Abstracts
The reproductive biology of Palythoa caribaeorum (Duchassaing & Michelotti 1860) and Protopalythoa variabilis (Duerden 1898) was studied through monthly samples from tagged colonies from June 1996 to June 1997, in São Sebastião channel, São Paulo, Brazil (45º26'W, 23º50'S). The gametogenesis was similar to that of other zoanthids as shown by histological preparations. Oocyte diameters and maturation stages of testis vesicles were evaluated on squash preparations. Both species showed sequential protogynic hermaphroditism, with high frequency of fertile polyps (83% in P. variabilis and 72% in P. caribaeorum), high frequency of colonies in female sex condition (65.3% of P. variabilis and 41.7% of P. caribaeorum), and apparently continuous gametogenesis. In P. caribaeorum, egg release was continuous and sperm release took place during half of the analyzed period. In P. variabilis, egg and sperm release occurred in April-May and February-March 1997, respectively.
Anthozoa; Zoanthidea; Palythoa; Protopalythoa; sexual reproduction
A biologia reprodutiva de Palythoa caribaeorum (Duchassaing & Michelotti 1860) e Protopalythoa variabilis (Duerden 1898) foi estudada por amostras mensais de colônias etiquetadas de junho de 1996 a junho de 1997, no canal de São Sebastião, São Paulo, Brasil (45º26'W, 23º50'S). A gametogênese apresentou-se semelhante à de outros zoantídeos, como evidenciado em preparações histológicas. O diâmetro dos oócitos e os estágios de maturação dos folículos testiculares foram avaliados por preparações do tipo "squash". Ambas as espécies mostraram hermafroditismo seqüencial protogínico, com alta freqüência de pólipos férteis (83% em P. variabilis e 72% em P. caribaeorum) e de colônias na condição sexual feminina (65,3% para P. variabilis e 41,7% para P. caribaeorum), e, aparentemente, gametogênese contínua. Em P. caribaeorum, a liberação de oócitos foi contínua e a liberação de espermatozóides ocorreu durante metade do período analisado. Para P. variabilis, a liberação de oócitos e de espermatozóides ocorreu em abril-maio e fevereiro-março de 1997, respectivamente.
Anthozoa; Zoanthidea; Palythoa; Protopalythoa; reprodução sexuada
Reproductive biology of Palythoa caribaeorum and Protopalythoa variabilis (Cnidaria, Anthozoa, Zoanthidea) from the southeastern coast of Brazil
Biologia reprodutiva de Palythoa caribaeorum e Protopalythoa variabilis (Cnidaria: Anthozoa: Zoanthidea) da costa sudeste do Brasil
Boscolo, H. K.; Silveira, F. L.
Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo, Rua do Matão, travessa 14, n. 321, CEP 05508-900, Cidade Universitária, São Paulo, SP, Brazil
Correspondence to Correspondence to Helena K. Boscolo Departamento de Zoologia Instituto de Biociências, Universidade de São Paulo Rua do Matão, travessa 14, n. 321 CEP 05508-900, Cidade Universitária, São Paulo, SP, Brazil E-mail: helenakr@ib.usp.br; fldsilve@usp.br
ABSTRACT
The reproductive biology of Palythoa caribaeorum (Duchassaing & Michelotti 1860) and Protopalythoa variabilis (Duerden 1898) was studied through monthly samples from tagged colonies from June 1996 to June 1997, in São Sebastião channel, São Paulo, Brazil (45º26'W, 23º50'S). The gametogenesis was similar to that of other zoanthids as shown by histological preparations. Oocyte diameters and maturation stages of testis vesicles were evaluated on squash preparations. Both species showed sequential protogynic hermaphroditism, with high frequency of fertile polyps (83% in P. variabilis and 72% in P. caribaeorum), high frequency of colonies in female sex condition (65.3% of P. variabilis and 41.7% of P. caribaeorum), and apparently continuous gametogenesis. In P. caribaeorum, egg release was continuous and sperm release took place during half of the analyzed period. In P. variabilis, egg and sperm release occurred in April-May and February-March 1997, respectively.
Key words: Anthozoa, Zoanthidea, Palythoa, Protopalythoa, sexual reproduction.
