Abstracts
African beetles Onthophagus gazella from both sexes were analyzed by electrophoresis for an investigation of esterase isozymes using alpha-naphthyl propionate and methylumbelliferyl propionate as substrates. Only one of the esterases (Est. 6) reacted with one of the substrates (alpha-naphthyl propionate). Six areas of activity were found, two of them being polymorphic (Est. 3 and Est. 4). For presence of Est. 3, 337 individuals were analyzed, including descendants of 32 controlled crossings: two alleles were identified, whose frequencies are Est. 3A = 0.447 and Est. 3B = 0.553. The population is in equilibrium for this locus (qui-square = 4.18; 0.2 > P > 0.1). For Est. 4, 338 individuals, descendants of 32 controlled crossings, were analysed. In this case, three alleles were identified whose frequencies are: Est. 4A = 0.277; Est. 4B = 0.661; and Est. 4C = 0.062. The population is not in equilibrium for this locus (qui-square = 40.259; p < 0.001). Two esterases were detected only in the pupal stage and another one in larvae. Of the 23 loci analyzed in these insects up to now, 3 are polymorphic (13%), which indicates very low variability in the population here studied.
beetle; isozyme; esterase
Foram analisados adultos de ambos os sexos do besouro africano Onthophagus gazella por eletroforese, para investigação de isozimas de esterases, utilizando como substratos alfa-naftil propionato e metil umbeliferil propionato. Somente uma das esterases (Est. 6) reagiu apenas com um dos substratos (alfa-naftil propionato). Foram encontradas seis regiões de atividades diferentes, sendo duas polimórficas (Est. 3 e Est. 4). Na região de Est. 3, foram analisados 337 indivíduos, incluindo descendentes de 32 cruzamentos controlados, e identificados 2 alelos, cujas freqüências são: Est. 3A = 0,447 e Est. 3B = 0,553. A população está em equilíbrio para esse loco (qui-quadrado = 4,18; 0,2 > p > 0,1). Na região de Est. 4, foram analisados 338 indivíduos, incluindo descendentes de 32 cruzamentos controlados, e identificados 3 alelos, cujas freqüências são: Est. 4A = 0,277; Est. 4B = 0,661; e Est. 4C = 0,062. A população não está em equilíbrio para esse loco (qui-quadrado = 40, 259; p < 0,001). Foram detectadas duas regiões de esterases características da fase de pupa e uma região que aparece somente na larva. Do total de 23 locos amostrados até o momento nesses insetos, 3 são polimórficos (13%), o que indica variabilidade genética muito baixa na população estudada.
besouro; isozima; esterase
AFRICAN DUNG BEETLE Onthophagus gazella FABRICIUS (COLEOPTERA: SCARABAEIDAE) ESTERASE ISOZYMES
MARTINS, E.1 and CONTEL,E. P. B.2
1School of Medicine of the Triângulo Mineiro, Praça Manoel Terra, 330, CEP 38015-050, Uberaba, MG, Brazil
2Departamento de Genética, Escola de Medicina de Ribeirão Preto, Avenida Bandeirantes, 3900, CEP 14049-900, Ribeirão Preto, SP, Brazil
Correspondence to: Elizabeth Martins, Rua General Osório, 1104, CEP 33081-090, Uberaba, MG, Brazil, e-mail: martins@mednet.com.br
Received June 21, 2000 Accepted September 26, 2000 Distributed November 30, 2001
(With 2 figures)
ABSTRACT
African beetles Onthophagus gazella from both sexes were analyzed by electrophoresis for an investigation of esterase isozymes using alpha-naphthyl propionate and methylumbelliferyl propionate as substrates. Only one of the esterases (Est. 6) reacted with one of the substrates (alpha-naphthyl propionate). Six areas of activity were found, two of them being polymorphic (Est. 3 and Est. 4). For presence of Est. 3, 337 individuals were analyzed, including descendants of 32 controlled crossings: two alleles were identified, whose frequencies are Est. 3A = 0.447 and Est. 3B = 0.553. The population is in equilibrium for this locus (qui-square = 4.18; 0.2 > P > 0.1). For Est. 4, 338 individuals, descendants of 32 controlled crossings, were analysed. In this case, three alleles were identified whose frequencies are: Est. 4A = 0.277; Est. 4B = 0.661; and Est. 4C = 0.062. The population is not in equilibrium for this locus (qui-square = 40.259; p < 0.001). Two esterases were detected only in the pupal stage and another one in larvae. Of the 23 loci analyzed in these insects up to now, 3 are polymorphic (13%), which indicates very low variability in the population here studied.
