Abstract
Even today, an effective diagnostic test for aspergillosis in penguins is unknown, being the gold standard post-mortem examinations. The fungal antigen galactomannan (GM) has been used as a biomarker of disease in humans and is detected by the Platelia Aspergillus EIA (BioRad)®, a commercial kit based on the sandwich ELISA technique. It is standardized for use in neutropenic patients, however studies have demonstrated its usefulness also possible for birds. The aim of our study was to evaluate the effectiveness of Platelia Aspergillus EIA® test (BioRad-US) in the diagnosis of aspergillosis in Magellanic penguins, determining sensitivity, specificity, and positive and negative predictive values for different cut-off points. Were included in the study, blood serum samples (n = 29) Magellanic penguins in captivity that died by aspergillosis. Detection of GM was performed following manufacturer's instructions and the GM index was obtained by dividing the average value of OD of the duplicate of the clinical sample by duplicate OD of the average value of the cut-off sample provided by the kit. Through information database results were obtained for the presence of anti-Aspergillus fumigatus antibodies detected by agar gel immunodiffusion (AGID) for all serum samples. Results were analyzed using chi-square test and Kruskal-Wallis from SPSS 20.0, IBM®. ROC curve was obtained and from this, rates of sensitivity, specificity, positive and negative predictive values were also calculated based on four different cutoff points (0.5, 1.0, 1.5 and 2.0). The serum GM index did not differ between animals of the case and control group (pkw =0.097). In determining the ROC curve for serum GM detection the value of area under the curve was 0.635. From the values determined by the coordinate of the curve, four different cut points (0.5, 1.0, 1.5 and 2.0) were analyzed, resulting in sensitivity rates ranging from 86.2 to 34.5% % and specificity between 87% and 26.1%. By comparing the serum GM index in group case as the presence or absence of antibodies detected by AGID was found p=0.503. The detection of GM the Platelia Aspergillus EIA® test seems is not be useful for the diagnosis of aspergillosis in naturally infected penguins.
Keywords:
galactomannan; immunoenzimatic test; mycosis; birds
Resumo
Ainda hoje, um teste diagnóstico eficaz para aspergilose em pinguins não é conhecido, sendo o padrão-ouro os exames post-mortem. O antígeno fúngico galactomanana (GM) tem sido utilizado como biomarcador da doença em humanos, sendo detectado pelo Platelia Aspergillus EIA (BioRad)®, um kit comercial que se baseia na técnica ELISA sanduíche. É padronizado para utilização em pacientes neutropênicos, no entanto estudos tem demonstrado sua possível utilidade também para aves.O objetivo de nosso estudo foi avaliar a eficácia do teste Platelia Aspergillus EIA® (BioRad-US) no diagnóstico da aspergilose em pinguins-de-Magalhães, determinando sensibilidade, especificidade e valores preditivos positivos e negativos em diferentes pontos de corte. Foram incluídas no estudo, amostras de soro sanguíneo (n=29) de pinguins-de-Magalhães em cativeiro que vieram a óbito por aspergilose. A detecção de GM foi realizada seguindo instruções do fabricante e o índice de GM foi obtido dividindo o valor da média da DO da duplicata da amostra clínica pelo valor da média da DO da duplicata da amostra de cut-off fornecida pelo kit. Através de informações em banco de dados foram obtidos resultados sobre a presença de anticorpos anti-Aspergillus fumigatus, detectada por Imunodifusão em gel de ágar (IDGA) em todas as amostras séricas. Os resultados foram analisados utilizando-se teste de qui-quadrado e Kruskal-Wallis a partir do programa estatístico SPSS 20.0, IBM®. Curva ROC foi obtida e a partir desta, taxas de sensibilidade, especificidade, valores preditivo positivo e negativo foram igualmente calculados considerando quatro diferentes pontos de corte (0.5, 1.0, 1.5 e 2.0). O índice de GM sérica não diferiu entre os animais do grupo caso e controle (pKW = 0.097). Na determinação da curva ROC para detecção de GM sérica o valor da área sobre a curva foi de 0.635. A partir dos valores determinados pelas coordenadas da curva, quatro diferentes pontos de corte (0.5, 1.0, 1.5 e 2.0) foram analisados, resultando em taxas de sensibilidade variando de 86.2% a 34.5%, e de especificidade entre 87% e 26.1%. Ao comparar o índice de GM sérica nos animais do grupo caso quanto a presença ou não de anticorpos detectados pela IDGA foi encontrado p=0.503. A detecção de GM pelo teste Platelia Aspergillus EIA® não parece ser útil para o diagnóstico da aspergilose em pinguins naturalmente infectados.
