Abstracts
Preliminary studies showed that dorsal artery contraction mediated by acetylcholine (ACh) is blocked with indomethacin in intertidal fish (G. laevifrons). Our objective was to characterize the cholinergic pathway in several artery vessels of the I. conceptionis. Afferent and efferent branchial, dorsal and mesenteric arteries were dissected of 6 juvenile specimens, isometric tension studies were done using doses response curves (DRC) for Ach (10–13 to 10–3 M), and cholinergic pathways were obtained by blocking with atropine or indomethacin. CRC to ACh showed a pattern of high sensitivity only in efferente branchial artery and low sensibility in all vessels. Furthermore, these contractions were blocked in the presence of atropine and indomethacin in all vessels. Our results corroborate previous results observed in intertidal species that contraction induced by acetylcholine is mediated by receptors that activate a cyclooxygenase contraction pathway.
intertidal fish; vascular reactivity; acetylcholine; atropine; indomethacin; cycloxygenase
Estudos preliminares mostraram que a contração da artéria dorsal mediada por acetilcolina (ACh) é bloqueada com indometacina em peixes marinhos (G. laevifrons). Nosso objetivo foi caracterizar a via colinérgica em várias artérias de I. conceptionis. Artérias aferentes e eferentes branquiais, dorsais e mesentéricas foram dissecadas de 6 espécimes juvenis. Os estudos de tensão isométrica foram feitos utilizando-se a curva dose - resposta (CDR) para Ach (10–13 a 10–3M), e identificaram-se as vias colinérgicas, bloqueando com atropina e indometacina. CRC para ACh mostrou um padrão de alta sensibilidade na artéria eferentes branquiais e baixa sensibilidade em todos os vasos sanguineos. Essas contrações foram bloqueadas na presença de atropina e indometacina em todas as artérias avaliadas. Nossos resultados confirmam que a contração induzida por acetilcolina é mediada por receptores muscarínicos que ativam ciclo-oxigenase.
peixes marinos; reatividade vascular; acetilcolina; atropina; indometacina; ciclo-oxigenase
1 Introduction
Physiological constraints are important determinants of the distributions limits of
species and populations; however, processes associated with environmental tolerance
explaining, at the local scale, differential habitat use or, species distribution
patterns remain poorly understood. Indeed, latitude, altitude and depth all
constitute gradients, which can generate physiological diversity. Among populations,
variation in physiological traits can be environmental induced, through non-genetic,
reversible mechanisms of phenotypic flexibility or acclimatization. In line with
this, it is a transitory species Girella laevifrons (intertidal
specie) that only inhabits these environments during the juvenile stage and later
migrates to the subtidal zone (Pulgar et al.,
1999Pulgar, J., Bozinovic, F. and Ojeda, FP., 1999. Behavioral
thermoregulation in the intertidal fish Girella laevifrons
(Kyphosidae): the effect of starvation. Marine and
Freshwater Behaviour and Physiology, vol. 32, no. 1, p. 27-38.
http://dx.doi.org/10.1080/10236249909379035.
http://dx.doi.org/10.1080/10236249909379...
). Intertidal fish represent a complex organism tolerated to extreme
conditions in the microhabitats of pools during low tide. Previous results obtained
in our laboratory, performed in the dorsal artery in, showed a powerful
vasoconstriction induced by acetylcholine mediated by cyclooxygenase (Urriola-Urriola and Moraga, 2008Urriola-URRIOLA, N. and Moraga, FA., 2008. Comparative study of
cholinergic responses in doral artery between intertidal fish Girella
laevifrons and subtidal fish Isacia
conceptionis.Revista de Farmacologia de Chile, vol. 1, no. 1, p.
94.),
furthermore, studies performed in several vessels such as dorsal, mesenteric,
afferent and efferent branchial arteries in G. laevifrons, suggest
a presence of two muscarinic receptors of high and low sensibility to acetylcholine
and a coupled mechanism between muscarinic receptors and vasoconstriction mediated
by cycloxygenase (Moraga and Urriola-Urriola,
2014Moraga, FA. and Urriola-Urriola, N., 2014. Vascular function in
arteries of intertidal fish (). Girella
laevifronsKyphosidaeBrazilian Journal of Biology,
vol. 74, no. 3, p. 739-743. PMid: 25296227.). Our results are partially agreed with previous studies performed
in fish shown that acetylcholine induced contraction in all the species studied so
far (Small et al., 1990Small, SA., MacDonald, C. and Farrell, AP., 1990. Vascular
reactivity of the coronary artery in rainbow trout (Oncorhynchus mykiss). The
American Journal of Physiology, vol. 258, no. 6 Pt 2, p. R1402-R1410.
PMid:2360689.; Olson and Villa, 1991Olson, KR. and Villa, J., 1991. Evidence against endothelium-derived
relaxing factor(s) in trout vessel. The American Journal of Physiology, vol.
260, no. 29, p. 925-933.; Miller and Vanhoutte, 1992Miller, VM. and Vanhoutte, PM., 1992. Endothelium-dependent vascular
responsiveness: evolutionary aspects. In RYAN, US. and RUVANYI, GM. (Eds.).
Endothelial regulation of vascular tone. New York: Marcel Dekker Inc. p.
3-20.; Evans and Gunderson, 1998aEvans, DH. and Gunderson, MP., 1998a. Functional characterization of
a muscarinic receptor in the smooth muscle of the shark (Squalus
acanthias). Experimental. Biology Online, vol. 3, p.
