Abstracts
Remobilization and re-utilization of 15N and 85Rb labelled nitrogen and potassium reserves for new growth and fruit formation was studied under greenhouse conditions using both normal and deficient young coffee plants. It was found that K reserves are used in higher proportion than is stored N by fruits and other organs. The export of N by organs of residence in the normal plants obeyed the following proportions of the total: leaves 47%-58%, branches and flower buds 21%-27%, roots 21%-32%. The corresponding figures in the case of deficient plants were: leaves 49%-65%, branches and flower buds 21%-27%, roots 14%-25%. Re-utilization of K took place in the following proportions in the normal plants: leaves 54%-64%, branches and flower buds 20%-21%, roots 30%-41%. In K deficient plants the figures were: leaves 62%-79%, branches and flower buds 1.2%-4.4%, roots 20%-33%. In tissues formed after the initiation of flowering buds, the demand for N is met by reserves as follows: normal plants: fruits 20.6%-24.8%, leaves 15.6%-19.4%, twigs 19%-20.5%; deficient plants: fruits 43.5%-48.5%, leaves 48.1%-51.9%, twigs 46%-53%. The K needs for new tissues are met in the order: normal plants: fruits 40%-45.8%, leaves 27%-37.6%, twigs 26%-33.1%; deficient plants: fruits 65.7%-81.5%, leaves 52.6%-68.4%, twigs 62%-86.1%. Fruits represent the main sink for both N and K. Re-utilization of both elements is higher in the case of deficient plants.
re-utilization; remobilization; nitrogen; potassium; rubidium; coffee plants; 15N; 85Rb
A remobilização do N (15N) e do K (85Rb) de reserva e seu uso pelas diferentes partes do cafeeiro (Coffea arabica cv. Catuaí Vermelho) no período reprodutivo, particularmente pelos frutos, foram estudados sob condições normais e de carência de N ou K. O K de reserva, comparado ao N de reserva, é utilizado em maior proporção pelos frutos e demais órgãos do cafeeiro. A exportação do N pelos órgãos de reserva foi a seguinte: plantas normais - folhas (47%-58%); ramos + gemas florais (20%-21%); raízes (21%-32%). Plantas deficientes - folhas (49%-65%); ramos + gemas florais (21%-27%); raízes (14%-25%). A remobilização do K de reserva ocorreu na seguinte proporção: plantas normais - folhas (54%-64%); ramos + gemas florais (20%-21%); raízes (30%-41%). Plantas deficientes - folhas (62%-79%); ramos + gemas florais (1,2%-4,4%); raízes (20%-33%). Em tecidos lançados após a iniciação da gema floral, a demanda por N é coberta pelas reservas do cafeeiro com o seguinte porcentual: plantas normais - frutos (20,6%-24,8%), folhas (15,6%-19,4%), ramos (19,0%-20,5%); plantas deficientes: frutos (43,5%-48,5%); folhas (48,1%-51,9%); ramos (46,0%-53,0%). Para o K em plantas normais: frutos (40,0%-45,8%), folhas (27,0%-37,6%), ramos (26%-33,1%); plantas deficientes: frutos (65,7%-81,5%); folhas (52,6%-68,4%); ramos (62,0%-86,1%).
