Abstracts
In this study the Q10 coefficients of heterotrophic activities were measured during aerobic decomposition of Utricularia breviscapa Wright ex Griseb from Óleo lagoon (21° 36' S and 49° 47' W), Luiz Antonio, SP. The bioassays were set up with fragments of U. breviscapa and incubated with lagoon water at distinct temperatures (15.3, 20.8, 25.7 and 30.3 °C). Periodically for 95 days, the concentrations of dissolved oxygen were determined in the bioassays. The results of the temporal variation of dissolved oxygen were fitted to a first-order kinetic model. The stoichiometric relations were calculated on the basis of these fittings. In general, the results allowed us to conclude: i) the oxygen/carbon stoichiometric relations (O/C) varied in function of temperature and time. The temporal variations of the O/C observed in the decomposition of U. breviscapa, suggest that, in the initial phases of the process, low organic carbon concentrations were enough to generate great demands of oxygen, ii) the oxygen consumption coefficients (k d) presented low variation in function of increasing temperature, iii) the increment of the temperature induced a higher consumption of oxygen (COmax) and iv) the simulations indicate that during summer, temperature activates the metabolism of decomposing microbiota.
Q10; stoichiometric coefficient; oxygen uptake; aquatic macrophyte; mathematical model
Neste estudo, foram discutidos os coeficientes Q10 das atividades heterotróficas durante a decomposição aeróbia de Utricularia breviscapa Wright ex Griseb da lagoa do Óleo (21° 36' S e 47° 49' O), Luiz Antônio, SP. Os bioensaios foram realizados com fragmentos de U. breviscapa e água da lagoa sendo incubados em diferentes temperaturas (15,3, 20,8, 25,7 e 30,3 °C). Periodicamente, por 95 dias, as concentrações de oxigênio dissolvido foram determinadas nos bioensaios. Os resultados da variação temporal de oxigênio dissolvido foram ajustados a um modelo cinético de primeira ordem. As relações estequiométricas foram calculadas com base nesses ajustes. De modo geral, os resultados permitiram concluir: i) as relações estequiométricas (O/C) variaram em função da temperatura e do tempo. As variações temporais dos coeficientes estequiométricos observadas na decomposição de U. breviscapa sugerem que, nas fases iniciais do processo, concentrações baixas de carbono orgânico são suficientes para gerar grandes demandas de oxigênio; ii) os coeficientes de consumo de oxigênio (k d) apresentaram baixa variação em função do incremento da temperatura; iii) o incremento da temperatura induziu um maior consumo de oxigênio (COmáx); e iv) as simulações indicaram que, durante o verão, a temperatura favoreceu o aumento do metabolismo da microbiota decompositora.
Q10; coeficientes estequiométricos; consumo de oxigênio; macrófita aquática; modelo matemático
ECOLOGY
Q10 of heterotrophic activity during aerobic decomposition of Utricularia breviscapa and its effect on carbon cycling in a tropical lagoon
Q10 da atividade heterotrófica durante a decomposição aeróbia de Utricularia breviscapa e seu efeito na ciclagem do carbono da lagoa do óleo
Cunha-Santino, MB.I, * * e-mail: cunha_santino@ufscar.br, irineu@ufscar.br ; Bianchini Júnior, I.II, * * e-mail: cunha_santino@ufscar.br, irineu@ufscar.br
IDepartamento de Hidrobiologia, Universidade Federal de São Carlos - UFSCar, Via Washington Luiz, Km 235, CP 676, CEP 13565-905, São Carlos, SP, Brazil
IIPrograma de Pós-Graduação em Ecologia e Recursos Naturais, Universidade Federal de São Carlos - UFSCar, Via Washington Luiz, Km 235, CP 676, CEP 13565-905, São Carlos, SP, Brazil
ABSTRACT
In this study the Q10 coefficients of heterotrophic activities were measured during aerobic decomposition of Utricularia breviscapa Wright ex Griseb from Óleo lagoon (21° 36' S and 49° 47' W), Luiz Antonio, SP. The bioassays were set up with fragments of U. breviscapa and incubated with lagoon water at distinct temperatures (15.3, 20.8, 25.7 and 30.3 °C). Periodically for 95 days, the concentrations of dissolved oxygen were determined in the bioassays. The results of the temporal variation of dissolved oxygen were fitted to a first-order kinetic model. The stoichiometric relations were calculated on the basis of these fittings. In general, the results allowed us to conclude: i) the oxygen/carbon stoichiometric relations (O/C) varied in function of temperature and time. The temporal variations of the O/C observed in the decomposition of U. breviscapa, suggest that, in the initial phases of the process, low organic carbon concentrations were enough to generate great demands of oxygen, ii) the oxygen consumption coefficients (kd) presented low variation in function of increasing temperature, iii) the increment of the temperature induced a higher consumption of oxygen (COmax) and iv) the simulations indicate that during summer, temperature activates the metabolism of decomposing microbiota.
