Abstracts
The objective of this study was to perform a malacological assessment at the Ibirité reservoir watershed in the metropolitan region of Belo Horizonte (Minas Gerais) and to evaluate the natural infestation rate of Biomphalaria straminea (Gastropoda: Planorbidae)by Schistosoma mansoni (Platyhelminthes: Trematoda) and Chaetogaster limnaei (Oligochaeta: Naididae). The samples were collected from July to August 2002. The B. straminea individuals collected were kept in the laboratory; the natural infestation rate by S. mansoni and C. limnaei was assessed weekly. The malacological assessment identified fivemollusk species present in the Ibirité reservoir watershed: B. straminea, Physa marmorata, Lymnea sp., Melanoides tuberculatus,and Pomacea austrum. Laboratory observations showed that the B. straminea individuals were infected by C. limnaei rather than S. mansoni. Although there was no infection of B. straminea by S. mansoni,presence of B. straminea in itself merits close attention due to possible risk of human schistosomiasis by the local population.
Biomphalaria straminea; Chaetogasterlimnaei; malacological assessment; bioindicators; schistosomiasis
O objetivo deste estudo foi realizar um levantamento malacológico na bacia do reservatório de Ibirité, na região metropolitana de Belo Horizonte (Minas Gerais), e avaliar a taxa de infestação natural de Biomphalaria straminea (Gastropoda: Planorbidae)por Schistosoma mansoni (Platyhelminthes: Trematoda) e Chaetogaster limnaei (Oligochaeta: Naididae). As amostragens foram realizadas de julho a agosto de 2002. Os espécimes de B. straminea coletados foram levados para o laboratório e as avaliações da taxa de infestação foram realizadas semanalmente. O levantamento malacológico identificou cinco espécies de moluscos presentes na bacia do reservatório: B. straminea, Physa marmorata, Lymnea sp., Melanoides tuberculatus e Pomacea austrum. As observações em laboratório mostraram que os espécimes de B. straminea estavam infectados somente por C. limnaei. Apesar de não ter sido encontrado S. mansoni, a presença desse planorbídeo deve ser vista com atenção, em decorrência da possibilidade de infecção com esquistossomose humana para a população local.
Biomphalaria straminea; Chaetogasterlimnaei; levantamento malacológico; bioindicadores; esquistossomose
Malacological assessment and natural infestation of Biomphalaria straminea (Dunker, 1848) by Schistosoma mansoni (Sambon, 1907) and Chaetogaster limnaei (K. von Baer, 1827) in an urban eutrophic watershed
Levantamento malacológico e infestação natural de Biomphalaria straminea (Dunker, 1848) por Schistosoma mansoni (Sambon, 1907) e Chaetogaster limnaei (K. von Baer, 1827) em uma bacia hidrográfica eutrofizada urbana (MG, Brasil)
Callisto, M.I; Moreno, P.I,III; Gonçalves, J. F. Jr.I,III; Ferreira, W. R.I; Gomes, C. L. Z.II
ILaboratório de Ecologia de Bentos, Departamento de Biologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, C.P. 486, CEP 30161-960, Belo Horizonte, MG, Brazil
IIGabriel Passos Refinaria, Petrobrás, Rod. Fernão Dias, BR 381, km 427, C.P. 21, CEP 32530-000, Betim, MG, Brazil
IIIEstudante de graduação do Programa em Ecologia, Conservação e Manejo da Vida Silvestre, Universidade Federal de Minas Gerais
Correspondence to Correspondence to Marcos Callisto Laboratório de Ecologia de Bentos, Departamento de Biologia Geral Instituto de Ciências Biológicas Universidade Federal de Minas Gerais C.P. 486 CEP 30161-960, Belo Horizonte, MG, Brazil e-mail: callisto@icb.ufmg.br
ABSTRACT
The objective of this study was to perform a malacological assessment at the Ibirité reservoir watershed in the metropolitan region of Belo Horizonte (Minas Gerais) and to evaluate the natural infestation rate of Biomphalaria straminea (Gastropoda: Planorbidae)by Schistosoma mansoni (Platyhelminthes: Trematoda) and Chaetogaster limnaei (Oligochaeta: Naididae). The samples were collected from July to August 2002. The B. straminea individuals collected were kept in the laboratory; the natural infestation rate by S. mansoni and C. limnaei was assessed weekly. The malacological assessment identified fivemollusk species present in the Ibirité reservoir watershed: B. straminea, Physa marmorata, Lymnea sp., Melanoides tuberculatus,and Pomacea austrum. Laboratory observations showed that the B. straminea individuals were infected by C. limnaei rather than S. mansoni. Although there was no infection of B. straminea by S. mansoni,presence of B. straminea in itself merits close attention due to possible risk of human schistosomiasis by the local population.