Key words: beetle, isozyme, esterase.
RESUMO
Isozimas de esterases do besouro africano Onthophagus gazella Fabricius (Coleoptera: Scarabaeidae)
Foram analisados adultos de ambos os sexos do besouro africano Onthophagus gazella por eletroforese, para investigação de isozimas de esterases, utilizando como substratos alfa-naftil propionato e metil umbeliferil propionato. Somente uma das esterases (Est. 6) reagiu apenas com um dos substratos (alfa-naftil propionato). Foram encontradas seis regiões de atividades diferentes, sendo duas polimórficas (Est. 3 e Est. 4). Na região de Est. 3, foram analisados 337 indivíduos, incluindo descendentes de 32 cruzamentos controlados, e identificados 2 alelos, cujas freqüências são: Est. 3A = 0,447 e Est. 3B = 0,553. A população está em equilíbrio para esse loco (qui-quadrado = 4,18; 0,2 > p > 0,1). Na região de Est. 4, foram analisados 338 indivíduos, incluindo descendentes de 32 cruzamentos controlados, e identificados 3 alelos, cujas freqüências são: Est. 4A = 0,277; Est. 4B = 0,661; e Est. 4C = 0,062. A população não está em equilíbrio para esse loco (qui-quadrado = 40, 259; p < 0,001). Foram detectadas duas regiões de esterases características da fase de pupa e uma região que aparece somente na larva. Do total de 23 locos amostrados até o momento nesses insetos, 3 são polimórficos (13%), o que indica variabilidade genética muito baixa na população estudada.
Palavras-chave: besouro, isozima, esterase.
INTRODUCTION
Onthophagus gazella (Scarabaeidae, Scarabaienae), which became known as the African beetle, has been imported in Brazil by the National Center of Beef Cattle Research (CNPGC) of the Brazilian Agricultural Research (Embrapa) Company of Campo Grande (MS), as part of a program seeking the biological control of the hornfly, Haematobia irritans (Nascimento et al., 1990).
Electrophoretic techniques for isozyme separation have produced genetic markers for various practical applications (Wagner & Selander, 1974; Hartl & Clark, 1989).
Some enzymatic loci are frequently polymorphic, while others are rarely present as two or more alleles. Esterases, many of which present little specificity to substracts, are enzymes with large variability among insects and other organisms. Healy et al. (1991) identified 22 different esterases in Drosophila melanogaster, by combining polyacrilamide gel electrophoresis with isoelectric focusing. Oakeshott et al. (1993) classified more than 30 esterases in D. melanogaster based on two-dimensional electrophoretic analysis, substrate specificities, and esterase inhibitors. The results led to a genetic map of 15 different genes of this specie, 12 of them found in two chromosomic sites. More than twelve alleles were identified in esterase loci of local populations of several species of Lepidoptera (Lima & Contel, 1990) and Diptera (Tsakas & Krimbas, 1970). In populations of the cricket Gryllus integer, 24 non-silent alleles were detected in an esterase locus (Wagner & Selander, 1974). This great variability of esterases was also found in bees (Frohlich et al., 1990; Bitondi & Mestriner, 1983), Anopheles darlingi (Santos et al., 1985), Anopheles triannulatus (Santos et al., 1992), Anopheles gambiae (Vernick et al., 1988), Paratheresia claripalpis (Marin & Mestriner, 1985), Aedes aegypti (Dinardo-Miranda & Contel, 1996), Spodoptera frugiperda (Lima & Contel, 1990), and other insects belonging to several orders (Prabhakaran & Kamble, 1994; Vaughan et al., 1995).
