Abstract
Plants that have potential as alternative food source (floral nectar, pollen and plant tissues) to the boll weevil during the intercropping season were evaluated considering the prevalent conditions of Cerrado in the Central Brazil. Initially, we tested the nutritional adequacy for the survival of the insect of flower resource (pollen and nectar) provided by eight plant species (fennel, mexican sunflower, castor bean, okra, hibiscus, sorghum, pigeonpea and sunn hemp). Subsequently, we tested if the resources provided by the selected plants continued to be exploited by the boll weevil in the presence of cotton plant, its main food source average longevity of boll weevil adults was significantly longer when they were fed on hibiscus’ flowers (166.6 ± 74.4) and okra flowers (34.7 ± 28.9) than when they fed on flowers of other six species. Subsequently, the preference of the boll weevil in the use of resources was compared between okra or hibiscus and cotton plants, in dual choice experiments. Boll weevils preferred plants of the three species in the reproductive stages than those in vegetative stages. Although the cotton plant in the reproductive stage was the most preferred plant of all, boll weevils preferred flowering okra and hibiscus than cotton at the vegetative stage.
Keywords:
Anthonomus grandis; Malvacea; okra; hibiscus
Resumo
Plantas que podem ser uma fonte potencial de recursos alimentares (néctar floral, pólen e tecidos vegetais) para o bicudo do algodoeiro durante a entressafra foram avaliadas, considerando as condições ambientais prevalentes na região de Cerrado do Brasil Central. Primeiro, testamos a adequação nutricional dos recursos (pólen e néctar) fornecidos por oito espécies de plantas (erva-doce, margaridão, mamona, quiabo, hibisco, sorgo, feijão guandu e crotalária) como único recurso alimentar para a sobrevivência do inseto. Posteriormente, nós avaliamos se os recursos fornecidos pelas plantas selecionadas continuaram a serem explorados pelo bicudo na presença do algodoeiro, seu recurso alimentar principal. A longevidade média do bicudo do algodoeiro foi significativamente maior quando eles foram alimentados com flores de hibisco (166,6 ± 74,4) e quiabo (34,7 ± 28,9) do que quando alimentados com flores das outras seis espécies. Em seguida, a preferência do bicudo no uso de recursos foi comparada contrastando o quiabo e o hibisco com o algodão, em experimentos de dupla escolha. Os bicudos preferiram as plantas das três espécies na fase reprodutiva em relação àquelas em estádios vegetativos. Embora a preferência por plantas de algodão na fase reprodutiva tenha sido maior, os bicudos preferiram plantas em floração de quiabo e hibisco quando estas foram contrastadas com o algodão na fase vegetativa.
Palavras-chave:
Anthonomus grandis; Malvacea; quiabo; hibisco
1 Introduction
The boll weevil, Anthonomus grandis Boheman 1843 (Coleoptera: Curculionidae), a serious pest of upland cotton, Gossypium hirsutum L. (Malvaceae), has caused severe damage to cotton production in the Americas for decades. Adult boll weevils prefer the reproductive structures, where they feed and lay eggs and where the larvae develop to pupal stage (Showler, 2004Showler, A.T., 2004. Influence of cotton fruit stages as food sources on boll weevil (Coleoptera: Curculionidae) fecundity and oviposition. Journal of Economic Entomology, vol. 97, no. 4, pp. 1330-1334. http://dx.doi.org/10.1093/jee/97.4.1330. PMid:15384345.
http://dx.doi.org/10.1093/jee/97.4.1330...
). Due to its feeding inside the reproductive structures, boll weevil attacks suppress the production of cotton fibers (Degrande, 1991Degrande, P.E., 1991. Aspectos biológicos do bicudo. In: E.P. DEGRANDE, ed. Bicudo do algodoeiro: manejo integrado. Campo Grande: UFMS, pp. 11-27.). Additionally, this species has a high reproductive capacity and is capable of rapid population growth (Cross, 1973Cross, W.H., 1973. Biology, control, and eradication of the boll weevil. Annual Review of Entomology, vol. 18, no. 1, pp. 17-46. http://dx.doi.org/10.1146/annurev.en.18.010173.000313.
http://dx.doi.org/10.1146/annurev.en.18....
) and therefore, the small population that survives the dry season in Central Brazil can infest and severely damage the next cotton crop. The biological and behavioral traits of the boll weevil hinder the implementation of control measures because this species is largely protected from natural enemies, adverse environmental conditions and insecticide spraying (Ramalho and Wanderley, 1995Ramalho, F.S. and Wanderley, P.A., 1995. Ecology and management of the boll weevil in South American cotton. American Entomologist, vol. 42, no. 1, pp. 41-47. http://dx.doi.org/10.1093/ae/42.1.41.
http://dx.doi.org/10.1093/ae/42.1.41...
; Santos et al., 2013Santos, R.L., Neves, R.C.S., Colares, F. and Torres, J.B., 2013. Parasitóides do bicudo Anthonomus grandis e predadores residentes em algodoeiro pulverizado com caulim. Semina: Ciências Agrárias, vol. 34, pp. 3463-3474.; Neves et al., 2014Neves, R., Colares, F., Torres, J.B., Santos, R.L. and Bastos, C.S., 2014. Rational practices to manage boll weevils colonization and population growth on family farms in the semiárido region of Brazil. Insects, vol. 5, no. 4, pp. 818-831. http://dx.doi.org/10.3390/insects5040818. PMid:26462942.
http://dx.doi.org/10.3390/insects5040818...
).
The great genetic variability and phenotypic plasticity of the boll weevil makes it able to adapt to a wide variety of environmental conditions, allowing the expansion of its geographical distribution beyond its center of origin (Central America) (Showler, 2009Showler, A.T., 2009. Roles of host plants in boll weevil range expansion beyond tropical Mesoamerica. American Entomologist, vol. 55, no. 4, pp. 234-242. http://dx.doi.org/10.1093/ae/55.4.234.
http://dx.doi.org/10.1093/ae/55.4.234...
). Populations of boll weevil native from Mesoamerican region, for example, presented dormancy, characterized by a physiological state in which the insect does not feed or reproduce at all during the harsh winter (Showler 2007Showler, A.T., 2007. Subtropical boll weevil ecology. American Entomologist, vol. 53, no. 4, pp. 240-249. http://dx.doi.org/10.1093/ae/53.4.240.
http://dx.doi.org/10.1093/ae/53.4.240...
, 2009Showler, A.T., 2009. Roles of host plants in boll weevil range expansion beyond tropical Mesoamerica. American Entomologist, vol. 55, no. 4, pp. 234-242. http://dx.doi.org/10.1093/ae/55.4.234.
http://dx.doi.org/10.1093/ae/55.4.234...
). In subtropical regions, under low temperate conditions during the winter, a minor portion of boll weevil population move into surrounding habitats where they survive and reproduce during the winter in small patches of cotton volunteer plants or survive feeding on other plants in the local vegetation in a stage of reproductive dormancy, especially in areas of citrus and cactus fruit, common in the region of Texas - USA (Spurgeon et al., 2003SPURGEON, D.W., SAPPINGTON, T.W. and SUH, C.P., 2003. A system for characterizing reproductive and diapause morphology in the boll weevil (Coleoptera: Curculionidae). Annals of the Entomological Society of America, vol. 96, no. 1, pp. 1-11. http://dx.doi.org/10.1603/0013-8746(2003)096[0001:ASFCRA]2.0.CO;2.
http://dx.doi.org/10.1603/0013-8746(2003...
