Abstracts
This paper describes nematode infection in the cultured freshwater fish Leporinus macrocephalus (Osteichthyes: Anostomidae) collected at Batatais, São Paulo State, Brazil. Of a total of 32 examined fish, 21 (65%) were infected with Goezia leporini n. sp. (Nematoda: Anisakidae) with mean infection intensity of 4.1 parasites. The nematodes presented total length greater than G intermedia, G. holmesi, G. pelagia, G. minuta, G. kliksi, G. sinamora, G. nonipapillata, G. alii, G. moraveci, G. brasiliensis, and G. brevicaeca. The main difference was a great number of preanal papillae in males when compared to G. brasiliensis and G. brevicaeca. The present description also differs from that of G. brasiliensis with respect to spicule length and distance of vulva from the anterior extremity.
Nematoda; Anisakidae; Goezia leporini n. sp.; cultured fish; Leporinus macrocephalus
Este trabalho descreve a infecção por nematóide em peixe de água doce cultivado Leporinus macrocephalus (Osteichthyes: Anostomidae) do município de Batatais, São Paulo, Brasil. Os peixes apresentavam aglomeração nas bordas do viveiro, entrada da água, inapetência, letargia, perda do equilíbrio, palidez do sangue e dos órgãos e ascite. De 32 peixes examinados, 21 (65%) estavam infectados com Goezia leporini n. sp. (Nematoda: Anisakidae), com intensidade média de 4,1 parasitos. Os nematóides apresentaram maior comprimento total do que G intermedia, G. holmesi, G. pelagia, G. minuta, G. kliksi, G. sinamora, G. nonipapillata, G. alii, G. moraveci, G. brasiliensis e G. brevicaeca. A principal diferença foi o maior número de papilas pré-anais nos machos do que o observado em G. brasiliensis e G. brevicaeca. Essa descrição também difere de G. brasiliensis no comprimento do espículo e na distância da vulva até a extremidade anterior.
Nematoda; Anisakidae; Goezia leporini n. sp.; peixe cultivado; Leporinus macrocephalus
A new nematode species Goezia leporini n. sp. (Anisakidae) from cultured freshwater fish Leporinus macrocephalus (Anostomidae) in Brazil
Uma nova espécie de nematóide Goezia leporini n. sp. (Anisakidae) de peixe de água doce cultivado Leporinus macrocephalus (Anostomidae) no Brasil
Martins, M. L.; Yoshitoshi, E. R.
Departamento de Aqüicultura, CCA, Universidade Federal de Santa Catarina, Florianópolis, SC, Brazil
Correspondence Correspondence to Maurício Laterça Martins Departamento de Aqüicultura, CCA, UFSC Rod. SC 404, km 3, Itacorubi, C.P. 476 CEP 88040-900, Florianópolis, SC, Brazil e-mail: mlaterca@cca.ufsc.br
ABSTRACT
This paper describes nematode infection in the cultured freshwater fish Leporinus macrocephalus (Osteichthyes: Anostomidae) collected at Batatais, São Paulo State, Brazil. Of a total of 32 examined fish, 21 (65%) were infected with Goezia leporini n. sp. (Nematoda: Anisakidae) with mean infection intensity of 4.1 parasites. The nematodes presented total length greater than G intermedia, G. holmesi, G. pelagia, G. minuta, G. kliksi, G. sinamora, G. nonipapillata, G. alii, G. moraveci, G. brasiliensis, and G. brevicaeca. The main difference was a great number of preanal papillae in males when compared to G. brasiliensis and G. brevicaeca. The present description also differs from that of G. brasiliensis with respect to spicule length and distance of vulva from the anterior extremity.
Key words: Nematoda, Anisakidae, Goezia leporini n. sp., cultured fish, Leporinus macrocephalus.