The main objective of our work was to investigate the genetic loci responsible for isozyme production from controlled crossings of Onthophagus gazella maintained in terraria, and in the population that colonized the pastures of the Getúlio Vargas Experimental Farm of Uberaba (MG). Martins & Contel (1998) previously analyzed the presence of malic enzyme (ME), isocitrate dehydrogenase (IDH), glycerol phosphate dehydrogenase (GPDH), and leucine-aminopeptidase (LAP) in O. gazella. In the present paper, we describe two new esterase isozymes using two different substrates, in various O. gazella developmental phases, and two polymorphic loci in its adult forms.
MATERIAL AND METHODS
Controlled crossings
O. gazella were grown and bred as previously described (Martins & Contel, 1997). In order to carry out genetic studies with controlled crossing, a couple of insects were maintained in a terrarium. Newborn males and females were transfered and fed in two separated (independent) terraria for seven days, after which the insects were submitted to experimental cross-breeding. Eggs, larvae, and pupae were collected from the "pears" of the terraria. Young adult forms were transfered to a new terrarium and fed before being collected. Different samples were harvested in small glass flasks or vials and stored froozen at 20ºC for further analysis.
Isozymes analyses
Individual beetle samples of the controlled crossing were ground in 0.5 ml of distilled water. The extracts were clarified by centrifugation. Aliquots of the different supernatants were loaded on starch gel and submitted to electrophoresis analysis. After the run, the gel was sliced in two parts in order to be stained and reacted with two different substrates. Electrophoretic conditions and reaction color development were done according to Dinardo-Miranda (1994).
RESULTS
To investigate esterase isozymes in beetles, O. gazella, controlled crossings of males and females were performed in terraria. The parents and descendants were harvested at different developmental stages and enzymatic activity tests were done to analyze esterease expression at the protein level. Total protein extracts were prepared and submitted to electrophoresis in Tris-acetate buffer at pH 7.2 and 7.6. The gels were stained in the presence of alpha-naphthyl and methylumbelliferyl propionate. Under these conditions, six different esterase areas could be characterized, with both alpha-naphthyl (Figs. 1 and 2) and methylumbelliferyl propionates:
Est. 1: shown as fastest, narrow, clear, and without variation area; Est. 2: shown as a large band, strongly stained, also seemingly invariable in mobility; Est. 3: characterized by moderate mobility, as one or two areas indicating three genotypes, according to the hypothesis of a locus with two alleles; Est. 4: appears as the expression of three codominant alleles of a locus; Est. 5 and Est. 6: esterases that can be observed, independently of age and of sex, when migration exceeds 5 hours or electric current of larger intensity is used. In these conditions, Est. 1 and Est. 2 cannot be visualized and Est. 3 band is barely seen. Est. 6 does not appear with methylumbelliferyl propionate (Fig. 2). Analyzing the Est. 3 area, of males and females of O. gazella, two phenotypes were observed, revealed as either one or two activity areas.
Observing the descendants of 32 controlled crossings, the presence of a locus with two codominant alleles (Est. 3A and Est. 3B) was verified. The 6 possible types of crossings were found: out of 64 total individuals used in the matings, 37 had their genotypes determined and 26 deduced (Table 1).
Based on the electrophoretic profiles obtained of 337 animals, the allele frequencies calculated were 0.447 for Est. 3A and 0.553 for Est. 3B.
The qui-square test showed that the studied population is in genetic equilibrium (qui-square = 4.18; 0.2 > p > 0.1). We did not observe any difference in segregation between the sexes, meaning numbers are approximately similar for males and females.