reutilização; remobilização; nitrogênio; potássio; rubídio; cafeeiro; 15N; 85Rb
Studies on mineral nutrition of the coffee plant (Coffea arabica L. cv. Catuaí Vermelho). LXIV. Remobilization and re-utilization of nitrogen and potassium by normal and deficient plants
Estudos sobre a nutrição mineral do cafeeiro (Coffea arabica L. cv. Catuaí Vermelho). LXIV. Remobilização e reutilização de nitrogênio e potássio por plantas normais e deficientes
Lima Filho, O. F. deI; Malavolta, E.II
IEmbrapa Agropecuária Oeste, C.P. 661, CEP 79804-970, Dourados, MS, Brazil
IICentro de Energia Nuclear na Agricultura, CENA, Universidade de São Paulo, C.P. 96, CEP 13400-970, Piracicaba, SP, Brazil
Correspondence Correspondence to Eurípedes Malavolta Centro de Energia Nuclear na Agricultura, CENA, Universidade de São Paulo, C.P. 96 CEP 13400-970, Piracicaba, SP, Brazil e-mail: mala@cena.usp.br
ABSTRACT
Remobilization and re-utilization of 15N and 85Rb labelled nitrogen and potassium reserves for new growth and fruit formation was studied under greenhouse conditions using both normal and deficient young coffee plants. It was found that K reserves are used in higher proportion than is stored N by fruits and other organs. The export of N by organs of residence in the normal plants obeyed the following proportions of the total: leaves 47%-58%, branches and flower buds 21%-27%, roots 21%-32%. The corresponding figures in the case of deficient plants were: leaves 49%-65%, branches and flower buds 21%-27%, roots 14%-25%. Re-utilization of K took place in the following proportions in the normal plants: leaves 54%-64%, branches and flower buds 20%-21%, roots 30%-41%. In K deficient plants the figures were: leaves 62%-79%, branches and flower buds 1.2%-4.4%, roots 20%-33%. In tissues formed after the initiation of flowering buds, the demand for N is met by reserves as follows: normal plants: fruits 20.6%-24.8%, leaves 15.6%-19.4%, twigs 19%-20.5%; deficient plants: fruits 43.5%-48.5%, leaves 48.1%-51.9%, twigs 46%-53%. The K needs for new tissues are met in the order: normal plants: fruits 40%-45.8%, leaves 27%-37.6%, twigs 26%-33.1%; deficient plants: fruits 65.7%-81.5%, leaves 52.6%-68.4%, twigs 62%-86.1%. Fruits represent the main sink for both N and K. Re-utilization of both elements is higher in the case of deficient plants.
Key words: re-utilization, remobilization, nitrogen, potassium, rubidium, coffee plants,15N,85Rb.
RESUMO
A remobilização do N (15N) e do K (85Rb) de reserva e seu uso pelas diferentes partes do cafeeiro (Coffea arabica cv. Catuaí Vermelho) no período reprodutivo, particularmente pelos frutos, foram estudados sob condições normais e de carência de N ou K. O K de reserva, comparado ao N de reserva, é utilizado em maior proporção pelos frutos e demais órgãos do cafeeiro. A exportação do N pelos órgãos de reserva foi a seguinte: plantas normais folhas (47%-58%); ramos + gemas florais (20%-21%); raízes (21%-32%). Plantas deficientes folhas (49%-65%); ramos + gemas florais (21%-27%); raízes (14%-25%). A remobilização do K de reserva ocorreu na seguinte proporção: plantas normais folhas (54%-64%); ramos + gemas florais (20%-21%); raízes (30%-41%). Plantas deficientes folhas (62%-79%); ramos + gemas florais (1,2%-4,4%); raízes (20%-33%). Em tecidos lançados após a iniciação da gema floral, a demanda por N é coberta pelas reservas do cafeeiro com o seguinte porcentual: plantas normais frutos (20,6%-24,8%), folhas (15,6%-19,4%), ramos (19,0%-20,5%); plantas deficientes: frutos (43,5%-48,5%); folhas (48,1%-51,9%); ramos (46,0%-53,0%). Para o K em plantas normais: frutos (40,0%-45,8%), folhas (27,0%-37,6%), ramos (26%-33,1%); plantas deficientes: frutos (65,7%-81,5%); folhas (52,6%-68,4%); ramos (62,0%-86,1%).
Palavras-chave: reutilização, remobilização, nitrogênio, potássio, rubídio, cafeeiro, 15N, 85Rb.
INTRODUCTION
Coffee plants have high N and K requirements (Catani & Moraes, 1958; Malavolta et al., 1963; Catani et al., 1967; Correa et al., 1983). There is a close relationship among nitrogen supply, number of leaves, and number of flower buds (Dierendonck, 1959). Whereas adequate tissue N levels are favorable for starch and other carbohydrate production needed for fruit formation and growth, in deficient plants symptoms develop particularly when the berries grow (Malavolta, 1986).
Potassium also plays a major role in coffee plant physiology especially during fruit growth and maturation. The K quantity exported at harvest exceeds that of N which helps to explain why it can become limiting after a few years (Mitchell, 1988). A good correlation exists between the K status, as measured by leaf content, and stored starch and yield. When tissue K is adequate the proportion of floats and branches with symptoms of overbearing decreases (Glander, 1958; Malavolta, 1986).
