Abstracts
The conservationist community is giving special attention to epigean insects due to their importance in the preservation of terrestrial habitats. This work analysed the diversity, richness, abundance and similarity at the soil surface of Coleoptera composition among five environments: native forest, native grassland, Pinus elliottii plantation, Eucalyptus saligna plantation and degraded area by soil use in southern Brazil, from October 2004 to October 2005. A total of 1,812 individuals were collected, attributed to 45 morph-species and 14 families. The higher richness and abundance were observed in native forest (31 species and 782 individuals) and the lower richness and abundance in degraded area (14 species, 86 individuals). Scarabaeidae was the richest family captured, with nine morph-species, and the most frequent family was Nitidulidae (1,113 individuals). According to the Shannon-Wiener index, the degraded area had smaller diversity in relation to the native forest, E. saligna and Pinus elliottii plantations. No difference in diversity between evaluated areas was found for the Simpson diversity index. The most dominant site was the degraded. The correlation between the total number of morph-species captured was not different to the degraded area and the P. elliottii monoculture (r = 0.47) and the correlation between the total individuals number was not significant between native forest and native grassland (r = 0.46) and between degraded areas and the other sites. According to the Jaccard Similarity Index, the greatest similarity for the organism composition occurred between P. elliottii plantation and E. saligna plantation, presenting 74% of overlap.
beetles; species richness; abundance; estimates
A comunidade conservacionista tem fornecido atenção especial aos insetos epigeicos devido à sua importância na preservação de habitats terrestres. Este trabalho avaliou a diversidade, riqueza, abundância e similaridade da composição dos Coleópteros da superfície dos solos em cinco ambientes: mata nativa, campo nativo, plantação Pinus elliottii e Eucalyptus saligna e área degradada pelo uso do solo no sul do Brasil, entre outubro de 2004 e outubro de 2005. Foram coletados 1.812 indivíduos, atribuídos a 45 morfoespécies e 14 famílias. Verificou-se maior riqueza e abundância na mata nativa (31 espécies, 782 indivíduos) e menor riqueza e abundância na área degradada (14 espécies, 86 indivíduos). A família com maior riqueza foi Scarabaeidae, com nove morfoespécies e a família mais abundante foi Nitidulidae (1.113 indivíduos). De acordo com o índice de diversidade de Shannon-Wiener, a área degradada apresentou menor diversidade em relação à floresta nativa e às plantações de E. saligna e Pinus elliottii. Nenhuma diferença na diversidade entre as áreas avaliadas foi encontrada para o índice de diversidade de Simpson. A mata nativa e o campo nativo apresentaram as maiores estimativas de riqueza. O ambiente de maior dominância foi a área degradada. A correlação entre o número total de morfoespécies capturadas na área degradada e na monocultura de P. elliottii não foi diferente (r = 0,47) e a correlação entre o número total de indivíduos entre a mata nativa e o campo nativo (r = 0,46) e entre a área degradada e os outros ecossistemas não diferiu. Conforme o índice de similaridade de Jaccard, as áreas mais semelhantes quanto à composição de coleópteros foram a plantação de P. elliottii e E. saligna, apresentando 74% de sobreposição.
besouros; riqueza de espécies; abundância; estimativas
ECOLOGY
Diversity of the families of Coleoptera captured with pitfall traps in five different environments in Santa Maria, RS, Brazil
Diversidade de Coleoptera em cinco diferentes ambientes no sul do Brasil
Fagundes, CK.I; Di Mare, RA.II,* * e-mail: ram13@terra.com.br ; Wink, C.III; Manfio, D.IV
IInstituto Nacional de Pesquisas da Amazônia INPA, Av. André Araújo, 2936, CEP 69060-001, Manaus, AM, Brazil
IILaboratório de Biologia Evolutiva, Universidade Federal de Santa Maria UFSM, Faixa de Camobi, Km 9, CEP 97105-900, Santa Maria, RS, Brazil
IIILaboratório de Física do Solo, Departamento de Solos, Universidade Federal de Santa Maria UFSM, Faixa de Camobi, Km 9, CEP 97105-900, Santa Maria, RS, Brazil
IVLaboratórios de Sistemática e Bioecologia de Coleoptera, Universidade Federal do Paraná UFPR, CP 19020, CEP 81531-980, Curitiba, PR, Brazil
ASBTRACT
The conservationist community is giving special attention to epigean insects due to their importance in the preservation of terrestrial habitats. This work analysed the diversity, richness, abundance and similarity at the soil surface of Coleoptera composition among five environments: native forest, native grassland, Pinus elliottii plantation, Eucalyptus saligna plantation and degraded area by soil use in southern Brazil, from October 2004 to October 2005. A total of 1,812 individuals were collected, attributed to 45 morph-species and 14 families. The higher richness and abundance were observed in native forest (31 species and 782 individuals) and the lower richness and abundance in degraded area (14 species, 86 individuals). Scarabaeidae was the richest family captured, with nine morph-species, and the most frequent family was Nitidulidae (1,113 individuals). According to the Shannon-Wiener index, the degraded area had smaller diversity in relation to the native forest, E. saligna and Pinus elliottii plantations. No difference in diversity between evaluated areas was found for the Simpson diversity index. The most dominant site was the degraded. The correlation between the total number of morph-species captured was not different to the degraded area and the P. elliottii monoculture (r = 0.47) and the correlation between the total individuals number was not significant between native forest and native grassland (r = 0.46) and between degraded areas and the other sites. According to the Jaccard Similarity Index, the greatest similarity for the organism composition occurred between P. elliottii plantation and E. saligna plantation, presenting 74% of overlap.
Keywords: beetles, species richness, abundance, estimates.
RESUMO
A comunidade conservacionista tem fornecido atenção especial aos insetos epigeicos devido à sua importância na preservação de habitats terrestres. Este trabalho avaliou a diversidade, riqueza, abundância e similaridade da composição dos Coleópteros da superfície dos solos em cinco ambientes: mata nativa, campo nativo, plantação Pinus elliottii e Eucalyptus saligna e área degradada pelo uso do solo no sul do Brasil, entre outubro de 2004 e outubro de 2005. Foram coletados 1.812 indivíduos, atribuídos a 45 morfoespécies e 14 famílias. Verificou-se maior riqueza e abundância na mata nativa (31 espécies, 782 indivíduos) e menor riqueza e abundância na área degradada (14 espécies, 86 indivíduos). A família com maior riqueza foi Scarabaeidae, com nove morfoespécies e a família mais abundante foi Nitidulidae (1.113 indivíduos). De acordo com o índice de diversidade de Shannon-Wiener, a área degradada apresentou menor diversidade em relação à floresta nativa e às plantações de E. saligna e Pinus elliottii. Nenhuma diferença na diversidade entre as áreas avaliadas foi encontrada para o índice de diversidade de Simpson. A mata nativa e o campo nativo apresentaram as maiores estimativas de riqueza. O ambiente de maior dominância foi a área degradada. A correlação entre o número total de morfoespécies capturadas na área degradada e na monocultura de P. elliottii não foi diferente (r = 0,47) e a correlação entre o número total de indivíduos entre a mata nativa e o campo nativo (r = 0,46) e entre a área degradada e os outros ecossistemas não diferiu. Conforme o índice de similaridade de Jaccard, as áreas mais semelhantes quanto à composição de coleópteros foram a plantação de P. elliottii e E. saligna, apresentando 74% de sobreposição.