Key words:Biomphalaria straminea, Chaetogasterlimnaei, malacological assessment, bioindicators, schistosomiasis.
RESUMO
O objetivo deste estudo foi realizar um levantamento malacológico na bacia do reservatório de Ibirité, na região metropolitana de Belo Horizonte (Minas Gerais), e avaliar a taxa de infestação natural de Biomphalaria straminea (Gastropoda: Planorbidae)por Schistosoma mansoni (Platyhelminthes: Trematoda) e Chaetogaster limnaei (Oligochaeta: Naididae). As amostragens foram realizadas de julho a agosto de 2002. Os espécimes de B. straminea coletados foram levados para o laboratório e as avaliações da taxa de infestação foram realizadas semanalmente. O levantamento malacológico identificou cinco espécies de moluscos presentes na bacia do reservatório: B. straminea, Physa marmorata, Lymnea sp., Melanoides tuberculatus e Pomacea austrum. As observações em laboratório mostraram que os espécimes de B. straminea estavam infectados somente por C. limnaei. Apesar de não ter sido encontrado S. mansoni, a presença desse planorbídeo deve ser vista com atenção, em decorrência da possibilidade de infecção com esquistossomose humana para a população local.
Palavras-chave:Biomphalaria straminea, Chaetogasterlimnaei, levantamento malacológico, bioindicadores, esquistossomose.
INTRODUCTION
The rapid urbanization rate of Brazilian cities has resulted in progressively deteriorating sanitary conditions and increased poverty levels of the human population. This situation has greatly challenged public health authorities, created new health problems, and increased the demand for public health services. The spread of human schistosomiasis is attributed not only to migration of infected people into non-infected areas, but also to invertebrate host (Biomphalaria spp.) dispersion. Epidemiological studies often fail to consider the social, economic, and behavioral characteristics of the region when, in fact the watershed should be considered as a study unit (Barbosa et al., 2000).
The natural infestation rates of Biomphalaria spp. by S. mansoni oscillates in freshwater ecosystems from as high as 4% in Biomphalaria straminea,andup to 52% in Biomphalaria glabrata. The overall infestation rate is related to the abundance of Biomphalaria in the area (Favre et al., 2002).
A promising new path for biological control of the snail intermediate hosts of human schistosomiasis is the introduction of other snail species, such as Melanoides tuberculatus (Pointier, 2001; Giovanelli et al.,2002), that act only as natural competitors, and whose competitive pressure on Biomphalaria spp. can lead to the latter's extinction. M. tuberculatus and Biomphalaria spp. have similar diets, feeding on detritus, associated microorganisms (microalgae and bacteria), and fine particulate organic matter (Pointier et al., 1991; De Marco, 1999). M. tuberculatus is an exotic species from Asia that has adapted very well to the climatic conditions of Brazil and can now be found in the benthic malacofauna of many permanent and stable lacustrine ecosystems. In Minas Gerais State, the introduction of M. tuberculatus hasresulted in strong competitive pressure for space, as observed in Dom Helvécio Lake at Rio Doce State Park (De Marco, 1999) and in Lagoa Santa lake in the carstic region (Lagoa Santa Municipality) (Freitas et al., 1994). Recently, Guimarães (2001) observed the reduction and extinction of the B. straminea population in a small lagoon in the Municipality of Esmeraldas (metropolitan region of Belo Horizonte) after the appearance of M. tuberculatus.