; Spurgeon and Raulston, 2006Spurgeon, D.W. and Raulston, J.R., 2006. Boll weevil (Coleoptera: Curculionidae) adult diapause responses to selected environmental and dietary conditions. Annals of the Entomological Society of America, vol. 99, no. 6, pp. 1085-1100. http://dx.doi.org/10.1603/0013-8746(2006)99[1085:BWCCAD]2.0.CO;2.
http://dx.doi.org/10.1603/0013-8746(2006...
; Showler and Abrigo, 2007Showler, A.T. and Abrigo, V., 2007. Common subtropical and tropical non pollen food sources of the boll weevil (Coleoptera: Curculionidae). Environmental Entomology, vol. 36, no. 1, pp. 99-104. http://dx.doi.org/10.1093/ee/36.1.99. PMid:17349122.
http://dx.doi.org/10.1093/ee/36.1.99...
; Showler, 2009Showler, A.T., 2009. Roles of host plants in boll weevil range expansion beyond tropical Mesoamerica. American Entomologist, vol. 55, no. 4, pp. 234-242. http://dx.doi.org/10.1093/ae/55.4.234.
http://dx.doi.org/10.1093/ae/55.4.234...
, 2012Showler, A.T., 2012. The conundrum of chemical boll weevil control in subtropical regions. In: F. PERVEEN, ed. Insecticides: pest engineering. Croatia: InTech, pp. 437-448.). They stay there until the cotton plants begin to produce cotton squares in the next crop season.
It is known that insect populations living in different regions present subtle differences in their population’s response according to particular environmental conditions, especially in relation to temperature and resource availability (Tauber and Tauber, 1976TAUBER, M.J. and TAUBER, C.A., 1976. Insect seasonality: diapause maintenance, termination, and postdiapause development. Annual Review of Entomology, vol. 21, no. 1, pp. 81-107. http://dx.doi.org/10.1146/annurev.en.21.010176.000501.
http://dx.doi.org/10.1146/annurev.en.21....
). Although population outbreaks of boll weevil would be associated with agricultural expansion in the tropics, boll weevil naturally occurs in South America since before the extensive cultivation of cotton in the region, their presence has been ignored until 1949, due to the scarce weevil collecting, especially in the native areas (Scataglini et al., 2000Scataglini, M.A., Confalonieri, V.A. and Lanteri, A.A., 2000. Dispersal of the cotton boll weevil (Coleoptera: Curculionidae) in South America: evidence of RAPD analysis. Genetica, vol. 108, no. 2, pp. 127-136. http://dx.doi.org/10.1023/A:1004163820446. PMid:11138940.
http://dx.doi.org/10.1023/A:100416382044...
). Nevertheless, the knowledge about survival mechanisms of the boll weevil during overwinter under tropical conditions, host plants and life history are still incipient. Such lack of information makes quite difficult the proposition of efficient management techniques alternative to the massive application of insecticides.
Recent studies start unveiling the peculiar characteristics of the populations’ adaptive responses of boll weevil in the tropics. These populations during the winter, when cotton is not available, present only reproductive dormancy (Paula et al., 2013Paula, D.P., Claudino, D., Timbó, R.V., Miranda, J.E., Bemquerer, M.P., Ribeiro, A.C.J., Sujii, E.R., Fontes, E.M.G. and Pires, C.S.S., 2013. Reproductive dormancy in boll-weevil from populations of the midwest of Brazil. Journal of Chemical Ecology, vol. 106, no. 1, pp. 86-96. PMid:23448019.), remains active and feeding on pollen from other plants such as Compositae (Asteraceae), Solanaceae, Euphorbiaceae, Amaranthaceae, Leguminosae and Smilacaceae (Ribeiro et al., 2010Ribeiro, P.D.A., Sujii, E.R., Diniz, I.R., Medeiros, M.A., Salgado-Labouriau, M.L., Branco, M.C., Pires, C.S.S. and Fontes, E.M.G., 2010. Alternative food sources and overwintering feeding behavior of the boll weevil, Anthonomus grandis boheman (Coleoptera: Curculionidae) under the tropical conditions of Central Brazil. Neotropical Entomology, vol. 39, no. 1, pp. 28-34. http://dx.doi.org/10.1590/S1519-566X2010000100005. PMid:20305896.
http://dx.doi.org/10.1590/S1519-566X2010...
; Cuadrado, 2002Cuadrado, G.A., 2002. Anthonomus Boheman (Coleoptera: Curculionidae) en la zona central y sur oeste de misiones, Argentina: polen como fuente alimenticia y su relación con el estado fisiológico en insectos adultos. grandisNeotropical Entomology, vol. 31, no. 1, pp. 121-132. http://dx.doi.org/10.1590/S1519-566X2002000100017.
http://dx.doi.org/10.1590/S1519-566X2002...
). These plants apparently serve only for feeding and shelter, not being used for boll weevil reproduction (Cross et al., 1975Cross, W.H., Lukefahr, M., Fryxell, P.A. and Burke, H.R., 1975. Host plants of the boll weevil. Environmental Entomology, vol. 4, no. 1, pp. 19-26. http://dx.doi.org/10.1093/ee/4.1.19.
http://dx.doi.org/10.1093/ee/4.1.19...
; Gabriel, 2002Gabriel, D., 2002. Avaliação de malváceas cultivadas como hospedeiras alternativas para a reprodução do bicudo do algodoeiro. Arquivos do Instituto Biologico, vol. 69, no. 3, pp. 69-76.). Although none of these plant species are comparable to cotton in maintaining and increasing populations of boll weevil, they could keep the adults until they find cotton fields (Lukefahr et al., 1986Lukefahr, M.J., Barbosa, S. and Braga Sobrinho, R., 1986. Plantas hospedeiras do bicudo com referência especial a flora brasileira. In: S. BARBOSA, M. J. LUKEFAHR and R. BRAGA SOBRINHO, eds. O bicudo do algodoeiro. Brasília: EMBRAPA –DDT, pp. 275-285.). Thereby, the small population that survives from the dry season can infest and severely damage the next cotton crop (Ribeiro et al., 2006Ribeiro, P., Diniz, I.R., Sujii, E.R., Pires, C.S.S. and Fontes, E.M.G., 2006. Estimativa da população de Anthonomus Grandis Boheman, 1843 (Coleoptera:Curculionidae) na safra e entressafra do algodoeiro. Brasília: Embrapa Recursos Genéticos eBiotecnologia. 22 p. Boletim de Pesquisa e Desenvolvimento, no. 150.).
The high diversification of the landscape found in the tropics opens up a range of environmental choices that may explain the persistence of remnant populations in the environment during the overwinter. This scenario is, for example, found in central Brazil, considered of great importance for the production of cotton in Brazil, the largest area (65.5%), with the highest production (65.1%) in country (2013/2014 crop year) (CONAB, 2014COMPANHIA NACIONAL DE ABASTECIMENTO – CONAB, 2014 [viewed 25 July 2014]. Acompanhamento da safra brasileira: 20013/14 [online]. Brasília. Available from: http://www.conab.gov.br/OlalaCMS/uploads/arquivos/14_01_10_10_12_36_boletim_portugues_dezembro_2013.pdf
http://www.conab.gov.br/OlalaCMS/uploads...