RESUMO
A biologia reprodutiva de Palythoa caribaeorum (Duchassaing & Michelotti 1860) e Protopalythoa variabilis (Duerden 1898) foi estudada por amostras mensais de colônias etiquetadas de junho de 1996 a junho de 1997, no canal de São Sebastião, São Paulo, Brasil (45º26'W, 23º50'S). A gametogênese apresentou-se semelhante à de outros zoantídeos, como evidenciado em preparações histológicas. O diâmetro dos oócitos e os estágios de maturação dos folículos testiculares foram avaliados por preparações do tipo "squash". Ambas as espécies mostraram hermafroditismo seqüencial protogínico, com alta freqüência de pólipos férteis (83% em P. variabilis e 72% em P. caribaeorum) e de colônias na condição sexual feminina (65,3% para P. variabilis e 41,7% para P. caribaeorum), e, aparentemente, gametogênese contínua. Em P. caribaeorum, a liberação de oócitos foi contínua e a liberação de espermatozóides ocorreu durante metade do período analisado. Para P. variabilis, a liberação de oócitos e de espermatozóides ocorreu em abril-maio e fevereiro-março de 1997, respectivamente.
Palavras-chave: Anthozoa, Zoanthidea, Palythoa, Protopalythoa, reprodução sexuada.
INTRODUCTION
The zoanthids are frequent organisms within shallow water communities along the coast of Brazil, in which Zoanthus spp. and Palythoa spp. are as common (Rohlfs de Macedo & Belém, 1994) as they are in other tropical reefs of the world (Ryland & Babcock, 1991). At the southeastern coast of Brazil there are numerous colonies of Protopalythoa variabilis (Duerden 1898) (non Palythoa variabilis).
The systematics of zoanthids is even today considered confusing. Both colonies and polyps are highly variable within species. Many published descriptions lack essential diagnostic information, which makes comparisons difficult. Recent work using molecular genetics (Burnett et al., 1997) suggests that many nominate species really represent no more than synonyms.
Studies on reproductive biology are few (reviewed by Ryland, 1997) and mainly consider Brachycnemina (i.e., Palythoa, Protopalythoa, Zoanthus). Muirhead et al. (1986) studied two deep-sea species of Macrocnemina (Epizoanthus spp.), which might be expected to exhibit differences in comparison with the shallow water brachycnemic species.
The goal of this study is to clarify sexual reproduction of Palythoa caribaeorum (Duchassaing & Michelotti 1860) and Protopalythoa variabilis (Duerden 1898) by describing the gametogenesis and focusing on monthly variation of the gonad profile within colonies to determine the frequency of fertile polyps and the reproduction rate of polyps and colonies. This is the first study on sexual reproduction of Zoanthidea for the South Atlantic, in which the methods employed include quantitative evaluation of observed testis vesicles. In Brazil, other research on zoanthids species has centered on composition (Rohlfs de Macedo & Belém, 1994) and aspects of asexual reproduction of Palythoa caribaeorum (see Moreno, 1999).
MATERIAL AND METHODS
Colonies of P. caribaeorum and P. variabilis sampled were living in the shallow rocky bottom of the São Sebastião Channel (SSC), São Paulo, Brazil, in the vicinity of the Centro de Biologia Marinha, University of São Paulo (CEBIMar - USP) (45º26'W, 23º50'S). The colonies occur mainly on the rocky shores in from 1 to 3 m depths. The colonies of P. caribaeorum cover large areas and the orange-brownish polyps (~1 cm height x 0.5 cm diameter) interconnect by a thick coenenchyme out of which only the oral discs appear. The colonies of P. variabilis are brownish with polyps (~3 cm height x 1 cm diameter) interconnected by a mat of basal coenenchyme (Boscolo, 2000).
Samples from tagged colonies were obtained at five stations monthly between June 1996 and June 1997. The five stations were called: (A) located between Itacuçê and Guaecá point (one colony of P. caribaeorum); (B) Saco Grande bay (one colony of P. caribaeorum); (C) south side of Segredo beach (two colonies of P. caribaeorum and two colonies of P. variabilis) (C1 and C2, respectively); (D) north side of Segredo beach (two colonies of P. caribaeorum) (D1 and D2); and (E) Cabras island (two colonies of P. variabilis) (E1 and E2) (Fig. 1). Each sample consisted of 20 polyps, 10 from the center and 10 from the margin of each colony. Only half of the collected polyps were studied; the remaining samples have been deposited in the Cnidaria collection of Museu Nacional, Universidade Federal do Rio de Janeiro (MNRJ 3415 to 3666).