Palavras-chave: besouros, riqueza de espécies, abundância, estimativas.
1. Introduction
Insects are the largest group among animals, occupying several ecological niches, being considered very important in the dynamics of natural ecosystems (Borror et al., 1992; Kim, 1993; Gullan and Cranston, 1996; Thomazini and Thomazini, 2000). These organisms exhibit great diversity in terms of species and colonisation of habitats, are easy to sample (Rosenberg et al., 1986) and present high sensitivity to environmental interference. Therefore, the composition of this group reflects the performance of the ecosystem (Correia and Pinheiro, 1999) and is fundamental in the analysis of the landscape structure.
The quality and quantity of litter are related to the richness and composition of epigean insects, since these characteristics may create different conditions on the soil surface (Loranger et al., 1998). The abundance and the structure of the edaphic insect communities depend on the alimentary supply and on the environmental properties conditioned by the local vegetation (Dunxiao et al., 1999). More complex environments support larger diversity of niches, resulting in a greater number of fauna nesting sites and feeding areas (Savolainen and Vepsäläinen., 1988). Thus, the structural diversity of the vegetation is highly correlated to the diversity of arthropods, and it is used in the understanding of disturbances caused by the simplifications of the natural ecosystems (Hutcheson, 1990; Loranger et al., 1998). Generally, the changes in abundance, diversity and composition of indicative species that depend on particular resources of the system may evaluate and explain the results of disturbance (Lewinsohn, 2001; Thomazini and Thomazini, 2002). According to Buchs (2003), biological diversity, in particular, is one of the most important tools in monitoring of health and functioning of ecosystems.
Coleoptera is the largest order of insects, with about 400 thousand species worldwide, representing 30% of animals and about 40% of all insects (Lawrence and Britton, 1991; Lawrence and Newton, 1995; Costa, 2000). The success of the group is due, mainly, to the presence of hardened fore wings, the ability to consume a wide variety of materials and holometabolia (Daly et al., 1998; Costa, 2000), characteristics that have enabled the conquest of different environments during their evolution.
Insects of this order are important in decomposition, nutrient cycling, pollination, seed dispersal and control of other animal populations (Costa, 2000; Davis et al., 2001). Furthermore, beetles are indicators of soil properties (Dunxiao et al., 1999), temperature and humidity variation of the environments (Stork and Eggleton, 1992), forest disruptions (Halffter and Favila, 1993; Davis et al., 2001) and environmental disturbances in the landscape structure (Buchs, 2003). According to Brown Junior (1997), this order satisfies all requirements to be used for environmental bioindication (Marinoni and Dutra, 1997).
This study aimed to evaluate the differences in the composition and structure of epigean Coleoptera communities in five different environments in Santa Maria, RS, Brazil. The following were analysed: a) the richness and abundance variability of Coleoptera families between environments; b) the existence of patterns on the presence of families in each environment; c) if these patterns may be related to the of degree human actions in ecosystems; d) the structure of communities in relation to the trophic level occupied by the Coleoptera families; e) the relationship between the time period and the increase in the number of Coleoptera species; f) richness and diversity estimates and uniformity values of the sample areas; and g) the similarity between the investigated areas based on species abundance and richness of Coleoptera families.
2. Material and Methods
2.1. Characteristics of the study locations
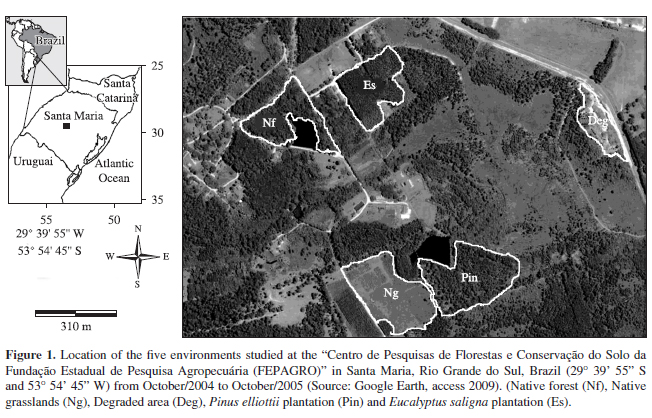
The study was developed in the "Centro de Pesquisas de Florestas e Conservação do Solo da Fundação Estadual de Pesquisa Agropecuária (FEPAGRO)", Santa Maria, Rio Grande do Sul (29° 43' 12" S and 53° 43' 01" W) (Figure 1). According to Maluf (2000) the climate is classified as Temperate Superhumid (Te Su) with an annual mean temperature of 19.2 °C. Rainfall is more or less evenly distributed throughout the year, with an annual mean of 1,708 mm (Maluf, 2000).
Five quarterly collections were made between October 2004 and October 2005 in five different environments. Native grassland: characterised by the homogeneity of the vegetation, with strong predominance of grasses. Degraded area (reject): where the soil does not present its surface layer, being completely naked, compacted, and having suffered the strong action of erosion processes. A 15-year-old Pinus elliottii plantation: showing almost absentee sub-forest vegetation, and litter with a thickness around ten centimetres. A 30-year-old Eucalyptus saligna plantation: showing considerable vegetation in the sub-forest and litter layer with an approximate thickness of five centimetres. In the native forest studied, the families Anacardiaceae, Annonaceae, Bignoniaceae, Caesalpinaceae, Fabaceae, Mimosaceae, Moraceae and Myrtaceae are found. This area is distinguished by the largest heterogeneity and density of the vegetation. The litter layer is around two centimetres thick. The size of each area evaluated and the minimum distance between them was calculated with the program Image J 1:41 (Rasband, 2009), from satellite images, obtained by Google Earth, with a point of view of two kilometres of altitude and with a mean total area of 140 ha (Table 1).
2.2. Sampling method
In all environments, eight collecting points were sampled. The traps were installed in a straight line, 15 m apart and 15 m from the fragment border. The insects were collected with "pitfall traps", constructed with PET bottles, presenting four openings of 5 × 7 cm. The openings were located at 20 cm from the base of the bottle. The traps were buried with the openings at the ground level, permitting the entry of the entomofauna. Each trap contained formalin to 10% and they were kept in the sample areas for two days. After this period, they were transported to the laboratory to identify the material.
2.3. Identification and data analysis
After being mounted on entomological pins, the insects were separated and quantified at the family level (Borror et al., 1992; Lawrence et al., 1999), and morph-species level. Although not conventional, the analyses were performed through the abundance of Coleoptera at the family level (Marinoni and Dutra, 1997). Pielou (1975) and Magurran (1988) consider it valid. The Kruskal-Wallis test was employed to evaluate the differences in richness and abundance among the Coleoptera families in the investigated environments. To compare the addition of species during the study period, a rarefaction curve was utilised, because the sample size was different among the studied environments (Moreno, 2001).