The remobilization and re-utilization of certain nutrients is an important metabolic feature during development or in cases of seed germination, under stress conditions in the period of vegetative growth and the reproductive stage as well and, in the case of perennials, before leaf fall. As indicated by deficiency symptoms which develop in the leaves, the degree of both N and K mobilization is large (Marschner, 1995).
There is a lack of studies dealing with N and K transport from organs of residence towards other ones, either vegetative or reproductive or both, under normal or deficiency conditions. The development of isotopic techniques has allowed better understanding of the mechanism involved in the uptake and transport of nutrients directly related to productivity increase and sustainability. In the present contribution the isotopic dilution technique was used with the stable isotopes 15N for nitrogen and 85Rb for potassium. 15N is intensively used as a tracer in soil-plant system studies. It has also been shown that 85Rb, a stable isotope, can be used as a tracer for K (Calvache et al., 1990).
Hydrated potassium and rubidium have a similar ionic radius and both cations occupy the same binding sites on the plasma membrane of root cells (Erdei & Trivedi, 1991). Although Rb cannot replace K in its metabolic roles, rubidium is capable of replacing potassium in proton transfer at the tonoplast level (Nakaoji et al., 1991; Marschner, 1995).
86Rb has been used in biological research as a tracer for K (Pettersson & Strid, 1989; Hughes et al., 1990; Kuhlmann, 1990; Peng et al., 1990; Gussarsson & Jensen, 1992). The use of 42K in experiments of short and medium duration is practically impossible due its unsuitable half-life (12.4 hours as opposed to 18.7 days for 86Rb), and low specific activity (Vose, 1980). The stable isotope 85Rb, on the other hand, has proved to be a safe and adequate tracer, notwithstanding the experimental period length. Calvache et al. (1991), for instance, were able to quantify available K for a potato crop by using 85Rb, thereby showing the possibility of using this isotope to replace potassium. However, the literature makes no reference to studies on K re-utilization by plant tissue involving the use 85Rb.
The objectives of the present study were the following: to assess N and K export from the organs of residence as sources, to leaves, stem, and root, before flower differentiation; re-utilization of both elements by different coffee plant organs, between flowering and fruit maturation, by normal and N and K deficient young plants.
MATERIAL AND METHODS
The experiment was conducted in a greenhouse, beginning in November 1995. Thirty-six 14-month-old coffee plants (Coffea arabica cv. Catuaí Vermelho, IAC H2077-2-5-81) were transferred into 5 L earthenware pots containing continuously aerated nutrient solution. The containers were treated inside with waterproof paint and covered with black plastic to prevent alga growth and water loss by evaporation. Nutrient solution initial concentrations were the following in millimols: KNO3-5.0; Ca(NO3)2.4H2O-3.0; NH4H2PO4-1.0; MgSO4.7H2O-1.0; NH4NO3-2.0. Micronutrients were supplied as: KCl-50; H3BO3-25; MnSO4.H2O-2.0; ZnSO4.7H2O-2.0; CuSO4.5H2O-0.5; H2MoO4 (85% MoO3)-0.5; Fe ethilenediamine tetraacetic acid EDTA-20 mM. Concentrations are given in micromols.
Plants were labelled with either 15N or 85Rb in June-July 1996. Two applications of 0.96 g 85RbCl and of 1.32 g (15NH4)2SO4 with 8% 15N in excess were made. At the same time unlabelled plants were grown for determining natural Rb abundance in the various organs (Calvache et al., 1991).
Before flowering, at the onset of reproductive dormant buds, 4 plants were harvested (first harvest). Leaves, branches (orthotropic and plagiotropic), flower buds, and roots were analyzed, and 15N, total N, 85Rb, and K determined. The labelled nutrient solutions were replaced by unlabelled ones in the remaining pots, and the roots were washed with distilled water. Twelve plants, corresponding to treatment 1, were grown in full strength nutrient solution, including the usual N and K rates. The next twelve plants, i.e., treatment 2, were given nutrient solution with one fourth of the N concentration. The remaining twelve plants, treatment 3, received nutrient solution with one fourth of the usual K level. The final harvest took place in July 1997 when the majority of fruits were ripe, and the plants were 2 years 9 month old. Leaves and older branches originating in the flush previous to the first harvest were collected separately from those formed afterwards. Green fruits, mature fruits, and roots were harvested separately as well. The various plant parts were dried at oC 70 and weighed, and 15N, total N, 85Rb, and K were determined.
