Abstract
Biomphalaria amazonica is a planorbid species considered a potential host of Schistosoma mansoni. It is widely distributed in the Neotropical zone, particularly in the North and Centre-West of Brazil and in the North of Bolivia. The aim of the present study was to determine the host-parasite relationship between B. amazonica and S. mansoni (BH and SJ strains). Specimens of B. amazonica and their snail-conditioned water were examined in terms of their ability to attract miracidia. The infectivity of the mollusks was determined by exposing them to 20 miracidia of both strains. Sporocyst development and amebocyte reactions were studied after each mollusk specimen was exposed to 100 miracidia. Although no cercariae were eliminated, specimens of B. amazonica proved capable of attracting 77% of the miracidia they were exposed to. Viable sporocysts with no amebocyte reaction were found 96 hours after the exposure to miracidia. These results indicate the susceptibility of B. amazonica to the BH and SJ strains of S. mansoni, and therefore demonstrate the importance of this planorbid species as a potential vector of the trematode in the areas where it occurs.
Keywords:
schistosomiasis; susceptibility; BH strain; SJ strain
Resumo
Biomphalaria amazonica é uma espécie de planorbídeo considerada vetora potencial do Schistosoma mansoni. É amplamente distribuída na zona neotropical, especialmente no Norte e Centro-Oeste do Brasil e Norte da Bolívia. O presente trabalho teve por objetivo estudar a relação parasito-hospedeiro entre B. amazonica e S. mansoni (linhagens BH e SJ). Espécimes de B. amazonica e sua água de condicionamento foram examinados em relação à sua capacidade de atração miraxonal. A infectividade dos moluscos foi testada expondo-os a 20 miracídios de ambas as linhagens. A viabilidade dos esporocistos e o desenvolvimento de reações amebocitárias foram estudados após cada molusco ser exposto a 100 miracídios. Apesar de não eliminarem cercárias, B. amazonica provou ser capaz de atrair 77% dos miracídios a que foram expostos. Esporocistos viáveis sem reação amebócitaria foram encontrados 96 horas após a exposição aos miracídios. Esses resultados indicam a suscetibilidade de B. amazonica às linhagens BH e SJ de S. mansoni e, portanto, demonstram a importância desta espécie de planorbídeo como um vetor potencial do trematodeo na área onde ele ocorre.
Palavras-chave:
esquistossomose; susceptibilidade; linhagem BH; linhagem SJ
1 Introduction
In Brazil, three planorbid species are regarded as natural hosts of Schistosoma mansoni (Sambon, 1907SAMBON, L.W., 1907. Descriptions of some new species of animal parasites. Proceedings of the Zoological Society of London, vol. 77, no. 2, pp. 282-283. http://dx.doi.org/10.1111/j.1096-3642.1907.tb01817.x.
http://dx.doi.org/10.1111/j.1096-3642.19...
): Biomphalaria glabrata (Say, 1818Say, T., 1818. Account of two new genera, and several new species, of fresh water and land shells. Journal of the Academy of Natural Sciences of Philadelphia, vol. 1, no. 2, pp. 276-284.); Biomphalaria straminea (Dunker, 1848Dunker, W., 1848. Diagnoses specierum novarum generis Planorbis collectionis Cumingianae. Proceedings of the Zoological Society of London, vol. 16, pp. 40-43.) and Biomphalaria tenagophila (D’Orbigny, 1835D’orbigny, A., 1835. Synopsis terrestrium et fluviatilium molluscorum, in suo per American meridionalem itinere collectorum. Magazine Zoological., vol. 5, no. 61-62, pp. 1-44.). Biomphalaria amazonica (Paraense, 1966Paraense, W.L., 1966. Biomphalaria amazonica and , two new species of neotropical planorbid mollusks B. cousini.Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 26, no. 2, pp. 115-126. PMid:5998785.) is considered a potential vector of S. mansoni because it has released cercariae in experimental infections (Corrêa and Paraense, 1971Corrêa, L.R. and Paraense, W.L., 1971. Susceptibility of with two strains of Biomphalaria amazonicaSchistosoma mansoni.Revista do Instituto de Medicina Tropical de São Paulo, vol. 13, no. 6, pp. 387-390. PMid:5124648.; Paraense and Corrêa, 1985Paraense, W.L. and Corrêa, L.R., 1985. Further experiments on susceptibility of to Biomphalaria amazonicaSchistosoma mansoni.Memórias do Instituto Oswaldo Cruz, vol. 80, no. 3, pp. 259-262. http://dx.doi.org/10.1590/S0074-02761985000300001. PMid:3939249.
http://dx.doi.org/10.1590/S0074-02761985...
; Fernandez and Thiengo, 2006Fernandez, M.A. and Thiengo, S.C., 2006. Susceptibility of and from Manso Dam, Mato Grosso, Brazil to infection with three strains of Biomphalaria amazonicaBiomphalaria occidentalisSchistosoma mansoni.Memórias do Instituto Oswaldo Cruz, vol. 101, suppl. 1, pp. 235-237. http://dx.doi.org/10.1590/S0074-02762006000900036. PMid:17308775.
http://dx.doi.org/10.1590/S0074-02762006...
). This species is widely distributed in the North and Centre-West of Brazil (Paraense and Corrêa, 1985Paraense, W.L. and Corrêa, L.R., 1985. Further experiments on susceptibility of to Biomphalaria amazonicaSchistosoma mansoni.Memórias do Instituto Oswaldo Cruz, vol. 80, no. 3, pp. 259-262. http://dx.doi.org/10.1590/S0074-02761985000300001. PMid:3939249.
http://dx.doi.org/10.1590/S0074-02761985...