Keywords: Q10, stoichiometric coefficient, oxygen uptake, aquatic macrophyte, mathematical model.
RESUMO
Neste estudo, foram discutidos os coeficientes Q10 das atividades heterotróficas durante a decomposição aeróbia de Utricularia breviscapa Wright ex Griseb da lagoa do Óleo (21° 36' S e 47° 49' O), Luiz Antônio, SP. Os bioensaios foram realizados com fragmentos de U. breviscapa e água da lagoa sendo incubados em diferentes temperaturas (15,3, 20,8, 25,7 e 30,3 °C). Periodicamente, por 95 dias, as concentrações de oxigênio dissolvido foram determinadas nos bioensaios. Os resultados da variação temporal de oxigênio dissolvido foram ajustados a um modelo cinético de primeira ordem. As relações estequiométricas foram calculadas com base nesses ajustes. De modo geral, os resultados permitiram concluir: i) as relações estequiométricas (O/C) variaram em função da temperatura e do tempo. As variações temporais dos coeficientes estequiométricos observadas na decomposição de U. breviscapa sugerem que, nas fases iniciais do processo, concentrações baixas de carbono orgânico são suficientes para gerar grandes demandas de oxigênio; ii) os coeficientes de consumo de oxigênio (kd) apresentaram baixa variação em função do incremento da temperatura; iii) o incremento da temperatura induziu um maior consumo de oxigênio (COmáx); e iv) as simulações indicaram que, durante o verão, a temperatura favoreceu o aumento do metabolismo da microbiota decompositora.
Palavras-chave: Q10, coeficientes estequiométricos, consumo de oxigênio, macrófita aquática, modelo matemático.
1. Introduction
Microbial metabolism is the sum of all biochemical reactions that are regulated by several extrinsic (pH, temperature, nutrient availability, osmotic pressure and redox potential) and intrinsic (co-enzymes and enzymes) conditioning factors. Temperature is one of the most important environmental variables that act directly on the metabolism of organisms. The metabolism of organisms reacts to temperature effect by an exponential rise (expressed by Q10), reaching a peak (at optimum temperature metabolism) and a rapid decline in heterotrophic capacity. The metabolic dependence of any organisms in relation to temperature (Q10) is determined by different types of enzymes that act on the respiration process. During respiration, the increase in the activation energy of enzymes can have a positive or negative effect (Strakraba et al., 1999). Besides the temperature, the differences among Q10 values can also express the several conditioning factors of decomposition interdependence: i) adaptability potential of microorganisms, ii) physiologic versatility (e.g. growth rate and enzyme production), iii) the microbial community diversity and iv) density and biomass of microorganisms. It is possible that this parameter is also influenced by the resource availability and by the specificity of the decomposing microbiota to a substrate (Cunha-Santino, 2003).
Regarding decomposition in aquatic environments, bacteria and fungi are the major organisms that mediate the degradation process (Dias et al., 2007). The decomposition rates depend on abiotic factors including nutrients availability (López et al., 1998), supply of electron acceptors (Kučinskien and Krev, 2006), temperature (Mendelssohn et al., 1999; Carvalho et al., 2005) and pH (López-Archilla et al., 2001). Other factors such as plant detritus availability, chemical composition and morphological structure (Gessner, 2000), nutritional C:N:P ratio of plants (Enriques et al., 1993), molecular mass (Boonchan et al., 2000), origin (Jonsson et al., 2001), detritus size (Bianchini Jr. and Cunha-Santino, 2006) also affect the decomposition rates.
Decomposition is processed by microorganisms through aerobic and anaerobic metabolism, the predominant type of catabolic process is driven by environmental conditions (e.g. redox potential and oxygen availability) that select organisms. Usually, anaerobic metabolism predominates in sediment and aerobic metabolism prevails in the water column since a positive budget of dissolved oxygen exists.
Considering the range of in situ variation of temperature due to seasonality of an oxbow lagoon located in a subtropical region, this study aimed at discussing the carbon cycling during decomposition of Utricularia breviscapa Wright ex Griseb in relation to Q10 of heterotrophic activity from decomposing microbiota.
2. Materials and Methods
2.1. Sampling site description and material collection
To assess the Q10 (or van't Hoff equation) of heterotrophic activity from aerobic decomposition, bioassays with Utricularia breviscapa Wright ex Griseb and water from Óleo Lagoon were accomplished. The Óleo Lagoon (21° 36' S and 47° 49' W) belongs to a set of oxbow lagoons in the floodplain of Mogi-Guaçu River, located within the Jataí Ecological Station (Luiz Antonio, São Paulo, Brazil). The Óleo Lagoon is a small and shallow oxbow lagoon presenting a seepage drainage (area: 19.470 m2, volume: 49.613 m3 and Zmean: 2.55 m), the dimensions of this environment are 710.0 m long and 60.0 m wide (Petracco, 2006). This lagoon is an oligo-mesotrophic system with N-nitrate concentration of 9.0 ± 4.7 μg.L-1, N-nitrite of 0.9 ± 0.1 μg.L-1 (Wisniewski et al., 2000), N-ammonium of 24.0 ± 8.8 μg.L-1 and P-phosphate of 62.4 ± 46.4 μg.L-1 (Pezzato, 2007). The water temperature varies annually from 18.3 ± 1.8 °C in July to 28.8 ± 1.8 °C in December (Cunha-Santino and Bianchini Jr., 2008).
