infestation of plants took place at 28 days following transplantation (8-10 leaves per plant). For the cage experiments, 20 M. persicae fourth-instars were transferred to the abaxial surfaces of the 2nd and 3rd fully extended youngest leaves (10 nymphs per leaf) of each plant. For the open-field experiment, around four infested leaf pieces (1.0 x 1.0 cm) from the stock rearing cucumber plants were fixed to each plant in the field and infestation level was adjusted to 20 fourth-instars per plant in the following day.
First experiment with cages: screening of fungal isolates
Isolates IBCB 66, PL 63 and CG 864 of B. bassiana, obtained from the Germplasm Bank at EMBRAPA Genetic Resources and Biotechnology, were originally isolated from cadavers of the coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae), a leaf-cutting ant, Atta sp. (Hymenoptera: Formicidae), and the black coconut bunch weevil, Homalinotus coriaceus (Gyllenhal) (Coleoptera: Curculionidae), respectively. All isolates were previously shown to be virulent toward aphids under lab conditions. Nymphs of Myzus persicae were immersed for 5 s in a 1.0 x 108 conidia/ml suspension, transferred to 9-cm Petri dishes containing a cabbage leaf onto a 3% agar-water layer. Petri dishes were sealed and kept for 10 days in an incubator (25 ± 2ºC, 72 ± 10% RH, and 12:12h light:dark photoperiod). Following conidiogenesis on the host surface, each pathogen was isolated and cultured twice on potato dextrose agar (PDA) (Acumed, Lansing, USA). Aerial B. bassiana conidia were mass produced in polypropylene bags containing cooked parboiled rice as semi-solid medium (Leite et al 2003). In order to obtain pure conidia, batches of fungus-containing substrate were first dehydrated in desiccators containing silica gel for seven days at room temperature. Subsequently, the colonized substrate was sieved (100-mesh sieve) under agitation (250 rpm), and the harvested conidia, with an initial viability of 92-98%, were stored at 8ºC until needed.
Selection of fungal isolates was performed in November 2004, with plants grown in plastic pots kept in plastic cages (90 cm x 60 cm x 80 cm) screened with voile fabric. Cages were arranged in six transects (blocks) with four cages (treatments) each, and kept 5 m apart. Each screened cage hold two potted cabbage plants. Plants were sprayed (20 ml suspension/plant) at days four and nine after the artificial infestation with aphids by means of a garden sprayer. Fungal isolates were prepared in an aqueous suspension (0.01% Tween 80 + 0.01% v/v Agral) at a concentration of 1.0 x 108 viable conidia/ml. Temperature and relative humidity inside three cages were monitored continuously with dataloggers (Klima Logger, TFA, Germany). Rainfall was monitored by a weather station located approximately 2 km from the field site.
The performance of B. bassiana isolates was evaluated by comparing the population density of M. persicae to a control treatment (0.01% Tween 80 + 0.01% Agral). The aphid population was assessed one day before and 14 days after the first application by inspecting the entire plant for the presence of nymphs and adults.
Second experiment with cages: assessment of formulation types
The experiment was carried out in December 2004, and cabbage plants grown in plastic pots were kept in screened cages similar to those previously described. The experiment was set up in a randomized block design with five replicates for each treatment, each replicate represented by one screened cage with two cabbage plants. Aerial conidia of isolate CG 864 produced in cooked rice were formulated as oil dispersions prepared with either a commercial emulsifiable oil (Natur'l Oil, Stoller do Brasil, Cosmopolis, Brazil) or proprietary adjuvants provided by a biopesticide company (Bthek Biotecnologia, Brasilia, Brazil).
The following treatments were applied: 1) aqueous conidial suspension prepared with surfactants (0.01% Tween 80 + 0.01% v/v Agral), 2) emulsifiable oil (Natur'l Oil) at 0.3% v/v, 3) proprietary adjuvants at 0.3% v/v, 4) oil dispersion 1 (conidia + Natur'l Oil), 5) oil dispersion 2 (conidia + adjuvants), 6) control (0.01% Tween 80 + 0.01% v/v Agral) and, 7) the chemical insecticide imidacloprid (Confidor 700 GRDA, Bayer S/A, Sao Paulo, Brazil) at a dosis equivalent to 0.02 g a.i./plant/spray. Oil dispersions were diluted in water just before use, adjusting the final concentration of surfactants to 0.3% v/v. For B. bassiana preparations and their adjuvants, plants were sprayed three times, starting at day four following aphid infestation (32 days post-transplanting). Conidial dosis per plant, spraying equipment and the interval between applications were as described for the previous experiment. The insecticide imidacloprid was applied to a single treatment and only once at the same day mycoinsecticide treatments were first sprayed. Temperature and relative humidity inside cages were monitored as earlier described.