; Fernandez and Thiengo, 2006Fernandez, M.A. and Thiengo, S.C., 2006. Susceptibility of and from Manso Dam, Mato Grosso, Brazil to infection with three strains of Biomphalaria amazonicaBiomphalaria occidentalisSchistosoma mansoni.Memórias do Instituto Oswaldo Cruz, vol. 101, suppl. 1, pp. 235-237. http://dx.doi.org/10.1590/S0074-02762006000900036. PMid:17308775.
http://dx.doi.org/10.1590/S0074-02762006...
; Coimbra Junior et al., 1984Coimbra JUNIOR, C.E.A., Santos, R.V. and Smanio NETO, L., 1984. Potencial endêmico da esquistossomose para o Estado de Rondônia, Brasil. Revista de Saúde Pública, vol. 18, pp. 510-515.; Dorval and Silva, 1990Dorval, M.E.C. and Silva, R.P., 1990. Biomphalaria amazonica Paraense, 1966 in the state of Mato Grosso do Sul, Brazil (Mollusca, Pulmonata, Planorbidae). Memórias do Instituto Oswaldo Cruz, vol. 85, no. 1, pp. 117-118. http://dx.doi.org/10.1590/S0074-02761990000100021. PMid:2215223.
http://dx.doi.org/10.1590/S0074-02761990...
) and has also been found in Bolivia (Pointier et al., 2002Pointier, J.P., Paraense, W.L., Dejong, R.J., Loker, E.S., Bargues, M.D. and Mas-Coma, S., 2002. A potential snail host of schistosomiasis in Bolivia: . Biomphalaria amazonica Paraense 1966Memórias do Instituto Oswaldo Cruz, vol. 97, no. 6, pp. 793-796. http://dx.doi.org/10.1590/S0074-02762002000600007. PMid:12386698.
http://dx.doi.org/10.1590/S0074-02762002...
). The regions in Brazil where this species occurs have high migration rates (Coura and Amaral, 2004Coura, J.R. and Amaral, R.S., 2004. Epidemiological and control aspects of schistosomiasis in Brazilian endemic areas. Memórias do Instituto Oswaldo Cruz, vol. 99, no. 5, suppl. 1, pp. 13-19. PMid:15486629.), which favors the appearance of foci of S. mansoni schistosomiasis. This study presents the results of an assessment of the attraction of miracidia of S. mansoni (BH and SJ strains) (Paraense and Corrêa, 1963Paraense, W.L. and Corrêa, L.R., 1963. Sobre a ocorrência de duas raças biológicas de no Brasil. Schistosoma mansoniCiência e Cultura, vol. 15, pp. 245-246.) to Biomphalaria amazonica and their ability to penetrate this species. Miracidia of S. mansoni are attracted to mollusks in general, particularly to their vectors (Kloetzel, 1958KLOETZEL, K., 1958. Observações sobre o tropismo do miracídio do pelo molusco . Schistosoma mansoniAustralorbis glabratusBrazilian Journal of Biology = Revista Brasileira de Biologia, vol. 18, pp. 223-232.). Substances that are able to attract miracidia can be found in hemolymph and snail-conditioned water (SCW), where mollusks live (Chernin, 1970Chernin, E., 1970. Behavioral responses of miracidia of and other trematodes to substances emitted by snails. Schistosoma mansoniThe Journal of Parasitology, vol. 56, no. 2, pp. 287-296. http://dx.doi.org/10.2307/3277659. PMid:5445826.
http://dx.doi.org/10.2307/3277659...
). For both B. glabrata and B. tenagophila, the evolution of sporocysts inside the mollusk after the penetration of miracidia has been studied in detail (Pan, 1965Pan, C., 1965. Studies on the host-parasite relationship between Schistosoma mansoni and the snail Australorbis glabratus.The American Journal of Tropical Medicine and Hygiene, vol. 14, no. 6, pp. 931-976. PMid:5840648.; Guaraldo et al., 1981Guaraldo, A.M.A., Magalhães, L.A., Rangel, H.A. and Pareja, G., 1981. Evolução dos esporocistos de Schistosoma mansoni (Sambon, 1907) em (Say, 1818) e (D’Orbigny,1835). Biomphalaria glabrataBiomphalaria tenagophilaRevista de Saúde Pública, vol. 15, no. 4, pp. 436-448. http://dx.doi.org/10.1590/S0034-89101981000400008. PMid:7336119.
http://dx.doi.org/10.1590/S0034-89101981...
). In specimens that are more susceptible to infection, sporocysts develop without facing major tissue immune reactions, whereas specimens that are more resistant to infection are encapsulated by amebocytes and killed (Guaraldo et al., 1981Guaraldo, A.M.A., Magalhães, L.A., Rangel, H.A. and Pareja, G., 1981. Evolução dos esporocistos de Schistosoma mansoni (Sambon, 1907) em (Say, 1818) e (D’Orbigny,1835). Biomphalaria glabrataBiomphalaria tenagophilaRevista de Saúde Pública, vol. 15, no. 4, pp. 436-448. http://dx.doi.org/10.1590/S0034-89101981000400008. PMid:7336119.
http://dx.doi.org/10.1590/S0034-89101981...
). The mollusks can die either because of the high number of invading miracidia during the infection or because of the development of sporocysts that cause physiological and metabolic changes during the hosts’ immune responses. Tissues can also be compromised during the development of cercariae and before they are eliminated (Coelho, 1970Coelho, M.V., 1970. O Parasito: . In: Schistosoma mansoniA.S. CUNHA. Esquistossomose mansoni. São Paulo: Editora da Universidade de São Paulo, pp. 1-12.). Since an assessment of the mollusk-parasite relationship in a laboratory is the first step in the epidemiological study of schistosomiasis in a certain area, the aim of the present study was to determine the ability of B. amazonica to attract miracidia, the infectivity and mortality of mollusks in relation to the miracidia of the BH and SJ strains, and the amebocyte reactions to primary sporocysts.