3.). However, Ach-mediated
vasoconstriction suggest that a coupled Ach-COX contraction described in our study
in fish is similar to that reported in human hypertension or hypertension model in
rats (Vanhoutte et al., 2005Vanhoutte, PM., Feletou, M. and Taddei, S., 2005.
Endothelium-dependent contractions in hypertension. British Journal of
Pharmacology, vol. 144, no. 4, p. 449-458.
http://dx.doi.org/10.1038/sj.bjp.0706042. PMid:15655530
http://dx.doi.org/10.1038/sj.bjp.0706042...
; Shi et al., 2008Shi, Y., Man, RY. and Vanhoutte, PM., 2008. Two isoforms of
cyclooxygenase contribute to augmented endothelium-dependent contractions in
femoral arteries of 1-year-old rats. Acta Pharmacologica Sinica, vol. 29, no. 2,
p. 185-192. http://dx.doi.org/10.1111/j.1745-7254.2008.00749.x.
PMid:18215347
http://dx.doi.org/10.1111/j.1745-7254.20...
). In order the evaluate the
presence of this response we are evaluated in other specie Isacia
conceptionis is an abundant represent of our coast. This specie have
behaviour from the open coast and deep near of 50 m, live over rocky and sandy
bottoms, contraries to the behavior described previously in
G.laevifrons. Respect with this, I.
conceptionis feeds on small crustaceans such as isopods and amphipods
but also on polychaetes and algae (Medina et al.,
2004Medina, M., Araya, M. and Vega, C., 2004. Alimentación y relaciones
tróficas de peces costeros de la zona norte de Chile. Invest. Mar, vol. 32, no.
1, p. 33-47. http://dx.doi.org/10.4067/S0717-71782004000100004.
http://dx.doi.org/10.4067/S0717-71782004...
). It geographical distribution in the South American pacific coast
since Lobos (06° 24’ 48.2” S and 80° 51’ 6.7” W, Perú) to Talcahuano (36° 43’ 30” S
and 73° 6’ 40” W, Chile) (Sánchez, 1997Sánchez, AC., 1997. Listado taxonomico de las especies marinas
identificadas en los océanos Pacífico y Atlántico (Caribe) de Nicaragua.
Managua: Ministerio de Economía y Desarrollo/MEDE PESCA. 28 p.).
Considering that ambient conditions are stable, we can indicate that have a minor
than stress condition in comparison with G.laevifrons. In respect,
we proposed that the marine fish (Isacia conceptionis) of open
coast, could be express an acetylcholine pathway of lower vasoconstriction response.
Our objective is determining the role of acetylcholine pathway in the vascular
function in a marine fish.
2 Material and Methods
2.1 Animals
Six juvenile I.conceptionis were extracted of the Totoralillo bay (30° 17’ S, 71° 31’ W) south of Coquimbo, Chile. All specimens were taken to the laboratory at the Universidad Católica del Norte and maintained for 3-5 days in filtered recirculation containers of fresh water at 15 °C. Afterward, corporal mass (150 ± 10 g) and longitude oral-tail (21 ± 0.8 cm) were measured for each specimen. Prior to experimentation, each specimen was anaesthetized with benzocaine (1:1000) added to the container to transport and sacrificed by decapitation.
2.2 Bath organ physiology
After decapitation, arterial vessels were carefully dissected from the following
areas: branchial afferent (ABA), branchial efferent (EBA), dorsal (DA) and
mesenteric (MA) and placed in cold (4 °C) physiological saline solution (PSS).
The PSS contained (in g/L): NaCl 7.37, KCl 0.31, KH2PO4
0.46, Na2HPO4 2.02, MgSO4 0.14,
CaCl2 0.1, glucose 0.9 with pH adjusted to 7.8 (Olson and Villa, 1991Olson, KR. and Villa, J., 1991. Evidence against endothelium-derived
relaxing factor(s) in trout vessel. The American Journal of Physiology, vol.
260, no. 29, p. 925-933.). Individual
arterial ring segments of 2 mm length were mounted in a four channel small
vessel wire myograph (model 610M Danish Myotech, Denmark). The vessels were
threaded onto two tungsten wires of 40 μm in diameter and attached to a force
transducer and a micrometer for isometric measurements. All signals were
acquired by a system acquisition (Powerlab 8sp, ADInstrument, Australia) and the
data collected on a personal computer for further analysis. After mounting the
rings, the arterial segments were incubated in PSS at 15 °C and gassed with air
for 30 min. Each vessel segment was stretched to its optimal diameter,
i.e. the diameter at which it developed a contraction
response to PSS-K+, using a diameter–tension protocol as previously
described for mammalian small arteries (Stassen
et al., 1997Stassen, FR., Raat, NJ., Brouwers-Ceiler, DL., Fazzi, GE., Smits,
JF. and De Mey, JG., 1997. Angiotensin II induces media hypertrophy and
hyperreactivity in mesenteric but not epigastric small arteries of the rat.
Journal of Vascular Research, vol. 34, no. 4, p. 289-297.
http://dx.doi.org/10.1159/000159236. PMid:9256089
http://dx.doi.org/10.1159/000159236...
). In this way, the myograph permitted direct measurement
of vessel wall tension while the internal diameter was controlled.
Following an equilibration period of at least 30 min, doses response curves (DRC) were performed for KCl (5.6-125 mM) and the cholinergic agonist acetylcholine (Ach) at concentrations ranging from 10–13 at 10–3 mol/L. Afterward, DRC to Ach were performed in vessels pre-incubated for 30 min with Atropine (At, 10–5M), an Ach receptor antagonist; and indomethacin (IND, 10–5 M), a cyclooxygenase (COX) inhibitor. Between experiments, the arterial preparations were allowed to recover for at least 30-60 min to return to resting basal tension.