The diversity analysis among the ecosystems were evaluated by the following diversity indexes: Shannon-Wiener (H'), which consider equal weight to the rare and abundant species and Simpson's index (1-D), which is characterized by being sensitive to changes in the most abundant species composition (Peet, 1974) were employed. The differences between these indexes were calculated using the Student test (t-test).
The uniformity among the coleopterans caught in the five sampling areas was calculated with the Berger and Parker index. Non-parametric tests, Jackknife one and two were used to estimate the number of species in the studied environments, based on abundance data. The relationship between the "singletons" and "doubletons" was utilised to estimate the richness and a Bootstrap procedure of 1.000 random samples and confidence interval of 95% was employed.
Spearman correlation was applied to group areas of larger similarity based on coleopterans species abundance. This analysis was processed with the Past program version 1.32 (Hammer et al., 2004). In the grouping of similar environments based on the coleopterans families found in each one, the Jaccard similarity index was used, which considers the presence and absence of species. The UPGMA method was used to group the areas. The evaluation of the trophic groups of the coleopteran families registered in the five environments was made according to Marinoni et al. (2001). The normality of all data was tested by the Shapiro-Wilk method. All tests considered a significance of 0.05.
3. Results
3.1. Richness and abundance
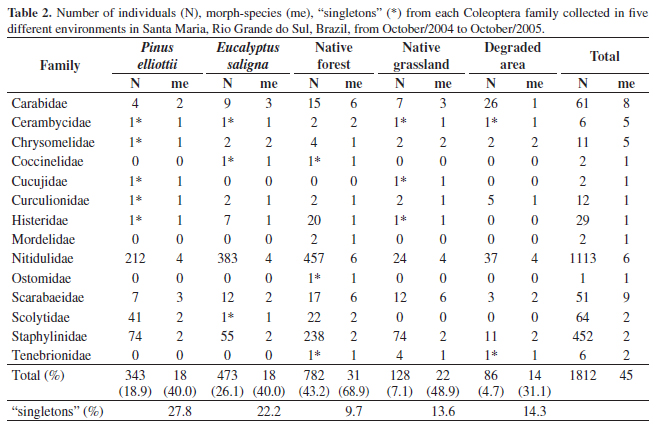
In the five survey areas, 1,812 beetles were caught, distributed in 45 morph-species from 14 different families (Table 2). The "singletons" frequency in the areas ranged from 9.7% in native forest to 27.8% in P. elliottii plantation. The degraded area showed the lowest number of "singletons". The native forest had the highest richness, presenting 31 morph-species (68.9%), while the lowest richness occurred in the degraded area. At this site, only 14 morph-species (31.1%) were found. The native forest also showed the greatest number of individuals (n = 782), including 43.2% of the total captured beetles, followed by E. saligna monoculture, with 473 individuals (26.1%). The lowest abundance of beetles was found in the degraded area, where only 86 specimens (4.7%) were captured.
Among the families recorded, Scarabaeidae was the family that exhibited the highest number of morph-species (9), followed by Carabidae (8) and Nitidulidae (6). These three families, with Chrysomelidae and Cerambycidae were responsible for 73.3% (33) of the total richness. However, the most abundant family among the environments studied were Nitidulidae (n = 1.113), representing 61.4% of the total specimens sampled and Staphylinidae, representing 24.9% (n = 452) of the individuals sampled. Only these two families composed 86.4% of the total beetles collected.
Analysing each study area separately, Nitidulidae was the richest family among the ecosystems. However, in the native forest, Scarabaeidae and Carabidae had the same number of morph-species as the supracited family (6). Exceptionally, in native grassland, Scarabaeidae was the richer family (6). The Nitidulidae family was also the most abundant family in all ecosystems, except in native grassland where the Staphylinidae represented the most of the captured individuals. Mordelidade and Ostomidae families were registered only in the native forest, while the Cucujidae family occurred only in the P. elliottii plantation and native grassland. The Cicindela genus (Carabidae - Cicindelinae) occurred only in native forest and in the E. saligna area. Twenty-six individuals were captured of the same morph-specie of the Carabidae in the degraded area. Among the families that did not occur in the degraded area are: Coccinelidae, Cucujidae, Histeridae, Mordelidae, Ostomidae and Scolytidae. The last three families also did not appear in native grassland.
3.2. Trophic groups
Of the 14 sampled families, four have an exclusively carnivorous habit, three are herbivores, two are herbivores or detritivore, one is fungivore or detritivore, one is fungivore or herbivore and three possess varied habits. The percentage of each group is given in Table 3.
3.3. Species accumulation curve
The graph of the species collected shows a decrease in the number of the new species of beetles captured in each sample period (Figure 2). In the first sample, the total number of individuals captured exceeded 50% of the morph-species found during the study period in all five areas.
3.4. Richness estimates
Jackknife two was the species richness estimate which resulted in the largest values for all the areas the whole sampling period (Table 4) was analysed. The bootstrap procedure resulted in the smallest values. The native forest and native grassland presented the highest richness estimates and the degraded area the smallest ones for both jackkinfe one and jackknife two.
3.5. Diversity indexes and uniformity
Diversity indexes show some differences among the five sites (Table 4). For the Shannon-Wiener index, native forest (2.26), P. elliottii (2.17) and E. saligna monocultures (2.17) are the ecosystems with the largest diversity. Simpson's index shows the same pattern. The values obtained for these sites are not different for both diversity indexes (Table 5). The degraded area exhibited a significantly smaller diversity in relation to the other investigated areas (except to the native grassland) for the Shannon-Wiener index. However, for the Simpson index, the degraded area did not differ in relation to the diversity from other sites. The most dominant site was the degraded area (BP = 0.29) (Table 4) and native forest had the lowest dominance (BP = 0.19), coinciding with the high diversity found in this site.
3.6. Structure of the communities
There was no significant difference in abundance among Coleoptera families collected in the environments assessed (Table 6). However, a significant difference was identified in Coleoptera richness captured between E. saligna monoculture and native forest, and between E. saligna monoculture and native grassland.
The correlation between the total number of morph-species found was not different to the degraded area and P. elliottii monoculture (r = 0.47) and the correlation between the total number of individuals was not significant between native forest and native grassland (r = 0.46) and between the degraded area and others sites (Table 7).
Cluster analysis from the Jaccard similarity index revealed that P. elliottii and E. saligna plantations had the most similar composition of organisms, showing an overlap of 74% (Figure 3).