2 Material and Methods
2.1 Species and strains
Specimens of B. amazonica were obtained from mollusks collected in Barão de Melgaço, Mato Grosso, Brazil (16° 11’ 40” S 55° 58’ 03” W) and kindly provided by Ph.D. Wladimir Lobato Paraense. The strains of S. mansoni used were the sympatric species of SJ (B. glabrata) and BH (B. tenagophila) (Paraense and Corrêa, 1963Paraense, W.L. and Corrêa, L.R., 1963. Sobre a ocorrência de duas raças biológicas de no Brasil. Schistosoma mansoniCiência e Cultura, vol. 15, pp. 245-246.).
2.2 Attraction of miracidia
In order to analyze the attraction of miracidia to mollusks and their snail-conditioned water (Chernin, 1972Chernin, E., 1972. Penetrative activity of miracidia stimulated by exposure to snail conditioned water. Schistosoma mansoniThe Journal of Parasitology, vol. 58, no. 2, pp. 209-212. http://dx.doi.org/10.2307/3278071. PMid:5022853.
http://dx.doi.org/10.2307/3278071...
), a glass apparatus consisting of two circular, 30-mm diameter chambers, linked by a channel (40mm long and 11mm wide), was used (Brasio et al., 1985Brasio, B.C., Magalhães, L.A., Miller, J. and Carvalho, J.F., 1985. Atração de miracídios de Schistosoma mansoni por hospedeiros invertebrados: comportamento de miracídios frente a girinos de Hylafuscovaria. Revista de Saúde Pública, vol. 19, no. 1, pp. 18-27. http://dx.doi.org/10.1590/S0034-89101985000100003. PMid:4081607.
http://dx.doi.org/10.1590/S0034-89101985...
). The specimen of B. amazonica was introduced into one chamber of the apparatus, which was filled with dechlorinated water. The mollusks had a diameter of between 5 and 7mm. For the SCW tests, a sample of SCW was added to one of the chambers and then 10 miracidia were placed in the center of the channel filled with dechlorinated water. After twenty minutes, the number of larvae that had moved to each compartment was counted. Ten mollusk specimens and ten different samples of SCW, for the BH and SJ strains of S. mansoni, were used.
2.3 Infectivity and mortality of mollusks
Thirty specimens of B. amazonica (diameter of 5 to 7-mm) were individually exposed to 20 miracidia of S. mansoni (BH and SJ strains) for two hours at a temperature of 28 °C under 60-W incandescent light. Susceptibility was determined from the fourth week after contact with miracidia, when cercariae were eliminated after the mollusks were exposed to light and heat. These observations were carried out until the 16th week after the infection. Every week, the water in the flasks was changed and the number of dead mollusks was registered. Feeding consisted of lettuce ad libitum.
2.4 Histopathological analysis of the cephalic region
For each strain of S. mansoni, twelve specimens of B. amazonica were individually exposed to one hundred miracidia. The mollusks were divided into groups of three specimens, according to the infection period (24 h, 48 h, 72 h and 96 h), and were fixed in Bouin’s fluid for 72 h (Guaraldo et al., 1981Guaraldo, A.M.A., Magalhães, L.A., Rangel, H.A. and Pareja, G., 1981. Evolução dos esporocistos de Schistosoma mansoni (Sambon, 1907) em (Say, 1818) e (D’Orbigny,1835). Biomphalaria glabrataBiomphalaria tenagophilaRevista de Saúde Pública, vol. 15, no. 4, pp. 436-448. http://dx.doi.org/10.1590/S0034-89101981000400008. PMid:7336119.
http://dx.doi.org/10.1590/S0034-89101981...
). The cephalic region immediately above the mantle collar was separated and serial histological sections (5 μm thickness) were stained with Gomori trichrome (Guaraldo et al., 1981Guaraldo, A.M.A., Magalhães, L.A., Rangel, H.A. and Pareja, G., 1981. Evolução dos esporocistos de Schistosoma mansoni (Sambon, 1907) em (Say, 1818) e (D’Orbigny,1835). Biomphalaria glabrataBiomphalaria tenagophilaRevista de Saúde Pública, vol. 15, no. 4, pp. 436-448. http://dx.doi.org/10.1590/S0034-89101981000400008. PMid:7336119.
http://dx.doi.org/10.1590/S0034-89101981...
). Optical microscopy analysis revealed the development of primary sporocysts and an amebocyte reaction to larvae.
2.5 Statistical analysis
SAS® software (SAS, 2012SAS Institute Inc. – SAS, 2012 [viewed 14 February 2012]. User’s manual [online]. Cary: SAS. Available from: http://support.sas.com/onlinedoc/913/docMainpage.jsp
http://support.sas.com/onlinedoc/913/doc...
) was used to compare the data obtained for B. amazonica with those obtained in a similar situation with B. glabrata and B. tenagophila, after exposure to their sympatric strains (BH and SJ, respectively).
