Abstracts
Adenosine is an important signaling molecule for many cellular events. Adenosine deaminase (ADA) is a key enzyme for the control of extra- and intra-cellular levels of adenosine. Activity of ADA was detected in hemolymph of B. glabrata and its optimum assay conditions were determined experimentally. The pH variation from 6.2 to 7.8 caused no significant change in ADA activity. Using adenosine as a substrate, the apparent Km at pH 6.8 was 734 µmols.L-1. Highest activity was found at 37ºC. Standard assay conditions were established as being 15 minutes of incubation time, 0.4 µL of pure hemolymph per assay, pH 6.8, and 37ºC. This enzyme showed activities of 834 ± 67 µmol.min-1.L-1 (25ºC) and 2029 ± 74 µmol.min-1.L-1 (37ºC), exceeding those in healthy human serum by 40 and 100 times, respectively. Higher incubation temperature caused a decrease in activity of 20% at 43ºC or 70% at 50ºC for 15 minutes. The ADA lost from 26 to 78% of its activity when hemolymph was pre-incubated at 50ºC for 2 or 15 minutes, respectively. Since the ADA from hemolymph presented high levels, it can be concluded that in healthy and fed animals, adenosine is maintained at low concentrations. In addition, the small variation in activity over the 6.2 to 7.8 range of pH suggests that adenosine is maintained at low levels in hemolymph even under adverse conditions, in which the pH is altered.
adenosine deaminase; Biomphalaria glabrata; hemolymph
A adenosina é uma molécula sinalizadora de muitos eventos celulares. A adenosina desaminase (ADA) é enzima chave para o controle dos níveis intra e extra celulares de adenosina. A atividade da ADA foi detectada em hemolinfa de B. glabrata e suas condições ótimas de ensaio foram determinadas experimentalmente. A variação do pH de 6,2 até 7,8 não causou mudança significativa na atividade. O Km aparente foi de 734 µmoles L-1, usando adenosina como substrato. A maior atividade foi encontrada usando 37ºC como temperatura de incubação. As condições de ensaio padrão foram então estabelecidas como sendo 15 minutos de tempo de incubação, 0,4 µL de hemolinfa por ensaio, pH 6.8 e 37ºC de temperatura de incubação. A enzima apresentou atividades de 834 ± 67 µmols.min-1.L-1 (25ºC) e 2029 ± 74 µmols.min-1.L-1 (37ºC), em torno de 40 e 100 vezes maiores que os níveis encontrados em soro de humanos sadios. Em temperaturas superiores, essa atividade cai 20% a 43ºC e 70% a 50ºC, em 15 minutos. A ADA perde 26 a 78% de sua atividade quando a hemolinfa é pré-incubada a 50ºC de 2 a 15 minutos, respectivamente. Considerando os altos níveis de ADA encontrados pode-se inferir que, em animais sadios e alimentados, a adenosina é mantida em baixas concentrações na hemolinfa. Tendo a atividade da enzima permanecido constante frente à larga faixa de pH testada, sugere-se que a ADA pode atuar com eficiência mesmo em situações adversas que determinem variações no pH da hemolinfa.