In the laboratory, the animals were anesthetized in a 1:1 solution of 7.5% magnesium chloride and seawater (until not reacting to touch), fixed in 4% formaldehyde solution in seawater, and dissected under a stereomicroscope to remove the complete mesenteries or macrosepta.
The mesenteries were dehydrated in an ethanol series, cleared in xylene, and wax-embedded. Serial sections of 7 µm were rehydrated with an ethanol series and stained with Weigert hematoxylin + Mallory trichromic (Mahoney, 1973), a suitable stain for basophilic and acidophilic oocytes. Six slides, 3 of P. variabilis and 3 of P. caribaeorum, were deposited in the Cnidaria collection, MNRJ 3667 to 3672. The histological treatment made the oocytes shrink to approximately 50%-60% of their size, so that preserved oocytes of P. variabilis up to 400 µm in diameter measured no more than 160 µm in the histological sections.
Squash preparations of the mesenteries were studied with a Wild M20 microscope so as to measure the oocytes and estimate the percentage of total vesicle area occupied by spermatozoa. From each sample the diameters of 50 oocytes having a visible nucleolus were measured using an ocular micrometer. Non-circular cells were measured by the average of the longest and shortest dimensions. Samples with fewer than 10 oocytes were characterized as sterile. Divisions between oocyte size classes were set at 45 µm intervals. The Kolmogorov-Smirnov 2-sample analyses (a = 5%) (Sokal & Rohlf, 1995) tested differences between sets of oocyte diameter measurements for different regions of the same colony. When the null hypothesis (Ho: d21 = d22) was rejected, the set of oocyte diameter measurements remained separated. The testis vesicles (n = 50 per sample) were studied and categorized by subjectively evaluating the percentage of total vesicle area occupied by spermatozoa (head and tail).
We also analyzed the percentage of fertile polyps in every reproductive state in relation to the total number of polyps, and the reproductive state of the polyps and colonies by the percentage found in female, male, hermaphroditic, or sterile condition.
RESULTS
Palythoa caribaeorum
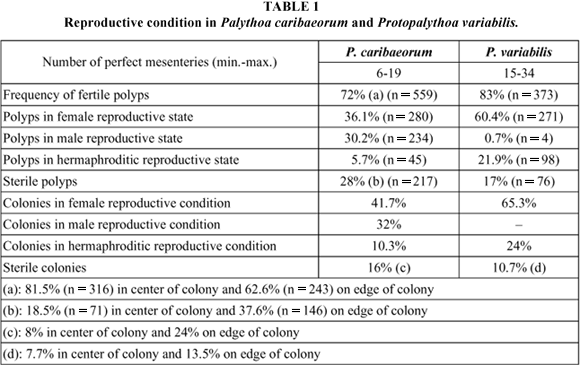
The polyps of P. caribaeorum had from 6 to 19 perfect mesenteries with gonads (Table 1) and were 5 mm in length.
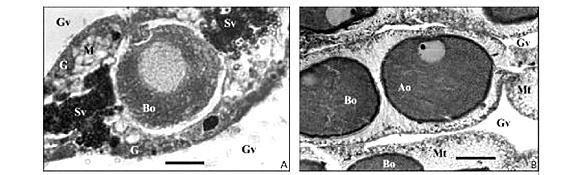
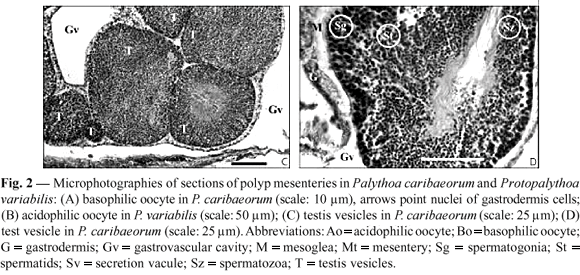
The oocytes appear as endodermal cells with nuclei near the gastrodermal layer (Fig. 2A and 2B), and having a maximum diameter of 51.25 µm in the histological sections (basophilic, 112.5 µm in squash); all were basophilic. The testis vesicles spread extended from the ciliary band to the aboral region of the mesenteries. These vesicles were clear, with the central lumen even more translucent. The histological sections showed three layers: spermatogonia-spermatocytes, spermatids, and spermatozoa (Fig. 2C and 2D).
