Abstracts
The aim of this work was to study biological aspects and the life cycle of Hylesia Metapyrrha in a laboratory. Laboratorial breeding was made at 25 ± 1 °C, 70 ± 10% UR and 14 hours of photophase, feeding the larvae with guava leaves (Psidium guayava L. - Myrtaceae). Time was evaluated on the days of all the development stages; morphometry was evaluated in millimeters and the pupa’s mass in grams. The eggs were disposed in groups and covered by urticating abdominal hair. The incubation period lasted 52 days. The larvae, with gregarious habits, presented background black coloration, yellowish scoli and two orange longitudinal lines above and below the spiracles, during the development which lasted an average period of 74.59 days and went through seven instars. The pre-pupa and the pupa stages lasted on average 8.82 and 50.56 days, respectively; the female pupae presented a duration, weight and size which was significantly bigger. The adult stage lasted on average 5.50 days with periods of pre, post and oviposition of 2.30, 1.90 and 1.00 days, respectively. This study broadens the knowledge of the immature stages, biological, morphological and behavioral aspects, until then restricted to the morphology and to registers of the occurrence of the adult forms.
Biology; dermatitis; entomology; erucism; lepidopterism
O presente estudo objetivou estudar aspectos biológicos e o ciclo de vida de Hylesia metapyrrha em laboratório. Para tanto, foi realizada uma criação laboratorial a 25 ± 1 °C, 70 ± 20% UR e 14 horas de fotofase, alimentando-se as lagartas com folhas de goiabeira (Psidium guajava L - Myrtaceae). Para todas as fases de desenvolvimento, foram avaliados o tempo em dias, a morfometria em milímetros e, para as pupas, a massa, em gramas. Também foram feitas observações sobre características morfológicas e etológicas. Os ovos, de formato subcilíndrico, são dispostos em grupos e recobertos por cerdas abdominais urticantes, o período de incubação foi de 52,00 dias. As lagartas, de hábito gregário, apresentam coloração de fundo negra, escolos amarelados e duas linhas longitudinais laranja dispostas acima e abaixo dos espiráculos, durante o desenvolvimento que teve um período médio de 74,59 dias, passaram por sete ínstares. As fases de pré-pupa e pupa duraram em média 8,82 e 50,56 dias, respectivamente, sendo que as pupas do sexo feminino apresentaram duração, peso e tamanho significativamente maiores que as dos machos. A fase adulta durou em média 5,50 dias, com períodos de pré, pós e oviposição de 2,30, 1,90 e 1,00 dias, respectivamente. Este estudo amplia os conhecimentos sobre as fases imaturas, aspectos biológicos, morfológicos e comportamentais até então restritos apenas à morfologia e registros da ocorrência das formas adultas.
Biologia; dermatite; entomologia; erucismo; lepidopterismo
BIOLOGY
Biological aspects of Hylesia metapyrrha (Lepidoptera; Saturniidae; Hemileucinae), in laboratory
Aspectos biológicos de Hylesia metapyrrha Walker (Lepidoptera; Saturniidae; Hemileucinae), em laboratório
Specht, A.I, II, * * e-mail: aspecht@ucs.br ; Formentini, AC.I; Corseuil, E.III
ILaboratório de Biologia, Departamento de Ciências Exatas e da Natureza, Campus Universitário da Região dos Vinhedos, Universidade de Caxias do Sul, Alameda João Dal Sasso, 800, CEP 95700-000, Bento Gonçalves, RS, Brazil
IIInstituto de Biotecnologia, Universidade de Caxias do Sul, Cidade Universitária, CEP 95070-560, Caxias do Sul, RS, Brazil
IIILaboratório de Entomologia, Faculdade de Biociências, Pontifícia Universidade Católica do Rio Grande do Sul, CP 1429, CEP 90619-900, Porto Alegre, RS, Brazil
ABSTRACT
The aim of this work was to study biological aspects and the life cycle of Hylesia Metapyrrha in a laboratory. Laboratorial breeding was made at 25 ± 1 °C, 70 ± 10% UR and 14 hours of photophase, feeding the larvae with guava leaves (Psidium guayava L. Myrtaceae). Time was evaluated on the days of all the development stages; morphometry was evaluated in millimeters and the pupas mass in grams. The eggs were disposed in groups and covered by urticating abdominal hair. The incubation period lasted 52 days. The larvae, with gregarious habits, presented background black coloration, yellowish scoli and two orange longitudinal lines above and below the spiracles, during the development which lasted an average period of 74.59 days and went through seven instars. The pre-pupa and the pupa stages lasted on average 8.82 and 50.56 days, respectively; the female pupae presented a duration, weight and size which was significantly bigger. The adult stage lasted on average 5.50 days with periods of pre, post and oviposition of 2.30, 1.90 and 1.00 days, respectively. This study broadens the knowledge of the immature stages, biological, morphological and behavioral aspects, until then restricted to the morphology and to registers of the occurrence of the adult forms.