4. Discussion
The greater coleopterans richness and coleopterans abundance in forest is related to the benefits obtained by these organisms from the soil moisture and microclimate stability offered by forests (Butterfield et al., 1995). Panzer and Schwartz (1998) observed that the plant species richness explained more than 49% of the variance of the insect species richness among the studied areas. The abundance of beetles found in E. saligna plantation may be associated to the ecological plasticity of some species (Butterfield et al., 1995) and to the amount of litter in this area, originated from the great sub-forest presence. This characteristic stimulates the beetles that are responsible for nutrient cycling and organic matter decomposition. However, the richness in E. saligna plantation is different from native area and native grassland. This result may have reflected the low nutritional quality of soil in this environment, since the eucalypt leaves have a high concentration of essential oils (Rice, 1974; Silva, 1978). Furthermore, this site presented a discontinuous canopy and high solar radiation (Ferreira and Marques, 1998). The lack of vegetation in the degraded area may have contributed to reduce the richness and abundance of the beetle species due to the low availability of food resources (Thomazini and Thomazini, 2002) and to the decrease of soil moisture at this site (Stork and Eggleton, 1992). Perner and Malt (2003) reported that beetles have clear reactions to microclimate and soil moisture changes.
Among the identified coleopteran families, Scarabaeidae presented the highest number of morph-species. These beetles are important in organic matter recycling (Kim, 1993) and in the seed dispersals (Davis and Sutton, 1998). Meanwhile, the Nitidulidae family was the most abundant, followed by Staphylinidae. The abundance of Nitidulidae in the study areas is due mainly to the different habits of its species. They can distribute themselves in the most diverse ecosystems, fuelling up on different materials (Audino et al., 2007). The abundance of Staphylinidae, however, is associated with its large diversity. This group is among the most common and important organisms of soil fauna and they are found in almost all types of ecosystems (Bohac, 1999).
Considering all the environments, Nitidulidae was the family that had the highest abundance and Scarabaeidae the highest richness. On the other hand, they did not obtain the same morph-species composition and dominance among the sites investigated. The vegetation density and structure of each ecosystem might have exerted an important influence on these guidelines. In P. elliottii and E. saligna monocultures, there possibly prevailed the species favoured by dry environments (Bohac, 1999). When the sites were analysed separately, there were some changes in this pattern. In native grassland, Staphylinidae was the most abundant group and in E. saligna and P. elliottii plantation and degraded area, Nitidulidae exhibited the highest richness. These differences in coleopteran composition and number reflect the different environmental requirements of the group based in the ecosystem structure and plant composition.
Some groups had higher richness or appeared only in poor soils, such as the Cucujidae family, which was observed only in P. elliottii plantation and in native grassland. Kevan (1999) associated the Carabidae diversity with the vegetation richness, but one morph-specie of Carabidae was found only in the degraded area. Steinborn and Meyer (1994) and Eyre et al. (2003) reported that soil type and soil moisture are factors of great influence in the communities of this family. Thus, Cucujidae and Cicindelinae might have obtained benefits from the lack of competition in those sites (Stork and Eggleton, 1992). Although Carabidae beetles are characterised by their great variety of species in the ecosystems (Lövei and Suderland, 1996), in the present study, they were not abundant. The low abundance of this group might have occurred due to its gregarious distribution in response to the pattern of resources availability (Paarmann et al., 2001).
Based on the lack of an asymptote in the rarefaction curve, it was observed that were not collected all beetles species in the study areas. The acquisition of such data requires a greater sampling effort (Soberón and Llorente, 1993). The use of other methodologies certainly would contribute with an asymptote.
The composition of beetles in each environment differs due to the needs, trophic level and behaviour of each group (Nouhuys, 2005). Most collected species belonged to families exclusively carnivorous as observed by Marinoni et al. (2001). These kinds of beetles depend on the diversity of animal products of an ecosystem, which in turn depend on other organisms and so on. Thus, Nouhuys (2005) suggested that in a community it is expected that organisms from higher trophic levels are more sensitive to environmental changes. Our study supports the author's idea, because only one exclusively carnivorous family (Carabidae - Cicindelinae) was recorded in the degraded area.
The herbivore and detritivore arthropods have received considerable attention, because they are organisms of high impact on ecosystems. Detritivores are responsible for increasing the flow of nutrients in the soil, while herbivores are responsible for the input energy. The activity of these organisms, therefore, influences the whole ecosystem (Crossley et al., 1992). Detritivores require environments with relatively dense vegetation and soils with thick layers of leaf litter (Iannuzzi et al., 2003). The phytophages are particularly dependent on the physical structure and floristic composition of their habitats (Brown Junior, 1997). Thus, the units of landscape of this study should have influenced, especially, Cerambycidae, Curculionidae and Mordelidae diversity. This hypothesis may be verified with the use of a methodology to capture species that occur in another stratum of vegetation.
The greater richness and greater diversity of insects found in native forest is related to its complex structure, which includes many plants species, vertical stratification and interconnected canopies (Elton, 1973). This environment provides the beetles with a greater diversity of food, more stable microclimate, higher humidity and greater quantity of refuges against predators (Vallejo et al., 1987). There was no difference in Coleoptera diversity found in native forest and E. saligna plantation. This result may reflect the ability of E. saligna plantation to support distinct species originated from the great sub-forest presence (Butterfield et al., 1995), since this environment presented an inferior amount of beetles in relation to the native forest. Monoculture areas that have a discontinuous canopy, like the P. elliotti site studied, allows a large sunshine input, high evaporation and strong impact of rain on the soil, causing the reduction of the soil fauna (Ferreira and Marques, 1998). The variation of the species diversity is influenced also by other factors such as phylogenetic diversity (May, 1990) and endemism (Jetz et al., 2004).
As the pattern observed by Odum (1985), the high dominance of beetle species in the degraded area reflected a significant difference between the richness estimate in this environment and in the native grassland and native forest. The degraded area provides a lower number of insect species due to its low diversity of plants and the high compression of the first centimetres of soil, damaging the animals that move among its particles (Dorst, 1973).
The large presence of plant material in native forest soil and E. saligna monoculture should have allowed the coexistence of a larger number of morph-species beetles in these sites. In contrast, there was no correlation in the total number of Coleoptera species captured between the degraded area and the P. elliottii monoculture, and there was no correlation in the total number of individuals collected between native forest and native grassland and between degraded area and the other sites. Thus, the abundance and richness of beetles in the studied environments seems to depend not only on the composition and on plant structure, but on the quantity of plant product in each ecosystem.
The most similar areas in the composition of Coleoptera species were E. saligna and P. elliottii plantations. The similarity in their vegetable physiognomies characterised by structural homogeneity may explain this result. Areas with Eucalyptus plantation have many opportunistic ant species and low occurrence of rare species (Ramos et al., 2001). Moreover, the smaller similarity between native grassland and native forest should reflect differences in floristic composition and vertical structure of these ecosystems. Furthermore, Coleoptera composition in the degraded area differs from others, possibly because of the naked soil and the little food resources available to the beetles.
Epigean beetles show changes in the landscape and respond to environmental impacts through their close relationship with the processes that occur in soils and their susceptibility to interference in ecosystems. This study reinforces that the Coleoptera order may reflect the condition of each environment. Thus, it is necessary to identify taxa for each stress factor of the ecosystem and for different intensities of these factors. With this such knowledge, we will be able to carry out the appropriate management of environments with only a minor degree of disturbance.