Assessment of mycoinsecticides under open-field conditions
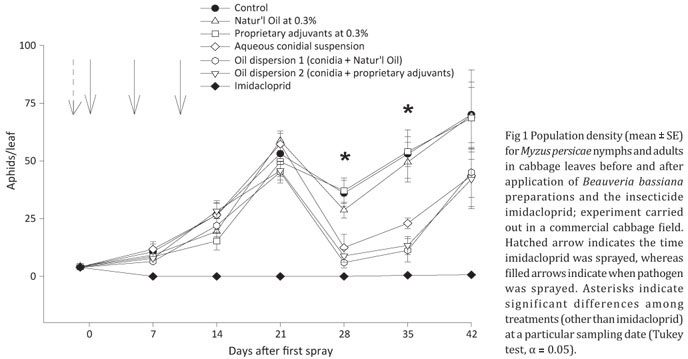
The experiment was conducted between January and February 2005 in a commercial cabbage field at Federal District, without the use of cages. The experimental site consisted of 28 plots measuring 1.5 m x 4.1 m, each consisting of four rows 0.50 m apart and 0.45 m wide, and totaling 40 plants. A 3 m long uncultivated space was kept between plots and blocks. Seven treatments, referred to in the second cage experiment, were applied at different doses. Isolate CG 864 was applied three times at 200 L/ha in a concentration of 5.0 x 1010 conidia/L (1.0 x 1013 viable conidia/ha), beginning four days after the artificial infestation of plants with aphids. A 5-day interval between sprays was adopted, and applications were performed with a backpack sprayer equipped with a hollow cone spray nozzle (ceramic nozzle Micron HC3, Pulsar Tecnologia, Sao Paulo, Brazil) with a pressure equivalent to 2.88 kgf/m2. Just as in the previous experiment, imidacloprid was applied once at 210 g a.i./ha. Temperature, relative humidity and rainfall during the experiment were obtained from a weather station installed 2 km away from the field site. The experimental design was set up in randomized blocks with four replicates (plots). Aphid population was assessed one day before and 7, 14, 21, 28, 35 and 42 days after the first spraying, and 15 plants were randomly inspected per plot on each date.
Statistical analyses
The data from the first cage experiment with B. bassiana isolates were log (x+1)-transformed prior to ANOVA, and means were compared by Tukey test or paired t-test at α = 0.05. The relative control efficiency (%) was calculated using the formula of Henderson & Tilton (1955), based on the population levels of aphids in the control plot and other treatments on the first and last assessments. For the second cage experiment and the field experiment with commercial cabbage, data were log (x+1)-transformed prior to MANOVA for repeated measurements over time, and means were compared within and between evaluation dates by using the Tukey's test ( α = 0.05). The relative control efficiency (%) was calculated as previously mentioned. All analyses were done using the statistical packages available in SAS software (SAS Institute 2001).
Results and Discussion
In the first cage experiment, the initial aphid infestation did not differ among treatments (F3,15 = 1.18, P = 0.35) (Table 1). However, colonies of M. persicae were significantly smaller (F3,15 = 8.51, P < 0.01) in plants sprayed with isolates CG 864 and PL 63 14 days after treatment, but not with isolate IBCB 66. PL 63 and CG 864 were the most promising isolates, with 57-60% relative control efficiency (Table 1). The efficiency observed for the isolate ICBC 66 disagrees from Loureiro & Moino Jr (2006), who reported this isolate as very active against M. persicae. However, this discrepancy might be related to the different experimental conditions used, as several other factors, such as culture conditions, formulation, temperature and host plant are known to affect virulence of a given isolate (Butt & Goettel 2000, Lacey & Kaya 2007, Yeo et al 2003, Santoro et al 2007).