Keywords: Biology, dermatitis, entomology, erucism, lepidopterism.
RESUMO
O presente estudo objetivou estudar aspectos biológicos e o ciclo de vida de Hylesia metapyrrha em laboratório. Para tanto, foi realizada uma criação laboratorial a 25 ± 1 °C, 70 ± 20% UR e 14 horas de fotofase, alimentando-se as lagartas com folhas de goiabeira (Psidium guajava L - Myrtaceae). Para todas as fases de desenvolvimento, foram avaliados o tempo em dias, a morfometria em milímetros e, para as pupas, a massa, em gramas. Também foram feitas observações sobre características morfológicas e etológicas. Os ovos, de formato subcilíndrico, são dispostos em grupos e recobertos por cerdas abdominais urticantes, o período de incubação foi de 52,00 dias. As lagartas, de hábito gregário, apresentam coloração de fundo negra, escolos amarelados e duas linhas longitudinais laranja dispostas acima e abaixo dos espiráculos, durante o desenvolvimento que teve um período médio de 74,59 dias, passaram por sete ínstares. As fases de pré-pupa e pupa duraram em média 8,82 e 50,56 dias, respectivamente, sendo que as pupas do sexo feminino apresentaram duração, peso e tamanho significativamente maiores que as dos machos. A fase adulta durou em média 5,50 dias, com períodos de pré, pós e oviposição de 2,30, 1,90 e 1,00 dias, respectivamente. Este estudo amplia os conhecimentos sobre as fases imaturas, aspectos biológicos, morfológicos e comportamentais até então restritos apenas à morfologia e registros da ocorrência das formas adultas.
Palavras-chave: Biologia, dermatite, entomologia, erucismo, lepidopterismo.
1. Introduction
Some lepidoptera are important to human health due to the effects caused by the toxic substances that are produced and inoculated by their larvae as well as by the hair found on the abdomen of the adult females that cause dermatitis known as erucism and lepidopterism, respectively. In Brazil, the representatives of Saturniidae, Megalopygidae, Limacodidae and Arctiidae stand out (Moraes, 2003).
Among the Saturniidae, the Hemileucinae subfamily stands out as it has the greatest diversity of species of medical importance. The Hylesia Hübner (1820) genus presents 110 species, and is the second most diverse of the subfamily (Lemaire, 2002). It is well-known from a medical point of view as, besides the larvae having urticating action, the adult females have abdominal hair that detaches very easily, causing allergic reactions (Rotberg, 1971, Haddad and Cardoso, 2003).
Dermatitis caused by the representatives of this genus was originally described by Leger and Mouzels (1918) in French Guiana and was confirmed in Argentina by various studies by Dallas in 1926. It was described again by Boyé (1932) in Guiana and received the status of estival epidemics in Argentina by Jörg (1933). According to Hill et al. (1948) such dermatitis also had periodical notoriety due to many accidents which happened to crews on oil tankers that called at the fluvial port of Caripito and surroundings in Venezuela, when thousands of moths of Hylesia urticans Floch and Abonnenc, 1944, nowadays Hylesia metabus Cramer, 1775, (Lemaire, 2002) were attracted by the lights of the boats causing serious dermatitis. It was so serious that it caused union conflicts and was acknowledged as a labor disease. Similarly, Hylesia nigricans (Berg, 1982) in Argentina was treated as the cause of a labor disease due to the fact that the abdominal hair that covered the eggs during the egg laying period caused dermatitis to the people that cleaned fructiferous plants (Casalá et al., 1967). In Brazil, dermatitis epidemic outbreaks caused by Hylesia paulex Dognin, 1922 were reported from 1989 to 1991 in São Paulo (Cardoso et al., 1990; Glasser et al., 1993).