Palavras chave:
galactomanana; teste imunoenzimático; micoses; aves
1. Introduction
Aspergillus species have been known for decades as important pathogens of birds, leading to high mortality rates, including the Spheniscidae family, being about 90-95% of the cases of aspergillosis caused by Aspergillus section Fumigati ( Cray et al., 2009b CRAY, C., WATSON, T., RODRIGUEZ, M. and ARHEART, K.L., 2009b. Application of galactomannan analysis and protein electrophoresis in the diagnosis of aspergillosis in avian species. Journal of Zoo and Wildlife Medicine, vol. 40, no. 1, pp. 64-70. http://dx.doi.org/10.1638/2007-0138.1. PMid:19368241.
http://dx.doi.org/10.1638/2007-0138.1 ...
; Cabana, 2013 CABANA, A.L. 2013. Monitoramento sorológico para diagnóstico precoce da aspergilose em pinguins em cativeiro. Pelotas: Programa de Pós Graduação em Veterinária, Universidade Federal de Pelotas, 110 p. Dissertação de Mestrado. ; Xavier et al., 2011 XAVIER, M.O., AQUINO, V.R. and SEVERO, L.C., 2011. Galactomanana no diagnóstico de aspergilose invasiva. Revista Brasileira de Oncologia Clínica , vol. 7, no. 7, pp. 41-50. ).
The diagnosis ante-mortem of invasive aspergillosis (IA) in birds is limited and traditional techniques, such as blood tests, biochemical tests and imaging studies may reveal only nonspecific changes ( Xavier et al., 2011 XAVIER, M.O., AQUINO, V.R. and SEVERO, L.C., 2011. Galactomanana no diagnóstico de aspergilose invasiva. Revista Brasileira de Oncologia Clínica , vol. 7, no. 7, pp. 41-50. ; 2008 XAVIER, M.O., PASQUALOTTO, A.C., SOARES, M.P., et al 2008. Aspergillosis in penguins: gross lesions in 15 cases. 3rd ed. Miami: Advances Against Aspergillosis, 132 p. ; 2007 XAVIER, M.O., SOARES, M.P., MEINERZ, A.R.M., NOBRE, M.O., OSÓRIO, L.G., SILVA FILHO, R.P. and MEIRELES, M.C.A., 2007. Aspergillosis: a limiting factor during recovery of captive Magellanic penguins. Brazilian Journal of Microbiology, vol. 38, no. 3, pp. 480-484. http://dx.doi.org/10.1590/S1517-83822007000300018.
http://dx.doi.org/10.1590/S1517-8382200...
).
Mycological classic tests have low sensitivity and / or specificity, and use of serological tests, although indicated in the literature ( Cabana et al., 2015 CABANA, Â.L., XAVIER, M.O., POESTER, V., KLAFKE, G.B., BRUNO FILHO, P.L., MARTINS, A., SILVA FILHO, R.P. and MEIRELES, M.C.A., 2015. Serological monitoring of antibodies for an early diagnosis ofaspergillosis in captive penguins. Pesquisa Veterinária Brasileira, vol. 35, no. 6, pp. 573-578. http://dx.doi.org/10.1590/S0100-736X2015000600015.
http://dx.doi.org/10.1590/S0100-736X201...
; Tell, 2005 TELL, L.A., 2005. Aspergillosis in mammals and birds: impact on veterinary medicine. Journal of Exotic Pet Medicine, vol. 43, no. s1, suppl. 1, pp. 71-73. http://dx.doi.org/10.1080/13693780400020089. PMid:16110795.
http://dx.doi.org/10.1080/1369378040002...