For the PSS-K+ solution (125 mM KCl), NaCl was replaced by an equimolar amount of KCl. All chemicals were reagent grade and purchased from Sigma Chemical (St Louis, MO, USA). The following drugs used in the study: Ach, At and IND were purchased from Sigma Chemical (St Louis, MO, USA).
2.3 Data analysis and statistics
DRC were analyzed in terms of maximal response (Rmax), sensitivity (EC50 or pEC50) to different contractile agents by fitting the individual data with a nonlinear sigmoid regression curve (Prism 4.0, Graphpad, San Diego, CA, USA). Rmax was expressed as (N/m). Sensitivity was expressed as EC50 (the concentration of agonist at which 50% of Rmax was obtained) or as pEC50 (-logEC50).
All results were expressed as mean ± SEM. A two way ANOVA for repeated measurements was used for statistical analysis of physiological variables. Differences were considered significant when p<0.05 (Primer of Biostatistical v 3.0, Mc Graw Hill).
3 Results
The calculated internal diameter for all arteries is shown in Table 1, resume an optimal diameter determined in each arteries segments of each vascular territories were of a similar internal diameter.
3.1 Response to potassium chloride (KCl)
Table 2 provides a summary for the parameter EC50 and Rmax obtained from the analysis of the arterial response curves. No differences were observed in the EC50 in any of the arteries evaluated. In contrast, Rmax increased significantly in the DA and ABA when compared to those obtained from MA and EBA.
3.2 Responses to acetylcholine
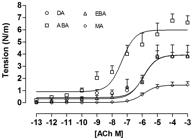
Comparison of all vascular beds showed that ABA had a higher tension to Ach (Figure 1). DRC revealed a pattern of higher sensitivity with lower doses (10–10 – 10–8 M) of Ach and lower sensitivity with higher doses (10–6 – 10–3 M) of Ach in ABA and minor magnitude in DA. Lower concentrations of Ach produced an increase in tension near to 38% of the maximal response in these vessels. Table 2 summarizes the pEC50 and Rmax values obtained from analysis of the arterial curves. A major pEC50 was observed in ABA as compared to EBA and MA. However, a higher Rmax was found in the ABA as compared to those observed in the EBA, MA and DA (p<0.05). Furthermore, EBA and DA as a major Rmax than MA (Table 2).
Acetylcholine dose-response curves of isolated rings from: afferent branchial artery (ABA; open squares); efferent branchial artery (EBA, open triangle); mesenteric artery (MA, open rhombus) and dorsal artery (DA, open circles). Each point represents the mean±S.E.M.
3.3 Response blockade with Atropine
In order to evaluate if the arterial contraction observed in the presence of Ach was blocked with At (10–5 M), DRC to Ach were performed in all arterial vessels. The data shows that the contractions induced by ACh were abolished in the presence of At in all vessels studied. Table 2, summarizes the pEC50 and Rmax values obtained from analysis of the arterial curves.
3.4 Response blockade with IND
In order to evaluate the mechanism of contraction induced by Ach, we blocked with IND (10–5 M) and DRC to Ach were performed in all arterial vessels. Contraction induced by Ach was abolished in presence of IND, in EBA and AM. In contrast, Rmax was maintained to ABA and DA. Table 2 summarizes the pEC50 and Rmax values obtained from analysis of the arterial curves.
4 Discussion
Our study demonstrates that marine fish I. conceptionis possess a vasoconstrictor mechanism mediated by Ach coupled to cyclooxygenase pathway. Furthermore, suggest the presence of two muscarinic receptors: one high of sensitivity and a second of lower sensitivity coupled to different mechanisms of activation, only in the ABA and DA. By other way, suggest functionally presence on one muscarinic receptor in EBA and MA territories.
4.1 Vascular response to acetylcholine
Our results show that all vessels studied (ABA, EBA, MA and DA) express a vasoconstriction mediated by Ach.
The vasoconstriction observed in I. conceptionis suggests the
presence of two receptor populations present in the ABA and DA. The first
response was characterized by a high sensitivity to a range of 10–10
to 10–8 M of Ach; this response was 38% of the maximum response
observed only in ABA and DA. These response of high sensitivity to Ach was
previously described in rings DA, ABA, EBA and MA in isometric studies of
vascular reactivity in Girella laevifrons (Moraga and Urriola-Urriola, 2014Moraga, FA. and Urriola-Urriola, N., 2014. Vascular function in
arteries of intertidal fish (). Girella
laevifronsKyphosidaeBrazilian Journal of Biology,
vol. 74, no. 3, p. 739-743. PMid: 25296227.), also in
perfused trunks in cod (Gadus morhua) spleen which displayed
contractions to Ach at concentrations between 10–10 and
2x10–9 M (Nilsson and Grove,
1974Nilsson, S. and Grove, DJ., 1974. Adrenergic and cholinergic
innervation of the spleen of the cod: Gadus morhua. European Journal of
Pharmacology, vol. 28, no. 1, p. 135-143.
http://dx.doi.org/10.1016/0014-2999(74)90124-1. PMid:4430318
http://dx.doi.org/10.1016/0014-2999(74)9...