Acknowledgements We are grateful to the colleagues who helped in the field activities and the beetle identification. Thanks to the "Centro de Pesquisas de Florestas e Conservação do Solo da Fundação Estadual de Pesquisa Agropecuária FEPAGRO" for providing access to areas for this study and to the "Fundo de Incentivo à Pesquisa"- FIPE, Universidade Federal de Santa Maria, for financing this work.
References
AUDINO, LD., NOGUEIRA, JM., SILVA, PG., NESKE, MZ., RAMOS, AHB., MORAES, LP. and BORBA, MFS., 2007. Identificação dos coleópteros Insecta: Coleoptera das regiões de Palmas município de Bagé e Santa Barbinha município de Caçapava do Sul, RS. Bagé: Embrapa Pecuária Sul. 92 p.
BOHAC, J., 1999. Staphylinid beetles as bioindicators. Agriculture, Ecosystems & Environment, vol. 74, no. 1/3, p. 357-372. doi:10.1016/S0167-8809(99)00043-2
BORROR, DJ., TRIPLEHORN, CA. and JOHNSON, NF., 1992. An introduction to the study of insects. 6rd ed. Orlando: Saunders College Publishing. 875 p.
BROWN JUNIOR, KS., 1997. Insetos como rápidos e sensíveis indicadores de uso sustentável de recursos naturais. In: MARTOS, HL. and MAIA, NB. (Eds.). Indicadores Ambientais. Sorocaba: PUC/Shell Brasil. 266 p.
BUCHS, W., 2003. Biodiversity and agri-environmental indicators-general scopes and skills with special reference to the habitat level. Agriculture, Ecosystems & Environment, vol. 98, no. 1/3, p. 35-78. doi:10.1016/S0167-8809(03)00070-7
BUTTERFIELD, J., LUFF, ML., BAINES, M. and EYRE, MD., 1995. Carabid beetle communities as indicators of conservation potential in upland forests. Forest Ecology and Management, vol. 79, no. 1/2, p. 63-77. doi:10.1016/0378-1127(95)03620-2
CORREIA, MEF. and PINHEIRO, LBA., 1999. Monitoramento da fauna do solo sob diferentes coberturas vegetais em um sistema integrado de produção agroecológica. Seropédica: Embrapa Agrobiologia. 15 p. no. 3.
COSTA, C., 2000. Estado de conocimiento de los Coleoptera Neotropicales. In MARTÍN-PIERA, F., MORRONE, JJ. and MELIA, A. (Eds.). Hacia un Proyecto CYTED para el Inventario y Estimación de la Diversidad Entomológica en Iberoamérica. Zaragoza: Sociedad Entomológica Aragonesa. 326 p.
CROSSLEY, BJ, MUELLER, R. and PERDUE, JC., 1992. Biodiverity of microathropods in agricultural soils: Relations to processes. In PAOLETTI, MG. and PIMENTEL, D. (Eds.). Biotic divers. in agroecosys. Amsterdam: Elservier. p. 37-46.
DALY, HV., DOYEN, JT, and PURCELL, AH., 1998 Introduction to Insect Biology and Diversity. Oxford: Oxford University Press. 680 p.
DAVIS, AJ. and SUTTON, SL., 1998. The effects of rainforest canopy loss on arboreal dung beetles in Borneo: implications for the measurement of biodiversity in derived tropical ecosystems. Drivers and Distribution, vol. 4, no. 4, p. 167-173. doi:10.1046/j.1472-4642.1998.00017.x
DAVIS, AJ., HOLLOWAY, JD., HUIJBREGTS, H., KRIKKEN, J., KIRK-SPRIGGS, AH. and SUTTON, SL., 2001. Dung beetles as indicators of change in the forests of northern Borneo. Journal of Applied Ecology, vol. 38, no. 3, p. 593-616. doi:10.1046/j.1365-2664.2001.00619.x
DORST, J., 1973. Antes que a natureza morra. São Paulo: Edgard Blucher/USP. 394 p.
DUNXIAO, H., CHUNRU, H., YALING, X., BANWANG, H., LIYUAN, H. and PAOLETTI, MG., 1999. Relationship between soil arthropods and soil properties in a Suburb of Qianjiang City, Hubei, China. Critical Reviews in Plant Sciences, vol. 18, no. 3, p. 467-473. doi:10.1016/S0735-2689(99)00378-0
ELTON, CS., 1973. The structure of invertebrate populations inside neotropical rain forest. Journal of Animal Ecology, vol. 42, p. 55-103. doi:10.2307/3406
EYRE, MD., LUFF, ML., STANLEY, JR. and TELFER, MG., 2003. The relationship between British ground beetles Coleoptera, Carabidae and land cover. Journal of Biogeography, vol. 30, no. 5, p. 719-730. doi:10.1046/j.1365-2699.2003.00859.x
FERREIRA, RL. and MARQUES, MMGSM., 1998. A fauna de artrópodes de serapilheira de áreas de monocultura com Eucalyptus sp. e mata secundária heterogênea. Anais da Sociedade Entomológica do Brasil, vol. 27, no. 3, p. 395- 403.
GULLAN, PJ. and CRANSTON, PS., 1996. The Insects: an Outline of Entomology. London: Chapman & Hall. 19 p.
HALFFTER, G. and FAVILA, ME., 1993. The Scarabaeidae Insecta: Coleoptera an animal group for analyzing, inventorying and monitoring biodiversity in tropical rainforest and modified landscapes. Biology International, vol. 27, p. 15-21.
HAMMER, O., HARPER, DAT. and RYAN, PD., 2004. PaST - Palaeontological Statistics. Version 1.32. Available from: <http://folk.uio.no/ohammer/past/>. Access in: 10 jul. 2009.
HUTCHESON, J., 1990. Characterization of terrestrial insect communities using quantified, Malaise-trapped Coleoptera. Ecological Entomology, vol. 15, no. 2, p. 143-151. doi:10.1111/j.1365-2311.1990.tb00795.x
IANNUZZI, L., MAIA, ACD., NOBRE, CEB., SUZUKI, DK. and MUNIZ, FJA., 2003. Padrões locais de diversidade de Coleptera Insecta em vegetação de caatinga. In LEAL, IR., TABARELLI, M. and SILVA, JMC. (Eds.). Ecologia e conservação da caatinga. Recife: Editora da Universidade Federal de Pernambuco. p. 367-389.
JETZ, W, RAHBEK, C. and COLWELL, RK., 2004. The coincidence of rarity and richness and the potential signature of history in centres of endemism. Ecology Letters, vol. 7, no. 12, p. 1180-1191. doi:10.1111/j.1461-0248.2004.00678.x
KEVAN, PG., 1999. Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agriculture, Ecosystems & Environment, vol. 74, no. 1/3, p. 373-393.
KIM, KC., 1993. Biodiversity, conservation and inventory: Why insects matter. Biodiversity and Conservation, vol. 2, no. 3, p. 191-214. doi:10.1007/BF00056668
LAWRENCE, JF. and BRITTTON, EB., 1991. Coleoptera. In NAUMANN, I. (Ed.). The Insects of Australia. New York: Cornell University Press. p. 543-683.