To achieve the entire potential of U. breviscapa decomposition (soluble and non-soluble components of biomass), fresh living samples were collected from the littoral zone and washed with in situ water. In the laboratory, the plants were washed with tap water in order to remove adhered particles (periphyton, sediment and coarse material). Plant material was then dried at 50 °C, ground (0.2 cm < Φ < 1.32 cm) and homogenised. Prior to the assays settings, lagoon water samples were collected from the stand of U. breviscapa with a Van Dorn bottle. The water samples were filtered (Millipore; pore size: 0.45 μm).
2.2. Aerobic mineralisation experiment and mathematical model
The aerobic mineralisation of U. breviscapa was performed at four distinct temperatures (15.3 °C, 20.8 °C, 25.7 and 30.3 °C) in incubations (n = 2 for each temperature) prepared with fragments of U. breviscapa and filtered lagoon water samples (proportion: 200 mg.L-1 DW; Bianchini et al., 2008). To remove the background dissolved oxygen (DO) consumption, two control incubations (with water sample of lagoon) were also incubated for each temperature.
The incubations were oxygenated with filtered clean air for 1 hours until reaching saturation with DO and the initial DO concentrations of incubations were measured with a DOmeter (YSI model 58; Yellow Spring Instruments; precision: 0.03 mg.L-1). In sequence the incubations were closed and maintained under controlled temperature and in the darkness. During 95 days of experiment (n = 22 samplings), the DO concentrations were registered in the incubations. After the measurements, the incubations were covered again to avoid the processes of diffusion of atmospheric oxygen. In order to prevent anaerobiosis within incubations, the solutions where re-oxygenated when the DO concentrations were near 2.0 mg.L-1 until the oxygen reached saturation.
The carbon:oxygen (O/C) stoichiometric coefficients were estimated from the relations between the maximum consumption of oxygen (CO) observed from the accumulated DO fittings in this study and the amount of inorganic carbon generated from the parameterisation of the kinetic model during carbon aerobic mineralisation (dCM/dt) of U. breviscapa (Cunha-Santino and Bianchini Jr., 2008).
2.3. Kinetic model and statistical analysis
Considering that the consumption of oxygen is directly related to the oxidation of organic resource, and that this process is well-represented by first order kinetics models, the kinetic models proposed by Cunha-Santino et al. (2008) and Bianchini Jr. et al. (2008) were used. The DO concentrations from the mineralisation chambers were correct by subtraction of DO concentration from control chambers (with only Óleo Lagoon sample water). Kinetics fittings of CO were calculated using a non-linear regression with the iterative algorithm of Levenberg-Marquardt (Press et al., 1993). According to these procedures, the temporal changes in the CO were represented by Equation 1:
where: CO = consumption of oxygen concentrations accumulated value (mg.L-1); COmax = maximum oxygen consumption (mg.L-1), kd = deoxygenation coefficient (per day) and t = time (day).
The COmax were used to estimate Q10 values and the temperature effect (Equation 2) was described by Equation 3 (USEPA, 1985):
where: θ - temperature fitting coefficient (θ = Q10(0.1)).
The CO temporal variation were statically analysed using nonparametric analysis (Kruskal-Wallis) followed by Duncan's multiple comparison test in order to verify for significant differences among temperatures (p < 0.05).
3. Results
From the kinetic point of view, despite the temperature variation, the CO variation presented a pattern of accentuated consumption at the beginning of the mineralisation processes (from 20th to 30th days of incubation: Figure 1). After this period, a gradual decrease in the oxidations was verified, tending towards stabilisation, in the final phases. The increasing temperature raised the values of COmax, this parameter varied from 101.9 ± 5.89 (15.3 °C) to 337.7 ± 22.25 mg.g-1 DW (30.3 °C; Table 1). Considering the four incubation temperatures, the mean value of COmax during mineralisation of U. breviscapa was 229.8 mg.g-1 DW. Regarding COmax values in the four temperatures, the Q10 from oxygen uptake from U. breviscapa decomposition was 1.89 (Figure 1).