) it is not in routine use, as the gold standard is still restricted to histopathological and mycological post-mortem ( Cray et al., 2009a CRAY, C., REAVILL, D., ROMAGNANO, A., VAN SANT, F., CHAMPAGNE, D., STEVENSON, R., ROLFE, V., GRIFFIN, C. and CLUBB, S., 2009a. Galactomannan assay and plasma protein electrophoresis findings in psittacine birds with aspergillosis. Journal of Avian Medicine and Surgery, vol. 23, no. 2, pp. 125-135. http://dx.doi.org/10.1647/2007-041.1. PMid:19673459.
http://dx.doi.org/10.1647/2007-041.1 ...
, b CRAY, C., WATSON, T., RODRIGUEZ, M. and ARHEART, K.L., 2009b. Application of galactomannan analysis and protein electrophoresis in the diagnosis of aspergillosis in avian species. Journal of Zoo and Wildlife Medicine, vol. 40, no. 1, pp. 64-70. http://dx.doi.org/10.1638/2007-0138.1. PMid:19368241.
http://dx.doi.org/10.1638/2007-0138.1 ...
).
Modern techniques for the diagnosis of aspergillosis by direct detection of antigen galactomannan (GM) in clinical samples have been increasingly used for the diagnosis of IA in humans from different species. The GM is a polysaccharide present in the fungal cell wall of the genus Aspergillus , a family of derivatives galactofuranose antigens. Their release into the bloodstream occurs during the growth of hyphae in tissue invasion process ( Mennink-Kersten et al., 2004 MENNINK-KERSTEN, M.A., DONNELLY, J.P. and VERWEIJ, P.E., 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. The Lancet Infectious Diseases, vol. 4, no. 6, pp. 349-357. http://dx.doi.org/10.1016/S1473-3099(04)01045-X. PMid:15172343.
http://dx.doi.org/10.1016/S1473-3099(04...
; Nucci and Colombo, 2012 NUCCI, M., COLOMBO, A.L., 2012. Quando utilizar terapia empírica em doenças fúngicas invasivas? Rev. Panam. Infectol., vol. 14, no. 1, pp. 32-44. ). This molecule can be considered as an important biomarker for the determination of invasive fungal infections by Aspergillus spp. in different clinical samples to be water soluble ( Maertens et al., 2007 MAERTENS, J.A., KLONT, R., MASSON, C., THEUNISSEN, K., MEERSSEMAN, W., LAGROU, K., HEINEN, C., CRÉPIN, B., VAN ELDERE, J., TABOURET, M., DONNELLY, J.P. and VERWEIJ, P.E., 2007. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clinical Infectious Diseases, vol. 44, no. 10, pp. 1329-1336. http://dx.doi.org/10.1086/514349. PMid:17443470.
http://dx.doi.org/10.1086/514349 ...
; Xavier et al., 2011 XAVIER, M.O., AQUINO, V.R. and SEVERO, L.C., 2011. Galactomanana no diagnóstico de aspergilose invasiva. Revista Brasileira de Oncologia Clínica , vol. 7, no. 7, pp. 41-50. ).
The Platelia Aspergillus EIA® (Bio-Rad USA) is a commercially available diagnostic kit which is based on sandwich ELISA for detection of galactomannan ( Maertens et al., 2007 MAERTENS, J.A., KLONT, R., MASSON, C., THEUNISSEN, K., MEERSSEMAN, W., LAGROU, K., HEINEN, C., CRÉPIN, B., VAN ELDERE, J., TABOURET, M., DONNELLY, J.P. and VERWEIJ, P.E., 2007. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clinical Infectious Diseases, vol. 44, no. 10, pp. 1329-1336. http://dx.doi.org/10.1086/514349. PMid:17443470.
http://dx.doi.org/10.1086/514349 ...
; Xavier et al., 2011 XAVIER, M.O., AQUINO, V.R. and SEVERO, L.C., 2011. Galactomanana no diagnóstico de aspergilose invasiva. Revista Brasileira de Oncologia Clínica , vol. 7, no. 7, pp. 41-50. ).This test is standard for blood serum and bronchoalveolar lavage of human and neutropenic patients, and when performed serially, anticipates the diagnosis of aspergillosis within one week. Some limitations are described, and the rates of false-negative and false-positive results fluctuate around 10% ( Mennink-Kersten et al., 2004 MENNINK-KERSTEN, M.A., DONNELLY, J.P. and VERWEIJ, P.E., 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. The Lancet Infectious Diseases, vol. 4, no. 6, pp. 349-357. http://dx.doi.org/10.1016/S1473-3099(04)01045-X. PMid:15172343.
http://dx.doi.org/10.1016/S1473-3099(04...