3 Results
3.1 Attraction of miracidia
Biomphalaria amazonica (the mollusk and its SCW) attracted a significantly higher number of SJ miracidia than BH miracidia. When comparing the mollusk and its SCW, the latter showed a less intense attraction (Table 1). Since the strain-variable interaction was relevant (P˃0.0015), statistical data analysis was carried out considering the strains (BH and SJ) and the variables (SCW and mollusk). The results are shown in Tables 2 and 3. Specimens of B. amazonica attracted a significantly higher number of BH miracidia than their SCW. Conversely, SJ miracidia were attracted in a similar way to specimens of B. amazonica and their SCW (Table 2). The SCW of B. amazonica attracted a significantly higher number of SJ miracidia than BH miracidia, whereas specimens of B. amazonica showed no difference in the way they attracted miracidia (Table 3).
Attraction of BH and SJ miracidia strains of Schistosoma mansoni to Biomphalaria. amazonica (mollusc and Snail Conditioned Water, SCW).
Attraction of BH and SJ miracidia strains to Biomphalaria amazonica considering the variables Snail Conditioned Water (SCW) and mollusc.
Attraction of BH and SJ miracidia strains of Schistosoma mansoni to Snail Conditioned Water (SCW) or mollusc (Biomphalaria amazonica).
3.2 Infectivity and mortality
Four weeks after the mollusks had been exposed to BH and SJ miracidia, they were examined to determine the liberation of cercariae. In total, 13 specimens of B. amazonica that were exposed to the BH strain were alive. At the end of the experiment (16th week), only two of these mollusks were still alive and the tissue exhibited no sporocysts. Of the specimens of B. amazonica that were exposed to the SJ strain, 18 were alive in the 4th week and four survived until the 16th week. These mollusks did not exhibit SJ sporocysts. In the control groups, 26 specimens of B. glabrata were alive in the 4th week, but all of them had died by the 10th week. Conversely, 29 specimens of B. tenagophila were alive in the 4th week and 25 were still alive at the end of the experiment. These results can be seen in Figure 1.
Survival of the molluscs exposed to 20 miracidia of S. mansoni (BH and SJ strains). Ba: B. amazonica; Bg: B. glabrata; Bt: B. tenagophila.
Of the 60 specimens of B. amazonica that were exposed to S. mansoni, none eliminated cercariae during the experimental period (16 weeks).
Of the specimens of B. tenagophila that were alive in the 4th week post-infection, two eliminated cercariae (infection rate of 6.7%). Of the 30 specimens of B. glabrata that were exposed to BH miracidia, nine eliminated cercariae (infection rate of 30%).
3.3 Histopathological analysis of the cephalic region
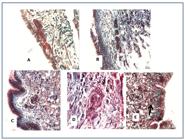
The number of sporocysts encapsulated by an amebocyte reaction was similar in all three species, although the number of viable larvae without an amebocyte reaction was significantly higher for B. glabrata (Figures 2A and 2B). A higher number of BH miracidia entered B. amazonica, and a significantly higher number of BH sporocysts were surrounded by an amebocyte reaction (Figure 2C). Ninety-six hours after the infection, viable sporocysts without an amebocyte reaction were seen in the three mollusk species (Figure 3). The largest sporocysts were found in B. glabrata (Figure 3C). In the case of B. amazonica, 53% of the sporocysts were viable. Figure 4 displays the viable sporocysts without an amebocyte reaction in B. amazonica. At the 96th hour, the size and number of germinative cells was similar for BH and SJ sporocysts (Figures 4A and 4B). The same aspects were observed for BH sporocysts in B. glabrata (Figure 4C). In B. tenagophila, the number of unviable sporocysts exceeded the number of viable ones (Figures 3B and 4D). Unviable sporocysts without an amebocyte reaction were observed in all three planorbid species (Figures 3 and 4E).
(A) Viable sporocysts with amoebocyte reaction; (B) Viable sporocysts without amoebocyte reaction in B. amazonica, B. glabrata and B.tenagophila; (C) Viability of BH and SJ sporocysts in B. amazonica (Vr: viable with reaction; Vs: viable without reaction; Ir: unviable with reaction and Is: unviable without reaction; columns with the same letter do not differ significantly amongst themselves).
Viability of sporocysts in B. amazonica, B. tenagophila and B.glabrata. (Vr: viable with reaction; Vs: viable without reaction; Ir: unviable with reaction; and Is: unviable without reaction; columns with the same letter do not differ significantly amongst themselves).
BH (A) and SJ (B) sporocysts of S. mansoni in B. amazonica 96 hours after the infection; (C) Viable BH sporocysts in B. glabrata; (D) Unviable sporocysts with amoebocyte reaction in B. tenagophila. Infection period of 96 hours; (E) Unviable BH sporocysts without amoebocyte reaction in B. amazonica. Stained with Gomori trichrome.
4 Discussion
Planorbid species with a physiology that allows the parasite to develop miracidia and produce cercariae are considered a vector of S. mansoni. Once the cercariae are eliminated in water, they can evolve into adult worms after penetrating the definitive host. This process of infecting the mollusk is genetically controlled and depends upon the genes of the mollusks being preadapted to the genes of the parasite.
Different species of snails of the genus Biomphalaria exhibit different degrees of susceptibility. Some species are more susceptible and others are more (or completely) refractory to infection by S. mansoni. Phylogenetic studies of Biomphalaria have demonstrated a co-evolution in the host/parasite relationship, indicating that the parasite exerts greater pressure when mollusks adapt to taxonomic groups. High susceptibility has been confirmed after the adaptation of the parasite to the mollusk over time (DeJong et al., 2001Dejong, R.J., Morgan, J.A., Paraense, W.L., Pointier, J.P., Amarista, M., Ayeh-Kumi, P.F., Babiker, A., Barbosa, C.S., Bremond, P., Pedro Canese, A., De Souza, C.P., Dominguez, C., File, S., Gutierrez, A., Incani, R.N., Kawano, T., Kazibwe, F., Kpikpi, J., Lwambo, N.J., Mimpfoundi, R., Njiokou, F., Noel Poda, J., Sene, M., Velasquez, L.E., Yong, M., Adema, C.M., Hofkin, B.V., Mkoji, G.M. and Loker, E.S., 2001. Evolutionary relationships and biogeography of (Gastropoda, Planorbidae) with implications regarding its role as host of the human bloodfluke, BiomphalariaSchistosoma mansoni.Molecular Biology and Evolution, vol. 18, no. 12, pp. 2225-2239. http://dx.doi.org/10.1093/oxfordjournals.molbev.a003769. PMid:11719572.
http://dx.doi.org/10.1093/oxfordjournals...