). Head isolation preparations in the icefish Chionodraco
hamatus (Pellegrino et al.,
2003Pellegrino, D., Acierno, R. and Tota, B., 2003. Control of
cardiovascular function in the icefish Chionodraco hamatus: involvement of
serotonin and nitric oxide. Comparative Biochemistry and Physiology. Part A,
Molecular & Integrative Physiology, vol. 134, no. 2, p. 471-480.
http://dx.doi.org/10.1016/S1095-6433(02)00324-0. PMid:12547277
http://dx.doi.org/10.1016/S1095-6433(02)...
) exhibited a 30% maximum contraction response to Ach
(10–10 M) and this response was blocked by At (10–5
M). Moreover, in branchial circulation of the eel, a bimodal response to very
low concentrations of Ach (10–12-10–10 M) and a dilation
response at higher concentrations which produced a contraction, were both
blocked by atropine (10–5 M) (Pellegrino et al., 2002Pellegrino, D., Sprovieri, E., Mazza, R., Randall, DJ. and Tota, B.,
2002. Nitric oxide-cGMP-mediated vasoconstriction and effects of acetylcholine
in the branchial circulation of the eel. Comparative Biochemistry and
Physiology. Part A, Molecular & Integrative Physiology, vol. 132, no. 2, p.
447-457. http://dx.doi.org/10.1016/S1095-6433(02)00082-X.
PMid:12020661
http://dx.doi.org/10.1016/S1095-6433(02)...
). This effect could suggest the presence of
two muscarinic receptors with different sensitivities and functions. These first
and second response only was observed ABA in our preparation at concentrations
between 10–6 and 10–3 is in agreement with a low
sensitivity. In our preparation, EBA, MA and DA, we only observe the response
equivalent to low sensibility to Ach (10–6 to 10–3 M). The
range described in our study was similar to that described in vascular ring
preparations between the ranges of 10–8 to 10–6 M
(coronary, celiac mesenteric, central aorta, efferent branchial artery) in trout
(Olson and Villa, 1991Olson, KR. and Villa, J., 1991. Evidence against endothelium-derived
relaxing factor(s) in trout vessel. The American Journal of Physiology, vol.
260, no. 29, p. 925-933.; Small et al., 1990Small, SA., MacDonald, C. and Farrell, AP., 1990. Vascular
reactivity of the coronary artery in rainbow trout (Oncorhynchus mykiss). The
American Journal of Physiology, vol. 258, no. 6 Pt 2, p. R1402-R1410.
PMid:2360689.) and in the ventral
aorta in sharks (Evans and Gunderson,
1998aEvans, DH. and Gunderson, MP., 1998a. Functional characterization of
a muscarinic receptor in the smooth muscle of the shark (Squalus
acanthias). Experimental. Biology Online, vol. 3, p.
3., bEvans, DH. and Gunderson, MP., 1998b. A prostaglandin, not NO,
mediates endothelium-dependent dilation in ventral aorta of shark (Squalus
acanthias). The American Journal of Physiology, vol. 274, no. 4 Pt 2, p.
R1050-R1057. PMid:9575968.). In addition, all
previously described studies found only one component in the CRC to Ach. In our
study, both responses (high and low sensitivities) were abolished by the use of
At (10–5 M). This evidence is corroborated by other studies in trout,
shark and eel (Nilsson and Grove, 1974Nilsson, S. and Grove, DJ., 1974. Adrenergic and cholinergic
innervation of the spleen of the cod: Gadus morhua. European Journal of
Pharmacology, vol. 28, no. 1, p. 135-143.
http://dx.doi.org/10.1016/0014-2999(74)90124-1. PMid:4430318
http://dx.doi.org/10.1016/0014-2999(74)9...
;
Small et al., 1990Small, SA., MacDonald, C. and Farrell, AP., 1990. Vascular
reactivity of the coronary artery in rainbow trout (Oncorhynchus mykiss). The
American Journal of Physiology, vol. 258, no. 6 Pt 2, p. R1402-R1410.
PMid:2360689.; Pellegrino et al., 2002Pellegrino, D., Sprovieri, E., Mazza, R., Randall, DJ. and Tota, B.,
2002. Nitric oxide-cGMP-mediated vasoconstriction and effects of acetylcholine
in the branchial circulation of the eel. Comparative Biochemistry and
Physiology. Part A, Molecular & Integrative Physiology, vol. 132, no. 2, p.
447-457. http://dx.doi.org/10.1016/S1095-6433(02)00082-X.
PMid:12020661
http://dx.doi.org/10.1016/S1095-6433(02)...
; Pellegrino et al., 2003Pellegrino, D., Acierno, R. and Tota, B., 2003. Control of
cardiovascular function in the icefish Chionodraco hamatus: involvement of
serotonin and nitric oxide. Comparative Biochemistry and Physiology. Part A,
Molecular & Integrative Physiology, vol. 134, no. 2, p. 471-480.
http://dx.doi.org/10.1016/S1095-6433(02)00324-0. PMid:12547277
http://dx.doi.org/10.1016/S1095-6433(02)...
; Moraga and Urriola-Urriola, 2014Moraga, FA. and Urriola-Urriola, N., 2014. Vascular function in
arteries of intertidal fish (). Girella
laevifronsKyphosidaeBrazilian Journal of Biology,
vol. 74, no. 3, p. 739-743. PMid: 25296227.),
supporting the presence of muscarinic receptors in fish vasculature.