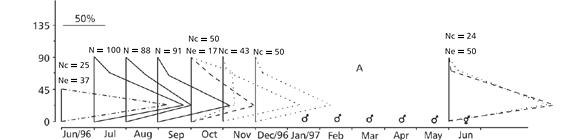
From the beginning of the sample period, the oocytes in the colonies preceded the testis vesicles, which in turn preceded the recovery of oocyte production. For a short period, all colonies presented a hermaphroditic condition (Fig. 3).
In October 1996, only two colonies (stations B and C1) had oocytes larger than 90 µm in diameter (max. 112.5 µm) (Figs. 3B and 3C1). All the other colonies showed oocytes ranging up to 90 µm. The entire number 2 colony at station D had been sterile for over 3 months, while its edges had been sterile for over 6 months (Fig. 3D2).
Testis vesicles developed over approximately 6 months. From December 1996 to January 1997, testis vesicles had only spermatogonia, and until the following period (February to May 1997) they were filled with spermatozoa (diameter of ~ 480 µm, in squash). A generally large proportion of these germ cells showed production and liberation until May 1997.
Protopalythoa variabilis
The polyps of P. variabilis, which were up to 15 mm in length, had from 15 to 34 perfect mesenteries with gonads (Table 1).
In most fertile mesenteries, the oocytes and testis vesicles occupied all of the gonad area. In squash preparations, oocytes of up to 450 µm in diameter and without zooxanthellae were observed. In histological preparations, the largest diameter of the basophilic oocytes was 53.25 µm, with a nucleus 21.75 µm in diameter and a 6 µm-diameter nucleolus. The average diameter of acidophilic oocytes was over 150 µm, with a 40 µm nucleus, and a 10 µm nucleolus. During the maturation of the oocyte, the nucleus migrated to its edge, next to the gastrodermis; the surrounding mesoglea thinned out and the gastrodermis thickened (Fig. 2B). The testis vesicles were similar to those of P. caribaeorum (see above) (Fig. 2C and 2D) except in size (P. variabilis with ~ 280 µm in diameter).
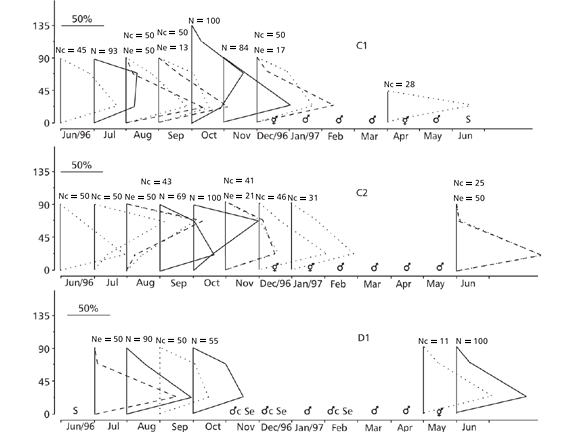
The oocyte size of colonies of P. variabilis increased gradually throughout the study periods in all stations (Fig. 5). From March to May 1997, only the two colonies of station E spawned or were sterile. The testis vesicles were being produced from November 1996 on and apparently were released in February-March 1997 (Fig. 6).
The oocytes and testis vesicles occurred together during 4-5 months, whereas the timing for spawning was not totally simultaneous (Figs. 5 and 6).
Reproductive patterns
Oocytes and testis vesicles of both species can co-occur in the same mesentery and are distinguishable either by their coloration (sperm, white; eggs, yellow) or by a short margin between the regions. In the female gonad, the mesoglea and gastrodermis are slender next to the site in which the oocyte nucleus is located.
Both species had high percentages of fertile polyps, although in P. caribaeorum the polyps in the central region of the colonies were more fertile than those on the edges. The colonies of P. caribaeorum showed the highest percentages of fertile polyps in August-September 1996 and in March 1997. From June 1996 to February 1997, all polyps of the majority of the colonies of P. variabilis were fertile (Fig. 7).