; Nucci and Colombo, 2012 NUCCI, M., COLOMBO, A.L., 2012. Quando utilizar terapia empírica em doenças fúngicas invasivas? Rev. Panam. Infectol., vol. 14, no. 1, pp. 32-44. ).
Although the ELISA sandwich test for GM detection is considered an important diagnostic tool for AI in humans. Studies have shown that can also contribute to the diagnosis of other species such as dogs, cattle and poultry ( Arca-Ruibal et al., 2006 ARCA-RUIBAL, B., WERNERY, U., ZACHARIAH, R., BAILEY, T.A., SOMMA, A., SILVANOSE, C. and MCKINNEY, P., 2006. Assessment of a commercial sandwich ELISA in the diagnosis of aspergillosis in falcons. The Veterinary Record, vol. 158, no. 13, pp. 442-444. http://dx.doi.org/10.1136/vr.158.13.442. PMid:16581995.
http://dx.doi.org/10.1136/vr.158.13.442...
; Billen et al., 2009 BILLEN, F., PEETERS, D., PETERS, I.R., HELPS, C.R., HUYNEN, P., MOL, P., MASSART, L., DAY, M.J. and CLERCX, C., 2009. Comparison of the value of measurement of serum galactomannan and Aspergillus-specific antibodies in the diagnosis of canine sino-nasal aspergilosis. Veterinary Microbiology, vol. 133, no. 4, pp. 358-365. http://dx.doi.org/10.1016/j.vetmic.2008.07.018. PMid:18768268.
http://dx.doi.org/10.1016/j.vetmic.2008...
; Cray et al., 2009a CRAY, C., REAVILL, D., ROMAGNANO, A., VAN SANT, F., CHAMPAGNE, D., STEVENSON, R., ROLFE, V., GRIFFIN, C. and CLUBB, S., 2009a. Galactomannan assay and plasma protein electrophoresis findings in psittacine birds with aspergillosis. Journal of Avian Medicine and Surgery, vol. 23, no. 2, pp. 125-135. http://dx.doi.org/10.1647/2007-041.1. PMid:19673459.
http://dx.doi.org/10.1647/2007-041.1 ...
; Franca et al., 2012 FRANÇA, M., CRAY, C. and SHIVAPRASAD, H.L., 2012. Serologic testing for aspergillosis in commercial broiler chickens and turkeys. Avian Diseases, vol. 56, no. 1, pp. 160-164. http://dx.doi.org/10.1637/9836-061911-Reg.1. PMid:22545542.
http://dx.doi.org/10.1637/9836-061911-R...
; Garcia et al., 2001 GARCIA, M.E., CABALLERO, M., CRUZADO, M., ANDRINO, M., GONZALEZ-CABO, J.F. and BLANCO, J.L., 2001. The value of the determination of anti-AspergillusIgG in the serodiagnosis of canine aspergillosis: Comparison with galactomannan detection. Journal of Veterinary Medicine, vol. 48, no. 10, pp. 743-750. http://dx.doi.org/10.1046/j.1439-0450.2001.00504.x. PMid:11846019.
http://dx.doi.org/10.1046/j.1439-0450.2...
; Garcia et al., 2008 GARCIA, M.E., CABALLERO, J., ALVAREZ-PEREZ, S. and BLANCO, J.L., 2008. Seroprevalence of Aspergillus fumigatus antibodies in bovine herds with a history of reprodutive disorders. Veterinary Medicine, vol. 53, no. 3, pp. 117-123. http://dx.doi.org/10.17221/1939-VETMED.
http://dx.doi.org/10.17221/1939-VETMED ...
; Guillot et al., 1999 GUILLOT, J., SARFATI, J., BARROS, M., CADORÉ, J.L., JENSEN, H.E. and CHERMETTE, R., 1999. Comparative study of serological tests for the diagnosis of equine aspergillosis. The Veterinary Record, vol. 145, no. 12, pp. 348-349. http://dx.doi.org/10.1136/vr.145.12.348. PMid:10530885.
http://dx.doi.org/10.1136/vr.145.12.348...