As opposed to those checked for the parameter COmax, the deoxygenation coefficients (kd) tended to decrease in function of the rising temperature: 0.074 ± 0.0083 day-1 (15.3 °C), 0.062 ± 0.0034 per day (20.8 °C), 0.055 ± 0.0024 per day (25.7 °C) and 0.051 ± 0.0052 per day (30.3 °C). The respective half-time (t1/2) for the incubations temperature were 9.4; 11.2; 13.6 and 13.4 days. Considering the high determination coefficients (r2: 0.97 to 0.99) from kinetic fitting to the experimental results (Table 1), the model used was adequate to represent the kinetic of consumptions of oxygen of U. breviscapa degradation.
The stoichiometric relations represent the oxygen consumptions temporal variation in relation to carbon. The results indicated that O/C varied in function of temperature and time (Figure 2). However, despite temperature increment, it was possible to identify a pattern of common variation. It was verified, in the initial phase, the increment of the values of the stoichiometric coefficients; the maximum values of O/C had been observed on the 5th day: 9.5 (15.3 °C), 16.2 (20.8 °C), 16.0 (25.7 °C) and 20.2 (30.3 °C); after, the stoichiometric coefficients decreased continuously until the end of the experiment (180 days), tending to zero.
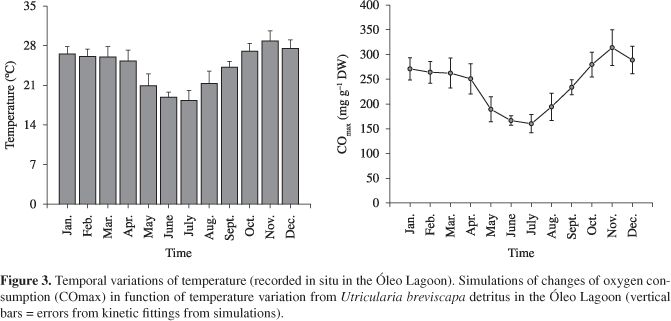
The annual variation of temperature of Óleo Lagoon showed a well defined seasonal pattern (Figure 3). The temperature ranged from 19.35 ± 1.26 °C in July (dry season) to 28.50 ± 1.71 °C in November (rainy season). The changes obtained by simulation of the aerobic mineralisation potential of carbon expressed by COmax (Figure 3) indicated higher value for deoxygenation in November (COmax = 313.9 mg.g-1 DW) and the lowest COmax occurred in July (160.3 mg.g-1 DW; Figure 3).
The statistical analysis indicated significant differences between the OC kinetics from incubations at 15.3 °C in relation to those at 25.7 °C (p < 0.01) and 30.3 °C (p < 0.001). No significant differences (p > 0.05) were observed among 20.8, 25.7 and 30.3 °C.
4. Discussion
Experiments that consider DO uptake have been frequently used to evaluate the heterotrophic metabolism in aquatic ecosystems (Berman et al., 2001; Strauss and Lamberti, 2002) and these assays are often accomplished in function of temperature variation (Panhota et al., 2006). In these studies the quantitative relations between the oxygen uptakes are implicit, and the CO2 concentration is approximately equivalent to the amount of consumed oxygen (Karl, 1986). The kinetic development of DO consumptions, independent of temperature incubation, were similar to that observed by Borsuk and Stow (2000). Considering that detritus of aquatic macrophytes are chemically heterogeneous, presenting a labile and refractory fractions (Cunha-Santino and Bianchini Jr., 2008) the oxidation of the labile fraction at the beginning of experiment prevails, generating high demands of dissolved oxygen. In aquatic environments, the oxidation of labile fraction takes place when senescence of macrophytes occurs; this process constantly liberates hydrosoluble substances into the water column. On the other hand, the reductions in the oxygen uptake were probably related to the mineralisation of the refractory fractions, i.e. cellulose and lignin (Esslemont et al., 2007) and also humic substances formation during decomposition of macrophytes (Rice, 1982).
The parameters kd from kinetic models of U.brevisapa aerobic mineralisation allowed the supposition that the oxygen consumptions are a short-term degradation process (t1/2 less than two weeks) and despite that the kd tended to decrease in function of temperature increasing, the parameter varied in the same order of magnitude.
During aerobic mineralisation of an algae, for the Staurastrum iversenii cells, a pattern of decrease in parameter kd in function of increasing temperature (Pacobahyba, 2002) was also observed. Overall, the OCmax and kd values varied in function of origin and phenological type of detritus (morphological structure, chemical composition), community of microorganisms, nutrient concentration and temperature (sensu Bianchini Jr., 2003). Although, when comparing the variation of kd and OCmax in function of temperature (Table 1), it is possible to infer that these parameters are independent and inversely proportional. The increase in temperature interferes in the total amount of CO but not in the velocity of this process.