adenosina desaminase; Biomphalaria glabrata; hemolinfa
Characterization of adenosine deaminase (ADA) in Hemolymph of Biomphalaria glabrata
Caracterização da adenosina desaminase (ADA) em Hemolinfa de Biomphalaria glabrata
Vale, M. R.I; Pereira, R. V.I; Almeida, S. M.I; Almeida, Y. M.II; Nunes, S. F. L. C.I
ILaboratório de Bioquímica - Departamento de Fisiologia e Farmacologia, Faculdade de Medicina - Universidade Federal do Ceará, Rua Cel. Nunes de Melo, 1127, CEP 60.430- 270- Rodolfo Teófilo, Fortaleza Ceará Brazil
IILaboratório de Parasitologia Departamento de Patologia, Faculdade de Medicina - Universidade Federal do Ceará, Caixa Postal 3163, CEP 60.430-270 Fortaleza-Ceará-Brazil
Correspondence toCorrespondence to Marcus Raimundo Vale Universidade Federal do Ceará Rua Cel. Nunes de Melo, 1127 CEP 60430-270, Rodolfo Teófilo, Ceará, Brazil e-mail: mvale@ufc.br
ABSTRACT
Adenosine is an important signaling molecule for many cellular events. Adenosine deaminase (ADA) is a key enzyme for the control of extra- and intra-cellular levels of adenosine. Activity of ADA was detected in hemolymph of B. glabrata and its optimum assay conditions were determined experimentally. The pH variation from 6.2 to 7.8 caused no significant change in ADA activity. Using adenosine as a substrate, the apparent Km at pH 6.8 was 734 µmols.L-1. Highest activity was found at 37ºC. Standard assay conditions were established as being 15 minutes of incubation time, 0.4 µL of pure hemolymph per assay, pH 6.8, and 37ºC. This enzyme showed activities of 834 ± 67 µmol.min-1.L-1 (25ºC) and 2029 ± 74 µmol.min-1.L-1 (37ºC), exceeding those in healthy human serum by 40 and 100 times, respectively. Higher incubation temperature caused a decrease in activity of 20% at 43ºC or 70% at 50ºC for 15 minutes. The ADA lost from 26 to 78% of its activity when hemolymph was pre-incubated at 50ºC for 2 or 15 minutes, respectively. Since the ADA from hemolymph presented high levels, it can be concluded that in healthy and fed animals, adenosine is maintained at low concentrations. In addition, the small variation in activity over the 6.2 to 7.8 range of pH suggests that adenosine is maintained at low levels in hemolymph even under adverse conditions, in which the pH is altered.
Keywords: adenosine deaminase, Biomphalaria glabrata, hemolymph.
RESUMO
A adenosina é uma molécula sinalizadora de muitos eventos celulares. A adenosina desaminase (ADA) é enzima chave para o controle dos níveis intra e extra celulares de adenosina. A atividade da ADA foi detectada em hemolinfa de B. glabrata e suas condições ótimas de ensaio foram determinadas experimentalmente. A variação do pH de 6,2 até 7,8 não causou mudança significativa na atividade. O Km aparente foi de 734 µmoles L-1, usando adenosina como substrato. A maior atividade foi encontrada usando 37ºC como temperatura de incubação. As condições de ensaio padrão foram então estabelecidas como sendo 15 minutos de tempo de incubação, 0,4 µL de hemolinfa por ensaio, pH 6.8 e 37ºC de temperatura de incubação. A enzima apresentou atividades de 834 ± 67 µmols.min-1.L-1 (25ºC) e 2029 ± 74 µmols.min-1.L-1 (37ºC), em torno de 40 e 100 vezes maiores que os níveis encontrados em soro de humanos sadios. Em temperaturas superiores, essa atividade cai 20% a 43ºC e 70% a 50ºC, em 15 minutos. A ADA perde 26 a 78% de sua atividade quando a hemolinfa é pré-incubada a 50ºC de 2 a 15 minutos, respectivamente. Considerando os altos níveis de ADA encontrados pode-se inferir que, em animais sadios e alimentados, a adenosina é mantida em baixas concentrações na hemolinfa. Tendo a atividade da enzima permanecido constante frente à larga faixa de pH testada, sugere-se que a ADA pode atuar com eficiência mesmo em situações adversas que determinem variações no pH da hemolinfa.
Palavras-chave: adenosina desaminase, Biomphalaria glabrata, hemolinfa.