; Jones and Orosz, 2000 JONES, M.P. and OROSZ, S.E., 2000. The diagnosis of aspergillosis in birds. Journal of Exotic Pet Medicine, vol. 9, no. 2, pp. 52-58. http://dx.doi.org/10.1053/AX.2000.4619.
http://dx.doi.org/10.1053/AX.2000.4619 ...
; Nucci and Colombo, 2012 NUCCI, M., COLOMBO, A.L., 2012. Quando utilizar terapia empírica em doenças fúngicas invasivas? Rev. Panam. Infectol., vol. 14, no. 1, pp. 32-44. ; Xavier et al., 2011 XAVIER, M.O., AQUINO, V.R. and SEVERO, L.C., 2011. Galactomanana no diagnóstico de aspergilose invasiva. Revista Brasileira de Oncologia Clínica , vol. 7, no. 7, pp. 41-50. ). However, there are protocols and indications for its use in penguins.
Due to the high incidence of aspergillosis in penguins determining high mortality rates in these animals in captivity as well as the difficulty of the definitive diagnosis of ante-mortem disease in this species, this study aim to evaluate the effective of Platelia Aspergillus EIA® test (Bio-Rad-US) the diagnosis of aspergillosis in naturally infected Magellanic penguins, determining sensitivity, specificity, and positive and negative predictive values for different cutoff points.
2. Material and Methods
They were included in the study, blood serum samples of Magellanic penguins that died of aspergillosis during the rehabilitation period in the Centro de Recuperação de Animais Marinhos of Rio Grande - CRAM-FURG (n=29). The samples are stored in the mycology laboratory- FAMED-FURG, and all cases were confirmed from post-mortem examinations with mycological culture and histopathological examination. As a control group, were included over 23 serum samples from healthy Penguins CRAM-FURG, which they were rehabilitated and released to their natural habitat. All samples were aliquoted in biosafety cabinet to prevent contamination by airborne conidia and found themselves stored at -20 ° C.
A single blood sample each animal was included and collected from venipuncture of the cephalic vein. All samples were obtained in a maximum period of 60 days (7-69) before death (case group) and / or release (control).
The GM detection was performed on all serum samples animals included in the study according to the manufacturer's instructions. In brief, 300ul of sample was added to 100ul of Platelia Aspergillus EIA® treatment solution into microtubes and subsequently arranged in the thermoblock for heat treatment for six minutes at 120° C. Then the wells were centrifuged at 10,000xg for 10 minutes. In sequence the strips were filled with 50ul conjugate and the sample supernatant and then the plate was incubated for 90 minutes at 37° C. Then the wells were centrifuged at 10,000xg for 10 minutes. In sequence the strips were filled with 50ul conjugate and the sample supernatant and then the plate was incubated for 90 minutes at 37° C. The reaction was terminated by addition of 1.5 N solution of sulfuric acid and reading the optical density (OD) at 450 nm with 620 nm reference filter. The tests were conducted in duplicate and reactions were all positive control samples used, and the negative cutoff point provided by the kits diagnostic. The GM index was obtained by dividing the average value of OD of the duplicate of the clinical sample by duplicate OD of the average value of the cut-off sample provided by the kit.
From the database Laboratório de Micologia, It was obtained information about the presence of anti-Aspergillus fumigatus antibodies detected by agar gel immunodiffusion (AGID) for all serum samples.
Results were analyzed using chi-square test and Kruskal-Wallis from SPSS 20.0, IBM®. ROC curve was obtained and from this, rates of sensitivity, specificity, positive and negative predictive values were also calculated based on four different cutoff points (0.5, 1.0, 1.5 e 2.0).