). Most areas present around the cultivated fields of cotton are a mix of native areas of Brazilian savannas, monocultures (such as soybeans), polycultures (maize, sorghum, beans, vegetables), as well as pastures. It is expected that each of these environments can provide different conditions or resources, which would be of greater or lesser degree, exploited by boll weevil in the absence of cotton plants.
In this work we seek to broaden the knowledge on the ecology and behavior of the boll weevil in tropical environmental conditions persisting in areas of Cerrado in Central Brazil, the Brazilian savannas. Considering their potential ecologically adaptive responses, we first seek to evaluate - thorough experimental essays in laboratory - which plant(s) can be a potentially source of food resources (floral nectar, pollen and / or plant tissues) to the boll weevil, during the overwinter. First, we tested for the nutritional adequacy of flowers resource (pollen and/ or nectar) provided by eight species for the survival of boll weevil adults. Subsequently, we evaluated whether the resources provided by some selected plants continued to be exploited by the boll weevil when the main resource (cotton plant) was also available. The purposes of these tests were: 1) to identify differences in preference for resource exploitation between plants evaluated in their different phenological stages; 2) to evaluate the visual effect of the reproductive structures of plants that were preferentially explored.
2 Material and Methods
2.1 Test of the nutritional adequacy of the flowers resources
Eight plant species were selected for evaluation as suppliers of flower resources for the boll weevil: anise (Foeniculum vulgare), mexican sunflower (Tithonia diversifolia), castor bean (Ricinus communis), sunn hemp (Crotalaria juncea), pigeonpea (Cajanus cajan), okra (Albeomoschus esculentus), hibiscus (Hibiscus rosa-sinensis) and sorghum (Sorghum bicolor). All plants chosen are cultivated in the central region of Brazil, have few requirements regarding field management, and can be cultivated from July to November, i.e. during the winter.
The suitability of food provision by the pre-selected plant species for boll weevil survival was evaluated in the laboratory. Inflorescences or flowers of different species of pre-selected plants were collected in the fields at Embrapa Genetic Resources and Biotechnology Research Center (15º 43’47.9S; 47º 53’59.3W), and Fazenda Malunga where they were organically cultivated (15º 58’49.8S; 47º 29’29.7W) in Brasilia, Federal District, thus ensuring that the resources used were free of synthetic products such as pesticides, which could influence the results of the tests. The collected material was placed in paper sacks and brought to the laboratory to be provided as a fresh resource for the boll weevil. Collection of the flowers was done once a week. They were collected with the stems and immediately inserted into previously moistened floral foam, then being taken to the laboratory where they were kept moist during the week until the next collection.
Newly emerged boll weevils from cotton squares free of chemical pesticides, collected in the field at Embrapa Genetic Resources and Biotechnology (15º 43’47.9S; 47º 53’59.3W), were placed individually inside 500 mL transparent plastic vials, and closed with voile. Insects were supplied with water and fed ad libitum with flowers from the pre-selected plants. Flowers were replaced every two days. Cotton squares were used as positive control to compare boll weevil survival. Number of replications varied from 22 to 58 according to plant species. Vials were kept under 25 ± 2 °C, 60% ± 10% RH and 13 h photophase. The experiments for all species were set up between October 2010 and June 2011, during the cotton season. The boll weevils were observed until the record of their death. To verify possible differences in survivorship among males and females, the sexes were then determined by observing the posterior edge of the 8thtergite of the specimens according to Sappington and Spurgeon (2000)Sappington, T.W. and Spurgeon, D.W., 2000. Preferred technique for adult sex determination of the boll weevil (Coleoptera: Curculionidae). Annals of the Entomological Society of America, vol. 93, no. 3, pp. 610-615. http://dx.doi.org/10.1603/0013-8746(2000)093[0610:PTFASD]2.0.CO;2.
http://dx.doi.org/10.1603/0013-8746(2000...
, under magnification of a stereomicroscope.
2.2 Dual-choice tests
Plants reveled by the first essay as being able to serve as food for the boll weevil, were contrasted with cotton plants in dual-choice tests. The okra, hibiscus and cotton plants used in these essays were cultivated in four-liter pots in the greenhouse and in experimental outdoor areas at Embrapa Genetic Resources and Biotechnology, Brasília, DF, Brazil. Cotton and okra plants with two to three leaves were used (approximately 20 days after germination) as vegetative stage samples (cv = cotton in vegetative stage; “ov” okra in a vegetative stages) well as hibiscus plants aged around 30 days (hv = hibiscus in vegetative stage). Cotton plants were considered to be in the reproductive stage as soon as they show flower squares around seven millimeters, up to when flowers appeared (approximately 40 days after germination). Cotton squares are very attractive to the boll weevil (Howard, 1921Howard, L.O., 1921. Studies in the biology of the Mexican cotton boll weevil on short-staple Upland, and Sea-Island cottons. Bulletin of the U.S. Department of Agriculture, vol. 926, pp. 1-44.; Everett and Earle, 1964Everett, T.R. and Earle, N.W., 1964. Boll weevil oviposition responses in cotton squares and various other substrates. Journal of Economic Entomology, vol. 57, no. 5, pp. 651-656. http://dx.doi.org/10.1093/jee/57.5.651.
http://dx.doi.org/10.1093/jee/57.5.651...
); thus, we used cotton plants with only squares (no flowers or fruits) (labeled “cs”) and plants with flower and squares to represent the reproductive stage of cotton (“cf”). We used plants with squares, flowers and fruits to represent the reproductive stage of okra (“of”) and hibiscus (“hf”). Only whole plants lacking symptoms of disease or herbivore attack were used in the experiments. The plants were tested only once. Plants growing in the greenhouse or in the field were compared within the same experiment.
Adult boll weevils of known age (one to four days after emergence) were collected from attacked squares in the field and from larvae fed an artificial diet (Monnerat et al., 1999Monnerat, R.G., Dias, S.R. and Sá, M.F.G., 1999. Dieta artificial para criação do bicudo do algodoeiro em laboratório. Revista Brasileira de Entomologia, vol. 5, pp. 36-40.) in a colony maintained in the laboratory of the Embrapa Genetic Resources and Biotechnology. The results of the pilot experiments showed no differences between the responses of boll weevils from the field and those reared on an artificial diet in the laboratory. We removed the emerged insects and determined their sexes using a stereomicroscope based on the morphology of the posterior edge of the 8th tergite, according to Sappington and Spurgeon (2000Sappington, T.W. and Spurgeon, D.W., 2000. Preferred technique for adult sex determination of the boll weevil (Coleoptera: Curculionidae). Annals of the Entomological Society of America, vol. 93, no. 3, pp. 610-615. http://dx.doi.org/10.1603/0013-8746(2000)093[0610:PTFASD]2.0.CO;2.
http://dx.doi.org/10.1603/0013-8746(2000...
). The males and females were differentiated by marks made with indelible ink and maintained for 24 hours without food before the experiment.