INTRODUCTION
The nucleoside adenosine is both a metabolic precursor of nucleic acids and an important signaling molecule involved in regulating various physiological processes, mainly due to its inhibitory action on the immune system (Cohen et al., 2002; Okusa et al., 2000; Bouma et al., 1996; Firestein et al., 1995). Adenosine deaminase (ADA) participates in purine metabolism where it decomposes either adenosine or 2-deoxy-adenosine, producing inosine or 2-deoxy-inosine, respectively. The physiological roles of ADA are directly related to control of adenosine concentrations in intra- and extra-cellular space. More recently, ADA was found to be implicated in an extra-enzymatic activity (ecto-ADA) by binding to CD26 in the surface of lymphocytes, apparently functioning as an extra-cellular signaling molecule (Franco et al., 1998). This enzyme has become an object of great interest also because ADA deficiency causes severe combined immunodeficiency disease (SCID) (Hirschhorn, 1995); in addition, it is an important marker for tuberculosis (Burgess et al., 2001; Valdes et al., 1995; Valdes et al., 1996; Chalhoub et al., 1996).
This enzyme has been studied in a variety of animals like rats (Blackburn et al., 2000), mice (Singh & Sharma, 1998), dogs (Altug and Agaoglu, 2000), mosquitoes (Ribeiro et al., 2001), flies (Charlab et al., 2000), and marine mollusks (Chen et al., 2000). Recently, our laboratory has characterized ADA iso-forms from caprine tissues (Rodrigues et al., 2000).
Since ADA plays significant roles in the immune systems of several animals, we have directed our study to this enzyme in infection models. One of these is infection of the mollusk Biomphalaria glabrata by Schistosoma mansoni, which can also infect man, and in some areas causes a significant public health problem (for a review, see Morgan et al., 2001).
Although a large number of papers have focused on B. glabrata, nothing has yet been published about ADA in its tissues. This work describes some characteristics of ADA activity in this mollusks hemolymph, and was undertaken as the first step in investigating the possible role of ADA during infection of B. glabrata by S. mansoni.
MATERIAL AND METHODS
Collection of B. glabrata hemolymph
Animals were obtained from the Biological Sciences Institute, Federal University of Minas Gerais, Brazil, and maintained at 25ºC in the Parasitology Laboratory of Faculty of Medicine, Federal University of Ceará, Brazil.
Fed animals with shell diameters of between 15 and 18mm were used. The hemolymph was obtained by perforating the shells with a collector constructed in our laboratory. This device consists of an eppendorf tube with a rubber stopper through which were two injection needles, one linked by tubing to another needle (to perforate the shell), which in turn is connected to a 10 mL syringe (to create negative pressure in the system).
After collection, the hemolymph was centrifuged at 10.000 x g (4ºC). The supernatant was diluted 50 times in cold phosphate buffer (50 mmol.L-1, pH 6.8) and used for ADA determination.
ADA assay
The ADA assay was based on the quantification of ammonium formed by deamination of adenosine catalysed by this enzyme (Giusti, 1974). Due to high levels of ADA activity in the hemolymph, the latter was diluted 50 times in assay buffer (see below) in order to maintain the volumes by the method. Thus, 20µL of diluted hemolymph corresponds to 0.4µL of pure hemolymph. The sample assay contained 200 µL of adenosine (2.2 mmol.L-1) and 20 µL of hemolymph (1:50, v:v), both diluted in phosphate buffer, 50 mmolL-1, pH 6.8 (assay buffer). This was incubated at 37ºC for 15 minutes. The reaction was interrupted by the addition of 600 µL of phenol/sodium nitroprussiate (106 / 0.17 mmol.L-1), followed by 600 µL of alcaline sodium hypochlorite (11 mmol.L-1 NaOCl in 125 mmol.L-1 NaOH), and incubated for 30 minutes at 37ºC. A blue color developed due to formation of indophenol blue; absorbance at 268 nm was proportional to the concentration of ammonium released from adenosine. In order to discount spontaneous, non-enzymatic adenosine deamination, a control tube containing only adenosine was run in parallel, but the diluted hemolymph (20 µL) was added after addition of phenol reagent. For quantification of ammonium, a standard tube containing a known concentration of ammonium sulphate (200 µL 75 µmol.L-1) and a blank tube containing only buffer to discount background ammonium in the buffer (200 µL), were submitted to the same conditions as above, including the addition of hemolymph (20 µL) after the phenol/nitroprussiate reagent.