As well as their great diversity, the species of this genus are very similar, with a marked sexual dimorphism that makes the specific identification difficult (Lemaire, 2002). This caused many reports of dermatitis outbreaks in Brazil to be notified without the due specific identification (Gusmão et al., 1961; Mascarenhas et al., 1980, Domingos et al., 1992; Glasser et al., 1993). As well as this identification difficulty, there is a significant gap concerning biology which is restricted to five species (Lemaire, 2002); in Brazil, the knowledge concerning Hylesia nanus (Walker, 1855) should be mentioned (Santos et al., 1988, 1996).
Hylesia metapyrrha (Walker, 1855), or Hylesia corevia Schaus, 1900, is endemic in the southeast and in the south of Brazil and in adjacent areas of Paraguay. In Brazil, there is a register of occurrences in the states of Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Santa Catarina (Lemaire, 2002) and Rio Grande do Sul (Biezanko, 1986; Corseuil et al., 2002).
Currently, what is known about H. metapyrrha is restricted to the geographical area, the morphology of adults, including the genitalia (Lemaire, 2002), and to the morphology of the urticating hair (Lamy and Lemaire, 1983). Due to the availability of specimens and considering the inexistence of information regarding the biology and their immature forms, this study was carried out.
2. Material and Methods
Breeding, which was done at the Laboratório de Biologia CARVI/UCS at 25 ± 1 °C, 70 ± 10% UR and 14 hours of photophase, started with two eggs laid by a female collected by the first author in Caxias do Sul, RS on March 10, 2004.
The egg laying was individually conditioned in Petry dishes lined with filter paper and dampened daily. In the egg stage, the time of incubation per egg laying and the morphology with the measurement of diameter and height were evaluated.
Due to the fact that the natural host plant of this species was unknown (Lemaire, 2002), leaves of plants from 23 families were offered to larvae, and they fed solely on guava leaves (Psidium guajava Linn. - Myrtaceae) which were offered daily throughout the whole larval development.
Due to a gregarious habit, six larvae groups were kept in 10 x 6 x 8 cm transparent glass containers until the pre-pupa stage. For growth evaluation, the measurements of the width of the cephalic capsules were used as a parameter. In order to achieve this, they were collected daily, during the replacement of food. The measurements were taken using a stereoscopic microscope with a micrometric ocular with an accuracy of a hundredth of a millimeter. To identify the instars, a frequency distribution was done and compared to the Dyar (1890) rule.
After they stopped feeding and dispersed, which characterizes the pre-pupa formation, they were put into glass recipients with a capacity of up to 500 mL until cocoons were formed and, later on, pupae or chrysalides.
The chrysalides were removed from their cocoons to identify the gender, compared to the Butt and Cantu (1962) schemes, for mass evaluation on a scale with accuracy of a hundredth of gram two days after transforming, and for measurements of greater length and width using a digital paquimeter with accuracy of a hundredth of millimeter. The chrysalides were kept individually in glass vials with a capacity of 500 mL, on filter paper, dampened daily, until the adults emerged to evaluate their duration.
When they emerged, the adults were put together in couples, conditioned in transparent cylindrical PVC containers with a diameter of 12 cm and a height of 18 cm; a Petry dish was placed on the base and the upper part was covered with white voile. The interior of these cages was lined with filter paper and a twig was placed inside them for oviposition.
It should be mentioned that specimens of each development stage have been added to the collection at the Laboratório de Biologia da UCS.
The existing specimens at the Museu Anchieta de Porto Alegre (MAPA) and at the Museu Entomológico Ceslau Biezanko at the Faculdade de Agronomia "Eliseu Maciel", Universidade Federal de Pelotas (MECB) were located and examined.
3. Results and Discussion
The eggs, in a subcylindrical format and with round ends, presented a light yellow coloration and not a very noticeable micropile at one of the ends, with diameter and height averages of 1.05 ± 0.01 mm and 1.55 ± 0.01 mm (n = 20), respectively.
The egg laying strongly adhered to the twigs used as a substrate, and each egg adhered to the other. It was difficult to part them without rupturing the chorion. The mass of eggs presented a subhemispherical format and was densely covered by intertwined urticating hair, deriving from the females abdomen, as described for the other genus representatives (Gardiner, 1982; Lemaire, 2002). Under the conditions studied, all of the egg laying (n = 10) presented an incubation period of 52 days which can be considered quite extensive for Lepidoptera; in relation to this, Lemaire (2002) reported that several species of this genus may spend the unfavorable periods, such as the dry or cold seasons, in diapause in the form of eggs and, according to Rodriguez et al. (2004), the fact that the egg laying is covered with abdominal hair hinders or makes predatory interventions more difficult, thus extending the period the eggs are in the environment.