The boll weevils were subjected to dual-choice experiments inside a transparent acrylic cage (90 × 80 × 45 cm) to test their food preference. Two plants were diagonally positioned at opposite ends of the cage, 45 cm from each other. Taking into account the possible combination of the plant species and their growth stages, a total of 19 tests were conducted, with 20 replicates of each. In each test, we placed five boll weevil pairs in the center of the cage between the two plant samples. We assessed the number of weevils on each plant 24 hours later. We noted “no response” when an adult boll weevil did not choose a plant and moved to crevices in the cage walls, or in the rare cases in which it escaped the cage. The insects that died were not considered for statistical analysis.
2.3 Visual effect of reproductive structures on the choice of the boll weevil
Dual choice test were also conducted to evaluate the visual effect of reproductive structures on the choice and orientation of the boll weevil. These tests were conducted only for treatments involving hibiscus with flowers and squares and cotton with squares. The procedures used for the test were similar to those described in subsection 2.2. However, seeking to isolate the visual effect of reproductive structures (flowers and cotton squares) and restrict the effect of volatile emitted inside the acrylic cage, the flowers of the contrasted plants were bagged with green tissue paper, blocking the view by boll weevil. The same number of bags put on plants in the reproductive stage was also put on the plants in the vegetative stage.
2.4 Statistical analysis
Survival curves were constructed for each plant species, being estimated by Kaplan-Meier analysis (Kaplan and Meier, 1958Kaplan, E.L. and Meier, P., 1958. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association, vol. 53, no. 282, pp. 457-481. http://dx.doi.org/10.1080/01621459.1958.10501452.
http://dx.doi.org/10.1080/01621459.1958....
) with Log-Rank and using Gehan’s Wilcoxon as post-hoc test (Gehan, 1965Gehan, E.A., 1965. A generalized wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika, vol. 52, no. 1-2, pp. 203-223. http://dx.doi.org/10.1093/biomet/52.1-2.203. PMid:14341275.
http://dx.doi.org/10.1093/biomet/52.1-2....
; Mantel, 1966Mantel, N., 1966. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer & Chemotherapy, vol. 50, no. 3, pp. 163-170. PMid:5910392.; Hosmer and Lemeshow, 2004Hosmer, D.W. and Lemeshow, S., 2004. Applied logistic regression. New York: John Wiley & Sons.). The plants that were able to keep at least 90% of the total boll weevil population alive for more than 10 days were considered as suitable for the adult insect. The average longevity was compared through non-parametric Kruskal Wallis test.
Dual choice data were analyzed using a Generalized Linear Model (GLM) with under a Poisson error distribution. To evaluate whether boll weevil have some preference to plants contrasted in different fenological stages we summed the number of choices per treatment and this number was used as a response variable and the treatment was used as explanatory variable. To test for statistical differences in the variable between the treatments we used Analysis of Deviance (ANODEV), with a Chi-square test, followed by a model residual analysis (Crawley, 2007Crawley, M.J., 2007. The R Book. West Sussex: John Wiley & Sons. 942 p.). The final model was compared with a null model by an ANODEV using a Chi-square test. If significant differences from the null model were achieved we accepted the final model (Crawley, 2007Crawley, M.J., 2007. The R Book. West Sussex: John Wiley & Sons. 942 p.).
3 Results
3.1 Selection of plants for testing as providers of resources to the boll weevil adults
Survival curves between males and females for each tested plant did not significantly differ (p>0.05), so the survival curves of the boll weevils fed with different plants were run without differentiating between males and females. The survival of the boll weevil on cotton was significantly larger than the survival of all the other tested plant species (Log-Rank test, all with p<0.05), except for hibiscus. The survival of the boll weevil on hibiscus was larger than that on cotton (Gehan’s Wilcoxon test with p<0.05) (Figure 1). In the laboratory experiments, most of the pre-selected alternative plants failed to meet the criterion of nutritional suitability for boll weevil survival (Figures 1a, 1b, 1c, 1d, 1e, 1h), except hibiscus and okra (Figures 1f and 1g), which kept 90% of boll weevil adults alive for more than 10 days. When flowers from these two plants were offered to the boll weevil, the maximum adult survival was 300 days for hibiscus and 86 days for okra.
Survival curves of the boll weevils when flowers of different plants were offered: (a) Fennel (n=40); (b) Mexican sunflower (n=40); (c) Castor bean (n=22); (d) Sunn hemp (n=58); (e) Pigeonpea (n=24); (f) Okra (n=40); (g) Hibiscus (n=40) and (h) Sorghum (n=40).
The average longevity of the boll weevil adults was significantly greater when they were fed on hibiscus flowers (166.6 ± 74.4), cotton square (126.72 ± 49.34) and okra flowers (34.7 ± 28.9) (Figure 2) than when they were fed with the other species (7.79 ± 1.91; KW-H(8;315) = 209.1293; p<0,001).
Average longevity of boll weevil adults when flowers of different plants were offered (Bars represent confidence interval of 0.95%).
3.2 Dual choice test: Hibiscus and Okra X Cotton
The percentage of responding insects varied as a function of the combination of the species of plant and their growth stages (Figure 3 and Figure 4). The presence of cotton with squares or flowers and hibiscus plants with flowers favored the insects actively choosing a food plant, while the presence of hibiscus plants in vegetative stages increased the non-response (Figure 3).
Level of response of adult boll weevils, Anthonomus grandis, according to the combination of different resource types offered in dual-choice tests. (cs = cotton with squares; cf = cotton with flowers; cv = cotton in vegetative stage; hf = hibiscus with flowers; hv = hibiscus in vegetative stage. *Significant difference with the null model by Analysis of Deviance (ANODEV)).
Level of response of adult boll weevils, Anthonomus grandis, according to the combination of different resource types offered in dual-choice tests. (cs = cotton with squares; cf = cotton with flowers; cv = cotton in vegetative stage; of = okra with flowers; ov = okra in vegetative stage. *Significant difference with the null model by Analysis of Deviance (ANODEV)).
A greater number of individuals actively sought the cotton plants with squares compared at other combinations of the okra and cotton plants in different phenological stages (Figure 4).
The boll weevils preferably visited reproductive hibiscus more frequently than vegetative cotton (p<0.001) and square cotton (p=0.004) (Figure 5). Only, when we offered flowering cotton and vegetative hibiscus, the boll weevils chose the cotton (p=0.001).
Percentage of boll weevil who chose the plants offered (cotton and hibiscus) in the dual choice tests. (cs = cotton with squares; cf = cotton with flowers; cv = cotton in vegetative stage; hf = hibiscus with flowers; hv = hibiscus in vegetative stage. *Significant difference with the null model by Analysis of Deviance (ANODEV)).