LAWRENCE, JF. and NEWTON, EB., 1995. Families and subfamilies of Coleoptera with selected genera, notes, references and data on family-group names. In PAPALUK, JF. and SLIPINSKI, SA. (Eds.). Biology, Phylogeny and Classification of Coleoptera. Varsovia: Museum I Institut Zoologii PAN. p. 779-1006.
LAWRENCE, JF., HASTINGS, AM., DALLWITZ, MJ., PAINE, TA. and ZURCHER, EJ., 1999. Beetles of the World: a key and information system for families and subfamilies. Melbourne: CSIRO Publishing. Cd-rom, Version 1.0.
LEWINSOHN, MT., 2001. Esboço de uma estratégia abrangente de inventários de biodiversidade. In GARAY, I. and DIAS, B. (Eds.). Conservação da biodiversidade em ecossistemas tropicais. Petrópolis: Editora Vozes. 2001. p. 376-384.
LORANGER, G., PONGE, JF., BLANCHART, E. and LAVELLE, P., 1998. Influence of agricultural practices on arthropod communities in a vertisol Martinique. European Journal of Soil Biology, vol. 34, no. 4, p. 157-165. doi:10.1016/S1164-5563(00)86658-3
LÖVEI, GL. and SUNDERLAND, KD., 1996. Ecology and behavior of ground beetles Coleoptera: Carabidae. Annual Review of Entomology, vol. 41, p. 231-256.
MAGURRAN, AE., 1988. Ecological diversity and its measurement. Princeton: Princeton University Press. 179 p.
MALUF, JRT., 2000. Nova classificação climática do estado do Rio Grande do Sul. Revista Brasileira de Agrometeorologia, vol. 8, p. 141-150.
MARINONI, RC. and DUTRA, RRC., 1997. Famílias de Coleoptera capturadas com armadilha malaise em oito localidades do Estado do Paraná, Brasil. Diversidades alfa e beta. Revista Brasileira de Zoologia, vol. 14, no. 3, p. 751-770.
MARINONI, RC., GANHO, NG., MONNE, ML. and MERMUDES, JRM., 2001. Hábitos alimentares em Coleoptera Insecta. Ribeirão Preto: Holos Editora. 63 p.
MAY, RM., 1990. Taxonomy as destiny. Nature, vol. 347, no. 6289, p. 129-130. doi:10.1038/347129a0
MORENO, JA., 2001. Métodos para medir la biodiversidad. Zaragoza: Unesco and Sociedad Entomologica Aragonesa. 83 p.
NOUHUYS, S., 2005. Effects of habitat fragmentation at different trophic levels in insect communities. Annales Zoologici Fennici, vol. 42, no. 4, p. 433-447.
ODUM, EP., 1985. Ecologia. São Paulo: Interamericana. 434 p.
PAARMANN, W., ADIS, J., STORK, N., GUTZMANN, B., STUMPE, P., STARITZ, B., BOLTE, H., KÜPPERS, S., HOLZKAMP, K., NIERS, C. and FONSECA, CRV., 2001. The structure of ground beetle assemblages Coleoptera: Carabidae at fig fruit falls Moraceae in terra firme rain forest near Manaus Brazil. Journal of Tropical Ecology, vol. 17, no. 4, p. 549-561. doi:10.1017/S0266467401001419
PANZER, R. and SCHWARTZ, MW., 1998. Effectiveness of a vegetation-based approach to insect conservation. Conservation Biology, vol. 12, no. 3, p. 693-702. doi:10.1046/j.1523-1739.1998.97051.x
PEET, RK., 1974. The measurement of species diversity. Annual Review of Ecology and Systematics, vol. 5, p. 285-307. doi:10.1146/annurev.es.05.110174.001441
PERNER, J. and MALT, S., 2003. Assessment of changing agricultural land use: response of vegetation, ground-dwelling spiders and beetles to the conversion of arable land into grassland. Agriculture, Ecosystems & Environment, vol. 98, no. 1/3, p. 169-181. doi:10.1016/S0167-8809(03)00079-3
PIELOU, EC., 1975. Ecological diversity. New York: John Wiley and Sons. 165 p.
RAMOS, F., MARTINS, I., FARIAS, JM., SILVA, ICS., COSTA, DC. and MIRANDA, AP., 2001. Oviposition and predation by Speciomerus revoili Coleoptera, Bruchidae on seeds of Acrocomia aculeate Arecaceae in Brasília, DF, Brazil. Brazilian Journal of Biology, vol. 61, no. 3, p. 449-454. doi:10.1590/S1519-69842001000300014
RASBAND, W., 2009. Image J 1.34 m. USA: National Institutes of Health. Available from: <http://rsb.info.nih.gov/ij/>. Access in: 01 mar 2009.
RICE, EL., 1974. Allelopathy. New York: Academic Press. 266 p.
ROSENBERG, DM., DANKS, HV. and LEHMKUHL, DM., 1986. Importance of insects in environmental impact assessment. Environmental Management, vol. 10, no. 6, p. 773-783. doi:10.1007/BF01867730
SAVOLAINEN, R. and VEPSÄLÄINEN, K., 1988. A competition hierarchy among boreal ants: impact on resource partitioning and community structure. Oikos, vol. 51, no. 2, p. 135-155. doi:10.2307/3565636
SILVA, ZL., 1978. Alelopatia e defesa de plantas. Boletim Geográfico, vol. 36, p. 90-96.
SOBERÓN, J. and LLORENTE, J., 1993. The use of species accumulation functions for the prediction of species richness. Conservation Biology, vol. 7, no. 3, p. 480-488. doi:10.1046/j.1523-1739.1993.07030480.x
STEINBORN, HA. and MEYER, H., 1994. Einfluß alternativer und konventioneller Landwirtschaft auf die Prädatorenfauna in Agrarökosystemen Schleswig-Holsteins Araneida, Coleoptera: Carabidae, Diptera: Dolichopodidae, Empididae, Hybotidae, Microphoridae. Faunistisch-ökologische Mitteilungen, vol. 6, p. 409-438.
STORK, NE. and EGGLETON, P., 1992. Invertebrates as determinants and indicators of soil quality. American Journal of Alternative Agriculture, vol. 7, no. 1/2, p. 38-47.
THOMAZINI, MJ. and THOMAZINI, APBW., 2000. A fragmentação florestal e a diversidade de insetos nas florestas tropicais úmidas. Rio Branco: Embrapa Acre. 21 p.
-, 2002. Levantamento de insetos e análise entomofaunística em floresta, capoeira e pastagem no Sudeste Acreano. Rio Branco: Embrapa Acre. 41 p.
VALLEJO, LR., FONSECA, CL. and GONÇALVES, DRP., 1987. Estudo comparativo da mesofauna do solo em áreas de Eucalyptus citriodora e mata secundária heterogênea. Revista Brasileira de Biologia = Brazilian Journal of Biology, vol. 47, p. 363-370.