). Although B. amazonica is listed among the mollusks that are susceptible to S. mansoni, its geographic distribution in a harmless region indicates that it is rarely exposed to parasite pressure. However, this situation can be modified through the selection of populations of trematodes that are pre-adapted to the mollusk species. Studies of B. glabrata and B. tenagophila sought to determine alterations in susceptibility and resistance to S. mansoni. The authors found that an increase in susceptibility was more easily obtained than an increase in resistance (Zuim et al., 2005Zuim, N.R.B., Zanotti-Magalhães, E.M., Magalhães, L.A. and Linhares, A.X., 2005. Seleção genética de e Biomphalaria glabrataBiomphalaria tenagophila visando a alteração da suscetibilidade e resistência ao Schistosoma manson.Revista da Sociedade Brasileira de Medicina Tropical, vol. 38, no. 5, pp. 387-390. http://dx.doi.org/10.1590/S0037-86822005000500004. PMid:16172752.
http://dx.doi.org/10.1590/S0037-86822005...
). Even in the case of hybrid mollusks (crosses between susceptible and resistant species or populations), complete resistance to schistosome infections has never been achieved (Rosa et al., 2005Rosa, F.M., Godard, A.L.B., Azevedo, V. and Coelho, P.M.Z., 2005. Biomphalaria tenagophilaSchistosoma mansoni: dominant character of the resistance to in descendants of cross breeding between resistant (Taim, RS) and susceptible (Joinville, SC) strains. Memórias do Instituto Oswaldo Cruz, vol. 100, no. 1, pp. 19-23. http://dx.doi.org/10.1590/S0074-02762005000100004. PMid:15867958.
http://dx.doi.org/10.1590/S0074-02762005...
; Teodoro et al., 2011Teodoro, T.M., Janotti-Passos, L.K., Carvalho, O.S., Grijalva, M.J., Baús, E.G. and Caldeira, R.L., 2011. Hybridism between Biomphalaria cousini and Biomphalaria amazonica and its susceptibility to Schistosoma mansoni.Memórias do Instituto Oswaldo Cruz, vol. 106, no. 7, pp. 851-855. http://dx.doi.org/10.1590/S0074-02762011000700011. PMid:22124558.
http://dx.doi.org/10.1590/S0074-02762011...
). Evidence of genetic polymorphism has been reported, including comparisons of the genetic variability of mollusks that are susceptible and refractory to parasitism (Abdel-Hamid et al., 1999Abdel-Hamid, A.H., Molfetta, J.B., Fernandez, V. and Rodrigues, V., 1999. Genetic variation between susceptible and non-susceptible snails to Schistosoma infection using random amplified polymorphic DNA analysis (RAPDs). Revista do Instituto de Medicina Tropical de São Paulo, vol. 41, no. 5, pp. 291-295. PMid:10602543.; Spada et al., 2002Spada, R.G.M., Silva, D., Abdel-Hamid, A.Z., Sobral Hamaguchi, S.S., Zuim, N.R.B., Magalhães, E.M.Z., Magalhães, L.A. and Paes, J.T.R., 2002. Genetic markers between snails susceptible and resistant to Schistosoma mansoni infection. Biomphalaria glabrataMemórias do Instituto Oswaldo Cruz, vol. 97, suppl. 1, pp. 53-58. http://dx.doi.org/10.1590/S0074-02762002000900012. PMid:12426595.
http://dx.doi.org/10.1590/S0074-02762002...
; Silva et al., 2004Silva, D., Spada, R.G., Sobral-Hamaguchl, S.S., Abdel-Hamid, Z., Zuim, N.R., Zanitti-Magalhães, E.M., Magalhães, L.A. and Ribeiro-Paes, J.T., 2004. Biomphalaria tenagophilaSchistosoma mansoni: Genetic variability within intermediate snail hosts susceptible and resistant to infection. Parasite, vol. 11, no. 1, pp. 43-49. PMid:15071826.). This highlights the importance of the heredity of susceptibility among snails and the maintenance of foci of the disease. Other studies have reported that a similar population of snails may respond differently when exposed to different strains of S. mansoni, and that some of these strains exhibit pre-adapted phenotypes that can affect the degree of parasitism, thereby enabling the potential expansion of the schistosomiasis to new locations, where snail populations are found (Teodoro et al., 2011Teodoro, T.M., Janotti-Passos, L.K., Carvalho, O.S., Grijalva, M.J., Baús, E.G. and Caldeira, R.L., 2011. Hybridism between Biomphalaria cousini and Biomphalaria amazonica and its susceptibility to Schistosoma mansoni.Memórias do Instituto Oswaldo Cruz, vol. 106, no. 7, pp. 851-855. http://dx.doi.org/10.1590/S0074-02762011000700011. PMid:22124558.
http://dx.doi.org/10.1590/S0074-02762011...