RESUMO
Este trabalho descreve a infecção por nematóide em peixe de água doce cultivado Leporinus macrocephalus (Osteichthyes: Anostomidae) do município de Batatais, São Paulo, Brasil. Os peixes apresentavam aglomeração nas bordas do viveiro, entrada da água, inapetência, letargia, perda do equilíbrio, palidez do sangue e dos órgãos e ascite. De 32 peixes examinados, 21 (65%) estavam infectados com Goezia leporini n. sp. (Nematoda: Anisakidae), com intensidade média de 4,1 parasitos. Os nematóides apresentaram maior comprimento total do que G intermedia, G. holmesi, G. pelagia, G. minuta, G. kliksi, G. sinamora, G. nonipapillata, G. alii, G. moraveci, G. brasiliensis e G. brevicaeca. A principal diferença foi o maior número de papilas pré-anais nos machos do que o observado em G. brasiliensis e G. brevicaeca. Essa descrição também difere de G. brasiliensis no comprimento do espículo e na distância da vulva até a extremidade anterior.
Palavras-chave: Nematoda, Anisakidae, Goezia leporini n. sp., peixe cultivado, Leporinus macrocephalus.
INTRODUCTION
In Brazil aquaculture has recently shown rapid expansion. This occurred particularly with respect to fish farming. However, poor water quality and bad fish nutrition, as well as infectious and parasitic fish diseases can cause imbalance in the host/parasite/environment system, culminating in economic losses (Békési, 1992; Martins & Romero, 1996). The most cultivated fish species in Brazil are Piaractus mesopotamicus Holmberg, 1887 (pacu); Colossoma macropomum Cuvier, 1818 (tambaqui); the hybrid tambacu (P. mesopotamicus male x C. macropomum female); Leporinus macrocephalus Garavello & Britski, 1988 (piauçu); Brycon cephalus Gunther, 1869 (matrinxã); Cyprinus carpio Linnaeus, 1758 (carp); and Oreochromis niloticus Trewavas, 1983 (red and black tilapia).
There are several species of Goezia Zeder, with 1800 parasites described from marine, brackish-water, and freshwater fishes. In Brazil, the main host type of G. spinulosa Diesing, 1839, is Arapaima gigas (Travassos et al., 1928). G. brasiliensis Moravec, Kohn, & Fernandes, 1994, have been reported from Brycon hillarii and Pseudoplatystoma corruscans and G. brevicaeca Moravec, Kohn, & Fernandes, 1994, from B. hillarii; and Goezia larvae from Raphiodon vulpinus, Serrasalmus marginatus, and Ageneiosus valenciennesi (Moravec et al., 1993).
A new species of Goezia was identified in Brazil in the farmed freshwater fish Leporinus macrocephalus.
MATERIAL AND METHODS
Thirty-two specimens of L. macrocephalus Garavello & Britski, 1988 (Osteichthyes: Anostomidae), 121.9 (20.5 to 537.0) g in weight and 18.4 (11.0 to 33.0) cm in total length, were cultured in ponds with a 6.7 fish/m2 density at a fish farm in Batatais, São Paulo State, Brazil. Observed fish were captured in July and August 1999. At the same time, water quality was assessed using Corning portable equipment for measuring electric conductivity, and pH and YSI equipment for measuring dissolved oxygen and water temperature.
Before dissection the fish were sacrificed by immersion in a 0.1% benzocaine solution. Body mucus and pieces of gills, kidney, liver, spleen, and heart were then compressed between a glass slide and a coverslip with a drop of 0.65% saline solution for microscopic observation. Stomach and intestines were opened and observed in a Petri dish containing saline solution. Nematodes were carefully collected from the stomach, fixed in AFA at 65°C, and preserved in alcohol 70% with 5% glycerine. Helminths were dehydrated and cleared in Faia creosote or glycerine, and en face preparations were performed. For uterus and ovijector observation and egg measurement, five females were carefully dissected. Twenty males, 20 females, and 40 eggs from dissected females were studied in a camera lucida for measurement. For scanning electron microscopy, the nematodes were fixed at room temperature in a 3% glutaraldehyde solution with a 0.1 M phosphate buffer (pH 7.4). Afterwards, they were dehydrated with serial concentrations of alcohol, dried with CO2, assembled, and coated with 20 nm gold paladium, following which they were examined with a JEOL JSM-5410 microscope. Parasite identification was made according to Anderson et al. (1989). Prevalence and mean intensity were calculated according to Bush et al. (1997). All measurements are in millimeters (average and range in parentheses) unless otherwise stated.