Immediately after the eclosion, the larvae spent 24 hours feeding from the chorion of the eggs. They presented gregarious habits during their whole development, and remained sheltered during the first two instars in the lower portion of the leaves of the host plant, eating only at night, which is consistent with the habits of the genus representatives (Gardiner, 1982; Lemaire, 2002). After the third instar, the larvae built a pouch joining the host plant leaves with silk yarns, which they used as a communal day shelter, as described by H. nanus (Santos et al., 1988, 1996) until the end of this stage.
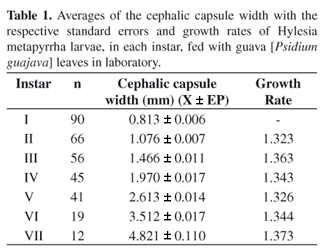
The larvae presented seven larval instars with an average growth rate of 1.345 (Table 1), very close to what is foreseen by Dyars (1890) rule.
Due to the gregarious habit, it was not possible to breed the larvae individually and the average duration of the larval period was 74.59 ± 0.88 days (n = 33), with a variation of 64 to 82 days.
The larvae morphology (Figure 1a) changed very little during their development; it is similar to the one described by the other species of the genus because they presented the pronotum scoli and the last uromere with twice the length of the others, but without the dilated end. They also differentiate from the other species of the genus due to the background coloration that is black and to the yellowish scoli, similar to the genus Leucanella Lemaire, 1969 larvae (Lemaire, 2002); in addition, there are two orange longitudinal stripes located above and below the spiracles.
There was 100% eclosion in the 167 eggs resulting from the two eggs having been laid, however, in the last instars, there was a great mortality rate, and only 33 pre-pupae were obtained, which represented a survival percentage of only 19.8%. This low survival rate may be related to several factors among which the insufficient suitability of the plant used as food is a factor that stands out. Regarding this factor, Lemaire (2002) reports that the few species whose biology is better known are polyphagians. However, the fact that the six groups fed themselves only from the guava plant, out of the representatives of 23 botanical families they had been offered, may indicate a certain preference. Another factor that has probably interfered with the development of this stage was their non adequacy to the laboratorial conditions for, as it is a species with a very long cycle, its development may be influenced by seasonal climatic variations, as observed from Tolype ventriosa Draudt, 1927 (Lepidoptera; Lasiocampidae) (Specht et al., 2004). With regards to environmental factors, Levesque et al. (2002), found evidence of the influence of temperature and luminosity on the larvae development of another lasiocampid, while Daly (1985) showed an influence of the breeding regimens on the growth rates and on morphometry in populations as well as among individuals from the same population.
When their development was complete, the larvae stopped eating, left the communal shelter and, individually, looked for locations for the cocoon formation (Figure 1b). This moment was considered the beginning of the pre-pupa stage (Figure 1c) that lasted until the next change, with an average duration of 8.82 ± 0.23 days (n = 33), up to the formation of the chrysalides inside the cocoon.
The pupa stage (Figure 1D) was characterized by the fact that the females lived significantly longer than the males, with 56.67 ± 2.14 days (n = 8) and 47.15 ± 1.91 (n = 8), respectively. Similarly, in relation to size, significant differences were observed with regards to the averages of greater length 25.25 ± 0.51 mm and 21.53 ± 0.31 mm; width 9.83 ± 0.19 mm and 8.86 ± 0.13 mm and mass 1.45 ± 0.09 g and 0.93 ± 0.06 g.
The adult stage lasted 5.50 ± 0.45 days (n = 16). The pre, post, and oviposition periods were, respectively, 2.30 ± 0.46; 1.90 ± 0.16 and 1.00 ± 0.00 days (n = 8). It has been observed that adults (Figure 1e, f), similarly to the other Hemileucinae representatives, do not eat so the larva needs to store all energy for the following stages (Lemaire, 2002). Notwithstanding the short longevity that was observed, the pre-oviposition time is proportionally more extensive due to the fact that it is meant to attract the opposite sex, copula and location of the host plant for the oviposition.
The adults size span was in accordance with the one described in Lemaire (2002), and females much bigger 65.19 ± 2.25 (n = 14) than males 47.49 ± 0.76 (n = 8) were observed.