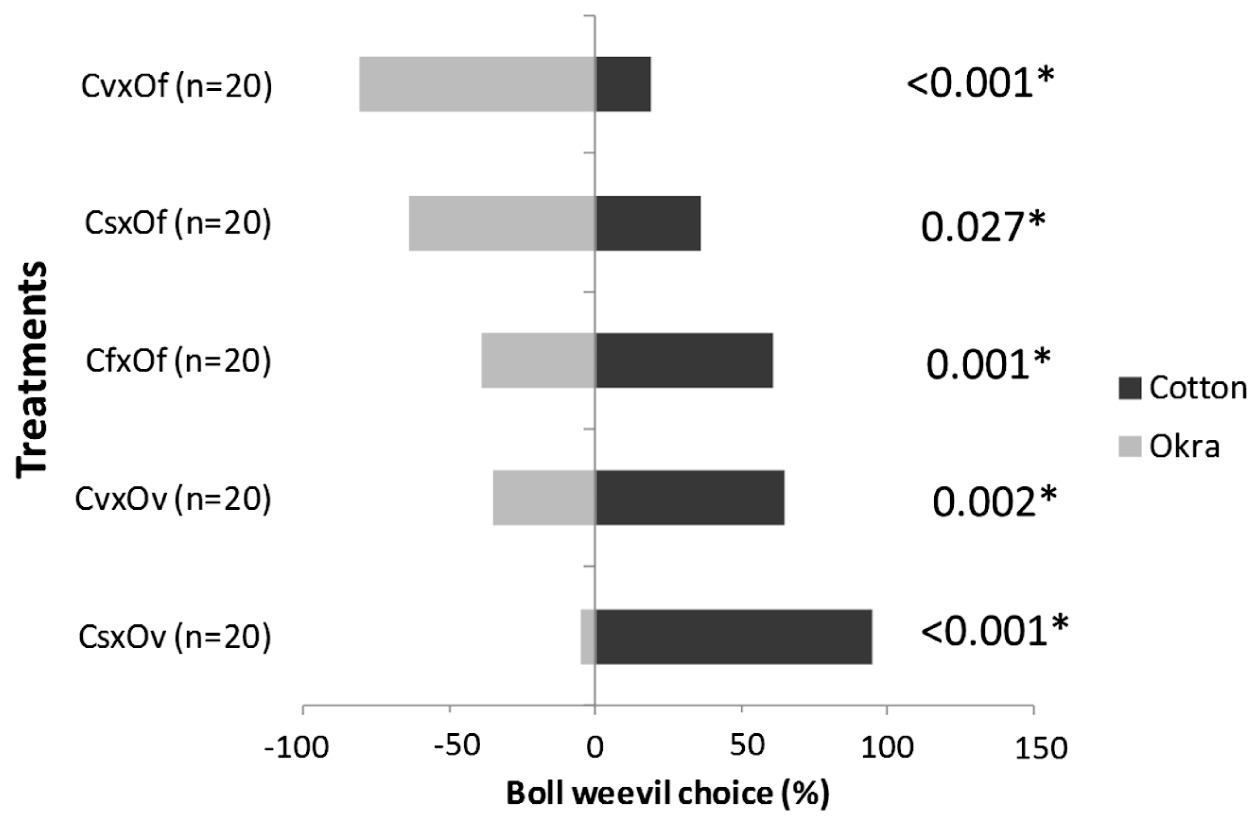
The boll weevils preferably visited vegetative cotton (p=0.002) and cotton with squares (p<0.001) more than vegetative okra (p<0.001) (Figure 6). When we offered flowering cotton and flowering okra, the boll weevils selected the flowering cotton (p=0.001) (Figure 4).However, when we offered flowering okra and cotton with squares and vegetative cotton the boll weevils more frequently selected okra (p=0.027 and p<0.001).
Percentage of boll weevil that chose the plants offered (cotton and okra) in the dual choice tests (cs = cotton with squares; cf = cotton with flowers; cv = cotton in vegetative stage; of = okra with flowers; ov = okra in vegetative stage. *Significant difference with the null model by Analysis of Deviance (ANODEV)).
When we offered two plants of the same species and of the same growth stage in the cage, the boll weevils consistently chose plants in the reproductive stage of cotton, hibiscus and okra (Figure 7). This result highlights that the reproductive structures of the species used in the test play an important role in the boll weevil behavior and may show attractiveness.
Percentage of boll weevil that chose plants of same species offered in different phenological stage, in the dual choice tests (cs = cotton with squares; cv = cotton in vegetative stage; of = okra with flowers; ov = okra in vegetative stage; hf = hibiscus with flowers; hv = hibiscus in vegetative stage. *Significant difference with the null model by Analysis of Deviance (ANODEV)).
3.3 Visual test
The boll weevils did not prefer plants in the reproductive stage when these structures were bagged, independently of the species and tested combination (Figure 8). This result indicates that the visualization of the reproductive structure may influence the boll weevil’s choice of a plant to visit, suggesting a possible feeding choice.
Percentage of boll weevil that chose the plants offered in different phenological stage with bagged reproductive structures, in the dual choice tests. (cf = cotton with flowers; cv = cotton in vegetative stage; hf = hibiscus with flowers; hv = hibiscus in vegetative stage).
4 Discussion
Amongst the eight plants tested in the laboratory as an alternative food for adult boll weevils, beyond cotton plant, only flowers of hibiscus and okra plants provided nutrition to this pest. Boll weevils have an evolutionary history with cotton that originated with the host shift from its Hampea ancestral host to Gossypium, the genera of the wild and domesticated cotton plants, early in the domestication process (Jones, 2001Jones, R.W., 2001. Evolution of the host plant associations of the species group (Coleoptera: Curculionidae): Phylogenetic tests of various hypotheses. Anthonomus grandisAnnals of the Entomological Society of America, vol. 94, no. 1, pp. 51-58. http://dx.doi.org/10.1603/0013-8746(2001)094[0051:EOTHPA]2.0.CO;2.
http://dx.doi.org/10.1603/0013-8746(2001...
). Both genera, Hibiscus and Abelmoschus, belong to Malvacea family, and indeed several studies have also shown that boll weevil adults primarily feed on plants from the Malvaceae family (Cuadrado, 2002Cuadrado, G.A., 2002. Anthonomus Boheman (Coleoptera: Curculionidae) en la zona central y sur oeste de misiones, Argentina: polen como fuente alimenticia y su relación con el estado fisiológico en insectos adultos. grandisNeotropical Entomology, vol. 31, no. 1, pp. 121-132. http://dx.doi.org/10.1590/S1519-566X2002000100017.
http://dx.doi.org/10.1590/S1519-566X2002...
; Cuadrado and Garralla, 2000Cuadrado, G.A. and Garralla, S.S., 2000. Plantas alimenticias alternativas del picudo de algodonero (. Anthonomus grandis Boh.) (Coleoptera: Curculionidae) em la Provincia de Formosa, Argentina. Anális palinológico del tracto digestivoAnais Sociedade Entomológica Brasileira, vol. 29, no. 2, pp. 245-255. http://dx.doi.org/10.1590/S0301-80592000000200006.
http://dx.doi.org/10.1590/S0301-80592000...
; Gabriel, 2002Gabriel, D., 2002. Avaliação de malváceas cultivadas como hospedeiras alternativas para a reprodução do bicudo do algodoeiro. Arquivos do Instituto Biologico, vol. 69, no. 3, pp. 69-76.; Jones et al., 1993Jones, R.W., Cate, J.R., Hernandez, E.M. and Sosa, E.S., 1993. Pollen feeding and survival of the boll weevil (Coleoptera: Curculionidae) on selected plant species in northeastern Mexico. Environmental Entomology, vol. 22, no. 1, pp. 99-108. http://dx.doi.org/10.1093/ee/22.1.99.
http://dx.doi.org/10.1093/ee/22.1.99...