RESULTS
Throughout the sampling period, water parameters showed normal values: electric conductivity 5.0-20.0 mS/cm, pH 6.3-7.3, dissolved oxygen 7.4-9.4 mg/I, and water temperature 14.0-19.0°C. Observed fish showed a 65% infection prevalence and a mean intensity of 4.1 parasites in the stomach. After dissection of infected fish, some nematodes could be seen passing through the stomach wall, in which helminths were sometimes found embedded.
Goezia leporini n.sp. (Nematoda: Anisakidae)
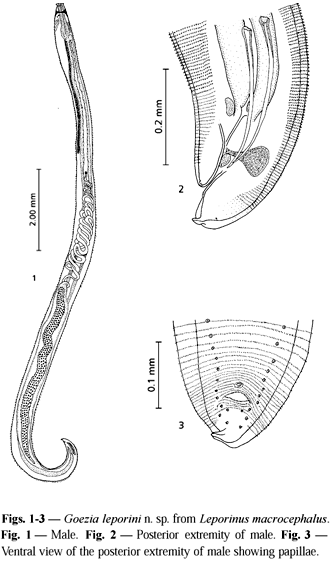
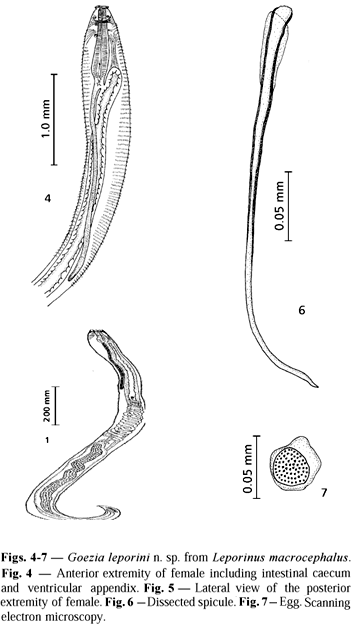
Description. Cylindrical and stout white-yellowish nematode. Females larger than males. Body cuticle with distinct transverse striations and cuticular spines. Spine rows more separated and spines longer when observed at ventriculus level. Anterior end flattened with three demarcated lips of approximately equal size. A smooth cuticle covering each lip was present. Dorsal lip with two double papillae situated laterally; each ventrolateral lip with one double papilla, one single papilla, and one lateral amphid. Internal edges of each lip provided with two protruding projections. Mouth separated from body by a slight constriction. One pair of cervical papillae (deirids) between the nerve ring and ventriculus. Esophagus clavate, ventriculus small (bulb) narrower than the widest level of esophagus. Excretory pore at the level of the nerve ring. Nerve ring surrounding the esophagus at the first third of its length. Ventricular appendix narrow, two to three times longer than the esophagus. Intestinal caecum reaching anteriorly certain level of esophagus. Male with two similar spicules. Female with didelphic and opisthodelphic uteri. Vulva without prominent labia slightly anterior to midbody and vagina directed posteriorly. Tail conical with digitiform process.
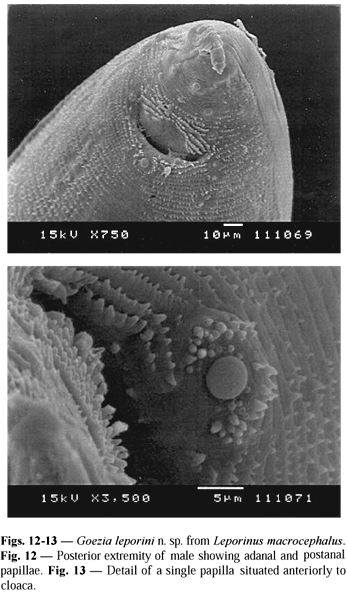
Male. Body 20.31 (13.57-34.08) long; maximum width 0.70 (0.45-1.06). Cervical papillae 0.36 (0.34-0.38) from the anterior extremity. Distance between two rings of spines at the ventriculus level 50.90 mm (47.00-54.90) and at cloaca level 3.90 mm (3.80-4.20). Spines at the ventriculus level 24.70 mm (24.00-28.00) long by 14.80 mm (11.60-16.70) wide at the base. Esophagus 1.23 (0.74-2.04) long by 0.24 (0.12-0.35) wide. Ventriculus 0.09 (0.08-0.12) long by 0.14 (0.12-0.15) wide. Nerve ring 0.34 (0.26-0.45) and excretory pore 0.34 (0.18-0.52) from the anterior extremity. Ventricular appendix 3.83 (2.20-9.29) long by 0.13 (0.06-0.20) wide. Intestinal caecum 0.21 (0.06-0.57) long by 0.18 (0.10-0.37) wide. Length ratio of intestinal caecum and ventricular appendix 1:18.2; between intestinal caecum and esophageal length 1:5.8. Right spicule 0.47 (0.34-0.56) long by 0.02 (0.02-0.03) wide; left spicule 0.48 (0.37-0.54) long by 0.02 (0.01-0.03) wide. A thin membrane was observed to surround the proximal extremity of each spicule. At the base of spicules near the cloaca level the presence of two rectal glands 0.08 by 0.06 was observed. Tail flexed ventrally, 0.13 (0.04-0.24) long (including digitiform process). Genital papillae 24 to 29 pairs: preanal pairs 18 to 23, adanal pairs 2, and postanal pairs 4 one pair being lateroventral. One single prominent median papilla 7.30 mm in diameter was observed on the anterior cloacal border.