Total N (Nt) was analyzed by the semi-micro Kjeldahl method as described by Malavolta et al. (1997). The isotopic determination of N contained in the samples was made by mass spectrometry, using a modified Dumas method according to Trivelin et al. (1973). The 15N analyses were made following there steps: a) conversion of the enriched nitrogen into ammonia; b) conversion of ammonia into N2;and c) determination of the isotopic dilution by mass spectrometry. The results of the N2 isotopic composition were obtained with mass members 28, 29, 30: I28 (14N14N); I29 (15N14N); I30 (15N15N). From the intensity (I) of the peaks (I = height in mm times sensitivity volts), the percent concentrations of 15N atoms was derived from the following equation: Atoms % 15N = [(I29 + 2 x I30)/(2 x I28 + 2 x I29 + 2 x I30)] x 100. This equation can also be written as: atoms % 15N = [(n. atoms 15N)/(n. atoms 14N + n. atoms 15N)] x 100. The extraction of both K and Rb from the plant material was made through nitric perchloric acid extraction. The K was determined by flame photometry and the Rb was assessed by atomic absorption (Malavolta et al., 1997).
The quantity of 15N accumulated in the mineral organs was calculated by the equation C = (A x Nt)/100, wherein C = 15N accumulated; A = atoms % 15N in excess in the organ; Nt = total nitrogen accumulated in the organ. Nitrogen remobilized or exported (NR) from the organs of reserve was calculated by the equation: NR = [C1 C2)/A1] x 100, wherein C1 = total 15N content in the organ of reserve immediately after the labelled period (initial harvest); C2 = total content of 15N in the organ of reserve at the moment of the final harvest (ripe berries); A1 = atoms of % of 15N in excess in the organ of reserve immediately after the labelling period (initial harvest).
The N percent re-utilized from the organs of reserve, in relation to total N accumulated in the organs formed before or after flower differentiation was estimated with the following expression: %U = [(Nt x A2)/A1] x 100, wherein Nt = total N in the organ under study; A1 = atoms % of 15N in excess in the organ of reserve immediately after the labelling period (initial harvest); A2 = atoms % of 15N in excess in the organ under study.
The abundance of Rb in the labelled samples was calculated with the equation: ab. Rb = [mol Rb/(mol Rb + mol K)] x 100. The excess abundance of Rb (ab.Rbexc) was estimated by using the expression ab.Rbexc = ab.Rb ab.Rbnat, wherein ab. Rbnat = natural abundance of Rb. The remaining calculations concerning K and Rb were similar to those described for nitrogen.
RESULTS AND DISCUSSION
Initial harvest
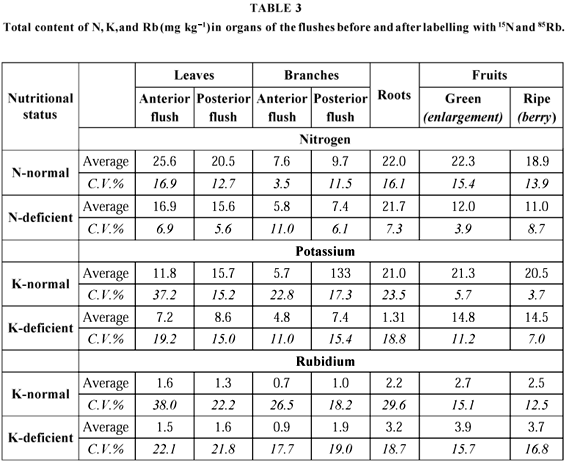
Table 1 shows data from the first harvest, before flowering, when all plant parts were labelled by 15N and 85Rb derived from the substrate. It can be seen that flower buds had a N level 30% higher than the leaves, whereas their K content was 31% lower. Nearly half of the plant N was in the leaves (45.9%); roots came next (32.9%). Branches and flower buds had 10.6% each. In the case of K, 53.1% was in the leaves, with 32.6% in the roots, whereas branches and flower buds had only 7.8% and 6.4%, respectively.