3. Results
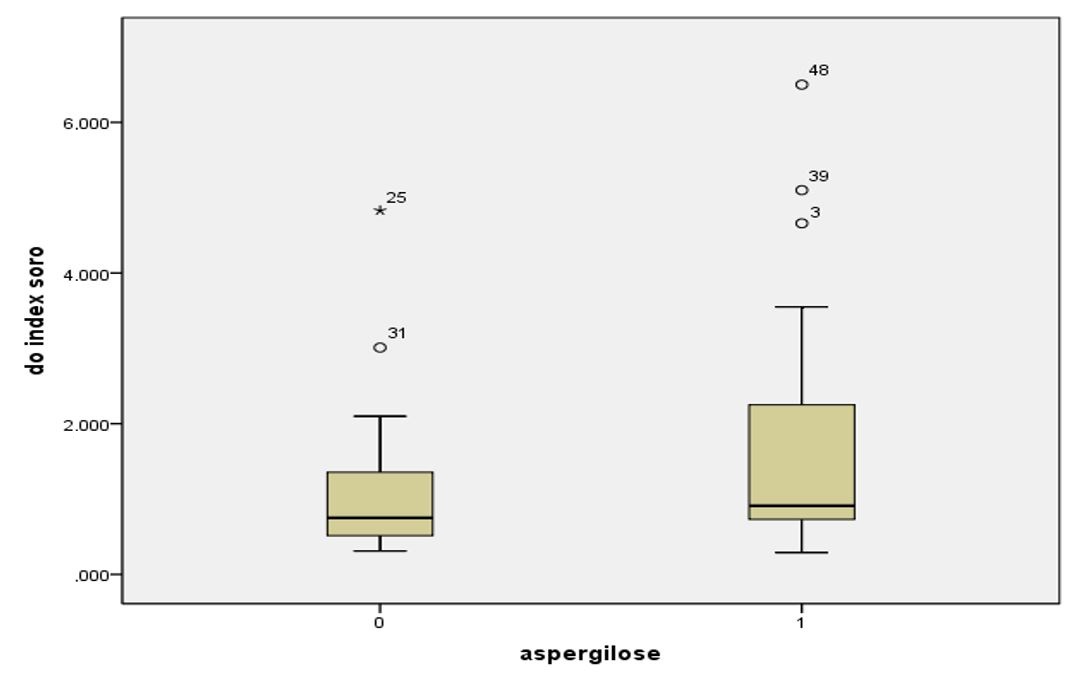
The serum GM index did not differ between animals in the case group and control (p KW = 0.097). The penguins with aspergillosis (n = 29) GM index ranged from 0.29 to 6.5, with a median of 0.91 and mean of 1.71, while the healthy animals (n = 23) the median was 0.75 and 1.13 average (varying from 0.31 to 4.83) ( Figure 1 ).
From these values it was determined the ROC (Receiver Operating Characteristic) for serum GM detection in the diagnosis of aspergillosis in penguins, where the x-axis (x) is the true positive (sensitivity) and the ordinate axis (y) is false positive (1- specificity) ( Figure 2 ). The analysis of the test demonstrated a precision index value of the area under the curve of 0.635 (0.482 to 0.788 CI) (p = 0.097).
From the values determined by the coordinate of the curve, four different cut points (0.5, 1.0, 1.5 and 2.0) were analyzed, resulting in sensitivity rates ranging from 86.2% to 34.5% and specificity between 91 3% and 26.1% ( Table 1 ).
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of serum GM detection in the diagnosis of aspergillosis in naturally infected penguins using different cut-off.
Of the 52 animals studied, only four had positive IGA, all of them belonging to the group case. Comparing the serum GM index in group case as the presence or absence of antibodies detected by AGID was no significant difference (p = 0.503), mean and median values of 2.59 and 1.76 (± 2.80), respectively, in animals with positive AGID, and 1.36 and 0.89 (± 1.20), respectively, in animals with antibodies to A. fumigatus.
4. Discussion
This study evaluated for the first time the applicability of the commercial Aspergillus EIA® Platelia kit for the diagnosis of aspergillosis in Magellanic penguins naturally infected, finding no significant difference in GM ratios between animals with and without the disease. A single study the this population with a similar was described by Cray et al. (2009b) CRAY, C., WATSON, T., RODRIGUEZ, M. and ARHEART, K.L., 2009b. Application of galactomannan analysis and protein electrophoresis in the diagnosis of aspergillosis in avian species. Journal of Zoo and Wildlife Medicine, vol. 40, no. 1, pp. 64-70. http://dx.doi.org/10.1638/2007-0138.1. PMid:19368241.
http://dx.doi.org/10.1638/2007-0138.1 ...
, however the authors included 56 birds with aspergillosis, of which only three were penguins, it is not possible to extrapolate the test efficacy results obtained by the authors for Sphenisciformes family.