Five muscarinic receptors in vascular smooth muscle have been identified by
functional, pharmacological and molecular studies in a variety of fish and
mammalian (agnathians, elasmobranch and teleosts) (Hulme et al., 1990HULME, EC., BIRDSALL, NJM. and BUCKLEY, NJ., 1990. Muscarinic
receptors subtypes. Annual Review of Pharmacology and Toxicology, vol. 30, p.
633-673.; Caulfield, 1993Caulfield, MP., 1993. Muscarinic receptors—characterization,
coupling and function. Pharmacology & Therapeutics, vol. 58, no. 3, p.
319-379. http://dx.doi.org/10.1016/0163-7258(93)90027-B.
PMid:7504306
http://dx.doi.org/10.1016/0163-7258(93)9...
; Eglen et al.,
1996Eglen, RM., Hegde, SS. and Watson, N., 1996. Muscarinic receptor
subtypes and smooth muscle function. Pharmacological Reviews, vol. 48, no. 4, p.
531-565. PMid:8981565.; Ehlert et al., 1997Ehlert, FJ., Ostrom, RS. and Sawyer, GW., 1997. Subtypes of the
muscarinic receptor in smooth muscle. Life Sciences, vol. 61, no. 18, p.
1729-1740. http://dx.doi.org/10.1016/S0024-3205(97)00433-5.
PMid:9365220
http://dx.doi.org/10.1016/S0024-3205(97)...
;
Evans and Gunderson, 1998aEvans, DH. and Gunderson, MP., 1998a. Functional characterization of
a muscarinic receptor in the smooth muscle of the shark (Squalus
acanthias). Experimental. Biology Online, vol. 3, p.
3.). In
fish, Ach induced contraction in all the species studied so far (Small et al., 1990Small, SA., MacDonald, C. and Farrell, AP., 1990. Vascular
reactivity of the coronary artery in rainbow trout (Oncorhynchus mykiss). The
American Journal of Physiology, vol. 258, no. 6 Pt 2, p. R1402-R1410.
PMid:2360689.; Olson and Villa, 1991Olson, KR. and Villa, J., 1991. Evidence against endothelium-derived
relaxing factor(s) in trout vessel. The American Journal of Physiology, vol.
260, no. 29, p. 925-933.; Miller and Vanhoutte, 1992Miller, VM. and Vanhoutte, PM., 1992. Endothelium-dependent vascular
responsiveness: evolutionary aspects. In RYAN, US. and RUVANYI, GM. (Eds.).
Endothelial regulation of vascular tone. New York: Marcel Dekker Inc. p.
3-20.; Evans
and Gunderson, 1998aEvans, DH. and Gunderson, MP., 1998a. Functional characterization of
a muscarinic receptor in the smooth muscle of the shark (Squalus
acanthias). Experimental. Biology Online, vol. 3, p.
3.). This contractile response was found even in
the presence or absence of endothelium (Olson
and Villa, 1991Olson, KR. and Villa, J., 1991. Evidence against endothelium-derived
relaxing factor(s) in trout vessel. The American Journal of Physiology, vol.
260, no. 29, p. 925-933.; Miller and
Vanhoutte, 1992Miller, VM. and Vanhoutte, PM., 1992. Endothelium-dependent vascular
responsiveness: evolutionary aspects. In RYAN, US. and RUVANYI, GM. (Eds.).
Endothelial regulation of vascular tone. New York: Marcel Dekker Inc. p.
3-20.; Evans and
Gunderson, 1998bEvans, DH. and Gunderson, MP., 1998b. A prostaglandin, not NO,
mediates endothelium-dependent dilation in ventral aorta of shark (Squalus
acanthias). The American Journal of Physiology, vol. 274, no. 4 Pt 2, p.
R1050-R1057. PMid:9575968.; Miller and
Vanhoutte, 2000Miller, VM. and Vanhoutte, PM., 2000. Prostaglandins but not nitric
oxide are endothelium-derived relaxing factors in the trout aorta. Acta
Pharmacologica Sinica, vol. 21, no. 10, p. 871-876.
PMid:11501036.). Furthermore, a pharmacological study done in sharks
found the presence of functional muscarinic receptors type 1 and 3, and non
functional muscarinic receptors types 2 and 4 (Evans and Gunderson, 1998aEvans, DH. and Gunderson, MP., 1998a. Functional characterization of
a muscarinic receptor in the smooth muscle of the shark (Squalus
acanthias). Experimental. Biology Online, vol. 3, p.
3.). By other way, in
mammals, Ach induces vascular smooth muscle relaxation by activation of
muscarinic receptors expressed in endothelial cells (Furchgott and Zawadzki, 1980FURCHGOTT, RF. and ZAWADZKI, JV., 1980. The obligatory role of the
endothelial cells in the relaxation of arterial smooth muscle by acetylcholine.
Nature, vol. 288, p. 373-376.). Molecular and
pharmacological approach has demonstrated the presence of muscarinic receptors
type 1-3 in arterial vessels (Norel et al.,
1996Norel, X., Walch, L., Costantino, M., Labat, C., Gorenne, I.,
Dulmet, E., Rossi, F. and Brink, C., 1996. M1 and M3 muscarinic receptors in
human pulmonary arteries. British Journal of Pharmacology, vol. 119, no. 1, p.
149-157. http://dx.doi.org/10.1111/j.1476-5381.1996.tb15688.x.
PMid:8872368
http://dx.doi.org/10.1111/j.1476-5381.19...
; Kawashima and Fujii,
2008Kawashima, K. and Fujii, T., 2008. Basic and clinical aspects of
non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and
their biological significance. Journal of Pharmacological Sciences, vol. 106,
no. 2, p. 167-173. http://dx.doi.org/10.1254/jphs.FM0070073.