In the second cage experiment, a sharp increase in M. persicae population was observed in control plants (Table 2). The population fluctuated over time (Wilk's λ = 0.29, F6,24 = 9.83, P < 0.01), but fluctuation was less pronounced in plants sprayed with either formulated or unformulated conidial preparations, and virtually zero in imidacloprid-treated cabbage plants. None of the adjuvants tested were toxic to aphids. Despite the apparently superior performance of formulated conidia in this cage experiment, no significant differences in population density of M. persicae were found between the aqueous conidial suspension treatment and oil dispersions (Table 2).
Noteworthy, control efficiency for unformulated conidia recorded in both cage experiments ranged from 60 to 71% at 14-28 daa, and no significant differences were observed among unformulated and formulated preparations in the second cage experiment (data not shown).
In the field experiment carried out in a commercial cabbage field (without cages), the population density of M. persicae did differ among treatments throughout the sampling period (Wilk's λ = 0.04, F30,58 = 2.38, P < 0.01) and, except for imidacloprid-treated plants, aphid populations rapidly grew in all plots (Fig 1). Significant differences in the mean densities of M. persicae among control and B. bassiana preparations were more evident at 28 and 35 daa (Fig 1). Among conidial preparations, significant differences were found only at 35 daa, when aphid colonies were smaller in plants sprayed with oil dispersions in relation to the aqueous (unformulated) conidial suspension (Fig 1). The imidacloprid treatment significantly differed from all other treatments, eliminating M. persicae colonies soon after its application, and keeping very low densities during the whole experimental period. The relative control efficiency followed the same trend (data not shown); imidacloprid was 98-100% efficient over the experiment duration, whereas control efficiency at 28-35 daa provided by B. bassiana preparations reached 76-83% and 57-65% for oil dispersions and unformulated conidia, respectively.
In tropical countries, control of pests with high reproductive potential by use of mycoinsecticides is a major challenge under field conditions. For instance, reduction in the average number of the aphid Diuraphis noxia (Kurdjumov) did not reach 80% in field trials in South Africa (Hatting et al 2004). In Brazil, only 60% control efficiency of Aphis sp. nymphs in an organic acerola plantation was reported following spraying of either B. bassiana or M. anisopliae (Medeiros et al 2007).
The B. bassiana oil dispersion based on Natur'l Oil (oil dispersion 1) was as efficient as the oil dispersion based on proprietary adjuvants (oil dispersion 2) (Fig 1). The impact of Beauveria bassiana on population density of this aphid became significant approx. four weeks following first spray in the commercial cabbage field, but the effect was short lived. The progressive reduction in the control efficiency of B. bassiana could be attributed, at least in part, to explosive growth rates of M. persicae populations starting at the 5th week after first spray. Additionally, poor results by the end of the experimental period were also affected by loss of residual effect by tested mycoinseticides, making horizontal transmission of the pathogen a low-impact event. Similar results were observed in other studies with aphids and other pests with high reproductive potential (Vandenberg et al 1998, Ying et al 2003) and, therefore, numerous reapplications would be necessary to keep density below economic threshold levels throughout the crop cycle.
The control efficiency afforded by the aqueous conidial suspension in the field experiment without cages was usually 10-20% lower than those of oil dispersions, and significant differences were evident in some sampling dates (data not shown). Interestingly, differences among formulation types were harder to detect in the previous cage experiment. It is likely that under field conditions unformulated preparations are less efficient than oil-formulated conidia (Wraight & Ramos 2002, Batta 2003). Entomopathogenic fungi sprayed on leaves are sensitive to adverse environmental factors such as sunlight, rain, humidity, leaf surface chemistry, and the phylloplane microbiota (Wraight et al 2001, Steinkraus 2006, Jaronski 2010). For instance, the inactivation of B. bassiana conidia by UV radiation has been broadly reported (Ignoffo & Garcia 1992, Inglis et al 1995, Fernandes et al 2007, Huang & Feng 2009). However, oil-formulated conidia are believed to be less susceptible to the effects of sunlight (Moore et al 1993, Alves et al 1998) and rainfall (Inglis et al 2000), and to display an increased adhesion and deposition on the hydrophobic cuticle of arthropods (Prior et al 1988, Ibrahim et al 1999). Therefore, enhanced control efficiency achieved by the use of oil dispersions over non-formulated conidia would be expected in the open-field cabbage experiment, in which a higher UV exposure compared to the cage experiments, and a total rainfall of 498.7 mm in 32 rainy days (data not shown), has most likely played an important role.