Female. Body 27.02 (20.31-32.65) long; maximum width 0.81 (0.31-1.12). Cervical papillae 0.49 (0.43-0.57) from the anterior extremity. Distance between two rings of spines at the level of the ventriculus 68.1 mm (62.7-74.5), at the level of the vulva 12.3 mm (9.8-14.7), and at the level of the anus 5.3 mm (4.9-5.8). Spines at the ventriculus level 20.80 mm (15.7-23.5) long by 12.90 mm (9.8-15.7) wide (at the base), and at the level of the vulva 8.50 mm (8.00-10.00) long by 4.00 mm (2.00-6.00) wide (at the base). Esophagus 1.45 (1.22-1.82) long by 0.33 (0.25-0.37) wide. Ventriculus 0.11 (0.08-0.14) long by 0.15 (0.12-0.18) wide. Nerve ring 0.38 (0.31-0.48) and excretory pore 0.32 (0.26-0.48) from the anterior extremity. Ventricular appendix 4.54 (2.61-6.33) long by 0.19 (0.10-0.31) wide. Intestinal caecum 0.30 (0.10-0.61) long by 0.26 (0.10-0.55) wide. Length ratio of intestinal caecum and ventricular appendix 1:14.9, and between intestinal caecum and esophagus 1:5.0. Vulva 11.59 (9.18-14.18) from anterior extremity. Ovijector short and muscular, 0.25 (0.01-0.43) long by 0.07 (0.05-0.11) wide, directed posteriorly. Uterus 2.68 (1.33-3.73) long. Tail 0.13 (0.04-0.24) long (including digitiform process). From dissected females the eggs enclosed in the uterus are spherical 32.7 mm (29-37) in diameter and provided with a smooth thin membrane 10.6 mm (6-14) thick.
Taxonomic summary
Type host: Leporinus macrocephalus
Garavello & Britski, 1988 (Anostomidae)
Local name: ''piauçu''
Site of infection: stomach
Type locality: Batatais, São Paulo State, Brazil, July 1999
Specimens deposited: Helminthological
Collection of Oswaldo Cruz Institute
(CHIOC number 34675), Av. Brasil 4365, 21045-900, Rio de Janeiro, Brazil
Etymology: the specific name of this species is proposed from the genus of the host
DISCUSSION
The fish studied belong to the most cultivated freshwater fish species in Brazil. Endoparasites of cultivated fish have previously enjoyed little research interest in Brazil and this is the first recording of mortality in farmed fish in Brazil probably caused probably by nematodes in the digestive tract, although fish was not specifically diagnosed for bacterial and viral disease. This may be affirmed because of macroscopical observation and the fact that nematodes were found perforating the stomach wall. There are several studies of the Goezia, but most of these focus on parasite description. Only low prevalences were reported in P. corruscans infected with G. spinulosa (Hamann, 1984) (8.33%), Mastacembelus armatus infected with G. moraveci De & Dey, 1992 (0.09%), and Goezia larvae from Rhamdia guatemalensis (Moravec et al., 1995) (0.12%). In this study, the massive presence of G. leporini n. sp. (prevalence 65%) was similar to that found by El-Darsh & Whitfield in 1999 (66.7%). Here, the nematodes were firmly attached to the stomach wall and evidence of their presence was associated with feeding and occurrences of secondary lesions in the host, as discussed by Deardorff & Overstreet (1980).