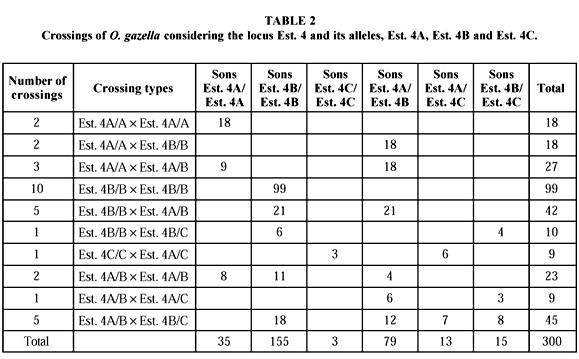
In regard to the locus Est. 4, six different phenotypes were detected which, in agreement with the proposed hypothesis, would be a result of the expression of 3 codominant alleles (Est. 4A, Est. 4B and Est. 4C). The homozygotes are shown by one activity area and the heterozygotes are shown by two. For this locus, 32 controlled crossings were also used, of which 15 depended on analyzes of parents and progeny; 6, on a single parent and progeny analyzes, and 11 on progeny analyzes. Ten out of 21 types of possible crossings were detected, which was expected, considering that the Est. C allele is rare (Table 2).
Allele frequencies for Est. 4 locus were estimated based on 338 individual analyzes: Est. 4A = 0.277; Est. 4B = 0.661; and Est. 4C = 0.062. The genetic equilibrium test led to a highly significant qui-square one (40.259; p < 0.001). This value may possibly be due to the low number of experimental crosses realized. An increase in the sample number could provide better frequencies for these alleles.
Some differences were observed in the esterase electrophoretic profiles performed during the developmental phases: the esterase activity was absent in eggs while in larva a characteristic area, which migrates between adult Est. 2 and Est. 3 was verified. The pre-pupae and the pupae possess their own esterases, of moderate mobilities, i. e., between adult Est. 4 and Est. 5, and characterized by two strong areas. If we consider every esterase observed in the several developmental phases of O. gazella, a total of at least 9 different loci would be involved in their syntheses, where 6 remain active in adult forms, 1 in the larvae, and 2 in the pupae.
DISCUSSION
The O. gazella population of Uberaba, MG, began in 1991, starting with 30 couples, as registered in the files of Getúlio Vargas Experimental Farm. Descendants of this group have colonized the pastures of the farm and possibly due to that there was a drastic decrease in population size. Only after aproximately 32 generations, insects used in the present work could be isolated in order to carry out the genetic studies described in this work.
Among the 23 loci sampled by electrophoresis (Martins & Contel, 1998; present paper), three presented more than one allele which means 1.217 alleles for locus [calculation according to Nei (1985)] and a proportion of polymorphic loci (Swofford & Selander, 1981) of 0.13.
This value is very low when compared with that calculated for populations of several species of Drosophila (58% to 71%) as described by Ayala & Powell (1972) and even for mammals (0.15), which were considered by Hartl & Clark (1989), as being those with least variability reported for allozymes.
Among the isozymes, the esterases showed larger variability. Different eletrophoretic profiles were found, according to the development phase, indicating genes whose activities are related to specific ages. Similar facts have already been reported for other insects, such as bees (Bitondi & Mestriner, 1983; Frohlich et al., 1990) and Drosophila (Healy et al., 1991). The esterases detected in the Coleopteron O. gazella can be classified, according to Oakeshott et al. (1993), as carboxyl-esterases since they preferentially react with methylumbelliferyl and alpha-naphthyl propionates.
Lack of specificity for substrates used in vitro is commonly observed in a great variety of organisms (Oakeshott et al., 1993). The Est. 6 of O. gazella showed lack of specificity for the methylumbelliferyl propionate substrate although another two areas can be visualized in some individuals with this substrate.
When Est. 3 detection was done on two progenies of controlled crossing, the phenotype Est. 3A/Est. 3A was not detected. In the first crossing, five individuals were expected with this phenotype (of the ten analyzed) and in the second crossing, phenotypes Est. 3A/Est. 3A and Est. 3A/Est. 3B were expected respectively in the 2.25 and 4.5 individuals.