In experiments under controlled conditions, the physiological state of decomposing microbiota is subject to variations, exhibiting two types of kinetic properties: intrinsic and extant (Grady et al., 1996). The intrinsic parameters depend on substrate nature and type of microorganisms, from chemical and physical conditions of incubations (Kovárová-Kovar and Egli, 1998). In opposition, the extant properties indicate the capabilities of microorganisms (cell history, intrinsic organism characteristic). The amplitude of temperature variation simulated during aerobic decomposition of U. breviscapa (from 15 to 30 °C) did not indicate an increase in mineralisation velocity (i.e. increase in metabolic rates) but act on the amount of oxygen consumed (i.e. oxygen yield for aerobic metabolism). Possibly, in this case, alternations within metabolic pathways occurred: i) the rise in temperature probably triggered the metabolic routes that consume more oxygen in metabolism, or ii) activate other processes that consume oxygen (e.g. nitrification), or iii) an increase in microbial density is favoured by increasing temperatures, and raised the number of microorganism populations increasing the oxygen uptake for the maintenance of aerobic metabolism.
During aerobic degradation, the oxidation of carbon by dissolved oxygen (acting as an oxidant agent) produces as end product stable inorganic compounds (like CO2 and water). In this case, it is assumed that all oxygen is used in the oxidation of organic carbon (Brezonik, 1994). Thus, the relation between consumed oxygen and oxidised carbon provides the stoichiometric coefficients. The stoichiometric relation (O/C) varied in function of time and temperature. However, independent of temperature variation, a pattern of temporal variation was observed, higher values of O/C were observed in the beginning of the experiment and with elapsed time the O/C relation tended to zero. Higher O/C ratio is attributed to labile fraction of detritus that usually correspond to dissolved organic matter formation (i.e. leachate). This fraction of detritus is composed of reactive substances that are rapidly incorporated into microbial metabolism and support biomass and catabolic processes (such as respiration). As aerobic degradation is processed, the refractory compounds dominate within detritus and the oxidations become a slow process. At this stage, a set of specific enzymes is necessary for the complete degradation of particulate debris.
Bioassays with litter, leaves, branches and barks showed the same pattern of O/C variation as verified in this study (Cunha-Santino and Bianchini Jr., 2002). The higher ratio of O/C were observed on the 3rd day of aerobic mineralisation (barks: 6.73; branches: 1.05; leaves: 2.45 and litter: 3.88). During decomposition of aquatic macrophytes under aerobic conditions the following O/C values were recorded: 5.03 (Montrichardia arborescens, maximum value: 17th day; Cunha-Santino et al., 2001), 11.88 (Eichhornia azurea, maximum value: 6th day; Cunha-Santino et al., 2003) and 11.05 (Salvinia auriculata, maximum value: 3rd day; Cunha-Santino and Bianchini Jr., 2001).
The variation of stoichiometric values were attributed mainly to: (i) chemical oxidations among the different organic compounds involved in the decomposition process; (ii) reactions mediated by enzymes; (iii) alterations within metabolic routes by different populations of microorganisms (e.g. Embden-Meyerhof-Parnas pathway and Entner-Doudoroff pathway); (iv) variation in number and species of microorganisms involved in decomposition; (v) variation on quality and quantity (labile and refractory fraction) from organic compounds available during degradation and (vi) amount of oxygen atom in detritus (Cunha-Santino, 2003).
The increase in stoichiometric relation in function of temperature is mainly attributed to an increase in metabolic activities of microbiota (e.g. positive effect on enzymes production) and favouring of chemical reactions by the rise in activation energy on the thermodynamic equilibrium. The Q10 is a coefficient that expresses the increase in metabolic activity by an increment in 10 °C.
Studies on leaf decomposition of Phragmites showed that oxygen consumption was significantly correlated with temperature (Q10 varied from 1.8 to 2.3; Andersen, 1978). Experiments of oxygen consumption in aquatic environments (USEPA, 1985) registered values of Q10 that varied from 1.22 to 4.05; in this compilation, it was suggested that Q10 of ca. 1.58 occurred in general, only at temperatures ranging between 20 and 30 °C, and that higher values were observed in processes developed at lower temperatures. The higher Q10 coefficient was observed at the lowest incubation temperature during mineralisation of U. breviscapa, the Q10 in the range 15.3 to 20.8 °C was 4.05; in the range of 20.8 to 25.7 °C, it was 1.41 and between 25.7 and 30.3 °C, it was 1.76. According to Kätterer et al. (1998), values of Q10 are near to 2 in the range of 5 to 35 °C. These values (ca. 2) are usually used in the simulations that deal with cycling in ecosystems. However, when considering one single substrate, the Q10 can be distinct from 2, therefore the effect of the optimum temperature acts differently on organisms in a community.