For both species, the largest percentage of polyps were female, but with different frequencies of fertile polyps between central and peripheral areas in the colonies of P. caribaeorum (Table 1). Male polyps were common in P. caribaeorum, but not in P. variabilis. Hermaphroditic polyps constituted the smallest percentage in P. caribaeorum; in P. variabilis they were the second most common. Sterile polyps of P. caribaeorum occurred more on the edges than within the central area of the colonies (Table 1: (a) and (b)). For both species, the largest percentages of sterile colony areas were at the edge of the colonies (Table 1: (c) and (d)).
DISCUSSION
Gametogenesis seems to be comparable to that of several species of zoanthids, for example: Palythoa tuberculosa (see Kimura et al., 1972), Epizoanthus abyssorum and Epizoanthus paguriphilus (see Muirhead et al., 1986), and Protopalythoa sp. (see Ryland & Babcock, 1991).
Male and female germinative products in Palythoa tuberculosa, and Protopalythoa sp. are different from those of P. variabilis and P. caribaeorum (Kimura et al., 1972; Karlson, 1981; Muirhead et al., 1986; Ryland & Babcock, 1991; Ryland, 1997). Gastrodermis cells may occur in the mesoglea area of oocytes of P. caribaeorum, allowing contact with the gastrodermis, the so-called trophonema (Dunn, 1975).
The size of testis vesicles filled with spermatozoa in P. caribaeorum is to date the largest recorded for any zoanthid. In Palythoa vestitus (see Cooke, 1976), Zoanthus pacificus (see Cooke, 1976), Zoanthus pulchellus (see Karlson, 1981), Protopalythoa sp. (see Ryland & Babcock, 1991), and Zoanthus solanderi (see Fadlallah et al., 1984) smaller sizes were observed but there are no comments as to whether these measurements were made on testis vesicles containing spermatozoa. Ryland (2000) observed that in Epizoanthus couchii, testis vesicles were smaller and filled only with spermatocytes.
The colonies of P. caribaeorum and of P. variabilis showed high percentages of fertile polyps in relation to what was observed in Protopalythoa sp. (39%) on the Great Barrier Reef (Australia) (Ryland & Babcock, 1991), and in Z. sociatus (16.5%) and Z. solanderi (5.2%) in Jamaica (Karlson, 1981). The variable frequency of fertile polyps in zoanthids may depend on local abiotic factors (Cooke, 1976; Karlson, 1981; Fadlallah et al., 1984; Ryland & Babcock, 1991). Karlson (1981) believed that the difference in fecundity was inversely proportional to polyp size in polyps of Zoanthus pulchellus, which are smaller than polyps of Z. sociatus and Z. solanderi. Although the polyps of Z. pulchellus are only half the size of polyps of P. variabilis, the percentage of fertile polyps is very similar (81% for Z. pulchellus; 83% for P. variabilis). Some colonies of P. variabilis had fewer fertile polyps, the same having previously been observed for Z. pacificus in Hawaii (Cooke, 1976).
In the hermaphroditic colonies of Zoanthus spp., polyps vary in reproductive activity (Karlson, 1981). This variability was also observed in polyps of P. caribaeorum and P. variabilis in the present study. Karlson (1981) argued that this variation in Zoanthus spp. could be a consequence of polyp mortality and colony regeneration.
The largest percentage of polyps were female, corroborating the hypothesis of Ryland (1997) that there are more polyps in the female state at any time, an expected trend because of the time needed to mature the eggs. Nevertheless, this hypothesis is not always valid because the reproductive state in polyps varies greatly. For Palythoa tuberculosa, Yamazato et al. (1973) reported a high percentage of sterile polyps, followed by polyps in the male reproductive condition.
Compared with the centers, the edge regions of colonies of P. caribaeorum showed a higher percentage of sterile polyps, something similar to that observed in Palythoa tuberculosa (see Yamazato et al., 1973). In addition, the edge region of colonies of both species showed a larger sterile-area percentage. As has been shown for interclonal border warrior anemones of Anthopleura elegantissima (Francis, 1976), some of the edge region areas for both species of zoanthids perhaps lack energy reserves for polyp gamete production. Moreno (1999) observed some strong antagonistic effects between border regions of colonies of P. caribaeorum in the same area as that of the present study.