The systemic action of imidacloprid and its prolonged residual effect on cabbage plants contributed to maintaining low levels of aphids throughout the experimental period. So far, most studies in tropical countries aiming to control aphids were carried out under lab conditions (Loureiro & Moino Jr 2006, Almeida et al 2007, Araujo Jr et al 2009). Our work represents a step ahead on the study of formulated B. bassiana-based mycoinsecticides for regulation of aphid populations in crucifers under field conditions, although control levels achieved were insufficient to recommend B. bassiana as a stand-alone control agent. Additional studies in order to enhance field persistence of fungal-based formulations under tropical conditions are required. Nevertheless, our results suggest the potential of this pathogen as a component of IPM strategies to be used in earlier stages of cabbage development, when aphid infestation is not high. Besides, mycoinsecticides can become a desirable tool to assist in the management of aphid resistance to conventional insecticides.
Acknowledgments
We are thankful to Dr Carlos Marcelo Soares (Bthek Biotecnologia Ltda., Brasilia, DF) for provision of proprietary adjuvants used in the studies, to CNPq for a scientific initiation scholarship (PIBIC) granted to S O D Oliveira, and FAP-DF for the financial support to our research project (FAPDF OF 184/2004).
Received 16 September 2010 and accepted 28 February 2011
Edited by Ítalo Delalibera Jr ESALQ/USP
- Alves RT, Bateman RP, Prior C, Leather SR (1998) Effects of simulated solar radiation on conidial germination of Metarhizium anisopliae in different formulations. Crop Prot 17: 675-679.
- Almeida GD de, Pratissoli D, Polanczyk RA, Holtz AM, Vicentini VB (2007) Determinação da concentração letal média (CL50) de Beauveria bassiana para o controle de Brevicoryne brassicae Idesia 25: 69-72.
- Araujo Jr JM, Marques EJ, Oliveira JV de (2009) Potential of Metarhizium anisopliae and Bauveria bassiana isolates and neem oil to control the aphid Lipaphis erysimi (Kalt.) (Hemiptera: Aphididae). Neotrop Entomol 38: 520-525.
- Batta YA (2003) Production and testing of novel formulation of the entomopathogenic fungus Metarhizium anisopliae (Metschinkoff) Sorokin (Deuteromycotina: Hyphomycetes). Crop Prot 22: 415-422.
- Butt TM, Goettel MS (2000) Bioassays of entomogenous fungi, p.141-195. In Navon A, Ascher KRS (eds) Bioassays of entomopathogenic microbes and nematodes. Wallingford, CABI Publishing, 324p.
- Faria MR de, Wraight SP (2007) Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control 4: 237-256.
- Fernandes EKK, Rangel DEN, Moraes AL, Bittencourt VREP, Roberts DW (2007) Variability in tolerance to UV-B radiation among Beauveria spp. isolates. J Invertebr Pathol 96: 237-243.
- Foster SP, Denholm I, Devonshire AL (2000) The ups and downs of insecticide resistance in peach-potato aphids (Myzus persicae) in the UK. Crop Prot 19: 873-879.
- Harrewijn P, Minks AK (1989) Integrated aphid management: General aspects, p.267-272. In Minks AK, Harrewijn P (eds) World crop pests - aphids: their biology, natural enemies and control. Vol. C. New York, Elsevier, 312p.
- Harrington R, van Emden HF (2007) (eds) Aphids as crop pests. London, CABI Publishing. 717p.
- Hatting JL, Wraight SP, Miller RM (2004) Efficacy of Beauveria bassiana (Hyphomycetes) for control of Russian wheat aphid (Homoptera: Aphididae) on resistant wheat under field conditions. Biocontrol Sci Technol 14: 459-473.
- Henderson CF, Tilton EW (1955) Tests with acaricides against the brown wheat mite. J Econ Entomol 48: 157-161.
- Huang BB, Feng MG (2009) Comparative tolerances of various Beauveria bassiana isolates to UV-B irradiation with a description of a modeling method to assess lethal dose. Mycopathol 168: 145-152.
- Ibrahim L, Butt TM, Beckett A, Clark SJ (1999) The germination of oil-formulated conidia of the insect pathogen, Metarhizium anisopliae Mycol Res 103: 901-907.
- Ignoffo CM, Garcia C (1992) Influence of conidial color on inactivation of several entomogenous fungi (Hyphomycetes) by simulated sunlight. Environ Entomol 21: 913-917.