; Showler and Abrigo, 2007Showler, A.T. and Abrigo, V., 2007. Common subtropical and tropical non pollen food sources of the boll weevil (Coleoptera: Curculionidae). Environmental Entomology, vol. 36, no. 1, pp. 99-104. http://dx.doi.org/10.1093/ee/36.1.99. PMid:17349122.
http://dx.doi.org/10.1093/ee/36.1.99...
; Ribeiro et al., 2010Ribeiro, P.D.A., Sujii, E.R., Diniz, I.R., Medeiros, M.A., Salgado-Labouriau, M.L., Branco, M.C., Pires, C.S.S. and Fontes, E.M.G., 2010. Alternative food sources and overwintering feeding behavior of the boll weevil, Anthonomus grandis boheman (Coleoptera: Curculionidae) under the tropical conditions of Central Brazil. Neotropical Entomology, vol. 39, no. 1, pp. 28-34. http://dx.doi.org/10.1590/S1519-566X2010000100005. PMid:20305896.
http://dx.doi.org/10.1590/S1519-566X2010...
). Probably, hibiscus and okra plants attracted and feed boll weevils because they are likely chemically and structurally more similar to cotton plants.
Despite the frequent records of pollen feeding by boll weevils, which are partly due to the fact that it is easier to detect pollen in the insect gut after dissection, they can also consume floral and extrafloral nectar and other plant tissues (Showler, 2008Showler, A.T., 2008. Longevity and egg development of adult female boll weevils fed exclusively on different parts and stages of cotton fruiting bodies. Entomologia Experimentalis et Applicata, vol. 127, no. 2, pp. 125-132. http://dx.doi.org/10.1111/j.1570-7458.2008.00679.x.
http://dx.doi.org/10.1111/j.1570-7458.20...
, 2009Showler, A.T., 2009. Roles of host plants in boll weevil range expansion beyond tropical Mesoamerica. American Entomologist, vol. 55, no. 4, pp. 234-242. http://dx.doi.org/10.1093/ae/55.4.234.
http://dx.doi.org/10.1093/ae/55.4.234...
). The nutritional value of these two resources is still unknown, as well as their isolated effect on boll weevil survival. Therefore, the consumption of pollen, nectar and other plant tissues by the boll weevil sensures their survival during the winter period (Showler and Abrigo, 2007Showler, A.T. and Abrigo, V., 2007. Common subtropical and tropical non pollen food sources of the boll weevil (Coleoptera: Curculionidae). Environmental Entomology, vol. 36, no. 1, pp. 99-104. http://dx.doi.org/10.1093/ee/36.1.99. PMid:17349122.
http://dx.doi.org/10.1093/ee/36.1.99...
).
The reproductive stages attracted more boll weevils than did vegetative ones, and flowers of hibiscus, okra and cotton plants are likely to be very attractive to boll weevils. In the other hand, the boll weevils did not prefer plants in the reproductive stage when these structures were bagged. Those results suggest that the visual stimuli may play an important role in guiding the boll weevil to select a resource. Showler (2002)Showler, A.T., 2002. Effects of kaolin-based particle film application on boll weevil (Coleoptera: Curculionidae) injury to cotton. Journal of Economic Entomology, vol. 95, no. 4, pp. 754-762. http://dx.doi.org/10.1603/0022-0493-95.4.754. PMid:12216817.
http://dx.doi.org/10.1603/0022-0493-95.4...
demonstrated that the boll weevil is less inclined to use kaolin-coated cotton plants for feeding and oviposition, indicating that visual stimuli are important for the recognition of cotton plants by cotton boll weevil. However, a study that characterized boll weevil responses to the volatiles released by cotton in different growth stages and herbivorous conditions in olfactometer, showed that A. grandis uses conspecific herbivore-induced volatiles enriched with the aggregation pheromone to locate host cotton plants and that terpenic compounds may be involved in their attractiveness (Magalhães et al., 2012Magalhães, D.M., Borges, M., Laumann, R.A., Sujii, E.R., Mayon, P., Caulfield, J.C., Midega, C.A.O., Khan, Z.R., Pickett, J.A., Birkett, M.A. and Blassioli-Moraes, M.C., 2012. Semiochemicals from herbivory induced cotton plants enhance the foraging behavior of the cotton boll weevil, Anthonomus grandis. Journal of Chemical Ecology, vol. 38, no. 12, pp. 1528-1538. http://dx.doi.org/10.1007/s10886-012-0216-5. PMid:23179097.
http://dx.doi.org/10.1007/s10886-012-021...
). This result suggests that the olfactory stimulus is also important probably at short distance. Therefore, the vision, combined with the smell, is an important component of the sensory system involved in the selectivity of host plants to insect herbivores process, and, also for boll weevil responding to silhouettes of different achromatic contrasts might include visual cues in their host plant location in the field (Hausmann et al., 2004Hausmann, C., Samietz, J. and Dorn, S., 2004. Visual orientation of overwintered (Coleoptera: Curculionidae). Anthonomus pomorumEnvironmental Entomology, vol. 33, no. 5, pp. 1410-1415. http://dx.doi.org/10.1603/0046-225X-33.5.1410.
http://dx.doi.org/10.1603/0046-225X-33.5...
).
Taken together, the results presented here provide important baseline information for developing a management system for the boll weevil, based on alternative food resources that can move the adults to a specific location where they can be control. These laboratory based results corroborate the results from Ribeiro et al. (2010)Ribeiro, P.D.A., Sujii, E.R., Diniz, I.R., Medeiros, M.A., Salgado-Labouriau, M.L., Branco, M.C., Pires, C.S.S. and Fontes, E.M.G., 2010. Alternative food sources and overwintering feeding behavior of the boll weevil, Anthonomus grandis boheman (Coleoptera: Curculionidae) under the tropical conditions of Central Brazil. Neotropical Entomology, vol. 39, no. 1, pp. 28-34. http://dx.doi.org/10.1590/S1519-566X2010000100005. PMid:20305896.
http://dx.doi.org/10.1590/S1519-566X2010...
, carried out in the field, that has shown that boll weevils can feed on alternative plants that occur in natural fields of the Central Brazil region. However, our results refine the information indicating that these alternative resources are mainly plant species that belongs to the Malvaceae family. Also, our work suggests that visual stimulus is important for the boll weevil’s ecological behavior of finding food during tropical winter, what allow them to survive across the intercrop period. These results will enable a more accurate delineation of the experiments required to test the attraction of the boll weevil to hibiscus and/or okra away from cotton in different experimentation scales (greenhouse and field scale). Successful boll weevil control with conventional methods has been shown to be costly and difficult to achieve, particularly for smallholders (Degrande, 1991Degrande, P.E., 1991. Aspectos biológicos do bicudo. In: E.P. DEGRANDE, ed. Bicudo do algodoeiro: manejo integrado. Campo Grande: UFMS, pp. 11-27.; Fontes et al., 2006Fontes, E.M.G., Ramalho, F.S., Underwood, E., Barroso, P.A.V., Simon, M.F., Sujii, E.R., Pires, C.S.S., Beltrão, N., Lucena, W.A. and Freire, E.C., 2006. The cotton agricultural context in Brazil. In: A. HILBECK, D.A. ANDOW and E.M.G. FONTES, eds. Environmental risk assessment of genetically modified organisms. Wallingford: CABI Publishing, vol. 2, pp. 21-66.). Knowing which species and development stages of the alternative food are most attractive to the boll weevil when compared to vegetative cotton, can help in the developmental of a control system that synchronize the different alternative species with cotton growth that ultimately, can reduce the insecticide sprays on the cotton culture in Central Brazil.