Absence of the phenotypes might be due either to the small number of insects analyzed or to a chance event or that the parents genotypes were not correctly or incorrect determination of the parent genotypes determined. The same crossings, however, supplied consistent data in relationship to locus Est. 4 indicating that these were well controlled.
Genetic equilibrium tests were done on Est. 3 and Est. 4 loci. The results suggested that Est. 3 locus remains in equilibrium, indicating that the population of O. gazella which we studied has already reached stability with respect to some loci. On the other hand, the two other loci, Est. 4 in the present work and ME (Martins & Contel, 1998), might be going through a selective process because they strayed from the expected in the collected data, considering that the number of insects analyzed is representative.
Onthophagus gazella can now be considered as an exotic species that colonizes the Brazilian Cerrado. From the original specimens maintained in Uberaba (MG), beetles were supplied to farms in three major areas of Minas Gerais and Southern Goiás. From Campo Grande (MS), couples of these insects were supplied to several Brazilian states. In Goiânia (GO), they are in the adaptation phase since 1992 (Lima & Godoi, 1994).
The population of Onthophagus gazella of Uberaba (MG) presents low genetic variability and is not in equilibrium in 2 of the 23 loci we studied. The estimated variability of esterase expression reported in our studies is possibly one of the lowest found in organisms reproducing sexually. A comparison of the O. gazella population with some others, Brazilian or not, will clarify if this species is really so homogeneous. Further analyzes should be done to elucidate this point because O. gazella has become cosmopolitan.
This is an initial stage of the biological study of these Coleopterons. We intend to analyze characteristics of populations of different areas. Since the species is now cosmopolitan, our studies will allow selection of populations of larger economic interest and evaluation of biological control programs that include use of this species.
Acknowledgments We are thankful to the managing managers of the Getúlio Vargas Experimental Farm, belonging to Epamig, on behalf of Zootecnists Reginério Soares de Faria and Leonardo de Oliveira Fernandes for having guaranteed conditions for the creation of the beetles; and to Prof. Doctor Roseli Aparecida Silva Gomes for having facilitated the accomplishment of part of the electrophoresis in her laboratory. This work was accomplished with the financial suport of Capes and CNPq.
- AYALA, F. J. & POWELL, J. R., 1972, Enzyme variability in the Drosophila willistoni group. VI. Levels of polymorphism and the physiological function of enzymes. Biochem. Genet., 7: 331-345.
- BITONDI, M. M. G. & MESTRINER, M. A., 1983, Esterase isozymes of Apis mellifera: Substrate and inhibition characteristics, developmental ontogeny, and electrophoretic variability. Biochem. Genet., 21: 985-1001.
- DINARDO-MIRANDA, L. L., 1994, Variabilidade protéica em populações naturais of Aedes aegypti (Linnaeus, 1762) (Diptera, Culicidae) Ph.D. Thesis, F.M.R.P., USP.
- DINARDO-MIRANDA, L. L. & CONTEL, E. P. B., 1996, Enzymatic variability in natural populations of Aedes aegypti (Diptera: Culicidae) from Brazil. J. Med. Entom., 33: 726-733.
- FROHLICH, D. R., BRINDLEY, W. A., BURRIS, T. E. & YOUSSEF, N. N., 1990, Esterase isozymes in the solitary bee, Megachile rotundata (Fab.): characterization, developmental multiplicity, and adult variability. Biochem. Genet., 28: 347-358.
- HARTL, D. L. & CLARK, A. G., 1989, Principles of Population Genetics 2. ed. Sinauer Associates, Inc., USA, 682p.
- HEALY, M. J., DUMANCIC, M. M. & OAKESHOTT, J. G., 1991, Biochemical and physiological studies of soluble esterases from Drosophila melanogaster Biochem. Genet., 29: 365-388.
- LIMA, L. M. K. S. & CONTEL, E. P. B., 1990, Electrophoretic analysis of 12 proteins in natural populations of Spodoptera frugiperda (Lepdoptera: Noctuidae). Rev. Bras. Genet., 13: 711-729.