PMid:18285657
http://dx.doi.org/10.1254/jphs.FM0070073...
). Additionally, studies performed in human pulmonary arteries
suggest the presence of muscarinic receptor type 3 in smooth muscle mediating
the Ach-induced contraction and muscarinic receptors type 1 are involved in the
endothelium-dependent Ach-induced relaxation (Norel et al., 1996Norel, X., Walch, L., Costantino, M., Labat, C., Gorenne, I.,
Dulmet, E., Rossi, F. and Brink, C., 1996. M1 and M3 muscarinic receptors in
human pulmonary arteries. British Journal of Pharmacology, vol. 119, no. 1, p.
149-157. http://dx.doi.org/10.1111/j.1476-5381.1996.tb15688.x.
PMid:8872368
http://dx.doi.org/10.1111/j.1476-5381.19...
). However, Ach-mediated vasoconstriction suggest
that a coupled Ach-COX contraction described in our study in fish is similar to
that reported in human hypertension or hypertension model in rats (Ge et al., 1995Ge, T., Hughes, H., Junquero, DC., Wu, KK., Vanhoutte, PM. and
Boulanger, CM., 1995. Endothelium-dependent contractions are associated with
both augmented expression of prostaglandin H synthase-1 and hypersensitivity to
prostaglandin H2 in the SHR aorta. Circulation Research, vol. 76, no. 6, p.
1003-1010. http://dx.doi.org/10.1161/01.RES.76.6.1003.
PMid:7758154
http://dx.doi.org/10.1161/01.RES.76.6.10...
; Vanhoutte et al., 2005Vanhoutte, PM., Feletou, M. and Taddei, S., 2005.
Endothelium-dependent contractions in hypertension. British Journal of
Pharmacology, vol. 144, no. 4, p. 449-458.
http://dx.doi.org/10.1038/sj.bjp.0706042. PMid:15655530
http://dx.doi.org/10.1038/sj.bjp.0706042...
; Shi et al., 2008Shi, Y., Man, RY. and Vanhoutte, PM., 2008. Two isoforms of
cyclooxygenase contribute to augmented endothelium-dependent contractions in
femoral arteries of 1-year-old rats. Acta Pharmacologica Sinica, vol. 29, no. 2,
p. 185-192. http://dx.doi.org/10.1111/j.1745-7254.2008.00749.x.
PMid:18215347
http://dx.doi.org/10.1111/j.1745-7254.20...
).
4.2 Acetylcholine-cyclooxygenases vasoconstriction pathway
Our results demonstrate that the Ach-mediated vasoconstriction in all the vessels studied (ABA, EBA, MA and DA) was abolished with At (10–5 M), indicating the presence of muscarinic receptors that promote vascular vasoconstriction in marine fish. However, when vessels were blocked by IND (10–5 M), the high sensitivity Ach-mediated vasoconstriction, previously described, was practically abolished in all the vessels studied, suggesting a coupling mechanism between activation of muscarinic receptors with the production of vasoconstrictor prostanoids mediated by COX in I. conceptionis. This effect did not modify the low sensitivity Ach-mediated vasoconstriction suggesting that this mechanism is mediated by muscarinic receptors in the vascular smooth muscle present in DA and ABA. In contrast, the low sensitivity Ach-mediated vasoconstriction was abolished in EBA and MA. Strategy to corroborate the participation of endothelium in the response described in our study is performing studies without endothelium in presence or absent of Ach plus COX inhibitor. Unfortunately, this design didn’t perform in our study.
In contrast, studies performed in shark (Squalus acanthias) or
trout, it has been shown that an endothelium-dependent dilation in the presence
of a calcium -ionophore (A-23187) promoted dilation by a prostaglandin and not
by NO (Evans and Gunderson, 1998bEvans, DH. and Gunderson, MP., 1998b. A prostaglandin, not NO,
mediates endothelium-dependent dilation in ventral aorta of shark (Squalus
acanthias). The American Journal of Physiology, vol. 274, no. 4 Pt 2, p.
R1050-R1057. PMid:9575968.; Olson and Villa, 1991Olson, KR. and Villa, J., 1991. Evidence against endothelium-derived
relaxing factor(s) in trout vessel. The American Journal of Physiology, vol.
260, no. 29, p. 925-933.; Miller and Vanhoutte, 2000Miller, VM. and Vanhoutte, PM., 2000. Prostaglandins but not nitric
oxide are endothelium-derived relaxing factors in the trout aorta. Acta
Pharmacologica Sinica, vol. 21, no. 10, p. 871-876.
PMid:11501036.) suggesting the
presence of dilation mediated by prostanoids (Evans and Gunderson, 1998bEvans, DH. and Gunderson, MP., 1998b. A prostaglandin, not NO,
mediates endothelium-dependent dilation in ventral aorta of shark (Squalus
acanthias). The American Journal of Physiology, vol. 274, no. 4 Pt 2, p.
R1050-R1057. PMid:9575968.; Miller
and Vanhoutte, 2000Miller, VM. and Vanhoutte, PM., 2000. Prostaglandins but not nitric
oxide are endothelium-derived relaxing factors in the trout aorta. Acta
Pharmacologica Sinica, vol. 21, no. 10, p. 871-876.