Extensive mortality in a freshwater population of Morone saxatilis was also related to the presence of Goezia sp. (Gaines & Rogers, 1972), as also observed in this work.
Features important in species differentiation have been based on size, shape, cuticular spines, caudal papillae of male, ratio between caecum/ventricular appendix and caecum/esophagus, excretory pore and nerve ring positions, distance of vulva from anterior end, and egg morphology.
There are three species described in Brazil: G. spinulosa in A. gigas collected from Amazonas and Pará (Travassos et al., 1928; Santos et al., 1979) and in A. ocellatus from Ceará (Freitas & Lent, 1946), and the G. brasiliensis in B. hilarii and P. corruscans and G. brevicaeca in B. hillarii from the Paraná River (Moravec et al., 1994).
The position and number of caudal papillae in males were different when compared with several other species (Table 1) and similar to one of G. brevicaeca (Moravec et al., 1994), although these authors did not report the presence of adanal papillae. The present description showed higher measurements of esophagus length, ventricular appendix length, caecum appendix, and caecum/oesophagus ratio in male specimens than those observed in G. brasiliensis and G. brevicaeca by Moravec et al. (1994). On the other hand, G. leporini n. sp. showed also higher measurements of esophagus length, ventricular appendix length, caecum appendix, and caecum/oesophagus ratio in females than those observed in G. kliksi Deardorf & Overstreet, 1980, and G. brasiliensis and G. brevicaeca (Moravec et al., 1994). It can also be inferred that G. leporini n. sp. has such a number of genital papillae in males, the highest distance of the vulva from the anterior extremity, and egg size in females when compared to G. brasiliensis described by Moravec et al. (1994).
The single prominent median papilla situated immediately anterior to the cloaca of G. leporini n. sp. was present only in G. spinulosa studied by Baylis (1927), and by scanning electron microscopy as shown by Costa et al. (1995). In addition, the present description showed lower spicule length than that observed in G. kliksi (Deardorff & Overstret, 1980) and in G. brasiliensis (Moravec et al., 1994). Nevertheless, a thin membrane surrounding the proximal spicule extremity was in according with that reported in G. spinulosa studied by Baylis (1927) and Santos et al. (1979), and G. nonipapillata Osorio-Sarabia, 1982. On the other hand, G. holmesi Sprent, 1978, and G. moraveci (De & Dey, 1992) have presented spicules with proximal and distal alae.
In accordance with these characteristics, the authors have defined a new species of Goezia, helpful in defining the effects and the seasonality of the nematode in the host.
Acknowledgments We are grateful to Claudia Aparecida Rodrigues (Laboratory of Electronic Microscopy, UNESP, Jaboticabal, São Paulo) and to Gastão Reis, Heloisa Laterça, and Paula Resende for correcting the manuscript translation.
Received June 16, 2002
Accepted December 5, 2002
Distributed August 31, 2003
- ANDERSON, R. C., CHABAUD, A. G. & WILMOTT, S., 1989, C.I.H. Keys to the nematode parasites of vertebrates CAB International, Wallingford, UK.
- BAYLIS, H. A., 1927, Some parasitic worms from Arapaima gigas (Teleostean fish) with a description of Philometra senticosa n. sp. (Filarioidea). Parasitology, 19: 35-47.
- BÉKÉSI, L., 1992, Evaluation of data on ichthyopathological analyses in the Brazilian Northeast. Ciência e Cultura, 44(6):400-403.
- BUSH, A. O., LAFFERTY, K. D., LOTZ, J. M. & SHOSTAK, W., 1997, Parasitology meets ecology on its own terms: Margolis et al revisited. J. Parasitol., 83(4): 575-583.
- COSTA, H. M. A., GUIMARÃES, M. P., CABRAL, D. D. & MUNDIM, M. J. S., 1995, Scanning electron microscopic observations on Goezia spinulosa (Diesing, 1839) (Nematoda: Anisakidae) from Arapaima gigas (Cuvier, 1817). Mem. Inst. Oswaldo Cruz, 90(6): 703-705.