An intimate association between the oligochaete worm Chaetogaster limnaei (K. von Baer, 1827) and many aquatic snail species has long been recognized as commensal or parasitic in several North American and European freshwater ecosystems (Conn, 1994; Barbour, 1977; Gale, 1973; Andrade & Campos, 1968; Ruiz, 1951; Von Baer, 1827). The presence of natural parasites in Biomphalaria spp. hinders and even stops infestation by S. mansoni. Annelidae like C. limnaei probably impede the colonization of S. mansoni miracidia in Biomphalaria spp. due to the large number of individuals that lodge inside the shells, thus diminishing the available space accessible to colonizing miracidia (Michelson, 1964). The vast majority of C. limnaei inhabit the mantle cavity of their host (Conn et al., 1994). At certain times of the year a large proportion of the worm population must find new hosts. This occurs when the worms hatch from their cocoons in early spring and when the host snails die (either during the winter, or after breeding in the summer). A constantly high infestation percentage of the host snail population indicates that dispersal from one host to another is highly efficient (Buse, 1972).
The objective of this study was to perform a malacological assessment at the Ibirité reservoir watershed in the metropolitan region of Belo Horizonte (MG) and to evaluate the natural infestation rate of B. straminea (Gastropoda: Planorbidae) by S. mansoni (Platyhelminthes: Trematoda) and C. limnaei (Oligochaeta: Naididae).
STUDY AREA
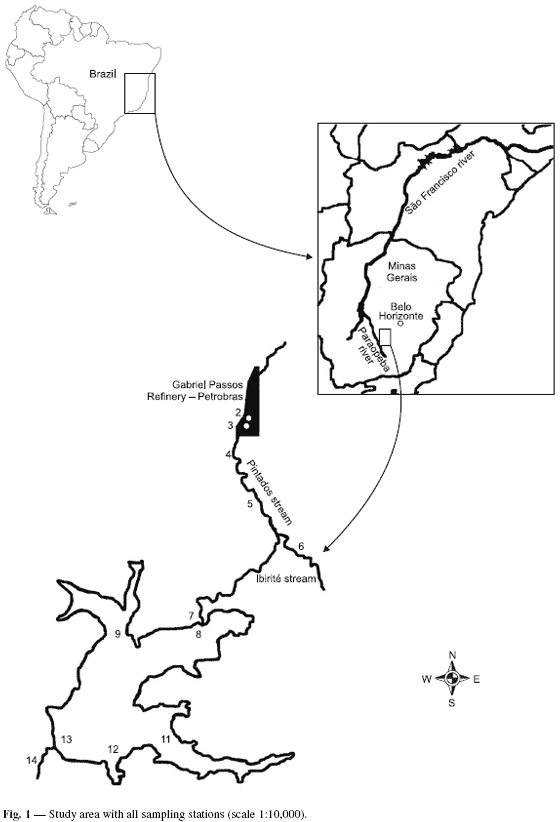
The Ibirité reservoir (19º07'00'', 20º02'30''S; 44º07'30'', 44º05'00''W) belongs to the Paraopeba river watershed, an affluent of the São Francisco river in Minas Gerais State, Brazil. The watershed is composed of the sub-basins of the Pintados, Retiro, and Do Onça streams, forming the Ibirité river. The Ibirité river dam was constructed in the 60 s, originating the Ibirité reservoir (Fundação João Pinheiro, 2001). The Ibirité river is a direct affluent of the Paraopeba river (Fig. 1).
The regional landscape is of convex hills with steep slopes. The rounding process of the slopes resulted in long alluvial deposits that presently fill the flat-bottom valleys. The average altitude is 950 m, with fluvial gaps ranging from 800 to 850 m and round hills that are approximately 1,000 m high. The climate is considered sub-humid tropical (Cwb), with summer rains (October to March) and a dry winter (April to September). Average annual temperature is c. 20ºC, with the coldest month averaging below 18ºC and the hottest, above 22ºC (Christofoletti, 1974). Tropical savanna (cerrado) is the regional vegetation type, with some sparse fragments of riparian vegetation. Surrounding areas are densely inhabited by slums. No domestic sewage treatment exists, and dejects are released in natura in the rivers of the Ibirité reservoir watershed.