- Inglis GD, Ivie TJ, Duke GM, Goettel MS (2000) Influence of rain and conidial formulation on persistence of Beauveria bassiana on potato leaves and Colorado potato beetle larvae. Biol Control 18: 55-64.
- Inglis DG, Goettel MS, Johnson DL (1995) Influence of ultraviolet light protectants on persistence of the entomopathogenic fungus, Beauveria bassiana Biol Control 5: 581-590.
- Jaronski ST (2010). Ecological factors in the inundative use of fungal entomopathogens. BioControl 55: 159-185.
- Lacey LA, Kaya HK (2007) (eds) Field manual of techniques in invertebrate pathology: application and evaluation of pathogens for control of insects and other invertebrate pests. 2nd ed. Dordrecht, Springer, 868p.
- Leite LG, Batista Filho A, Almeida JEM de, Alves SB (2003) Produção de fungos entomopatogênicos. Ribeirão Preto, A.S. Pinto, 92p.
- Loureiro ES, Moino Jr A (2006) Patogenicidade de fungos hifomicetos aos pulgões Aphis gossypii Glover e Myzus persicae (Sulzer) (Hemiptera: Aphididae). Neotrop Entomol 35: 660-665.
- Medeiros MB, Alves SB, Lopes RB, Barbosa A da S, Garcia MO, Berzaghi LM (2007) Associação de biofertilizante líquido e fungos entomopatogênicos no controle do pulgão Aphis sp. em aceroleira (Malpighia glabra L.). Rev Bras Agroecol 2: 821-824.
- Michereff Filho M, Faria M, Wraight SP, Silva KFAS (2009) Micoinseticidas e micoacaricidas no Brasil: como estamos após quatro décadas? Arq Inst Biol 76: 769-779.
- Moore D, Bridge PD, Higgins PM, Bateman RP, Prior C (1993) Ultra-violet radiation damage to Metarhizium flavoviride conidia and the protection given by vegetable and mineral oils and chemical sunscreens. Ann Appl Biol 122: 605-616.
- Prior C, Jollands P, Le Patourel G (1988) Infectivity of oil and water formulation of Beauveria bassiana (Deuteromycotina: Hyphomycetes) to the Cocoa weevil pest Pantorhytes plutus (Coleoptera: Curculionidae). J Invertebr Pathol 52: 66-72.
- Santoro PH, Neves PMOJ, Alexandre TM, Alves LFA (2007) Interferência da metodologia nos resultados de bioensaios de seleção de fungos entomopatogênicos para o controle de insetos. Pesq Agropec Bras 42: 483-489.
- SAS Institute (2001) SAS user's guide: statistics, version 8.2, 6th Editon. SAS Institute, Cary, NC, 943p.
- Steinkraus DC (2006) Factors affecting transmission of fungal pathogens of aphids. J Invertebr Pathol 92: 125-131.
- Vandenberg JD, Shelton AM, Wilsey WT, Ramos M (1998) Assessment of Beauveria bassiana sprays for control of Diamondback moth (Lepidoptera: Plutellidae) on crucifers. J Econ Entomol 91: 624-630.
- Wraight SP, Ramos ME (2002) Application parameters affecting field efficacy of Beauveria bassiana foliar treatments against Colorado potato beetle Leptinotarsa decemlineata Biol Control 23: 164-178.
- Wraight SP, Jackson MA, de Kock SL (2001) Production, stabilization and formulation of fungal biocontrol agents, p. 253-287. In Butt TM, Jackson C, Magan N (eds) Fungi as biocontrol agents: progress, problems and potential. Wallingford, CAB International, 416p.
- Yeo H, Pell JK, Alderson PG, Clark SJ, Pye BJ (2003) Laboratory evaluation of temperature effects on the germination and growth of entomopathogenic fungi and on their pathogenicity to two aphid species. Pest Manag Sci 59: 156-165.
- Ying SH, Feng MG, Xu ST, Ma ZB (2003) Field efficacy of emulsifiable suspensions of Beauveria bassiana conidia for control of Myzus persicae population on cabbage. Chin J Appl Ecol 14: 530-535.
Publication Dates
-
Publication in this collection
14 Sept 2011 -
Date of issue
Aug 2011
History
-
Received
16 Sept 2010 -
Accepted
28 Feb 2011