The excess abundance of 85Rb and the percent of excess 15N atoms in the several parts showed low coefficients of variation. There was high enrichment for both isotopes, made necessary due to the expected dilution caused by plant growth 10 months after labelling. Within this period plants with adequate N and K nutrition increased their dry matter fourfold.
Final harvest
Leaf analysis carried out at the final harvest in fruit-bearing branches of N or K deficient plants showed a 30% drop in N level, and one of 67% in K concentration in relation to the adequately supplied plants. Normal plants had 23 mg kg1 N and 25 mg kg1 K, whereas the deficient ones had 16 mg kg1 and 6 mg kg1, respectively. The more pronounced K-level drop was not accompanied by a proportional decrease in plant dry matter and fruit production. Average yield of the N and K deficient plants was similar. Meanwhile the vegetative biomass of the potassium deficient plants was higher than that of the N deficient ones (Table 2).
In all treatments the lowering of the N and K content in leaves produced either before or during the isotopic enrichment period, through the reproductive process was due to export into fruit and new organs during leaf senescence. Leaf fall also represented additional total N and K loss. Potassium and nitrogen are highly phloem mobile elements, and their re-utilization leads to rapid decline in their level in vegetative parts, thereby inducing earlier senescence (Marschner, 1995). Thus, nitrogen deficient plants grew less and produced fewer leaves, branches, and roots. Plants with adequate nutrition yielded, on the average, 56 g of dry fruits.
The Potassium deficient plants produced nearly 50% less and those deficient in nitrogen produced 40% below normal. There was, however, a marked lack of uniformity in fruit formation which caused a high coefficient of variation insofar as yield is concerned. For this reason the statistical analysis failed to show significant differences among treatments. The statistical test does not take into consideration the fact that 80% of the coffee plants adequately fed with both N and K yielded more than 10 g dry fruits, and 60% of such plants had a production higher than 50 grams. On the other hand, the N and K deficient treatments showed yields lower than 10 g in 46 and 58% of the parcels, respectively. Yields higher than 50 g were registered only in 18% and 17% of the N and K deficient plants, respectively.
Nutritional status at final harvest
Table III shows the concentrations of N, K, and Rb in the several organs as a function of the treatments. Nitrogen deficient plants suffered a 24% reduction of that element in leaves of the later flushing and in branches of both flushes. Leaves from the early flush had a 34% reduction in N level. The drop in fruit N averaged circa 45%. Root N was not affected by the limitation in N supply in the substrate. This is probably due to the fact that the main source of nutrients for phloem loading are branches and leaves, from which remobilization takes place, with reproductive and growing vegetative organs acting as drains. In the case of K deficient plants, leaves from the early flush and root as well showed an almost 40% drop in the content of that element. Leaves and branches of the later flush showed a 45% and 30% drop, respectively. The lowest reduction in K concentration, around 16%, took place in the branches of the early flush.
From Tables 2 and 3 the relationship between the quantities of N and K accumulated at the end of fruit ripening in the normal plants is derived. The K/N ratio was variable according to the organ considered and the period of its formation, i.e., before or after differentiation of flower buds: leaves from the early flush 0.5, leaves from the later flush 0.8; branches from the early flush 0.7, branches from the later flush 1.4; roots 0.9, fruits 1.1; general average 1.0. Before flowering the ratios were: 0.7 for leaves, 0.5 for branches, 0.6 for roots, and 0.4 for flowering buds; general average 0.6. This means that the N buildup in relation to K tends to decrease in the whole plant during the period of fruit growth. This finding is well defined in the branches of the later flush and in the roots. Before flowering, the total N content was 60% higher than that of K, whereas during the fruit ripening stage, the content of both nutrients was similar in the fruits and in the total as well.
Taking into account the existing reserves in the flower buds, there was an average 133% increase in the reproductive apparatus N content of the normal plants, against an almost 30% decrease in the deficient ones.
On the other hand, there a was a 530% increase in K content of the fruits of the well-supplied plants, and one of 127% in those from K deficient plants. The N quantity decrease which took place in the deficient plant reproductive organs between the stages of flowering buds and berry is due, in part, to the presence of few of the first, which had failed to develop, and mainly to the severe reduction in fruit N in relation to that of flower buds, which was 3.6 times lower, accompanied by a dry matter increase of only 180%.