Besides the female captured in March, which was the origin of this study, other material has only been found in collections, material that was found in Rio Grande do Sul, collected in April: Guarani das Missões, 05.IV.1933, C. Biezanko leg. 1 male (MECB); Pareci Novo, IV.1932, (without collector), 2 males (MAPA); Pareci Novo, 06.IV.1933, (without collector), 1 male (MAPA); Pareci Novo, 21.IV.1933, (without collector), 1 male (MAPA); Pelotas, 02.IV.1957, C. Biezanko leg. 1 male (MECB); Pelotas, 07.IV.1957, C. Biezanko leg. 1 male (MECB). Two Paraná specimens were also located in: Ponta Grossa, IV. 1955, F. Justus leg. 1 female (MECB) and Jaguariaiva, IV. 1955, F. Justus leg. 1 male (MECB).
Biezanko (1986) makes a reference to adults in the State only in April and Lemaire (2002) indicates an occurrence in the summer months and at the beginning of fall, from January to April, in other places where they were found.
The long life cycle at 25 °C of almost 200 days and the occurrence of adults, mainly at the end of the summer and beginning of fall, enabled us to estimate that in natural conditions and mainly in places with much lower temperatures, the biological cycle of this species may be similar to what has already been observed concerning H. nigricans which presents only one annual generation (Gardiner, 1982; Lemaire, 2002).
Acknowledgments We would like to thank FAPERGS for the scholarship for the second author (Proc. nº 02/508357) and financial support (Proc. nº 02/1739.6). We would also like to thank Eduardo José Ely e Silva (MECB) and Fernando Meyer (MAPA) for the support, who made the examination of the collections possible.
Received July 22, 2005
Accepted August 3, 2005
Distributed February 28, 2007
- BIEZANKO, CM., 1986. Adelocephalidae, Saturniidae, Mimallonidae, Lasiocampidae, Eupterotidae e Lymantriidae da Região Missioneira do Rio Grande do Sul. Rev. Centro de Ciências Rurais, vol. 16, no. 2, p. 89-112.
- BOYÉ, R., 1932. La papillonite guyanaise. Bull. Soc. Path. Exot., vol. 25, p. 1099-1100.
- BUTT, BA. and CANTU, E., 1962. Sex determination of lepidopterous pupae USDA, Washington, 7 pp. [ARS, 33-75].
- CARDOSO, JLC., BORGES FILHO, TS., CARNEIRO, ECG. and MORAES, RHP., 1990. Surto de dermatite por Hylesia paulex no litoral de São Paulo, Bertioga, verão de 1990. Mem. Inst. Butantan, vol. 52, suplemento, p. 82.
- CASALÁ, A., BIANCHI, C., NAVARRO, JVS., BIANCHI, O. and BALSA, R., 1967. Granulona de las manos por nidos de lepidópteros (Hylesia nigricans). Arch. Arg. Dermat., vol. 17, no. 4, p. 307-314.
- CORSEUIL, E., SPECHT, A. and LANG, C., 2002. Saturniídeos (Lepidoptera, Saturniidae) registrados para o Rio Grande do Sul, Brasil. I. Hemileucinae. Biociências, vol. 10, no. 2, p. 147-155.
- DALLAS, ED., 1926. Eritema producido por un lepidóptero. Rev. Soc. Entomol. Argent., vol. 1, no. 2, p. 63-64.
- DALY, HV., 1985. Insect morphometrics. Ann. Rev. Entomol., vol. 30, p. 415-438.
- DYAR, HG., 1890. The number of molts of lepidopterous larvae. Psyche, vol. 5, p. 420-422.
- DOMINGOS, MF., CARDOSO, JCC. and VALDERRAMA, RH., 1992. Hylesia sp. no litoral sul de São Paulo: relato do terceiro surto. Rev. Soc. Bras. Med. Trop., vol. 25, suplemento, p. 110.
- GARDINER, BOC. A Silkmoth Rearers Handbook 3th ed., Hanworth, The Amateur Entomological Society., 1982, 255p.
- GLASSER, CM., CARDOSO JLC., CARRÉRI-BRUNO, GC., DOMINGOS, MF., MORAES RHP. and CIARAVOL RMC., 1993. Surtos epidêmicos de dermatite causada por mariposas do gênero Hylesia (Lepidoptera: Hemileucidae) no Estado de São Paulo. Rev Saúde Pública, vol. 27, no. 3, p. 217-220.