The reservoir is going through a rapid artificial eutrophication process in which growth can be observed of primary producers that use the nutrients and accumulated organic matter. In different periods of the year occurrence of Microcystis aeroginosa and Eichhornia spp. blooms can also be detected. The water quality is very low, with high fecal contamination. Water temperatures are between 15.9ºC and 22.9ºC in the rivers, and 20.9ºC and 22.8ºC in the reservoir. Depth values are low in the rivers (0.1-0.8 m) and higher in the lbirité reservoir (5.0 m near the mouth of the river's inflow and 13.5 m close to the dam). The hypolimnion shows low oxygen concentrations (< 4.5 mg/L), pH values between 6.70 and 7.69 in the rivers and 6.50-7.00 in the reservoir. Electrical conductivity values were between 0.191 and 1.670 mS/cm in the rivers and below 0.360 in the bottom of the water column in the reservoir. Total alkalinity values were between 1.4 and 2.7 mEq/LCO2 in the rivers and below 1.3 and 1.8 mEq/LCO2 in the water column in the reservoir. The waters are clear, with turbidity values around 70 NTU at all sampling stations.
Sediments showed organic matter content between 1.32% and 16.80% DW in the rivers upstream from the reservoir, 13.04% DW downstream of the reservoir, and between 4.14 and 29.80% DW in the reservoir. Granulometric composition is typically sandy with heavy prevalence of coarse and medium sand in the rivers and silt-sand in the reservoir sediments.
MATERIAL AND METHODS
Samples were collected along the shoreline of the aquatic ecosystems in five sampling stations on the Pintados and Ibirité streams upstream from the reservoir, two sampling stations below the Gabriel Passos Refinery effluent treatment pool, six sampling stations in the reservoir, and one sampling station downstream from the reservoir (Fig. 1).
To evaluate the ecological conditions of the sampling stations located on the Pintados and Ibirité streams and their surroundings we used a rapid evaluation protocol of ecological conditions and habitat diversity in watershed stretches, as proposed by Callisto et al. (2002). This protocol evaluates not only the aquatic environment but also land use and occupation of the surrounding areas of the drainage watershed. The protocol evaluates: type of occupation of the water body margins; existence of erosion near or on the river margins, and bed siltation; anthropic activities in the surrounding areas; vegetal cover on the river bed; odor, oil presence, and water transparency; odor, oil presence and type of bottom; extension and frequency of rapids and pools; mud deposition; presence, stability, and extension of riparian vegetation; and presence of aquatic macrophytes. The final score of the protocol is obtained from the sum of the scores of each independent parameter, and reflects the ecological preservation status of the watershed stretches. Scores from 0 to 40 points represent impacted stretches, from 41 to 60 points represent altered stretches, and over 61 points, natural stretches.
Snails were collected with 7 cm tall metallic scoops, measuring 19 cm diameter at the bottom and 25 cm diameter at the opening, and containing 2 holes/cm2. Samples were collected along the stream margins or from floating vegetation (Typha domingensis, grass, etc.), sewage release areas, areas having higher concentrations of anglers, and near the employees' club of the Gabriel Passos Refinery. In each sampling station, three to seven distinct habitats were investigated, which involved a sampling effort of 10 to 20 scoops. Scoops were dipped into the water by the shore every meter at all sampling stations twice a month. Snail abundances were classified as rare (up to 5 mollusks/scoop), abundant (6 to 10 mollusks/scoop), and very abundant (more than 10 mollusks/scoop). In the laboratory the mollusks were macroscopically observed, identified, measured (maximum shell length and shell-opening diameter). They were then placed individually in small snap-cap glass recipients with natural, i.e., dechlorinated water, and left overnight until the next morning's examination, carried out with the help of a stereomicroscope. Identification of the mollusks was based on a report of Souza & Lima (1997). After the first examination, individuals negative for trematodes and oligochaetes were exposed to direct artificial illumination for 2 hours, followed by a second examination. After the examination period the mollusks were crushed and observed under a stereoscopic microscope for presence of S. mansoni and C. limnaei.