Studies that describe increments in metabolic rates in function of increasing temperatures are more common. For instance, Tartaglia (2001) found a Q10 of 2.80 during anaerobic mineralisation of refractory fractions of Eichhornia azurea. Antonio and Bianchini Jr. (2002) described the Q10 of glucose decay for aerobic process (1.12) and for anaerobic process (3.30). Kirchman and Rich (1997) found a Q10 of 2.4 for the consumption of glucose and mannose; according to the authors, the effect of the temperature in the carbon consumption presents important implications in the competition processes among microbial populations within the carbon cycle. Thus, the increases of the temperature regulate the concentrations of carbon through bacterial growth, by affecting the response time and the affinity of the bacteria for carbon. A revision of the metabolic dependence (e.g. growth, respiration) of some organisms in relation to the temperature (Strakraba, 1999) presented a range of Q10 variation from 1.17 to 7.79 (average = 2.31; n = 23); in this compilation the Q10 for decomposition mediated by bacteria in sediments was 1.71. Kätterer et al. (1998) suggest that the use of Q10, in decomposition experiments, is only applicable in the temperature range between 5 and 35 °C; temperatures ranging outside this interval deviate the Q10 significantly from 2; according to the authors, such variations of Q10 values (temporal and spatial) are possibly derived from optimum temperatures for metabolism of organisms involved in decomposition. Despite the great variation of response of the organisms in function of temperature, it is evident that each microbial community responds differently to the variations of this factor. Nicarlarot et al. (1994) found greater sensitivity to the effect of the temperature for holocellulose decomposing community (Q10 = 4.5) in detriment of glucose (Q10 = 2.9). Coûteaux et al. (2001) reported differences in the responses of microbial communities in relation to the temperature during decomposition of labile and refractory organic substance. Bunnell et al. (1977) studying litter decomposition, found different physiological responses of the community in relation to the substratum age; in this case, it was found that the litter of less than one year presented a Q10 of 8.79 and that of more than one year had a Q10 of 2.56; the authors attributed such a difference to the recalcitrant character of the oldest substrata.
Regarding the temporally aerobic mineralisation of U. breviscapa detritus in Óleo Lagoon and the experimental conditions adopted in this study (treatment of plant fragments and controlled conditions), the pressure on the DO in the water column is a short-term process since the half-time of labile fraction of detritus exerts a demand for higher concentration of oxygen for carbon oxidation during ca. two weeks after plant senescence. The pressure of the refractory fraction of detritus occurred basically on sediment once particulate detritus tended to sedimentation, and presented lower oxygen consumption in comparison to the labile fraction. In sediments, carbon degradation is processed by anaerobic routes considering that the redox profile of Óleo Lagoon sediment (Godinho, 2000) showed that the anaerobiosis was established in the surface layers (1 mm: -0.35 mV) and the redox potential decreased as depth increased (50 mm: -275 mV). In the warmer period, the temperature activates the metabolism of decomposing microbiota (i.e. Q10), increasing the OCmax over the water column of Óleo Lagoon; this fact is associated with low concentrations of DO in summer (Cunha-Santino and Bianchini Jr., 2008) and with low solubility of this gas in water due to the elevation of temperature.
Acknowledgements - The authors wish to thank the Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financing this work (Process n° 00/09297-6).
Received March 24, 2009
June 18, 2009
Distributed May 31, 2010 (With 3 figures)
- ANTONIO, RM. and BIANCHINI Jr., I., 2002. The effect of temperature on the glucose cycling and oxygen uptake rates in the Infernão lagoon water, state of São Paulo, Brazil. Acta Scientiarum, vol. 24, no. 2, p. 291-296.
- BERMAN, T., KAPLAN, B., CHAVA, S., VINER, Y., SHERR, BF. and SHERR, EB., 2001. Metabolically active bacteria in Lake Kinneret. Aquatic Microbial Ecology, vol. 23, no. 3, p. 213-224.
- BIANCHINI Jr., I., 2003. Modelos de crescimento e decomposição de macrófitas aquáticas. In THOMAZ, SM. and BINI, LM. (Eds.). Ecologia e manejo de macrófitas aquáticas Maringá: Eduem. p. 85-126.
- BIANCHINI Jr., I. and CUNHA-SANTINO, MB., 2006. The effect of the size of particles on mineralization of Oxycaryum cubense (Poepp. & Kunth) Lye. Revista Brasileira de Biologia = Brazilian Journal of Biology, vol. 66, no. 2B, p. 641-650.
- BIANCHINI Jr., I., CUNHA-SANTINO, MB. and PERET, AM., 2008. Oxygen demand during mineralization of aquatic macrophytes from an oxbow lagoon. Revista Brasileira de Biologia = Brazilian Journal of Biology, vol. 68, no. 1, p. 61-67.
- BOONCHAN, S., BRITZ, ML. and STANLEY, GA., 2000. Degradation and Mineralization of High-Molecular-Weight Polycyclic Aromatic Hydrocarbons by Defined Fungal-Bacterial Cocultures. Applied and Environmental Microbiology, vol. 66, no. 3, p. 1007-1019.
- BORSUK, ME. and STOW, CA., 2000. Bayesian parameter estimation in a mixed-order model of BOD decay. Water Research, vol. 34, no. 6, p. 1830-1836.
- BREZONIK, PL., 1994. Chemical kinetics and process dynamics in aquatic systems Boca Raton: Lewis. 754 p.