; Simões et al., 2013Simões, L.F., Camargo, E.A.F., Basto, L.A.D., Neves, M.F., Carvalho, J.F., Magalhães, L.A. and Zanotti-Magalhães, E.M., 2013. Susceptibility of Argentinean Biomphalaria tenagophila and and the possibility of geographic expansion of mansoni schistosomiasis. Biomphalaria straminea to infection by Schistosoma mansoniRevista da Sociedade Brasileira de Medicina Tropical, vol. 46, no. 5, pp. 611-616. http://dx.doi.org/10.1590/0037-8682-0131-2013. PMid:24142365.
http://dx.doi.org/10.1590/0037-8682-0131...
).
The ability to attract miracidia is the first factor to be considered in the establishment of the relationship between the trematode and its potential host. B. amazonica was able to attract approximately 77% of the miracidia it was exposed to (78% for BH miracidia and 75% for SJ miracidia). The SCW of B. amazonica attracted less BH miracidia than the mollusk itself. Components of this SCW may have reduced the attraction of BH miracidia, although no interference was related to the SJ miracidia, given that they were attracted in the same proportion as B. amazonica and its SCW. Brasio et al. (1985)Brasio, B.C., Magalhães, L.A., Miller, J. and Carvalho, J.F., 1985. Atração de miracídios de Schistosoma mansoni por hospedeiros invertebrados: comportamento de miracídios frente a girinos de Hylafuscovaria. Revista de Saúde Pública, vol. 19, no. 1, pp. 18-27. http://dx.doi.org/10.1590/S0034-89101985000100003. PMid:4081607.
http://dx.doi.org/10.1590/S0034-89101985...
reported that the SCW of B. glabrata attracted BH and SJ miracidia in a similar manner, although the SCW of B. tenagophila attracted a significantly higher number of SJ miracidia. While analyzing samples of the SCW of both species, the authors noticed a distinct composition. The histological study of the infection showed more penetration of BH miracidia, as displayed in Figure 2C. The same amount of viable sporocysts without an amebocyte reaction was found in B. amazonica and B. tenagophila. Ninety-six hours post-infection, the development patterns of the BH and SJ sporocysts in B. amazonica (Figures 4A and 4B) and of the BH sporocysts in B. glabrata were similar.
Concerning the survival of mollusks, B. amazonica had a shorter lifespan when exposed to the BH strain (Figure 1). Amebocyte reactions occurred in a significantly higher number around BH larvae (Figure 2C). The death of sporocysts was mainly followed by amebocyte reactions, although unviable sporocysts without an amebocyte reaction were observed in almost all specimens and infection periods (Figures 2C, 3 and 4E). Although an amebocyte reaction is the main defense mechanism (Lie et al., 1980Lie, K.J., Jeong, K.H. and Heyneman, D., 1980. Tissue reactions by Schistosoma mansoni in Biomphalaria glabrata.Annals of Tropical Medicine and Parasitology, vol. 74, no. 2, pp. 157-166. http://dx.doi.org/10.1080/00034983.1980.11687326. PMid:7436601.
http://dx.doi.org/10.1080/00034983.1980....
), the presence of degenerating larvae without an amebocyte reaction has been reported in mollusks that were simultaneously infected with S. mansoni and another trematode species (Balan et al., 1993Balan, D.L., Magalhães, L.A. and Piedrabuena, A.E., 1993. Aspectos imunológicos e parasitológicos em Biomphalaria tenagophila infectadas por Schistosoma mansoni e outros Digenea. Revista de Saúde Pública, vol. 27, no. 6, pp. 421-429. http://dx.doi.org/10.1590/S0034-89101993000600004. PMid:7997812.
http://dx.doi.org/10.1590/S0034-89101993...
). The greater damage to parasitized tissues, either from more penetration of BH miracidia or the amebocyte reaction of the mollusk, could explain the higher mortality of B. amazonica when it was exposed to the BH strain. The similar number of BH and SJ sporocysts in B. amazonica (Figures 2C and 3A) and the ability of this species to attract larvae allow us to conclude that it is susceptible to both strains of S. mansoni.
Based on these results, health surveillance and epidemiology centers located in the distribution area of B. amazonica should monitor the movements of people infected with S. mansoni, given that they could create foci of schistosomiasis in areas where the disease has not been reported as endemic.
Acknowledgements
This work was supported by FAPESP.
-
(With 4 figures)
References
- Abdel-Hamid, A.H., Molfetta, J.B., Fernandez, V. and Rodrigues, V., 1999. Genetic variation between susceptible and non-susceptible snails to Schistosoma infection using random amplified polymorphic DNA analysis (RAPDs). Revista do Instituto de Medicina Tropical de São Paulo, vol. 41, no. 5, pp. 291-295. PMid:10602543.
- Balan, D.L., Magalhães, L.A. and Piedrabuena, A.E., 1993. Aspectos imunológicos e parasitológicos em Biomphalaria tenagophila infectadas por Schistosoma mansoni e outros Digenea. Revista de Saúde Pública, vol. 27, no. 6, pp. 421-429. http://dx.doi.org/10.1590/S0034-89101993000600004 PMid:7997812.
» http://dx.doi.org/10.1590/S0034-89101993000600004 - Brasio, B.C., Magalhães, L.A., Miller, J. and Carvalho, J.F., 1985. Atração de miracídios de Schistosoma mansoni por hospedeiros invertebrados: comportamento de miracídios frente a girinos de Hylafuscovaria. Revista de Saúde Pública, vol. 19, no. 1, pp. 18-27. http://dx.doi.org/10.1590/S0034-89101985000100003 PMid:4081607.