RESULTS AND DISCUSSION
The waters of the Ibirité reservoir receive non-treated domestic sewage, agro-pasture effluents, and sediments carried along the watershed rivers. Not surprisingly, the characterization protocol of ecological conditions showed advanced environmental degradation in the Ibirité reservoir watershed, with direct consequences for habitat diversity, margin erosion, extinction of riparian vegetation, and water body siltation.
The malacological assessment identified five species that live in the waters of the Ibirité Reservoir: B. straminea, Physa cf. marmorata, Lymnaea sp., M. tuberculatus, and Pomacea haustrum. M. tuberculatus was numerically dominant in the reservoir, followed by P. haustrum, Physa cf. marmorata,and B. straminea (Table 1). The taxonomic composition and mollusk distribution in the reservoir did not exhibit significant spatial differences, reflecting the physical and chemical homogeneity of the ecosystem (F.A.R. Barbosa, personal communication). This fact probably favors the dominance of M. tuberculatus, which is an alien species with high adaptation capacity and also an efficient competitor (Guimarães et al., 2001; Giovanelli et al., 2002). Previous studies suggest the role of M. tuberculatus in diminishing the population of Biomphalaria spp., the invertebrate host of S. mansoni, on Santa Lucia Island (West Indies) and in Venezuela (Pointier, 1993; Pointier et al., 1994). These competition mechanisms require more study but it can already be affirmed that competition occurs when high density values of M. tuberculatus are reached and maintained (De Marco, 1999).
It was observed that, in the rivers flowing into the Ibirité reservoir, each stretch studied exhibited a distinct composition of mollusk species (Table 2). In the Pintados stream a longitudinal gradient was found along sampling stations 3, 4, and 5, where P. cf marmorata and B. straminea were the dominant species.
In the Ibirité stream adjacent to the urban area (station 6) only P. cf. marmorata was found, except in August 2002, marked by a rare occurrence of B. straminea. However, in the sampling station before the reservoir (station 7) there was a predominance of P. cf. marmorata and M. tuberculatus.
After the reservoir (station 14) there was a substitution of the dominant species in the malacofauna along the study period. In June, the dominant mollusk was M. tuberculatus. In July, this population started to decrease, allowing an increase in the population of B. straminea, which resulted in co-dominance. In August, the population of B. straminea started to dominate the malacofauna. During the last samplings, occasional rains, unusual for that period of the year, were observed. With the rain, flooding occurred in many swamp areas around the houses neighboring Ibirité reservoir, allowing the growth sites of B. straminea to overflow and the mollusks to be carried downstream.
The taxonomic composition of the malacological data from samples of the station 14 reflected the mollusk community structure that had colonized the Ibirité reservoir. Therefore, the changes observed in the abundance of mollusk species could be related to the rains that occurred at the end of the dry period of 2002.
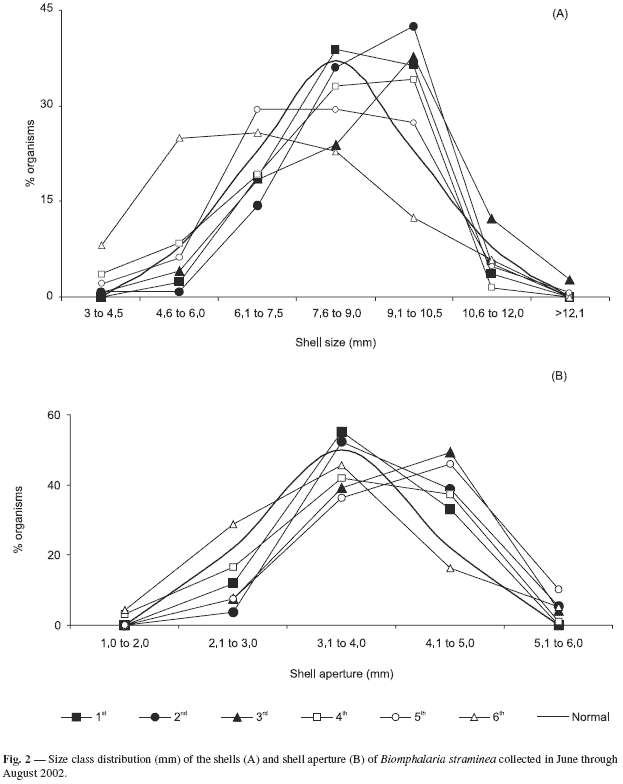
Shell size and aperture measures of B. straminea were distributed into classes (Fig. 2). This analysis suggested that in the dry period the population tends to have a normal distribution (p < 0.05), which means that a great number of adult individuals are present when compared to the young and old individuals. This tendency occurred in all periods except in the last sampling period, when the number of young individuals was higher. The class distribution curve of the size of shells collected in the sixth sampling period when more young individuals were found could have represented population recruitment.