The ADA thermostability was studied in two ways. First, the effect of assay temperature was analyzed by incubating the hemolymph (20 µL 1:50 v:v) in the presence of adenosine (2.2mmols.L-1) for 15 minutes from 25 to 50ºC. Second, the same effect was analyzed by pre-incubating the hemolymph alone (1:50, v:v) from 0 to 15 minutes at 50ºC, after which the ADA activity was measured.
The apparent Km value of ADA in hemolymph was determined based on the curve of the substrate calculated by a Lineweaver-Burk plot. The assay tubes containing 200 µL of adenosine in assay buffer (concentrations from 0 to 1 mmol.L-1) and 20 µL of hemolymph (1:50, v:v, in assay buffer) were incubated at 37ºC for 15 minutes. The quantification of ammonium was performed as described above.
The pH variation effect on ADA activity was tested under standard conditions, i.e., adenosine (200 µL 2.2 mmol.L-1) and hemolymph (20 µL 1:50, v:v), both diluted in an assay buffer having different pH (from 6 to 8), at 15 minutes of incubation time.
RESULTS
Standard conditions of hemolymph ADA were defined by studying ADA activity dependence on: time of incubation, pH, enzyme concentration, and substrate concentration. Figure 1A shows enzyme activity dependence on incubation time (0 60 minutes). Fifteen minutes was adopted as the standard incubation time throughout the work because it relates to a linear region of the curve and presents reliable absorbance values.
The effect of pH on ADA is presented in Figure 1B. As can be seen, there were no statistically significant differences between ADA measured in the pH range of 6.2 to 7.8 (Tukey test). However, pH 6.8 was adopted as the standard condition because it approximates ADA optimum pH in other animals and presents reliable absorbance values (Valdes et al., 1996; Rodrigues et al., 2000; Altug et al., 2000).
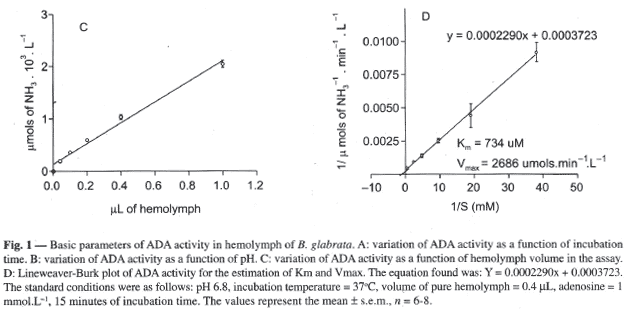
The gradual increase of hemolymph volume in the assay caused a linear increase in ADA activity, as demonstrated in Figure 1C. The standard volume of 20 µL (1:50, equivalent to 0.4 µL pure hemolymph in the figure; see methods) was chosen because it lays in the linear region of the curve, affords reliable spectrophotometer readings, and allows enough room for growing volume of substrate in determining the apparent Km (see below).
The Km and maximum velocity of ADA were estimated using a Lineweaver-Burk plot by the variation of substrate concentration from 0 to 2.1mmoles.L-1, as shown in Figure 1D. The curve equation (y=0.0002042.x + 0.0003723) gave an apparent Km of 734 µmol.L-1 and Vmax of 2686 µmol NH3.min-1.L-1 (s.e.m., n=6).
The increase of incubation temperature from 25 to 37ºC caused an increase in enzyme activity of from 834 + 67 to 2029 + 74 µmol.min-1.L-1 (s.e.m., n=8). However, further increase produced activity decrease (Figure 2A). Enzyme activity decay was observed when the incubation temperature was 43ºC or 50ºC (about 20% or 70%, respectively). The adopted incubation temperature was 37ºC because it resulted in the highest activity.