Remobilization and re-utilization of nitrogen and potassium
The main reserve of N (NR) which was used by the organs formed after flower bud initiation was the photosynthetic apparatus. Storage and remobilization or export of NR from the leaves contributed greatly to the growth of new organs and the reproductive organs. The period between anthesis and final expansion of fruits strongly drains minerals and photoassilates. In the coffee plant nitrate reduction activity increases considerably in the final stage of flower development, in the beginning of endosperm formation, and during the final phase of bean enlargement. During these physiological stages anabolism increases within the leaves, thereby meeting the demand for metabolites (Carelli et al., 1989).
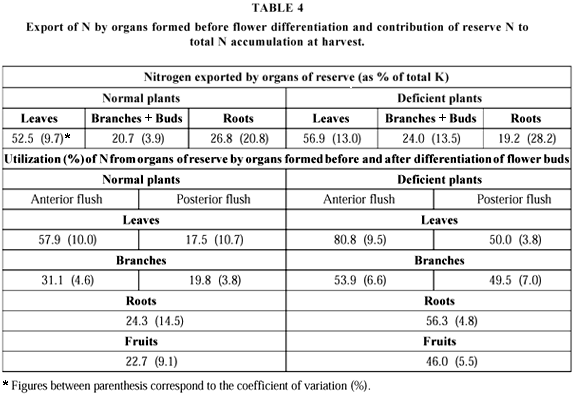
Leaves accounted for more than 50% of total NR exported both in normal (47%-58%) and N deficient plants (49%-65%). Branches (orthotropic and plagiotropic) and buds exported from 20% to 21% in normal plants, and 21% to 27% in the deficient ones. Roots contributed with 21% to 32% in plants with adequate N supply, and 14% to 25% in the deficient plants. In citrus plants Legaz et al. (1995) found 40%-50% for leaves, 15%-25% for branches, and 30%-35% for the roots. The NR contribution from each organ was, therefore, similar (Table 4).
Nutritional status influences both distribution of freshly absorbed elements and remobilização of previously acquired ones. These processes, therefore, play a fundamental role in the relationship between nutrient content and growth (Smith, 1986). The utilization of N derived from the reserve organs by those formed after flower initiation and differentiation in the deficient plants was markedly higher than in those well supplied with that element. In the organs of reserve of N deficient plants a higher proportion of the element was taken up before the differentiation of flower primordia. This suggests that in the deficient plants N absorbed from the nutrient solution was preferentially transported to growing organs in a proportion much higher than that found in the well nourished plants.
During fruit ripening NR is found mainly in the roots besides leaves from the later flush and fruits. The NR quantity in the fruits is a function of the load, reaching up to 25% of the total NR in the case of well-fed plants. In the deficient plants the value is lower by nearly 15%. The reproductive organs represent an important drain: root activity and nutrient uptake decrease because less carbohydrates are supplied, being preferentially directed to the fruits.
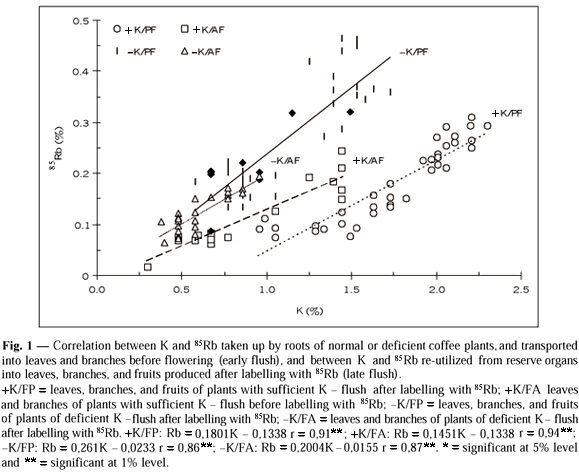
Immediately after 85Rb was withdrawn from the nutrient solution (first harvest), no correlation (r = 0.41ns) between concentrations of K and the isotope in the leaves was observed. In branches and roots, however, the correlation was high and significant (r = 0.90** for both organs). In the final harvest, regression analyses in various organs formed before or after the omission of 85Rb from the substrate presented high correlation between the contents of re-utilized 85Rb e K. It should be kept in mind that, for the organs of the late flush, these correlations involve both endogenous and exogenous K, whereas Rb is exclusively endogenous (Fig. 1).