- GUSMÃO, HH., FORATTINI, O. and ROTBERG, A., 1961. Dermatite provocada por lepidópteros do gênero Hylesia. Rev. Inst. Méd. Trop, vol. 3, no. 3, p. 114-120.
- HADDAD, V. and CARDOSO, JLC., 2003. Erucismo e Lepidopterismo. In CARDOSO, JLC., FRANÇA, FOS., WEN, FH., MÁLAQUE and V. HADDAD, CMS. (EDS.), Animais peçonhentos no Brasil biologia, clínica e terapêutica dos acidentes, Sarvier, São Paulo, SP.
- HILL, W., RUBENSTEIN, A. and KOVACS, J., 1948. Dermatitis resulting from contact with moths (genus Hylesia). Report of cases. J. A. M. A., vol. 138, no. 10, p. 737-742.
- JÖRG, ME., 1933. Nota previa sobre el princípio activo urticante de Hylesia nigricans (Lepidopt. Hemileucinae) y lãs dermitis provocadas por el mismo, p. 482-495. In: 8Ş Reunion de la Sociedad Argentina de Patologia Regional Del Norte, Jujuy. Anais. Buenos Aires. Universidade de Buenos Aires, 1063p.
- LAMY, M. and LEMAIRE, C., 1983. Contribution à la systématique des Hylesia: étude au microscope électronique à balayage des flechettes urticantes (Lep. Saturniidae). Bull. Soc. Entomol. France, vol. 88, no. 3/4, p. 176-192.
- LEGER, M. and MOUZELS, P., 1918. Dermatose prurigineuse determiné par des papillons saturnidés du genre Hylesia. Bull. Soc. Path. Exot, vol. 11, p. 104-116.
- LEMAIRE, C., 2002. The Saturniidae of America - Hemileucinae Goecke & Evers, Keltern, 3, v. 1388 p + 140 plates.
- LEVESQUE, KR., FORTIN, M. and MAUFFETTE, Y., 2002. Temperature and food quality effects on growth, consumption and post-ingestive utilization efficiencies of the forest tent caterpillar Malacosoma disstria (Lepidoptera: Lasiocampidae). Bull. Entomol. Res., vol. 92, no. 2, p. 127-136.
- MASCARENHAS, CS., VULCANO, MA. and PEREIRA, FS., 1980. Nova constatação de dermatite provocada por lepidópteros do gênero Hylesia Hübner. Lundiana, vol. 1, p. 143-148.
- MORAES, RHP. 2003. Lepidópteros de importância médica. In: JLC. Cardoso, FOS. França, FH. WEN, CMS. MÁLAQUE and V. HADDAD (eds.) Animais peçonhentos no Brasil biologia, clínica e terapêutica dos acidentes, Sarvier, São Paulo, SP.
- RODRIGUES, J., HERNANDÉZ, JV., FORNÉS, L., LUNDBERG, U., AROCHA-PIÑANGO, CL., and OSBORN, F., 2004. External morphology of abdominal setae from male and female Hylesia metabus adults (Lepidoptera: Saturniidae) and their function. Flo. Entomol, vol. 87, p. 30-36.
- ROTBERG, A., 1971. Lepidopterism in Brazil. In BÜCHERL, W. and BUCKLEY, EE. (Eds.) Venomous animals and their venoms. Vol. 3. Venomous invertebrates. New York, Academic Press, p. 157-168
- SANTOS, GP., ANJOS, N. and ZANUNCIO, JC., 1988. Biologia de Hylesia nanus (Walker, 1855) (Lepidoptera: Attacidae), desfolhadora da cutieira (Joannesia princeps: Euphorbiaceae). Rev. Ceres, vol. 35, no. 201, p. 479-485.
- SANTOS, GP., ZANUNCIO, TV., DIAS, OS. and ZANUNCIO, JC., 1996. Biologia de Hylesia nanus (Walker) (Lepidoptera: Attacidae). Ano. Soc. Entomol Brasil, vol. 25, no. 3, p. 479-482.
- SPECHT, A., FORMENTINI, AC. and CORSEUIL, E., 2004. Aspectos biológicos de Tolype ventriosa (Lepidoptera, Lasiocampidae) em laboratório. Biociências, vol. 12, no. 1, p. 37-42.
Publication Dates
-
Publication in this collection
07 May 2007 -
Date of issue
Feb 2007
History
-
Accepted
28 Feb 2007 -
Reviewed
03 Aug 2005 -
Received
22 July 2005