The pre-incubation of hemolymph alone at 50ºC for up to 15 minutes caused enzyme activity decay. Figure 2B shows that within 2 minutes of pre-incubation at 50ºC, the ADA lost about 26% of its activity (from 1733 + 123 µmol.min-1.L-1 - s.e.m., n=8, at t=0 to 1278 + 119 µmol.min-1.L-1, -s.e.m., n=8, at 2 min) and 78% when it was pre-incubated for 15 minutes (375 + 66 µmol.min-1.L-1 - s.e.m., n=8).
DISCUSSION
Under the conditions in which this work was done, the highest activity for hemolymph ADA in B. glabrata was observed at 37°C. However, the ambient temperature of the laboratory where the animals were kept was around 25°C, which should be the working temperature of the enzyme in the animal. Using 25ºC as the assay temperature, the ADA of the hemolymph presented activity about 40 times higher than ADA in human serum, which is 1020 µmols.min-1.L-1 (Giusti, 1974), and other animal sera assayed at 37°C (Sathar et al., 1999, Rodrigues et al., 2000). This could mean that in B. glabrata adenosine concentrations in hemolymph are maintained at very low levels.
Even though little or nothing is known about the role of adenosine in B. glabrata, by taking into account the role of this nucleoside in man and most animals, one may suggest that even a small increase of adenosine level can trigger important events in this animal. Thus, this level has to be strongly controlled. In addition, the large working pH range of ADA in hemolymph could also be interpreted as a factor involved in effectively controlling adenosine levels, even under extreme metabolic conditions.
It should be added that preliminary results of our laboratory work have demonstrated the existence of ADA iso-forms in the hepatopancreas of B. glabrata (Nunes et al., 2002).
The data obtained in this work represents a contribution to the study of ADA characteristics in B. glabrata, since it has determined some basic parameters of enzyme kinetics. These include incubation time, pH variation effects, apparent Km and Vmax, besides its thermostability.
The above data has encouraged us to go on with this investigation, and to continue the search for ADA iso-forms in other tissues of the mollusk.
Aknowledgments The second author thanks the Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP) for a scholarship. We are indebted to Dr. Vietla S. Rao for reviewing the manuscript.
Received June 23, 2003 - Accepted October 24, 2003 - Distributed May 31, 2005
- ALTUG, N., & AGAOGLU, T., 2000, Serum adenosine deaminase in dogs: its importance in experimental liver intoxication. Isr. Vet. Med., 55:130-134.
- BLACKBURN, M. R., VOLMER, J. B., THRASHER, J.L., ZHONG, H., CROSBY, J. R., LEE, J. J. & KELLEMS, R. E., 2000, Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J.Exp. Med., 192:159-170.
- BOUMA, M. G., VAN DEN WILDENBERG, F. A. & BUURMAN, W. A., 1996, Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am. J. Physiol. Cell. Physiol., 270: C522-C529.
- BURGESS L.J., SWANEPOEL C.G., & TALJAARD J.J., 2001, The use of adenosine deaminase as a diagnostic tool for peritoneal tuberculosis. Tuberculosis (Edinb), 81(3):243-248.
- CHALHOUB, M., CRUZ, A.A., MARCILIO, C. & NETTO, M.B., 1996, Value of determining the activity of adenosine deaminase (ADA) in the differential diagnosis of pleural effusions. Rev. Assoc. Med. Bras, 42: 139-146.
- CHARLAB, R., ROWTON, E.D. & RIBEIRO, J.M., 2000, The salivary adenosine desaminase from the sand fly Lutzomyia longipalpis Exp. Parasitol., 95:45-53.
- CHEN, X. Y., UCHIDA, H., MIGITAKA, Y., HAYASHI, T. & UWAJIMA, T., 2000, Isolation and characterization of adenosine deaminase in the adduct or muscle of marine bivalve molluscs. Fish. Sci., 66:748-754.