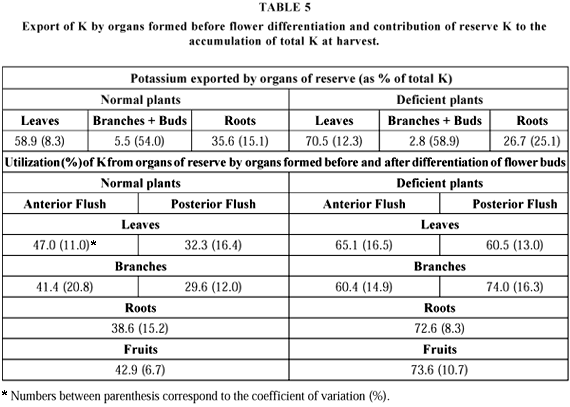
The export of reserve K (KR) occurred mainly from the leaves, and to a lesser extent, from the roots, as was the case with N. Branches and buds exported very little K. KR contributed 54% to 63.8% of the total exported in normal plants, and 61.8% to 79.2% in the deficient ones.
Roots from adequately supplied plants contributed 30.2% to 41.0% whereas those from deficient plants contributed 20% to 33.4% of the total. It follows that deficient plants tend to re-utilize preferentially leaf K (Table 5).
The average KR quantity in the plant at harvest time was equivalent to 36% of the total in the well-fed plants, against 67% in the deficient ones. In the first, KR in the fruits reached 14% of the total, and in the latter it reached 34%. These figures increase markedly when one considers the KR proportion in the fruit in relation to KR in the whole plant. In the plants without nutritional stress around 38% of the total KR was moved into the fruit. The corresponding value for the K deficient plants was 58%. These values are higher than those corresponding to the N balance.
Three-year-old plants show that 20.6% to 24.8% of the fruit demand is met by reserves which exist when flower buds begin to develop. These values, however, increased twofold (43.5% to 48.5%) when leaf N decreased by 30%. On the other hand, 40% to 45.7% of fruit K is covered by potassium reserves at the same period. This contribution reached 65.7% to 81.4% when leaf K decreased 67%. KR is used more intensively than NR by fruits and other organs of the coffee plant. These results point out the importance of N and K stored prior to flowering and exported and re-utilized during the reproductive cycle. The mathematical models which better demonstrate the relationship between utilization of N and K reserves and their leaf levels show it evens out in well-fed plants (Fig. 2).
Acknowledgments This work was supported by FAPESP (State Foundation for Support of Research, State of São Paulo, Brazil), grant 96/01857-5, and by the Brazilian Research Council (CNPq), grant 300934/95-5.
Received April 22, 2002
Accepted August 27, 2002
Distributed August 31, 2003
- CALVACHE, M., PINO, I. & BUNEDER, M., 1990, Posibilidades de uso de Rb-85 como trazador de potasio. Nucleotecnica, Santiago, 10(18): 43-45.
- CALVACHE, M., ESPINOSA, J., CÓRDOVA, J. & GANGOTENA, D., 1991, Determinación del potasio disponible en diferentes fuentes no marcadas (comerciales), utilizando Rb-85 como trazador. Nucleociencias, Quito, 2(2): 5-11.
- CARELLI, M. L. C., FAHL, J. I. & MAGALHÃES, A. C., 1989, Assimilação de nitrato durante o desenvolvimento reprodutivo de plantas de café. R. Bras. Ci. Solo, 13: 59-64.
- CATANI, R. A. & MORAES, F. R. P. de, 1958, A composição química do cafeeiro. Rev. Agr., Piracicaba, 33(1): 45-52.
- CATANI, R. A., PELLEGRINO, D., ALCARDE, J. C. & GRANER, C. A. F., 1967, Variação na concentração e na quantidade de macro e micronutrientes no fruto do cafeeiro durante o seu desenvolvimento. Anais da ESALQ, Piracicaba, 24: 249-263.
- CORREA, J. B., GARCIA, A. W. R. & COSTA, P. C. da, 1983, Extração de nutrientes pelos cafeeiros Mundo Novo e Catuaí. In: Congresso Brasileiro de Pesquisas Cafeeiras, 10, Poços de Caldas, 1983. Anais... Rio de Janeiro, IBC/GERCA, pp. 177-183.