The results of the natural infestation rates by parasites for B. straminea are presented in Table 3. The observations indicated an infestation of B. straminea with Chaetogaster limnaei (Annelida-Oligochaeta) instead of Schistosoma mansoni. Early naturalists considered C. limnaei to be a true parasite that was thought to feed upon slime produced by the host (Michelson, 1964). However, subsequent investigators demonstrated that the oligochaete fed mainly on microorganisms, and suggested that the worm be considered a commensal. Wagin (1931) also observed that the oligochaete ingested cercariae and suggested that C. limnaei might be of value in controlling transmission of trematodes such as Fasciola hepatica cercariae and Schistosoma mansoni miracidia.
Under field conditions, the intensity of Chaetogaster infestation in snails appears to vary considerably. Often, no more than ten Chaetogaster individuals were observed in each specimen of Lymnaea stagnalis and L. ovata,and more than 300 worms in a single specimen of L. stagnalis (Michelson, 1964). In our study, as many as 15 C. limnaei have been recovered from a single B. straminea. The highest infestations rates (up to 45 worms) were found in older and larger snails.
Figure 3 shows temporal trends in the prevalence of C. limnaei (i.e., percentage of gastropods infected) throughout the study period. (It is important to recognize that Chaetogaster-infectedsnails may be refractory to experimental trematode infestations and that laboratory snail colonies should be free from such infestations (Michelson, 1964; Graczyk, 1999).) A low number of gastropods harbored parasites other than C. limnaei. Less than 5% of the gastropods harbored hydracarinid larvae (water mites) in the mantle cavity. Larvae of the chironomid fly, Chironomus sp., were found attached to the outside of the shells.
A method by which Chaetogaster could localize and make contact with the host would be through attraction to the mucus of the host snail (Conn et al., 1994). A similar attraction has been observed in trematodes, suggesting that a reaction to chemical substances probably exists (Conn et al., 1995). It therefore appears that localization of the host by Chaetogaster is initiated by chemical substances from the snail, which is subsequently orientated by water currents (Buse, 1972). A study of the microscopic structure of Chaetogaster limnaei (Buse, 1968) indicated that it has sense cells in the prostomium, which could be responsible for detecting both chemical substances both in the surrounding medium and water currents. The nature of the association between C. limnaei and aquatic requires further investigation.
The presence of B. straminea, a species that shows a low rate of natural infestation by S. mansoni, should be taken as an alert signaling the possibility on the part of fishermen, swimmers, tourists, and the population in the affected area of finding themselves at risk for human schistosomiasis, which is an undisputable sign that public health conditions in the watershed are deteriorating. In such a situation, malacological monitoring programs should be designed and implemented so as to broaden the understanding of the biology of these mollusk species and also of the possible implications of these organisms for both the environment and the people living in it.
Future studies should examine the relationship between the patterns characterizing the B. straminea population and those of other native mollusks that may serve as hosts for C. limnaei. The latter, however, did not appear to be pathogenic with respect to the gastropod. However, because of the high prevalence and rates of infection of this worm in gastropods, further research on this point is warranted. In addition and more broadly, the present results suggest that further studies should explore differences in parasite/host patterns among various localities and habitats in the studied watershed.
In conclusion, this study demonstrated that the malacological fauna in the Ibirité reservoir watershed reflects a eutrophic process due to anthropogenic activities in the region and that C. limnaei,symbiotically associated with B. straminea,may have important implications with respect to biological control and/or changes in the epizootiology of native parasites in the Ibirité reservoir watershed.