- COHEN, E. S. LAW, W. R. EASINGTON, C. R. CRUZ, K. Q. NARDULLI, B. A. BALK, R. A. PARRILLO, J. E. & HOLLENBERG, S. M.,2002,Adenosine Deaminase Inhibition Attenuates Microvascular Dysfunction and Improves Survival in Sepsis. Am. J. Respir. Crit. Care Med, 166: 16 20.
- FIRESTEIN, G.S., BULLOUGH, D.A., ERION, M.D., JIMENEZ, R., RAMIREZ-WEINHOUSE, M., BARANKIEWICK, J., SMITH, C.W., GRUBER, H.E. & MULLANE, K.M., 1995, Inhibition of neutrophil adhesion by adenosine and an adenosine kinase inhibitor. J. Immunol., 154:326-334.
- FRANCO, R., VALENZUELA, A., LLUIS, C. & BLANCO, J., 1998, Enzymatic and extraenzymatic role of ecto-adenosine deaminase in lymphocytes. Immunol. Rev, 161:27-42.
- GIUSTI, G., 1974, Adenosine deaminase. pp. 1092-1099. In: Bergmeyer, Hans Ulrich (ed.), Methods of Enzymatic Analysis, Academic Press, New York.
- HIRSCHHORN, R., 1995, Adenosine deaminase deficiency: molecular basis and recent developments. Clin. Immunol, 76: S219-S227.
- MORGAN J.A., DEJONG R.J., SNYDER S.D., MKOJI G.M. & LOKER E.S., 2001, Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitol. 123 Suppl:S211-28
- NUNES S.F.L.C., PEREIRA R.V., VASCONCELOS M.P., ALMEIDA Y.M. & VALE, M.R. (2002), Isoformas de adenosina desaminase (ADA) em Biomphalaria glabrata. Anais da Reunião Anual da Federação das Sociedades de Biologia Experimental (FeSBE), Resumo 19.016
- OKUSA, M. D., LINDEN, J., HUANG, L., RIEGER, J. M., MACDONALD, T. L. & HUYNH, L. P., 2000, A2A adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion Am. J. Physiol. - Renal Physiol, 279(5): F809 - 818.
- RIBEIRO, J.M.C., CHARLAB, R. & VALENZUELA, J.G., 2001, The salivary adenosine desaminase activity of the mosquitoes Culex quinquefasciatus and Aedes aegypti. J. Exp. Biol., 204:2001-2010.
- RODRIGUES, L.F.S., FREIRE, G.H. & VALE, M.R., 2000, Multiple iso-forms of caprine adenosine desaminase. Isr. Vet. Med., 55:135-138.
- SATHAR, M.A., UNGERER, J. P., LOCKHAT, F., SIMJEE, A.E. & GOUWS, E., 1999, Elevated adenosine deaminase activity in patients with HIV and tuberculous peritonitis. Eur. J. Gastroenterol. Hepatol., 11:337-342.
- SINGH, L. S. & SHARMA, R., 1998, Alloxan diabetes regulates adenosine deaminase activity in mice: tissue- and age-specific correlation. Biochem. Mol. Biol. Int., 46:55-61.
- VALDES, L., ALVAREZ, D., SAN JOSE, E., JUANATEY, J.R., POSE, A., VALLE, J.M., SALGUEIRO, M & SUAREZ, J.R., 1995, Value of adenosine deaminase in the diagnosis of tuberculous pleural effusions in young patients in a region of high prevalence of tuberculosis. Thorax., 50: 600-603.
- VALDES, L., SAN JOSE, E., ALVAREZ, D. & VALLE, J.M., 1996, Adenosine deaminase (ADA) isoenzyme analysis in pleural effusions: diagnostic role and relevance to the origin of increased ADA in tuberculous pleurisy. Eur. Resp. J., 9: 747-751.
Correspondence to
Publication Dates
-
Publication in this collection
28 Nov 2005 -
Date of issue
May 2005
History
-
Accepted
24 Oct 2003 -
Received
23 June 2003