- DE, N. C. & DEY, J., 1992, A new species of the genus Goezia Zeder, 1800 (Nematoda: Anisakidae) from the fish, Mastacembelus armatus (Lacep.) from West Bengal, India. Syst. Parasitol., 22: 189-197.
- DEARDORFF, T. I. & OVERSTREET, R. M., 1980, Taxonomy and biology of North American species of Goezia (Nematoda: Anisakidae) from fishes, including three new species. Proc. Helminthol. Soc. Washington, 47(2): 192-217.
- EL-DARSH, H. E. M. & WHITFIELD, P. J., 1999, The parasite community infecting flounders, Platichthys flesus, in the tidal Thames. J. Helminthol., 73: 203-214.
- FREITAS, J. F. T. & LENT, H., 1946, Infestação de apaiaris ''Astronotus ocellatus'' (Agassiz) pelo nematódeo ''Goeza spinulosa'' (Diesing, 1839). Rev. Bras. Biol., 6(2): 215-222.
- GAINES, Jr. J. L. & ROGERS, W. A., 1972, Fish mortalities associated with Goezia sp. (Nematoda: Ascarididae) in Central Florida. Proc. 25th Ann. Conf. Southeast. Assoc. Game Fish Comm., pp. 496-497.
- HAMANN, M. I., 1984, Nematodos parasitos de peces pimelodidos del rio Paraná medio, Republica Argentina (Pisces, Pimelodidae). Neotropica, 30(83): 55-62.
- MARTINS, M. L. & ROMERO, N. G., 1996, Efectos del parasitismo sobre el tejido branquial en peces cultivados: estudio parasitológico e histopatológico. Rev. Bras. Zool., 93(2): 489-500.
- MORAVEC, F., KOHN, A. & FERNANDES, B. M. M., 1993, Nematode parasites of fishes of the Paraná River, Brazil. Part 2. Seuratoidea, Ascaridoidea, Habronematoidea and Acuarioidea. Folia Parasitol., 40: 115-134.
- MORAVEC, F., KOHN, A. & FEMANDES, B. M. M., 1994, Two new species of the genus Goezia, G. brasiliensis sp. n. and G. brevicaeca sp. n. (Nematoda: Anisakidae), from freshwater fishes in Brazil. Folia Parasitol., 41: 271-278.
- MORAVEC, F., VIVAS-RODRIGUEZ, C., SCHOLZ, T., MENDONZA-FRANCO, E., SCHMITTER-SOTO, J. J. & GONZÁLEZ-SOLÍS, D., 1995, Nematodes parasitic in fishes of cenotes (=sinkholes) of the Peninsula of Yucatán. Part 2. Larvae. Folia Parasitol., 42: 199-210.
- OSÓRIO-SARABIA, D., 1982, Descripción de una nueva especie del género Goezia Zeder, 1800 (Nematoda: Goeziidae) en peces de agua dulce de México. Ann. Inst. Biol., Univ. Nac. Aut. México, 52, Ser. Zoologia (1): 71-87.
- RASHEED, S., 1965, On a remarkable new nematode, Lappetascaris lutjani gen. et sp. nov. (Anisakidae: Ascaridoidea) from marine fishes of Karachi and an account of Thynnascaris inquies (Linton, 1901) n. comb. and Goezia intermedia n. sp. J. Helminthol., 39(4): 313-342.
- SANTOS, E., VICENTE, J. J. & JARDIM, C. R., 1979, Helmintos de peixes de rios amazônicos da Coleção Helmintológica do Instituto Oswaldo Cruz. lI. Nematoda. Atas Soc. Biol. Rio de Janeiro, 20: 11-19.
- SPRENT, J. F. A., 1978, Ascaridoid nematodes of amphibians and reptiles: Goezia. J. Helminthol., 52: 91-98.
- TRAVASSOS, L., ARTIGAS, P. T. & PEREIRA, C., 1928, Fauna helmintológica dos peixes de água doce do Brasil. Arch. Inst. Biol., São Paulo, 1: 5-67.
Publication Dates
-
Publication in this collection
20 Jan 2004 -
Date of issue
Aug 2003
History
-
Accepted
05 Dec 2002 -
Received
16 June 2002