When considering the sex condition of the species, it seems more appropriate to refer to the colony instead of the polyps (Ryland, 1997), although Kimura et al. (1972), Karlson (1981), and Fadlallah et al. (1984) considered only the polyps. The sex condition of a species is assessed by means of sequential sampling in tagged colonies, as has already been stressed by Yamazato et al. (1973). The colonies of P. variabilis and P. caribaeorum were fertile during the investigated period, with female, male, and hermaphroditic polyps. This characterizes a sequential hermaphroditism with an initial asynchronous oocyte production, followed by the development of testis vesicles. Hermaphroditic species are common within the brachycnemic zoanthids (Ryland, 1997). Protogynous hermaphroditism was also observed in colonies of P. tuberculosa and Protopalythoa sp. (Yamazato et al., 1973; Ryland & Babcock, 1991).
Some colonies of P. caribaeorum and P. variabilis have been proven sterile at certain times. Ryland (1997, 2000) has observed "non-reproductive" colonies among breeding zoanthids. Yamazato et al. (1973) believed that at certain times all colonies show great numbers of sterile polyps. Nevertheless, we consider that P. caribaeorum and P. variabilis showed continuous gametogenesis (sensu Giese & Pearse, 1974), with interruptions in gametogenesis among colonies. We interpret the two colonies of P. caribaeorum that had oocytes in a size class larger than 90 µm as demonstrating reproductive peaks that reflect subtle environmental fluctuations (Giese & Pearse, 1974).
The oocytes of P. variabilis were larger in size than those of any of the other species of zoanthids (see Yamazato et al., 1973; Cooke, 1976; Karlson, 1981; Fadlallah et al., 1984). They had no zooxanthellae such as those observed in Protopalythoa sp. before spawning (Ryland & Babcock, 1991). In P. caribaeorum, the largest oocyte observed was smaller than the oocytes observed by Fadlallah et al. (1984) in Panama.
For the investigative period, the hypothesis that P. caribaeorum continuously spawns is based on the fact that the largest oocyte ever observed measured 112.5 µm in diameter (squash), or 51.25 µm (histological sections). The largest size observed by Fadlallah et al. (1984) was 430 µm (squash). Considering that size reduction of preserved oocytes reaches around 60% after embedding (as observed for P. variabilis), in serial sections one expects the maximum diameter observed for P. caribaeorum to be ~ 172 µm. Therefore, the oocytes of P. caribaeorum may mature very rapidly into the acidophilus state and would then be quickly released, which is likely either in a space-limited mesentery and gastrovascular cavity or crowding caused by numerous developing eggs. Many broadcasting marine invertebrates are said to spawn primary oocytes (the expected acidophilus state of cells in this work), which are diploid cells that complete meiosis in the surrounding water or following fertilization (Giese & Pearse, 1974).
In this way, P. caribaeorum may attain more reproductive success by continuous egg shedding, which may be a strategy to minimize egg depletion and a protection against the effects of possible heavy predation in a single breeding season.
Contrary to P. caribaeorum, the colonies of P. variabilis showed a specific and apparently annual time for spawning, which occurs in April-May.
The spermatogenic cycle in P. caribaeorum presumably occurred over a period of ~ 6 months. The continuous production and liberation of testis vesicles observed possibly resulted from lack of space within both the mesentery and the gastrovascular cavity or by the crowded conditions caused by many developing testis vesicles. In P. variabilis, the spermatogenic cycle lasted approximately 5 months, with sperm release over 2 months. Spermatogenesis in both species is well delimited, suggesting seasonality.
In colonies of P. variabilis, the spermatogenic cycle was shorter than that of the egg cycle, which is similar to what was observed with species of Protopalythoa sp., and occurs because the testis vesicles mature more rapidly than the oocytes (Ryland & Babcock, 1991). The colonies of P. variabilis showed some synchrony between release of eggs and sperm.
In P. caribaeorum, oocyte production was generally interrupted during spermatogenesis. This may result from the great number of vesicles observed in the mesenteries, a feature that increases the chances of eggs released by other colonies of the local population to be fertilized (Babcock et al., 1992).