» http://dx.doi.org/10.1590/S0034-89101985000100003 - Chernin, E., 1970. Behavioral responses of miracidia of and other trematodes to substances emitted by snails. Schistosoma mansoniThe Journal of Parasitology, vol. 56, no. 2, pp. 287-296. http://dx.doi.org/10.2307/3277659 PMid:5445826.
» http://dx.doi.org/10.2307/3277659 - Chernin, E., 1972. Penetrative activity of miracidia stimulated by exposure to snail conditioned water. Schistosoma mansoniThe Journal of Parasitology, vol. 58, no. 2, pp. 209-212. http://dx.doi.org/10.2307/3278071 PMid:5022853.
» http://dx.doi.org/10.2307/3278071 - Coelho, M.V., 1970. O Parasito: . In: Schistosoma mansoniA.S. CUNHA. Esquistossomose mansoni. São Paulo: Editora da Universidade de São Paulo, pp. 1-12.
- Coimbra JUNIOR, C.E.A., Santos, R.V. and Smanio NETO, L., 1984. Potencial endêmico da esquistossomose para o Estado de Rondônia, Brasil. Revista de Saúde Pública, vol. 18, pp. 510-515.
- Corrêa, L.R. and Paraense, W.L., 1971. Susceptibility of with two strains of Biomphalaria amazonicaSchistosoma mansoni.Revista do Instituto de Medicina Tropical de São Paulo, vol. 13, no. 6, pp. 387-390. PMid:5124648.
- Coura, J.R. and Amaral, R.S., 2004. Epidemiological and control aspects of schistosomiasis in Brazilian endemic areas. Memórias do Instituto Oswaldo Cruz, vol. 99, no. 5, suppl. 1, pp. 13-19. PMid:15486629.
- D’orbigny, A., 1835. Synopsis terrestrium et fluviatilium molluscorum, in suo per American meridionalem itinere collectorum. Magazine Zoological., vol. 5, no. 61-62, pp. 1-44.
- Dejong, R.J., Morgan, J.A., Paraense, W.L., Pointier, J.P., Amarista, M., Ayeh-Kumi, P.F., Babiker, A., Barbosa, C.S., Bremond, P., Pedro Canese, A., De Souza, C.P., Dominguez, C., File, S., Gutierrez, A., Incani, R.N., Kawano, T., Kazibwe, F., Kpikpi, J., Lwambo, N.J., Mimpfoundi, R., Njiokou, F., Noel Poda, J., Sene, M., Velasquez, L.E., Yong, M., Adema, C.M., Hofkin, B.V., Mkoji, G.M. and Loker, E.S., 2001. Evolutionary relationships and biogeography of (Gastropoda, Planorbidae) with implications regarding its role as host of the human bloodfluke, BiomphalariaSchistosoma mansoni.Molecular Biology and Evolution, vol. 18, no. 12, pp. 2225-2239. http://dx.doi.org/10.1093/oxfordjournals.molbev.a003769 PMid:11719572.
» http://dx.doi.org/10.1093/oxfordjournals.molbev.a003769 - Dorval, M.E.C. and Silva, R.P., 1990. Biomphalaria amazonica Paraense, 1966 in the state of Mato Grosso do Sul, Brazil (Mollusca, Pulmonata, Planorbidae). Memórias do Instituto Oswaldo Cruz, vol. 85, no. 1, pp. 117-118. http://dx.doi.org/10.1590/S0074-02761990000100021 PMid:2215223.
» http://dx.doi.org/10.1590/S0074-02761990000100021 - Dunker, W., 1848. Diagnoses specierum novarum generis Planorbis collectionis Cumingianae. Proceedings of the Zoological Society of London, vol. 16, pp. 40-43.
- Fernandez, M.A. and Thiengo, S.C., 2006. Susceptibility of and from Manso Dam, Mato Grosso, Brazil to infection with three strains of Biomphalaria amazonicaBiomphalaria occidentalisSchistosoma mansoni.Memórias do Instituto Oswaldo Cruz, vol. 101, suppl. 1, pp. 235-237. http://dx.doi.org/10.1590/S0074-02762006000900036 PMid:17308775.
» http://dx.doi.org/10.1590/S0074-02762006000900036 - Guaraldo, A.M.A., Magalhães, L.A., Rangel, H.A. and Pareja, G., 1981. Evolução dos esporocistos de Schistosoma mansoni (Sambon, 1907) em (Say, 1818) e (D’Orbigny,1835). Biomphalaria glabrataBiomphalaria tenagophilaRevista de Saúde Pública, vol. 15, no. 4, pp. 436-448. http://dx.doi.org/10.1590/S0034-89101981000400008 PMid:7336119.
» http://dx.doi.org/10.1590/S0034-89101981000400008 - KLOETZEL, K., 1958. Observações sobre o tropismo do miracídio do pelo molusco . Schistosoma mansoniAustralorbis glabratusBrazilian Journal of Biology = Revista Brasileira de Biologia, vol. 18, pp. 223-232.
- Lie, K.J., Jeong, K.H. and Heyneman, D., 1980. Tissue reactions by Schistosoma mansoni in Biomphalaria glabrata.Annals of Tropical Medicine and Parasitology, vol. 74, no. 2, pp. 157-166. http://dx.doi.org/10.1080/00034983.1980.11687326 PMid:7436601.
» http://dx.doi.org/10.1080/00034983.1980.11687326 - Pan, C., 1965. Studies on the host-parasite relationship between Schistosoma mansoni and the snail Australorbis glabratus.The American Journal of Tropical Medicine and Hygiene, vol. 14, no. 6, pp. 931-976. PMid:5840648.