Acknowledgements
The authors thank the Embrapa Genetic Resources and Biotechnology Research Center, the National Council of Scientific and Technological Development (CNPq), the National Council for the Improvement of Higher Education (CAPES), the Minas Gerais State Foundation for Research Aid (FAPEMIG) and Fazenda Malunga for financial support and fellowships for the authors.
-
(With 8 figures)
References
- COMPANHIA NACIONAL DE ABASTECIMENTO – CONAB, 2014 [viewed 25 July 2014]. Acompanhamento da safra brasileira: 20013/14 [online]. Brasília. Available from: http://www.conab.gov.br/OlalaCMS/uploads/arquivos/14_01_10_10_12_36_boletim_portugues_dezembro_2013.pdf
» http://www.conab.gov.br/OlalaCMS/uploads/arquivos/14_01_10_10_12_36_boletim_portugues_dezembro_2013.pdf - Crawley, M.J., 2007. The R Book. West Sussex: John Wiley & Sons. 942 p.
- Cross, W.H., 1973. Biology, control, and eradication of the boll weevil. Annual Review of Entomology, vol. 18, no. 1, pp. 17-46. http://dx.doi.org/10.1146/annurev.en.18.010173.000313
» http://dx.doi.org/10.1146/annurev.en.18.010173.000313 - Cross, W.H., Lukefahr, M., Fryxell, P.A. and Burke, H.R., 1975. Host plants of the boll weevil. Environmental Entomology, vol. 4, no. 1, pp. 19-26. http://dx.doi.org/10.1093/ee/4.1.19
» http://dx.doi.org/10.1093/ee/4.1.19 - Cuadrado, G.A. and Garralla, S.S., 2000. Plantas alimenticias alternativas del picudo de algodonero (. Anthonomus grandis Boh.) (Coleoptera: Curculionidae) em la Provincia de Formosa, Argentina. Anális palinológico del tracto digestivoAnais Sociedade Entomológica Brasileira, vol. 29, no. 2, pp. 245-255. http://dx.doi.org/10.1590/S0301-80592000000200006
» http://dx.doi.org/10.1590/S0301-80592000000200006 - Cuadrado, G.A., 2002. Anthonomus Boheman (Coleoptera: Curculionidae) en la zona central y sur oeste de misiones, Argentina: polen como fuente alimenticia y su relación con el estado fisiológico en insectos adultos. grandisNeotropical Entomology, vol. 31, no. 1, pp. 121-132. http://dx.doi.org/10.1590/S1519-566X2002000100017
» http://dx.doi.org/10.1590/S1519-566X2002000100017 - Degrande, P.E., 1991. Aspectos biológicos do bicudo. In: E.P. DEGRANDE, ed. Bicudo do algodoeiro: manejo integrado. Campo Grande: UFMS, pp. 11-27.
- Everett, T.R. and Earle, N.W., 1964. Boll weevil oviposition responses in cotton squares and various other substrates. Journal of Economic Entomology, vol. 57, no. 5, pp. 651-656. http://dx.doi.org/10.1093/jee/57.5.651
» http://dx.doi.org/10.1093/jee/57.5.651 - Fontes, E.M.G., Ramalho, F.S., Underwood, E., Barroso, P.A.V., Simon, M.F., Sujii, E.R., Pires, C.S.S., Beltrão, N., Lucena, W.A. and Freire, E.C., 2006. The cotton agricultural context in Brazil. In: A. HILBECK, D.A. ANDOW and E.M.G. FONTES, eds. Environmental risk assessment of genetically modified organisms. Wallingford: CABI Publishing, vol. 2, pp. 21-66.
- Gabriel, D., 2002. Avaliação de malváceas cultivadas como hospedeiras alternativas para a reprodução do bicudo do algodoeiro. Arquivos do Instituto Biologico, vol. 69, no. 3, pp. 69-76.
- Gehan, E.A., 1965. A generalized wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika, vol. 52, no. 1-2, pp. 203-223. http://dx.doi.org/10.1093/biomet/52.1-2.203 PMid:14341275.
» http://dx.doi.org/10.1093/biomet/52.1-2.203 - Hausmann, C., Samietz, J. and Dorn, S., 2004. Visual orientation of overwintered (Coleoptera: Curculionidae). Anthonomus pomorumEnvironmental Entomology, vol. 33, no. 5, pp. 1410-1415. http://dx.doi.org/10.1603/0046-225X-33.5.1410
» http://dx.doi.org/10.1603/0046-225X-33.5.1410 - Hosmer, D.W. and Lemeshow, S., 2004. Applied logistic regression. New York: John Wiley & Sons.
- Howard, L.O., 1921. Studies in the biology of the Mexican cotton boll weevil on short-staple Upland, and Sea-Island cottons. Bulletin of the U.S. Department of Agriculture, vol. 926, pp. 1-44.
- Jones, R.W., 2001. Evolution of the host plant associations of the species group (Coleoptera: Curculionidae): Phylogenetic tests of various hypotheses. Anthonomus grandisAnnals of the Entomological Society of America, vol. 94, no. 1, pp. 51-58. http://dx.doi.org/10.1603/0013-8746(2001)094[0051:EOTHPA]2.0.CO;2
» http://dx.doi.org/10.1603/0013-8746(2001)094[0051:EOTHPA]2.0.CO;2 - Jones, R.W., Cate, J.R., Hernandez, E.M. and Sosa, E.S., 1993. Pollen feeding and survival of the boll weevil (Coleoptera: Curculionidae) on selected plant species in northeastern Mexico. Environmental Entomology, vol. 22, no. 1, pp. 99-108. http://dx.doi.org/10.1093/ee/22.1.99
» http://dx.doi.org/10.1093/ee/22.1.99 - Kaplan, E.L. and Meier, P., 1958. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association, vol. 53, no. 282, pp. 457-481. http://dx.doi.org/10.1080/01621459.1958.10501452
» http://dx.doi.org/10.1080/01621459.1958.10501452 - Lukefahr, M.J., Barbosa, S. and Braga Sobrinho, R., 1986. Plantas hospedeiras do bicudo com referência especial a flora brasileira. In: S. BARBOSA, M. J. LUKEFAHR and R. BRAGA SOBRINHO, eds. O bicudo do algodoeiro. Brasília: EMBRAPA –DDT, pp. 275-285.
- Magalhães, D.M., Borges, M., Laumann, R.A., Sujii, E.R., Mayon, P., Caulfield, J.C., Midega, C.A.O., Khan, Z.R., Pickett, J.A., Birkett, M.A. and Blassioli-Moraes, M.C., 2012. Semiochemicals from herbivory induced cotton plants enhance the foraging behavior of the cotton boll weevil, Anthonomus grandis. Journal of Chemical Ecology, vol. 38, no. 12, pp. 1528-1538. http://dx.doi.org/10.1007/s10886-012-0216-5 PMid:23179097.
» http://dx.doi.org/10.1007/s10886-012-0216-5 - Mantel, N., 1966. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer & Chemotherapy, vol. 50, no. 3, pp. 163-170. PMid:5910392.