Received December 29, 2009
Accepted April 22, 2010
Distributed May 31, 2011
- AUDINO, LD., NOGUEIRA, JM., SILVA, PG., NESKE, MZ., RAMOS, AHB., MORAES, LP. and BORBA, MFS., 2007. Identificação dos coleópteros Insecta: Coleoptera das regiões de Palmas município de Bagé e Santa Barbinha município de Caçapava do Sul, RS. Bagé: Embrapa Pecuária Sul. 92 p.
- BOHAC, J., 1999. Staphylinid beetles as bioindicators. Agriculture, Ecosystems & Environment, vol. 74, no. 1/3, p. 357-372. doi:10.1016/S0167-8809(99)00043-2
- BORROR, DJ., TRIPLEHORN, CA. and JOHNSON, NF., 1992. An introduction to the study of insects. 6rd ed. Orlando: Saunders College Publishing. 875 p.
- BROWN JUNIOR, KS., 1997. Insetos como rápidos e sensíveis indicadores de uso sustentável de recursos naturais. In: MARTOS, HL. and MAIA, NB. (Eds.). Indicadores Ambientais. Sorocaba: PUC/Shell Brasil. 266 p.
- BUCHS, W., 2003. Biodiversity and agri-environmental indicators-general scopes and skills with special reference to the habitat level. Agriculture, Ecosystems & Environment, vol. 98, no. 1/3, p. 35-78. doi:10.1016/S0167-8809(03)00070-7
- BUTTERFIELD, J., LUFF, ML., BAINES, M. and EYRE, MD., 1995. Carabid beetle communities as indicators of conservation potential in upland forests. Forest Ecology and Management, vol. 79, no. 1/2, p. 63-77. doi:10.1016/0378-1127(95)03620-2
- CORREIA, MEF. and PINHEIRO, LBA., 1999. Monitoramento da fauna do solo sob diferentes coberturas vegetais em um sistema integrado de produção agroecológica. Seropédica: Embrapa Agrobiologia. 15 p. no. 3.
- COSTA, C., 2000. Estado de conocimiento de los Coleoptera Neotropicales. In MARTÍN-PIERA, F., MORRONE, JJ. and MELIA, A. (Eds.). Hacia un Proyecto CYTED para el Inventario y Estimación de la Diversidad Entomológica en Iberoamérica. Zaragoza: Sociedad Entomológica Aragonesa. 326 p.
- CROSSLEY, BJ, MUELLER, R. and PERDUE, JC., 1992. Biodiverity of microathropods in agricultural soils: Relations to processes. In PAOLETTI, MG. and PIMENTEL, D. (Eds.). Biotic divers. in agroecosys. Amsterdam: Elservier. p. 37-46.
- DALY, HV., DOYEN, JT, and PURCELL, AH., 1998 Introduction to Insect Biology and Diversity Oxford: Oxford University Press. 680 p.
- DAVIS, AJ. and SUTTON, SL., 1998. The effects of rainforest canopy loss on arboreal dung beetles in Borneo: implications for the measurement of biodiversity in derived tropical ecosystems. Drivers and Distribution, vol. 4, no. 4, p. 167-173. doi:10.1046/j.1472-4642.1998.00017.x
- DAVIS, AJ., HOLLOWAY, JD., HUIJBREGTS, H., KRIKKEN, J., KIRK-SPRIGGS, AH. and SUTTON, SL., 2001. Dung beetles as indicators of change in the forests of northern Borneo. Journal of Applied Ecology, vol. 38, no. 3, p. 593-616. doi:10.1046/j.1365-2664.2001.00619.x
- DORST, J., 1973. Antes que a natureza morra. São Paulo: Edgard Blucher/USP. 394 p.
- DUNXIAO, H., CHUNRU, H., YALING, X., BANWANG, H., LIYUAN, H. and PAOLETTI, MG., 1999. Relationship between soil arthropods and soil properties in a Suburb of Qianjiang City, Hubei, China. Critical Reviews in Plant Sciences, vol. 18, no. 3, p. 467-473. doi:10.1016/S0735-2689(99)00378-0
- ELTON, CS., 1973. The structure of invertebrate populations inside neotropical rain forest. Journal of Animal Ecology, vol. 42, p. 55-103. doi:10.2307/3406
- EYRE, MD., LUFF, ML., STANLEY, JR. and TELFER, MG., 2003. The relationship between British ground beetles Coleoptera, Carabidae and land cover. Journal of Biogeography, vol. 30, no. 5, p. 719-730. doi:10.1046/j.1365-2699.2003.00859.x
- FERREIRA, RL. and MARQUES, MMGSM., 1998. A fauna de artrópodes de serapilheira de áreas de monocultura com Eucalyptus sp. e mata secundária heterogênea. Anais da Sociedade Entomológica do Brasil, vol. 27, no. 3, p. 395- 403.
- GULLAN, PJ. and CRANSTON, PS., 1996. The Insects: an Outline of Entomology. London: Chapman & Hall. 19 p.
- HALFFTER, G. and FAVILA, ME., 1993. The Scarabaeidae Insecta: Coleoptera an animal group for analyzing, inventorying and monitoring biodiversity in tropical rainforest and modified landscapes. Biology International, vol. 27, p. 15-21.
- HAMMER, O., HARPER, DAT. and RYAN, PD., 2004. PaST - Palaeontological Statistics Version 1.32. Available from: <http://folk.uio.no/ohammer/past/>. Access in: 10 jul. 2009.
- HUTCHESON, J., 1990. Characterization of terrestrial insect communities using quantified, Malaise-trapped Coleoptera. Ecological Entomology, vol. 15, no. 2, p. 143-151. doi:10.1111/j.1365-2311.1990.tb00795.x
- IANNUZZI, L., MAIA, ACD., NOBRE, CEB., SUZUKI, DK. and MUNIZ, FJA., 2003. Padrões locais de diversidade de Coleptera Insecta em vegetação de caatinga. In LEAL, IR., TABARELLI, M. and SILVA, JMC. (Eds.). Ecologia e conservação da caatinga. Recife: Editora da Universidade Federal de Pernambuco. p. 367-389.
- JETZ, W, RAHBEK, C. and COLWELL, RK., 2004. The coincidence of rarity and richness and the potential signature of history in centres of endemism. Ecology Letters, vol. 7, no. 12, p. 1180-1191. doi:10.1111/j.1461-0248.2004.00678.x
- KEVAN, PG., 1999. Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agriculture, Ecosystems & Environment, vol. 74, no. 1/3, p. 373-393.
- KIM, KC., 1993. Biodiversity, conservation and inventory: Why insects matter. Biodiversity and Conservation, vol. 2, no. 3, p. 191-214. doi:10.1007/BF00056668
- LAWRENCE, JF. and BRITTTON, EB., 1991. Coleoptera. In NAUMANN, I. (Ed.). The Insects of Australia New York: Cornell University Press. p. 543-683.