- BUNNELL, FL., TAIT, DEN., FLANAGAN, PW. and CLEVE, KV., 1977. Microbial respiration and substrate weight loss: I. A general model of the influence of abiotic variables. Soil Biology and Biochemistry, vol. 9, no. 1, p. 33-40.
- CARVALHO, P., THOMAZ, SM. and BINI, LM. 2005. Effects of temperature on decomposition of a potential nuisance species: the submerged aquatic macrophyte Egeria najas planchom (Hydrocharitaceae). Revista Brasileira de Biologia = Brazilian Journal of Biology, vol. 65, no. 1, p. 51-60.
- COÛTEAUX, MM., BOTTNER, P., ANDERSON, JM., BERG, B., BOLGE, T., CASALS, P., ROMANÝA, J., THIÉRY, J. and VALLEJO, RV., 2001. Decomposition of C-labelled standard plant material in a latitudinal transect of European coniferous forest: differential impact of climate on the decomposition of soil organic matter compartments. Biogeochemistry, vol. 54, no. 2, p. 147-170.
- CUNHA-SANTINO, MB., PACOBAHYBA, LD. and BIANCHINI Jr., I., 2001. Oxygen uptake from mineralization of Montrichardia arborescens In Anais do V Congresso de Ecologia do Brasil Porto Alegre: UFRG. (CD-ROM).
- CUNHA-SANTINO, MB. and BIANCHINI Jr., I., 2002. Estequiometria da decomposição aeróbia de galhos, cascas serapilheira e folhas. In ESPÍNDOLA, ELG., MAUAD, FF., SCHALCH, W., ROCHA, O., FELICIDADE, N. and RIETZLER, AC. (Eds.). Recursos hidroenergéticos: usos, impactos e planejamento integrado. São Carlos: Rima. p. 43-56.
- ______, 2003. Variação temporal da relação estequiométrica O/C na decomposição aeróbia de Eichhornia azurea (SW.) Kunth. In Resumos do II Workshop de Macrófitas Aquáticas Campo Grande: UFMS. p. 49.
- CUNHA-SANTINO, MB., 2003. Atividade enzimática, cinética e modelagem matemática da decomposição de Utricularia breviscapa da lagoa do Óleo (Estação Ecológica de Jataí, Luiz Antonio, SP). São Carlos: Universidade Federal de São Carlos. 140 p. [Tese de Doutorado]
- CUNHA-SANTINO, MB., GOUVÊA, SP., BIANCHINI Jr., I. and VIEIRA, AAH., 2008. Oxygen uptake during mineralization of photosynthesized carbon from phytoplankton of the Barra Bonita Reservoir: a mesocosm study. Revista Brasileira de Biologia = Brazilian Journal of Biology, vol. 68, no. 1, p. 123-130.
- CUNHA-SANTINO, MB. and BIANCHINI Jr., I., 2008. Carbon cycling potential from Utricularia breviscapa decomposition in a tropical oxbow lake (São Paulo, Brazil). Ecological Modelling, vol. 218, no. 3-4, p. 375-382.
- DIAS, M., ROYER, TV., LEFF, LG. 2007. Diversity of fungi, bacteria, and actinomycetes on leaves decomposing in a stream. Applied and Environmental Microbiology, vol. 73, no. 3, p.756-767.
- ENRIQUES, E., DUARTE, CM. and SAND-JENSEN, K., 1993. Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N:P content. Oecologia, vol. 94, no. 4, p. 457-471.
- ESSLEMONT, G., MAHER, W., FORD, P. and LAWRENCE, I., 2007. Riparian plant material inputs to the Murray River, Australia: composition, reactivity, and role of nutrients. Journal of Environmental Quality, vol. 36, no. 4, p. 963-974.
- GESSNER, MO., 2000. Breakdown and nutrient dynamics of submerged Phragmites shoots in the littoral zone of a hardwater lake. Aquatic Botany, vol. 66, no. 1, p. 9-20.
- GODINHO, MJL., 2000. Ecologia de microrganismos lacustres São Carlos: UFSCar. 52 p. (Relatório de aula prática)
- GRADY, CPL., SMETS Jr., BF. and BARBEAU, DS., 1996. Variability in kinetic parameter estimates: a review of possible causes and a proposed terminology. Water Research, vol. 30, no. 3, p. 742-748.
- KARL, DM., 1986. Determination of the in situ microbial biomass, viability, metabolism, and growth. In PONDEXTER, JS. and LEADBETTER, ER. (Eds.). Bacteria in nature: methods and special applications in bacterial ecology. New York: Plenum Press. p. 85-176.
- KÄTTERER, T., REICHSTEIN, M., ANDRÉN, O. and LOMANDER, A., 1998. Temperature dependence of organic matter decomposition: a critical review using literature data analyzed with different models. Biology Fertility Soils, vol. 27, no. 3, p. 258-262.