Acknowledgements This study was funded by grants from the Gabriel Passos Refinery, Petrobras S. A., with additional funding from the Brazilian National Council for Research (CNPq). We thank CAPES and FAPEMIG for the scholarships granted to the graduate students. The authors are indebted to Dr. Teofãnia Heloísa Dutra Amorim Vidigal for help with the identification of B. straminea species, Roney E. Silva for help with the identification of the species studied, and Dr. Adlane Villas-Boas who revised the English text. We also thank Dr. J. L. Atthayde for valuable criticism of a previous version of the manuscript and our colleagues of the Laboratório de Ecolologia de Bentos for technical assistance.
Received April 14, 2003 Accepted January 30, 2004 Distributed May 31, 2005
- ANDRADE, R. M. & CAMPOS, L. G., 1968, Natural infection of Biomphalaria straminea (Dunker, 1848) by oligochaetes of the genus Chaetogaster. Revista Brasileira de Malariologia e Doencas Tropicais, 21(1): 27-36.
- BARBOSA, C. S., PIERI, O. S., SILVA, C. B. & BARBOSA, F. S., 2000, Ecoepidemiologia da esquistossomose urbana na ilha de Itamaracá, Estado de Pernambuco. Rev. Saúde Pública, 34(4): 337-341.
- BARBOUR, M. T., 1977, Chaetogaster limnaei limnaei (Oligochaeta: Naididae) inhabiting the mantle cavity of the pill clam Sphaerium Transactions of the American Microscopical Society,96: 141-142.
- BUSE, A., 1968, A comparative study of the morphology, behavioral and ecology of Chaetogaster limnaei (von Baer) from several host species Ph.D. Thesis, University of Wales, 250p.
- BUSE, A., 1972, Behavioural aspects of the relationship of Chaetogaster limnaei (Oligochaeta: Naididae) with its gastropod host. Animal Behaviour, 20(2): 274-279.
- CALLISTO, M., FERREIRA, W., MORENO, P., GOULART, M. D. C. & PETRUCIO, M., 2002, Aplicação de um protocolo de avaliação rápida da diversidade de habitats em atividades de ensino e pesquisa (MG-RJ). Acta Limnologica Brasiliensia, 14(1): 91-98.
- CHRISTOFOLETTI, A., 1974, Geomorfologia São Paulo, Edgard Blucher, 120p.
- CONN, D. B., BABAPULLE, M. N., KLEIN, K. A. & RUSEN, D. A., 1994, Invading the invaders: infestation of Zebra mussels by native parasites in the St. Lawrence River. Proceedings of the Fourth International Zebra mussel Conference, Madison, Wisconsin, pp. 15-22.
- CONN, D. B., RICCIARDI, A., BABAPULLE, M. N., KLEIN, K. A. & ROSEN, D. A., 1995, Chaetogaster limnaei (Annelida: Oligochaeta) as a parasite of the zebra mussel Dreissena polymorpha, and the quagga mussel Dreissena bugensis (Mollusca: Bivalvia). Parasitol Res, 82(1): 1-7.
- DE MARCO, P. J., 1999, Invasion by the introduced aquatic snail Melanoides tuberculata (Muller, 1774) (Gastropoda: Prosobranchia: Thiaridae) of the Rio Doce State Park, Minas Gerais, Brazil. Stud Neotrop Fauna & Environm.,34: 186-189.
- Favre, T. C., Pieri, O. S., Zani, L. C., Ferreira, J. M., Domás, G. G., Beck, L. & Barbosa, C. S., 2002, A Longitudinal Study on the Natural Infection of Biomphalaria straminea and B. glabrata by Schistosoma mansoni in an Endemic Area of Schistosomiasis in Pernambuco, Brazil. Mem Inst Oswaldo Cruz, 97(4): 465-475.
- FREITAS, J. R., SANTOS, M. B. L., ROCHA, L. A. & BEDÊ, L. C., 1994, Competitive interactions among mollusks in urban reservoirs, ponds, and lakes. In: R. M. Pinto-Coelho, A. Giani & E. von Sperling (eds.), Ecology and human impact on lakes and reservoirs in Minas Gerais with special reference to future development and management strategies. pp. 165-188.