- LIMA, M. G. A. & GODOI, D. S., 1994, Adaptação do besouro sul-africano Onthophagus gazella após sua liberação em pastagens em Goiânia, GO. Annals of the IV Symposium of Biological Control. Embrapa/CPACT, p. 99.
- MARIN, A. A. & MESTRINER, M. A., 1985, Genetic polymorphism in Paratheresia claripalpis (Diptera, Tachinidae). Rev. Bras. Genet., 8: 291-302.
- MARTINS, E. & CONTEL, E. P. B., 1997, Dados biológicos da criação do besouro africano Onthophagus gazella Fabricius (Scarabaeidae) em terrários na Fazenda Experimental Getúlio Vargas de Uberaba, MG. Rev. Bras. Biol., 57: 403-409.
- MARTINS, E. & CONTEL, E. P. B., 1998, Isoenzimas do besouro africano Onthophagus gazella Fabricius (Scarabaeidae): Enzima málica (ME), Glicerol fosfato desidrogenase (GPDH), Isocitrato desidrogenase (IDH) e leucina-aminopeptidase (LAP). Rev. Bras. Biol., 58(1): 39-46.
- NASCIMENTO, Y. A., BIANCHIN, I. & HONER, M. R., 1990, Instruções para a criação do besouro africano Onthophagus gazella em laboratório. Campo Grande, MS. Embrapa/CNPGC. Release technical, n.33, 5p.
- NEI, M., 1985, Human evolution at the molecular level. In: T. OHTA & K. AOKI (eds.), Population Genetics and Molecular Evolution 1.ed. Japan Scientific Societies Press, Tokyo, pp. 41-64.
- OAKESHOTT, J. G., VAN PAPENRECHT, E. A., BOYCE, T. M., HEALY, M. J. & RUSSELL, R. J., 1993, Evolutionary genetics of Drosophila esterases. Genetica, 90: 239-268.
- PRABHAKARAN, S. K. & KAMBLE, S. T., 1994, Subcellular distribution and characterization of esterase isozymes from insecticide-resistant and susceptible strains of German cockroach (Dictyoptera: Blattelidae). J. Econ. Entom., 87: 541-545.
- SANTOS, J. M. M., CONTEL, E. P. B. & KERR, W. E., 1985, Biology of Amazonian mosquitoes. III. Esterase isozymes in Anopheles darlingi Acta Amazon., 15: 161-177.
- SANTOS, J. M. M. D., TADEI, W. P. & CONTEL, E. P. B., 1992, Biologia de anofelinos amazônicos. XIV. Isoenzimas de esterase em Anopheles triannulatus (Neiva and Pinto, 1922). Acta Amazon., 22: 219-228.
- SWOFFORD, D. L. & SELANDER, R. B., 1981, Byosis-1: a Fortran program for the comprehensive analysis of electrophoretic data in population genetics and systematics. J. Hered., 72: 281-283.
- TSAKAS, S. & KRIMBAS, C. B., 1970, Tehe genetics of Dacus oleae. IV. Relations between adult esterase genotypes and survival to organophosphate insecticides. Evolution, 24: 807-815.
- VAUGHAN, A., RODRIGUEZ, M. & HEMINGWAY, J., 1995, The independent gene amplification of electrophoretically indistinguishable B esterases from the insecticide-resistant mosquito Culex quinquefasciatus Biochem. J., 305: 651-658.
- VERNICK, K. D., COLLINS, F. H., SEELEY, D. C., GWADZ, R. W. & MILLER, L. H., 1988, The genetics and expression of an esterase locus in Anopheles gambiae Biochem. Genet., 26: 367-380.
- WAGNER, R. P. & SELANDER, R. K., 1974, Isozymes in insects and their significance. Ann. Rev. of Entomol., 19: 117-138.
Publication Dates
-
Publication in this collection
24 May 2002 -
Date of issue
Nov 2001
History
-
Accepted
26 Sept 2000 -
Received
21 June 2000