- Monnerat, R.G., Dias, S.R. and Sá, M.F.G., 1999. Dieta artificial para criação do bicudo do algodoeiro em laboratório. Revista Brasileira de Entomologia, vol. 5, pp. 36-40.
- Neves, R., Colares, F., Torres, J.B., Santos, R.L. and Bastos, C.S., 2014. Rational practices to manage boll weevils colonization and population growth on family farms in the semiárido region of Brazil. Insects, vol. 5, no. 4, pp. 818-831. http://dx.doi.org/10.3390/insects5040818 PMid:26462942.
» http://dx.doi.org/10.3390/insects5040818 - Paula, D.P., Claudino, D., Timbó, R.V., Miranda, J.E., Bemquerer, M.P., Ribeiro, A.C.J., Sujii, E.R., Fontes, E.M.G. and Pires, C.S.S., 2013. Reproductive dormancy in boll-weevil from populations of the midwest of Brazil. Journal of Chemical Ecology, vol. 106, no. 1, pp. 86-96. PMid:23448019.
- Ramalho, F.S. and Wanderley, P.A., 1995. Ecology and management of the boll weevil in South American cotton. American Entomologist, vol. 42, no. 1, pp. 41-47. http://dx.doi.org/10.1093/ae/42.1.41
» http://dx.doi.org/10.1093/ae/42.1.41 - Ribeiro, P., Diniz, I.R., Sujii, E.R., Pires, C.S.S. and Fontes, E.M.G., 2006. Estimativa da população de Anthonomus Grandis Boheman, 1843 (Coleoptera:Curculionidae) na safra e entressafra do algodoeiro. Brasília: Embrapa Recursos Genéticos eBiotecnologia. 22 p. Boletim de Pesquisa e Desenvolvimento, no. 150.
- Ribeiro, P.D.A., Sujii, E.R., Diniz, I.R., Medeiros, M.A., Salgado-Labouriau, M.L., Branco, M.C., Pires, C.S.S. and Fontes, E.M.G., 2010. Alternative food sources and overwintering feeding behavior of the boll weevil, Anthonomus grandis boheman (Coleoptera: Curculionidae) under the tropical conditions of Central Brazil. Neotropical Entomology, vol. 39, no. 1, pp. 28-34. http://dx.doi.org/10.1590/S1519-566X2010000100005 PMid:20305896.
» http://dx.doi.org/10.1590/S1519-566X2010000100005 - Santos, R.L., Neves, R.C.S., Colares, F. and Torres, J.B., 2013. Parasitóides do bicudo Anthonomus grandis e predadores residentes em algodoeiro pulverizado com caulim. Semina: Ciências Agrárias, vol. 34, pp. 3463-3474.
- Sappington, T.W. and Spurgeon, D.W., 2000. Preferred technique for adult sex determination of the boll weevil (Coleoptera: Curculionidae). Annals of the Entomological Society of America, vol. 93, no. 3, pp. 610-615. http://dx.doi.org/10.1603/0013-8746(2000)093[0610:PTFASD]2.0.CO;2
» http://dx.doi.org/10.1603/0013-8746(2000)093[0610:PTFASD]2.0.CO;2 - Scataglini, M.A., Confalonieri, V.A. and Lanteri, A.A., 2000. Dispersal of the cotton boll weevil (Coleoptera: Curculionidae) in South America: evidence of RAPD analysis. Genetica, vol. 108, no. 2, pp. 127-136. http://dx.doi.org/10.1023/A:1004163820446 PMid:11138940.
» http://dx.doi.org/10.1023/A:1004163820446 - Showler, A.T. and Abrigo, V., 2007. Common subtropical and tropical non pollen food sources of the boll weevil (Coleoptera: Curculionidae). Environmental Entomology, vol. 36, no. 1, pp. 99-104. http://dx.doi.org/10.1093/ee/36.1.99 PMid:17349122.
» http://dx.doi.org/10.1093/ee/36.1.99 - Showler, A.T., 2002. Effects of kaolin-based particle film application on boll weevil (Coleoptera: Curculionidae) injury to cotton. Journal of Economic Entomology, vol. 95, no. 4, pp. 754-762. http://dx.doi.org/10.1603/0022-0493-95.4.754 PMid:12216817.
» http://dx.doi.org/10.1603/0022-0493-95.4.754 - Showler, A.T., 2004. Influence of cotton fruit stages as food sources on boll weevil (Coleoptera: Curculionidae) fecundity and oviposition. Journal of Economic Entomology, vol. 97, no. 4, pp. 1330-1334. http://dx.doi.org/10.1093/jee/97.4.1330 PMid:15384345.
» http://dx.doi.org/10.1093/jee/97.4.1330 - Showler, A.T., 2007. Subtropical boll weevil ecology. American Entomologist, vol. 53, no. 4, pp. 240-249. http://dx.doi.org/10.1093/ae/53.4.240
» http://dx.doi.org/10.1093/ae/53.4.240 - Showler, A.T., 2008. Longevity and egg development of adult female boll weevils fed exclusively on different parts and stages of cotton fruiting bodies. Entomologia Experimentalis et Applicata, vol. 127, no. 2, pp. 125-132. http://dx.doi.org/10.1111/j.1570-7458.2008.00679.x
» http://dx.doi.org/10.1111/j.1570-7458.2008.00679.x - Showler, A.T., 2009. Roles of host plants in boll weevil range expansion beyond tropical Mesoamerica. American Entomologist, vol. 55, no. 4, pp. 234-242. http://dx.doi.org/10.1093/ae/55.4.234
» http://dx.doi.org/10.1093/ae/55.4.234 - Showler, A.T., 2012. The conundrum of chemical boll weevil control in subtropical regions. In: F. PERVEEN, ed. Insecticides: pest engineering. Croatia: InTech, pp. 437-448.
- Spurgeon, D.W. and Raulston, J.R., 2006. Boll weevil (Coleoptera: Curculionidae) adult diapause responses to selected environmental and dietary conditions. Annals of the Entomological Society of America, vol. 99, no. 6, pp. 1085-1100. http://dx.doi.org/10.1603/0013-8746(2006)99[1085:BWCCAD]2.0.CO;2
» http://dx.doi.org/10.1603/0013-8746(2006)99[1085:BWCCAD]2.0.CO;2 - SPURGEON, D.W., SAPPINGTON, T.W. and SUH, C.P., 2003. A system for characterizing reproductive and diapause morphology in the boll weevil (Coleoptera: Curculionidae). Annals of the Entomological Society of America, vol. 96, no. 1, pp. 1-11. http://dx.doi.org/10.1603/0013-8746(2003)096[0001:ASFCRA]2.0.CO;2
» http://dx.doi.org/10.1603/0013-8746(2003)096[0001:ASFCRA]2.0.CO;2 - TAUBER, M.J. and TAUBER, C.A., 1976. Insect seasonality: diapause maintenance, termination, and postdiapause development. Annual Review of Entomology, vol. 21, no. 1, pp. 81-107. http://dx.doi.org/10.1146/annurev.en.21.010176.000501
» http://dx.doi.org/10.1146/annurev.en.21.010176.000501
Publication Dates
-
Publication in this collection
01 Mar 2016 -
Date of issue
Apr-Jun 2016
History
-
Received
12 Aug 2014 -
Accepted
06 Feb 2015