PMid:11501036.). We proposed that are necessaries performing
studies of vascular function that evaluate others mechanisms that could be
explain this response, such as nitric oxide, monoxide or H2S role
dilation in vascular response in fish (Feng et
al., 2007Feng, J., Yano, K., Monahan-Earley, R., Morgan, ES., Dvorak, AM.,
Sellke, FW. and AIRD, WC., 2007. Vascular bed-specific endothelium-dependent
vasomomotor relaxation in the hagfish, Myxine glutinosa. American Journal of
Physiology - Regulatory, Integrative and Comparative Physiology, vol. 293, p.
R894-R900.; Dombkowski et al.,
2009Dombkowski, RA., Whitfield, NL., Motterlini, R., Gao, Y. and Olson,
KR., 2009. Effects of carbon monoxide on trout and lamprey vessels. American
Journal of Physiology. Regulatory, Integrative and Comparative Physiology, vol.
296, no. 1, p. R141-R149. http://dx.doi.org/10.1152/ajpregu.90507.2008.
PMid:19005018
http://dx.doi.org/10.1152/ajpregu.90507....
; Jennings and Donald.,
2010Jennings, BL. and Donald, JA., 2010. Mechanisms of nitric
oxide-mediated, neurogenic vasodilation in mesenteric resistance arteries of
toad Bufo marinus. American Journal of Physiology - Regulatory, Integrative and
Comparative Physiology, vol. 298, p. R767–R775,).
In conclusion, we propose that the marine fish I. conceptionis have muscarinic receptors type 1 and 3 with one muscarinic receptor having a high sensitivity that could be located in the endothelium coupled to COX that produce a prostanoid vasoconstrictor, and another muscarinic receptor having a low sensitivity located in the vascular smooth muscle. However, the type of muscarinic receptor and mechanisms underlying this response still needs to be evaluated.
Acknowledgements
We are grateful to technical assistance mr. Hervis Galleguillos and students of medicine mss Carolina Norero, Daniela Gonzalez, Natalia Soto and Marietta Nuñez that collaborate in the experiments. This research was partially supported by grant Bicentenario #31101042 and DGIP.
References
- Caulfield, MP., 1993. Muscarinic receptors—characterization, coupling and function. Pharmacology & Therapeutics, vol. 58, no. 3, p. 319-379. http://dx.doi.org/10.1016/0163-7258(93)90027-B. PMid:7504306
» http://dx.doi.org/10.1016/0163-7258(93)90027-B - Dombkowski, RA., Whitfield, NL., Motterlini, R., Gao, Y. and Olson, KR., 2009. Effects of carbon monoxide on trout and lamprey vessels. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, vol. 296, no. 1, p. R141-R149. http://dx.doi.org/10.1152/ajpregu.90507.2008. PMid:19005018
» http://dx.doi.org/10.1152/ajpregu.90507.2008 - Eglen, RM., Hegde, SS. and Watson, N., 1996. Muscarinic receptor subtypes and smooth muscle function. Pharmacological Reviews, vol. 48, no. 4, p. 531-565. PMid:8981565.
- Ehlert, FJ., Ostrom, RS. and Sawyer, GW., 1997. Subtypes of the muscarinic receptor in smooth muscle. Life Sciences, vol. 61, no. 18, p. 1729-1740. http://dx.doi.org/10.1016/S0024-3205(97)00433-5. PMid:9365220
» http://dx.doi.org/10.1016/S0024-3205(97)00433-5 - Evans, DH. and Gunderson, MP., 1998a. Functional characterization of a muscarinic receptor in the smooth muscle of the shark (Squalus acanthias). Experimental. Biology Online, vol. 3, p. 3.
- Evans, DH. and Gunderson, MP., 1998b. A prostaglandin, not NO, mediates endothelium-dependent dilation in ventral aorta of shark (Squalus acanthias). The American Journal of Physiology, vol. 274, no. 4 Pt 2, p. R1050-R1057. PMid:9575968.
- Feng, J., Yano, K., Monahan-Earley, R., Morgan, ES., Dvorak, AM., Sellke, FW. and AIRD, WC., 2007. Vascular bed-specific endothelium-dependent vasomomotor relaxation in the hagfish, Myxine glutinosa. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, vol. 293, p. R894-R900.
- FURCHGOTT, RF. and ZAWADZKI, JV., 1980. The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature, vol. 288, p. 373-376.
- Ge, T., Hughes, H., Junquero, DC., Wu, KK., Vanhoutte, PM. and Boulanger, CM., 1995. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circulation Research, vol. 76, no. 6, p. 1003-1010. http://dx.doi.org/10.1161/01.RES.76.6.1003. PMid:7758154
» http://dx.doi.org/10.1161/01.RES.76.6.1003 - Jennings, BL. and Donald, JA., 2010. Mechanisms of nitric oxide-mediated, neurogenic vasodilation in mesenteric resistance arteries of toad Bufo marinus. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, vol. 298, p. R767–R775,
- HULME, EC., BIRDSALL, NJM. and BUCKLEY, NJ., 1990. Muscarinic receptors subtypes. Annual Review of Pharmacology and Toxicology, vol. 30, p. 633-673.
- Kawashima, K. and Fujii, T., 2008. Basic and clinical aspects of non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and their biological significance. Journal of Pharmacological Sciences, vol. 106, no. 2, p. 167-173. http://dx.doi.org/10.1254/jphs.FM0070073. PMid:18285657
» http://dx.doi.org/10.1254/jphs.FM0070073 - Medina, M., Araya, M. and Vega, C., 2004. Alimentación y relaciones tróficas de peces costeros de la zona norte de Chile. Invest. Mar, vol. 32, no. 1, p. 33-47. http://dx.doi.org/10.4067/S0717-71782004000100004.