The reproduction of marine animals is influenced by various factors, e.g., temperature, salinity, and food availability, but it is unclear how these factors may interact in a series of complex events leading to gametogenesis and spawning (Pearse et al., 1991). Cooke (1976) believed that one, or a few of the factors above, triggers, but does not control, reproduction in P. vestitus and Z. pacificus. Fadlallah et al. (1984) suggested that repeated exposure to air during low tide, and consequent exposure of the colonies of P. caribaeorum and Z. sociatus to high temperatures, might be a cue to spawning. In the case of spawning in marine invertebrates, food availability, which in the rich upwelling waters near Cabo Frio, Brazil, favors just-released planktotrophic larvae, must also be taken into account (Ventura et al., 1997). Temperature seems to be the ultimate factor influencing the reproduction of Protopalythoa sp. in the Great Barrier Reef (GBR) in Australia, because no reproductive activity was observed while seawater temperature was either high or declining (Ryland & Babcock, 1991). But temperature is only one of many factors that determine the best breeding season (Grahame & Branch, 1985).
Unsettled issues involving the timing of gamete production in different invertebrate animals as well as continuous versus seasonal reproduction remain. The traditional assumption has been that under stable environmental conditions many invertebrates would present continuous reproduction (Giese & Pearse, 1974; Barnes, 1975). However, whereas the broadcasting stony corals of the GBR tend to have an annual gametogenic cycle with a definite spawning period (Harrison & Wallace, 1990), brooding stony corals at the same site tend to have multiple gametogenic cycles without a definite spawning period within each colony (Harrison & Wallace, 1990). But Harrison & Wallace (1990: 162) noted that "Reproductive cycles of some corals in which gametogenesis has been studied cannot be clearly interpreted". They also stated that occurrence of mature gametes or planulae could be a result of "...multiple gametogenic cycles within each coral, or asynchronous reproduction among members of the population".
The average monthly mean of the temperature for surface seawater in SSC (Table 2) showed the largest variation for the period November-December 1996. Maybe such a variation triggered the onset of spermatogenesis, as observed in four colonies of P. caribaeorum. A possibility also exists of a correlation between the higher temperatures in December-May (see Table 2) and that in the same period both species in all colonies were developing testis vesicles.
The local populations of P. caribaeorum and P. variabilis seem to follow the reproductive patterns already observed for some tropical species, with continuous annual gametogenesis coupled with a defined spawning period.
Acknowledgements During the fieldwork this research was supported by FAPESP (96/4983-1). We thank Caio Borghoff Gonçalves for the sampling. We thank the Directors, Prof. Drs. M. G. B. S. Moreira and J. C. de Freitas, and the staff of CEBIMar, at São Sebastião, SP, for their support. The first author thanks CAPES for a grant to finish her Master's Dissertation in Pós-graduação, Área Zoologia, IB, USP. Both authors thank CAPES for the funding the histology and photographic laboratories of the Zoology Department, IB, USP. We thank the staff of the Museu Nacional/UFRJ (Cnidaria) for providing the list of voucher specimens. We are indebted to Dr. J. S. Ryland (University of Wales, Swansea, UK) for providing us with important literature and suggestions, to Drs. D. O. Pires and E. Schlenz for helpful suggestions, and Dr. D. Fautin for her critical review and suggestions.
Received July 16, 2002 - Accepted October 16, 2003 - Distributed February 28, 2005
- BABCOCK, R., MUNDY, C., KEESING, J. & OLIVER, J., 1992, Predictable and unpredictable spawning events: in situ behavioural data from free-spawning coral reef invertebrates. Invert. Reprod. Dev., 22: 213-228.
- BARNES, H., 1975, Reproductive rhythms and some marine invertebrates: an introduction. Pubbl. Staz. Zool. Nap., 39: 8-25.
- BOSCOLO, H. K., 2000, Estimativa do comportamento reprodutor de zoantídeos (Cnidaria, Anthozoa, Hexacorallia) do canal de São Sebastião, SP MSc thesis, Universidade de São Paulo, Instituto de Biociências, 168p.
- BURNETT, W. J., BENZIE, J. A. H., BEARDMORE, J. A. & RYLAND, J. S., 1997, Zoanthids (Anthozoa, Hexacorallia) from the Great Barrier Reef and Torres Strait, Australia: systematics, evolution and a key to species. Coral Reefs, 16: 55-68.
- COOKE, W. J., 1976, Reproduction, growth and some tolerances of Zoanthus pacificus and Palythoa vestitus in Kaneohe Bay, Hawaii. pp. 281-288. In: G. O. Mackie (ed.), Coelenterate ecology and behavior Plenum Press, New York, 744p.