- DIERENDONCK, F. J. E., 1959, The manuring of coffee, cocoa, tea and tobacco Centro d'Étude de l'Ázote, Genebra.
- ERDEI, L. & TRIVEDI, S., 1991, Caesium/potassium selectivity in wheat and lettuce of different K+ status. J. Plant Physiol., 138:696-699.
- GLANDER, H., 1958, Conocimientos y experiencias obtenidas en la abonadura del café Verlagsgesellschaft für Ackerbau MBH (Bol. Verde n.8).
- GUSSARSSON, M. & JENSEN, P., 1992, Effects of copper and cadmium on uptake and leakage of K+ in birch (Betula pendula) roots. Tree Physiol., 11: 305-313.
- HUGHES, D. F., JOLLEY, V. D. & BROWN, J. C., 1990, Differential response of dicotyledonous plants to potassium-deficiency stress: iron-stress response mechanism. J. Plant Nutr., 13(11): 1405-1417.
- KUHLMANN, H., 1990, Importance of the subsoil for the K nutrition of crops. Plant Soil, 127(1): 129-136.
- LEGAZ, F., SERNA, M. D. & PRIMO-MILLO, E., 1995, Mobilization of the reserve N in citrus. Plant Soil, 173: 205-210.
- MARSCHNER, H., 1995, Mineral nutrition of higher plants Academic Press Inc., New York, 887p.
- MALAVOLTA, E., 1986, Nutrição, adubação e calagem para o cafeeiro. In: A. B. Rena, E. Malavolta, M. Rocha & T. Yamada (eds.), Cultura do cafeeiro: fatores que afetam a produtividade Potafós, Piracicaba, pp. 165-274.
- MALAVOLTA, E., GRANER, E. A., SARRUGE, J. R. & GOMES, L., 1963, Estudos sobre a alimentação mineral do cafeeiro. XI. Extração de macro e micronutrientes na colheita pelas variedades ''Bourbon Amarelo'', ''Caturra Amarelo'' e ''Mundo Novo''. Turrialba, 13(3): 188-189.
- MALAVOLTA, E., VITTI, G. C. & OLIVEIRA, S. A. de, 1997, Avaliação do estado nutricional das plantas: princípios e aplicações 2. ed. Potafós, Piracicaba, 319p.
- MITCHELL, H. W., 1988, Cultivation and harvesting of the arabica coffee tree. In: R. J. Clarke & R. Macrae (eds.), Coffee: volume 4: agronomy Elsevier Applied Science, London, 2: 43-90.
- NAKAOJI, K., HARADA, H., WAKIUCHI, N., SUEYOSHI, K., OJI, Y. & SHIGA, H., 1991, Proton-translocating inorganic pyrophosphatase in tonoplast vesicles from barley roots. Jap. J. Soil Sci. Plant Nutr., 62(4): 393-398.
- PENG, J. G., LUO, T. & CAI, A. Y., 1990, Tracer study on absorption and utilization rate of K fertilizers by rice plants. Fujian Agr. Sci. Tech., 4: 17-19.
- PETTERSSON, S. & STRID, H., 1989, Effects of aluminium on growth and kinetics of K+ (86Rb) uptake in two cultivars of wheat (Triticum aestivum) with different sensitivity to aluminium. Physiol. Plant., 76(3): 255-261.
- SMITH, F. W., 1986, Interpretation of plant analysis: concepts and principles. In: D. J. Reuter & J. B. Robinson (eds.), Plant analysis: an intepretation manual Inkata, Melbourne, 19: 1-12.
- TRIVELIN, P. C. O., SALATI, E. & MATSUI, E., 1973, Preparo de amostras para análise de 15N por espectrometria de massa CENA, Piracicaba, 41p. (Boletim Técnico, n.2).
- VOSE, P. B., 1980, Introduction to nuclear techniques in agronomy and plant biology Pergamon Press, New York, 391p.
Publication Dates
-
Publication in this collection
20 Jan 2004 -
Date of issue
Aug 2003
History
-
Accepted
27 Aug 2002 -
Received
22 Apr 2002