- LAWRENCE, JF. and NEWTON, EB., 1995. Families and subfamilies of Coleoptera with selected genera, notes, references and data on family-group names. In PAPALUK, JF. and SLIPINSKI, SA. (Eds.). Biology, Phylogeny and Classification of Coleoptera. Varsovia: Museum I Institut Zoologii PAN. p. 779-1006.
- LAWRENCE, JF., HASTINGS, AM., DALLWITZ, MJ., PAINE, TA. and ZURCHER, EJ., 1999. Beetles of the World: a key and information system for families and subfamilies. Melbourne: CSIRO Publishing. Cd-rom, Version 1.0.
- LEWINSOHN, MT., 2001. Esboço de uma estratégia abrangente de inventários de biodiversidade. In GARAY, I. and DIAS, B. (Eds.). Conservação da biodiversidade em ecossistemas tropicais Petrópolis: Editora Vozes. 2001. p. 376-384.
- LORANGER, G., PONGE, JF., BLANCHART, E. and LAVELLE, P., 1998. Influence of agricultural practices on arthropod communities in a vertisol Martinique. European Journal of Soil Biology, vol. 34, no. 4, p. 157-165. doi:10.1016/S1164-5563(00)86658-3
- LÖVEI, GL. and SUNDERLAND, KD., 1996. Ecology and behavior of ground beetles Coleoptera: Carabidae. Annual Review of Entomology, vol. 41, p. 231-256.
- MAGURRAN, AE., 1988. Ecological diversity and its measurement. Princeton: Princeton University Press. 179 p.
- MALUF, JRT., 2000. Nova classificação climática do estado do Rio Grande do Sul. Revista Brasileira de Agrometeorologia, vol. 8, p. 141-150.
- MARINONI, RC. and DUTRA, RRC., 1997. Famílias de Coleoptera capturadas com armadilha malaise em oito localidades do Estado do Paraná, Brasil. Diversidades alfa e beta. Revista Brasileira de Zoologia, vol. 14, no. 3, p. 751-770.
- MARINONI, RC., GANHO, NG., MONNE, ML. and MERMUDES, JRM., 2001. Hábitos alimentares em Coleoptera Insecta Ribeirão Preto: Holos Editora. 63 p.
- MAY, RM., 1990. Taxonomy as destiny. Nature, vol. 347, no. 6289, p. 129-130. doi:10.1038/347129a0
- MORENO, JA., 2001. Métodos para medir la biodiversidad. Zaragoza: Unesco and Sociedad Entomologica Aragonesa. 83 p.
- NOUHUYS, S., 2005. Effects of habitat fragmentation at different trophic levels in insect communities. Annales Zoologici Fennici, vol. 42, no. 4, p. 433-447.
- ODUM, EP., 1985. Ecologia. São Paulo: Interamericana. 434 p.
- PAARMANN, W., ADIS, J., STORK, N., GUTZMANN, B., STUMPE, P., STARITZ, B., BOLTE, H., KÜPPERS, S., HOLZKAMP, K., NIERS, C. and FONSECA, CRV., 2001. The structure of ground beetle assemblages Coleoptera: Carabidae at fig fruit falls Moraceae in terra firme rain forest near Manaus Brazil. Journal of Tropical Ecology, vol. 17, no. 4, p. 549-561. doi:10.1017/S0266467401001419
- PANZER, R. and SCHWARTZ, MW., 1998. Effectiveness of a vegetation-based approach to insect conservation. Conservation Biology, vol. 12, no. 3, p. 693-702. doi:10.1046/j.1523-1739.1998.97051.x
- PEET, RK., 1974. The measurement of species diversity. Annual Review of Ecology and Systematics, vol. 5, p. 285-307. doi:10.1146/annurev.es.05.110174.001441
- PERNER, J. and MALT, S., 2003. Assessment of changing agricultural land use: response of vegetation, ground-dwelling spiders and beetles to the conversion of arable land into grassland. Agriculture, Ecosystems & Environment, vol. 98, no. 1/3, p. 169-181. doi:10.1016/S0167-8809(03)00079-3
- PIELOU, EC., 1975. Ecological diversity. New York: John Wiley and Sons. 165 p.
- RAMOS, F., MARTINS, I., FARIAS, JM., SILVA, ICS., COSTA, DC. and MIRANDA, AP., 2001. Oviposition and predation by Speciomerus revoili Coleoptera, Bruchidae on seeds of Acrocomia aculeate Arecaceae in Brasília, DF, Brazil. Brazilian Journal of Biology, vol. 61, no. 3, p. 449-454. doi:10.1590/S1519-69842001000300014
- RASBAND, W., 2009. Image J 1.34 m. USA: National Institutes of Health. Available from: <http://rsb.info.nih.gov/ij/>. Access in: 01 mar 2009.
- RICE, EL., 1974. Allelopathy. New York: Academic Press. 266 p.
- ROSENBERG, DM., DANKS, HV. and LEHMKUHL, DM., 1986. Importance of insects in environmental impact assessment. Environmental Management, vol. 10, no. 6, p. 773-783. doi:10.1007/BF01867730
- SAVOLAINEN, R. and VEPSÄLÄINEN, K., 1988. A competition hierarchy among boreal ants: impact on resource partitioning and community structure. Oikos, vol. 51, no. 2, p. 135-155. doi:10.2307/3565636
- SILVA, ZL., 1978. Alelopatia e defesa de plantas. Boletim Geográfico, vol. 36, p. 90-96.
- SOBERÓN, J. and LLORENTE, J., 1993. The use of species accumulation functions for the prediction of species richness. Conservation Biology, vol. 7, no. 3, p. 480-488. doi:10.1046/j.1523-1739.1993.07030480.x
- STEINBORN, HA. and MEYER, H., 1994. Einfluß alternativer und konventioneller Landwirtschaft auf die Prädatorenfauna in Agrarökosystemen Schleswig-Holsteins Araneida, Coleoptera: Carabidae, Diptera: Dolichopodidae, Empididae, Hybotidae, Microphoridae. Faunistisch-ökologische Mitteilungen, vol. 6, p. 409-438.
- STORK, NE. and EGGLETON, P., 1992. Invertebrates as determinants and indicators of soil quality. American Journal of Alternative Agriculture, vol. 7, no. 1/2, p. 38-47.
- THOMAZINI, MJ. and THOMAZINI, APBW., 2000. A fragmentação florestal e a diversidade de insetos nas florestas tropicais úmidas. Rio Branco: Embrapa Acre. 21 p.
- -, 2002. Levantamento de insetos e análise entomofaunística em floresta, capoeira e pastagem no Sudeste Acreano. Rio Branco: Embrapa Acre. 41 p.
- VALLEJO, LR., FONSECA, CL. and GONÇALVES, DRP., 1987. Estudo comparativo da mesofauna do solo em áreas de Eucalyptus citriodora e mata secundária heterogênea. Revista Brasileira de Biologia = Brazilian Journal of Biology, vol. 47, p. 363-370.
Publication Dates
-
Publication in this collection
15 July 2011 -
Date of issue
May 2011
History
-
Received
29 Dec 2009 -
Accepted
22 Apr 2010