- Paraense, W.L., 1966. Biomphalaria amazonica and , two new species of neotropical planorbid mollusks B. cousini.Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 26, no. 2, pp. 115-126. PMid:5998785.
- Paraense, W.L. and Corrêa, L.R., 1963. Sobre a ocorrência de duas raças biológicas de no Brasil. Schistosoma mansoniCiência e Cultura, vol. 15, pp. 245-246.
- Paraense, W.L. and Corrêa, L.R., 1985. Further experiments on susceptibility of to Biomphalaria amazonicaSchistosoma mansoni.Memórias do Instituto Oswaldo Cruz, vol. 80, no. 3, pp. 259-262. http://dx.doi.org/10.1590/S0074-02761985000300001 PMid:3939249.
» http://dx.doi.org/10.1590/S0074-02761985000300001 - Pointier, J.P., Paraense, W.L., Dejong, R.J., Loker, E.S., Bargues, M.D. and Mas-Coma, S., 2002. A potential snail host of schistosomiasis in Bolivia: . Biomphalaria amazonica Paraense 1966Memórias do Instituto Oswaldo Cruz, vol. 97, no. 6, pp. 793-796. http://dx.doi.org/10.1590/S0074-02762002000600007 PMid:12386698.
» http://dx.doi.org/10.1590/S0074-02762002000600007 - Rosa, F.M., Godard, A.L.B., Azevedo, V. and Coelho, P.M.Z., 2005. Biomphalaria tenagophilaSchistosoma mansoni: dominant character of the resistance to in descendants of cross breeding between resistant (Taim, RS) and susceptible (Joinville, SC) strains. Memórias do Instituto Oswaldo Cruz, vol. 100, no. 1, pp. 19-23. http://dx.doi.org/10.1590/S0074-02762005000100004 PMid:15867958.
» http://dx.doi.org/10.1590/S0074-02762005000100004 - SAMBON, L.W., 1907. Descriptions of some new species of animal parasites. Proceedings of the Zoological Society of London, vol. 77, no. 2, pp. 282-283. http://dx.doi.org/10.1111/j.1096-3642.1907.tb01817.x
» http://dx.doi.org/10.1111/j.1096-3642.1907.tb01817.x - SAS Institute Inc. – SAS, 2012 [viewed 14 February 2012]. User’s manual [online]. Cary: SAS. Available from: http://support.sas.com/onlinedoc/913/docMainpage.jsp
» http://support.sas.com/onlinedoc/913/docMainpage.jsp - Say, T., 1818. Account of two new genera, and several new species, of fresh water and land shells. Journal of the Academy of Natural Sciences of Philadelphia, vol. 1, no. 2, pp. 276-284.
- Silva, D., Spada, R.G., Sobral-Hamaguchl, S.S., Abdel-Hamid, Z., Zuim, N.R., Zanitti-Magalhães, E.M., Magalhães, L.A. and Ribeiro-Paes, J.T., 2004. Biomphalaria tenagophilaSchistosoma mansoni: Genetic variability within intermediate snail hosts susceptible and resistant to infection. Parasite, vol. 11, no. 1, pp. 43-49. PMid:15071826.
- Simões, L.F., Camargo, E.A.F., Basto, L.A.D., Neves, M.F., Carvalho, J.F., Magalhães, L.A. and Zanotti-Magalhães, E.M., 2013. Susceptibility of Argentinean Biomphalaria tenagophila and and the possibility of geographic expansion of mansoni schistosomiasis. Biomphalaria straminea to infection by Schistosoma mansoniRevista da Sociedade Brasileira de Medicina Tropical, vol. 46, no. 5, pp. 611-616. http://dx.doi.org/10.1590/0037-8682-0131-2013 PMid:24142365.
» http://dx.doi.org/10.1590/0037-8682-0131-2013 - Spada, R.G.M., Silva, D., Abdel-Hamid, A.Z., Sobral Hamaguchi, S.S., Zuim, N.R.B., Magalhães, E.M.Z., Magalhães, L.A. and Paes, J.T.R., 2002. Genetic markers between snails susceptible and resistant to Schistosoma mansoni infection. Biomphalaria glabrataMemórias do Instituto Oswaldo Cruz, vol. 97, suppl. 1, pp. 53-58. http://dx.doi.org/10.1590/S0074-02762002000900012 PMid:12426595.
» http://dx.doi.org/10.1590/S0074-02762002000900012 - Teodoro, T.M., Janotti-Passos, L.K., Carvalho, O.S., Grijalva, M.J., Baús, E.G. and Caldeira, R.L., 2011. Hybridism between Biomphalaria cousini and Biomphalaria amazonica and its susceptibility to Schistosoma mansoni.Memórias do Instituto Oswaldo Cruz, vol. 106, no. 7, pp. 851-855. http://dx.doi.org/10.1590/S0074-02762011000700011 PMid:22124558.
» http://dx.doi.org/10.1590/S0074-02762011000700011 - Zuim, N.R.B., Zanotti-Magalhães, E.M., Magalhães, L.A. and Linhares, A.X., 2005. Seleção genética de e Biomphalaria glabrataBiomphalaria tenagophila visando a alteração da suscetibilidade e resistência ao Schistosoma manson.Revista da Sociedade Brasileira de Medicina Tropical, vol. 38, no. 5, pp. 387-390. http://dx.doi.org/10.1590/S0037-86822005000500004 PMid:16172752.
» http://dx.doi.org/10.1590/S0037-86822005000500004
Publication Dates
-
Publication in this collection
26 Sept 2016 -
Date of issue
Apr-Jun 2017
History
-
Received
14 Sept 2015 -
Accepted
04 Feb 2016