- KIRCHMAN, DL. and RICH, JH., 1997. Regulation of bacterial growth rates by dissolved organic carbon and temperature in the equatorial pacific ocean, Microbial Ecology, vol. 33, no. 1, p. 11-20.
- KOVÁROVÁ-KOVAR, K. and EGLI, T., 1998. Growth kinetics of suspended microbial cells: from single-substrate-controlled growth to mixed-substrate kinetics. Microbiology and Molecular Biology Reviews, vol. 62, no. 3, p. 646-665.
- KUINSKIEN, A. and KREV, A., 2006. Mineralization of organic matter in bottom sediments of the littoral zones of four Lithuanian lakes. Ekologija, vol. 1, p. 40-47.
- LÓPEZ, NI., DUARTE, CM., VALLESPINÓS, F., ROMERO, J. and ALCOVERRO, T., 1998. The effect of nutrients additions on bacterial activity in seagrass (Posidonia oceanica) sediments. Journal Experimental Marine Biology Ecology, vol. 224, no. 2, p. 155-166.
- LÓPEZ-ARCHILLA, AI., MARIN, I. and AMILS, R., 2001. Microbial community composition and ecology of an acidic aquatic environment: the Tinto River, Spain. Microbial Ecology, vol. 41, p. 20-35.
- MENDELSSOHN, IA., SORRELL, BK., BRIX, H., SCHIERUP, HH., LORENZEN, B. and MALTBY, E., 1999. Controls on soil cellulose decomposition along a salinity gradient in a Phragmites australis wetland in Denmark. Aquatic Botany, vol. 64, no. 3-4, p. 381-398.
- NICARLAROT, B., FAUVET, G. and CHENEBY, D., 1994. Carbon and nitrogen cycling through soil microbial biomass at various temperatures. Soil Biology and Biochemistry, vol. 26, no. 2, p. 253-261.
- PACOBAHYBA, LD., 2002. Decomposição de Staurastrum c.f. iversenii Nygaard var. americanum: efeitos da qualidade do recurso, da disponibilidade de oxigênio e da temperatura. São Carlos: Universidade Federal de São Carlos. 167 p. [Tese de Doutorado]
- PANHOTA, RS., CUNHA-SANTINO, MB. and BIANCHINI Jr., I., 2006. Consumos de oxigênio das mineralizações de lixiviados de Salvinia auriculata e de Utricularia breviscapa da lagoa do Óleo. In SANTOS, JE., PIRES, JSR. and MOSCHINI, LE. (Eds.). Estudos integrados em ecossistemas: Estação Ecológica de Jataí. São Carlos: EDUFSCar. p. 259-273.
- PEZZATO, MM., 2007. Macrófitas aquáticas submersas: fotossíntese, crescimento e variáveis abióticas da água. São Carlos: Universidade Federal de São Carlos. 118 p. [Tese de Doutorado]
- PETRACCO P., 2006. Efeito das variáveis abióticas na produção primária de Egeria najas e Utricularia breviscapa da lagoa do Óleo (Estação Ecológica de Jataí, Luiz Antônio, SP). São Carlos: Universidade Federal de São Carlos. 145 p. [Tese de Doutorado]
- PRESS, WH., TEUKOLSKY, SA., VETTERLING, WT. and FLANNERY, BP., 1993. Numerical recipes in C: the art of scientific computing. New York: Cambridge University Press. 994 p.
- RICE, DL., 1982. The detritus nitrogen problem: new observations and perspectives from organic geochemistry. Marine Ecology Progress Series, vol. 9, p. 153-162.
- STRAKRABA, M., JØRGENSEN, SE. and PATTEN, B., 1999. Ecosystem emerging: 2. Dissipation. Ecological Modelling, vol. 117, no. 1, p. 3-39.
- STRAUSS, EA. and LAMBERTI, GA., 2002. Effect on dissolved organic carbon quality on microbial decomposition and nitrification rates in stream sediments. Freshwater Biology, vol. 47, no. 1, p. 65-74.
- TARTAGLIA, D., 2001. O efeito da temperatura na degradação anaeróbia de Eichhornia azurea São Carlos: Universidade Federal de São Carlos. 40 p. [Monografia]
- United States Environmental Protection Agency - USEPA, 1985. Rates, constants and kinetics formulations in surface water quality modeling 2 ed. Athens: U.S. Government Printing Office.
- WISNIEWSKI, MJS., ROCHA, O., RIETZLER, AC. and RIETZLER, AC., 2000. Diversidade do zooplâncton nas lagoas marginais do rio Mogi-Guaçu: II Cladocera (Crustacea, Branchiopoda). In SANTOS, JE. and PIRES, JSR. (Eds.). Estudos integrados em ecossistemas: Estação Ecológica de Jataí. São Carlos: Rima. p. 559-586.
Publication Dates
-
Publication in this collection
14 June 2010 -
Date of issue
May 2010
History
-
Accepted
18 June 2009 -
Received
24 Mar 2009