- FUNDAÇÃO JOÃO PINHEIRO, 2001, Zoneamento urbano-ambiental para o entorno da REGAP e da UTE-ibiritermo. Belo Horizonte, 68p.
- GALE, W. F., 1973, Predation and parasitism as factors affecting Sphaerium transversum (Say) populations in Pool 19, Mississippi River. Research in Population Ecology,14: 169-187.
- GIOVANELLI, A., VIEIRA, M. V. & DA SILVA, C. L. P. A. C., 2002, Interaction between the intermediate host of schistosomiasis in Brazil Biomphalaria glabrata (Planorbidae) and a possible competitor Melanoides tuberculata (Thiaridae): I. Laboratory experiments. Mem Inst Oswaldo Cruz, Rio de Janeiro, 97(3): 363-369.
- GRACZYK, T. K. & FRIED, B., 1999, Development of Fasciola hepatica in the Intermediate Host. In: J. P. Dalton (ed.), Fasciolosis School of Biotechnology, Dublin City University, Republic of Ireland, pp. 31-46.
- GUIMARÃES, C. T., SOUZA, C. P. & SOARES, D. M., 2001, Possible competitive displacement of planorbids by Melanoides tuberculata in Minas Gerais, Brazil. Memórias do Instituto Oswaldo Cruz, 96: 173-176.
- MICHELSON, E. H., 1964, The protective action of Chaetogaster limnaei on snails exposed to Schistosoma mansoni The Journal of Parasitology,50(3): 441-444.
- POINTIER, J. P., 1993, The introduction of Melanoides tuberculata (Mollusca: Thiaridae) to the island of Saint Lucia (West Indies) and its role in the decline of Biomphalaria glabrata, the snail intermediate host of Schistosoma mansoni Acta Tropica, 54: 13-18.
- POINTIER, J. P., 2001, El control biológico de los moluscos vectores intermediarios de los esquistosomas: el ejemplo de la región del Caribe. Vitae: Academia Biomédica Digital Ed.: CAIBCO-Centro de Análise de Imagens Biomédicas Computadorizadas. Site: http://caibco.ucv.ve/Vitae/Vitaeocho/Articulos/ MedicinaTropical/ArchivosHTML/introduc.htm Junho/Agosto, número 8.
- POINTIER, J. P., TOFFART, J. L. & LEFÈVRE, M., 1991, Life tables of freshwater snails of the genus Biomphalaria (B. glabrata, B. alexandrina, B. straminea) and of one of its competitors Melanoides tuberculata under laboratory conditions. Malacologia, 33(1-2): 43-54.
- POINTIER, J. P., INCANI, R. N., BALZAN, C., CHROSCIECHOWSKI, P. & PRYPEHAN, S., 1994, Invasion of the rivers of the littoral central region of Venezuela by Thiara granifera and Melanoides tuberculata (Mollusca: Prosobranchia: Thiaridae) and the absence of Biomphalaria glabrata, snail host of Schistosoma mansoni The Nautilus, 107(4): 124-128.
- RUIZ, J. M., 1951, Nota sobre a cercariofagia de um oligochaeta do gênero Chaetogaster V. Baer 1827. Anais da Faculdade de Farmácia e Odontologia, USP, 9: 51-56.
- SOUZA, C. P. & LIMA, L. C., 1997, Moluscos de interesse parasitológico do Brasil Ed. Fiocruz, Rio de Janeiro, 79p.
- VON BAER, K. E., 1827, Beiträge zur Kenntnis der niedern Thiere. Nova Acta Caes. Acad. Leop. Car.,13: 523-762, 881-882.
- WAGIN, W. L., 1931, Chaetogaster limnaei K. Baer als Cercarienvertilger. Zool. Anz.,95: 55-59.
Correspondence to
Publication Dates
-
Publication in this collection
28 Nov 2005 -
Date of issue
May 2005
History
-
Accepted
30 Jan 2004 -
Received
14 Apr 2003