» http://dx.doi.org/10.4067/S0717-71782004000100004 - Miller, VM. and Vanhoutte, PM., 1992. Endothelium-dependent vascular responsiveness: evolutionary aspects. In RYAN, US. and RUVANYI, GM. (Eds.). Endothelial regulation of vascular tone. New York: Marcel Dekker Inc. p. 3-20.
- Miller, VM. and Vanhoutte, PM., 2000. Prostaglandins but not nitric oxide are endothelium-derived relaxing factors in the trout aorta. Acta Pharmacologica Sinica, vol. 21, no. 10, p. 871-876. PMid:11501036.
- Moraga, FA. and Urriola-Urriola, N., 2014. Vascular function in arteries of intertidal fish (). Girella laevifronsKyphosidaeBrazilian Journal of Biology, vol. 74, no. 3, p. 739-743. PMid: 25296227.
- Nilsson, S. and Grove, DJ., 1974. Adrenergic and cholinergic innervation of the spleen of the cod: Gadus morhua. European Journal of Pharmacology, vol. 28, no. 1, p. 135-143. http://dx.doi.org/10.1016/0014-2999(74)90124-1. PMid:4430318
» http://dx.doi.org/10.1016/0014-2999(74)90124-1 - Norel, X., Walch, L., Costantino, M., Labat, C., Gorenne, I., Dulmet, E., Rossi, F. and Brink, C., 1996. M1 and M3 muscarinic receptors in human pulmonary arteries. British Journal of Pharmacology, vol. 119, no. 1, p. 149-157. http://dx.doi.org/10.1111/j.1476-5381.1996.tb15688.x. PMid:8872368
» http://dx.doi.org/10.1111/j.1476-5381.1996.tb15688.x - Olson, KR. and Villa, J., 1991. Evidence against endothelium-derived relaxing factor(s) in trout vessel. The American Journal of Physiology, vol. 260, no. 29, p. 925-933.
- Pellegrino, D., Sprovieri, E., Mazza, R., Randall, DJ. and Tota, B., 2002. Nitric oxide-cGMP-mediated vasoconstriction and effects of acetylcholine in the branchial circulation of the eel. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, vol. 132, no. 2, p. 447-457. http://dx.doi.org/10.1016/S1095-6433(02)00082-X. PMid:12020661
» http://dx.doi.org/10.1016/S1095-6433(02)00082-X - Pellegrino, D., Acierno, R. and Tota, B., 2003. Control of cardiovascular function in the icefish Chionodraco hamatus: involvement of serotonin and nitric oxide. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, vol. 134, no. 2, p. 471-480. http://dx.doi.org/10.1016/S1095-6433(02)00324-0. PMid:12547277
» http://dx.doi.org/10.1016/S1095-6433(02)00324-0 - Pulgar, J., Bozinovic, F. and Ojeda, FP., 1999. Behavioral thermoregulation in the intertidal fish Girella laevifrons (Kyphosidae): the effect of starvation. Marine and Freshwater Behaviour and Physiology, vol. 32, no. 1, p. 27-38. http://dx.doi.org/10.1080/10236249909379035.
» http://dx.doi.org/10.1080/10236249909379035 - Shi, Y., Man, RY. and Vanhoutte, PM., 2008. Two isoforms of cyclooxygenase contribute to augmented endothelium-dependent contractions in femoral arteries of 1-year-old rats. Acta Pharmacologica Sinica, vol. 29, no. 2, p. 185-192. http://dx.doi.org/10.1111/j.1745-7254.2008.00749.x. PMid:18215347
» http://dx.doi.org/10.1111/j.1745-7254.2008.00749.x - Sánchez, AC., 1997. Listado taxonomico de las especies marinas identificadas en los océanos Pacífico y Atlántico (Caribe) de Nicaragua. Managua: Ministerio de Economía y Desarrollo/MEDE PESCA. 28 p.
- Small, SA., MacDonald, C. and Farrell, AP., 1990. Vascular reactivity of the coronary artery in rainbow trout (Oncorhynchus mykiss). The American Journal of Physiology, vol. 258, no. 6 Pt 2, p. R1402-R1410. PMid:2360689.
- Stassen, FR., Raat, NJ., Brouwers-Ceiler, DL., Fazzi, GE., Smits, JF. and De Mey, JG., 1997. Angiotensin II induces media hypertrophy and hyperreactivity in mesenteric but not epigastric small arteries of the rat. Journal of Vascular Research, vol. 34, no. 4, p. 289-297. http://dx.doi.org/10.1159/000159236. PMid:9256089
» http://dx.doi.org/10.1159/000159236 - Urriola-URRIOLA, N. and Moraga, FA., 2008. Comparative study of cholinergic responses in doral artery between intertidal fish Girella laevifrons and subtidal fish Isacia conceptionis.Revista de Farmacologia de Chile, vol. 1, no. 1, p. 94.
- Vanhoutte, PM., Feletou, M. and Taddei, S., 2005. Endothelium-dependent contractions in hypertension. British Journal of Pharmacology, vol. 144, no. 4, p. 449-458. http://dx.doi.org/10.1038/sj.bjp.0706042. PMid:15655530
» http://dx.doi.org/10.1038/sj.bjp.0706042
-
(With 1 figure)
Publication Dates
-
Publication in this collection
May 2015
History
-
Received
25 July 2013 -
Accepted
16 Jan 2014