- DUNN, D. F., 1975, Reproduction of the externally brooding sea anemone Epiactis prolifera Verrill, 1869. Biol. Bull., 148: 199-218.
- FADLALLAH, Y. H., KARLSON, R. H. & SEBENS, K. P., 1984, Comparative study of sexual reproduction in three species of Panamanian zoanthids (Coelenterata: Anthozoa). B. Mar. Sci., 35(1): 80-89.
- FRANCIS, L., 1976, Social organization within clones of the sea anemone Anthopleura elegantissima. Biol. Bull., 150: 361-376.
- GIESE, A. C. & PEARSE, J. S., 1974, Introduction: general principles. pp. 1-49. In: A. C. Giese & J. S. Pearse (eds.), Reproduction of marine invertebrates vol. I. Academic Press, London, 546p.
- GRAHAME, J. & BRANCH, G. M., 1985, Reproductive patterns of marine invertebrates. Oceanogr. Mar. Biol. Ann. Rev., 23: 373-398.
- HARRISON, P. L. & WALLACE, C. C., 1990, Reproduction, dispersal and recruitment of scleractinian corals. pp. 133-207. In: Z. Dubinsky (ed.), Coral reefs, ecosystems of the world 25 Elsevier, Amsterdam, 550p.
- KARLSON, R. H., 1981, Reproduction patterns on Zoanthus spp. from Discovery Bay, Jamaica. Proc. 4th Int. Coral Reef Symp., Manila, 2: 699-704.
- KIMURA, S., HASHIMOTO, Y. & YAMAZATO, K., 1972, Toxicity of the Zoanthid Palythoa tuberculosa. Toxicon, 10: 611-617.
- MAHONEY, R., 1973, Histological techniques. pp. 177-266. In: Laboratory techniques in Zoology 2nd. Butterworths & Co., London, 404p.
- MORENO, L. A. A., 1999, Ecologia da reprodução assexuada de Palythoa caribaeorum (Zoanthidea: Cnidaria) Ph.D. thesis, Universidade Estadual de Campinas, Instituto de Biologia, 234p.
- MUIRHEAD, A., TYLER, P. A. & THURSTON, M. H., 1986, Reproductive biology and growth of the genus Epizoanthus (Zoanthidea) from the north-east Atlantic. J. Mar. Biol. Assoc. U. K., 66(1): 131-143.
- PEARSE, J. S., MCCLINTOCK, J. B. & BOSCH, I., 1991, Reproduction of antarctic benthic marine invertebrates: tempos, modes, and timing. Am. Zool., 31: 65-80.
- ROHLFS DE MACEDO, C. M. R. & BELÉM, M. J. C., 1994, The genus Zoanthus in Brazil. 1. Characterization and anatomical revision of Zoanthus sociatus (Cnidaria, Zoanthinaria, Zoanthidae). Iheringia, 77: 135-144.
- RYLAND, J. S., 1997, Reproduction in Zoanthidea (Anthozoa: Hexacorallia). Invert. Reprod. Dev., 31: 177-188.
- RYLAND, J. S., 2000, Reproduction in British zoanthids, and an unusual process in Parazoanthus anguicomus. J. Mar. Biol. Assoc. U. K., 80: 943-944.
- RYLAND J. S. & BABCOCK, R. C., 1991, Annual cycle of gametogenesis and spawning in a tropical zoanthid, Protopalythoa sp. Hydrobiologia, 216/217: 117-123.
- SOKAL, R. R. & ROHLF, F. J., 1995, Biometry: the principles and practice of statistics in biological research 3rd ed., W. H. Freeman & Com., New York, 887p.
- VENTURA, C. R. R., FALCÃO, P. C., SANTOS, J. S. & FIORI, C. S., 1997, Reproductive cycle and feeding periodicity in the starfish Astropecten brasiliensis in the Cabo Frio upwelling ecosystem (Brazil). Invert. Reprod. Dev., 31: 135-141.
- YAMAZATO, K., YOSHIMOTO, F. & YOSHIHARA, N., 1973, Reproduction cycle in a zoanthid Palythoa tuberculosa Esper. Publ. Seto Mar. Biol. Lab., 20: 275-283.
Publication Dates
-
Publication in this collection
16 Nov 2005 -
Date of issue
Feb 2005
History
-
Accepted
16 Oct 2003 -
Received
16 July 2002