In domestic and wild birds this differents families and orders, including some species of raptors, Psittaciformes, Anseriformes and Galliniformes. Described studies regarding the effectiveness of GM detection to diagnosis of aspergillosis, and demonstrate sensitivity rates ranging from 12 to 67% and specificity ranging 73 and 95% using 0.5 as the cutoff point ( Arca-Ruibal et al., 2006 ARCA-RUIBAL, B., WERNERY, U., ZACHARIAH, R., BAILEY, T.A., SOMMA, A., SILVANOSE, C. and MCKINNEY, P., 2006. Assessment of a commercial sandwich ELISA in the diagnosis of aspergillosis in falcons. The Veterinary Record, vol. 158, no. 13, pp. 442-444. http://dx.doi.org/10.1136/vr.158.13.442. PMid:16581995.
http://dx.doi.org/10.1136/vr.158.13.442...
; Cray et al., 2009a CRAY, C., REAVILL, D., ROMAGNANO, A., VAN SANT, F., CHAMPAGNE, D., STEVENSON, R., ROLFE, V., GRIFFIN, C. and CLUBB, S., 2009a. Galactomannan assay and plasma protein electrophoresis findings in psittacine birds with aspergillosis. Journal of Avian Medicine and Surgery, vol. 23, no. 2, pp. 125-135. http://dx.doi.org/10.1647/2007-041.1. PMid:19673459.
http://dx.doi.org/10.1647/2007-041.1 ...
, b CRAY, C., WATSON, T., RODRIGUEZ, M. and ARHEART, K.L., 2009b. Application of galactomannan analysis and protein electrophoresis in the diagnosis of aspergillosis in avian species. Journal of Zoo and Wildlife Medicine, vol. 40, no. 1, pp. 64-70. http://dx.doi.org/10.1638/2007-0138.1. PMid:19368241.
http://dx.doi.org/10.1638/2007-0138.1 ...
; Dhama et al., 2013 DHAMA, K., CHAKRABORTY, S., VERMA, A.K., TIWARI, R., BARATHIDASAN, R., KUMAR, A. and SINGH, S.D., 2013. Fungal/mycotic diseases of poultry-diagnosis, treatment and control: a review. Pakistan Journal of Biological Sciences: PJBS, vol. 16, no. 23, pp. 1626-1640. PMid:24506030. ; Franca et al., 2012 FRANÇA, M., CRAY, C. and SHIVAPRASAD, H.L., 2012. Serologic testing for aspergillosis in commercial broiler chickens and turkeys. Avian Diseases, vol. 56, no. 1, pp. 160-164. http://dx.doi.org/10.1637/9836-061911-Reg.1. PMid:22545542.
http://dx.doi.org/10.1637/9836-061911-R...
; Fischer et al., 2014 FISCHER, D., VAN WAEYENBERGHE, L., CRAY, C., GROSS, M., USLEBER, E., PASMANS, F., MARTEL, A. and LIERZ, M., 2014. Comparison of diagnostic tools for the detection of aspergillosis in blood samples of experimentally infected falcons. Avian Diseases , vol. 58, no. 4, pp. 587-598. http://dx.doi.org/10.1637/10831-032714-Reg. PMid:25619004.
http://dx.doi.org/10.1637/10831-032714-...
).
These results do not match those found in our study with penguins, when considering this same cutoff value was detected high sensitivity rate (86.2%) but low specificity (26.1%). Similar rates of the authors mentioned above were found in our study only using a cutoff point four times (2.0), in this case the sensitivity was 34.5% and specificity of 91.3%.
Factors such as different animals species included in the studies, duration and development of aspergillosis in these birds, as well as clinical presentation of the disease (infection site) and immune response of different species of birds ( Deem, 2003 DEEM, S.L., 2003. Fungal diseases of birds of prey. The Veterinary Clinics of North America. Exotic animal practice, vol. 6, no. 2, pp. 363-376. http://dx.doi.org/10.1016/S1094-9194(03)00004-5. PMid:12827728.
http://dx.doi.org/10.1016/S1094-9194(03...
; Tell, 2005 TELL, L.A., 2005. Aspergillosis in mammals and birds: impact on veterinary medicine. Journal of Exotic Pet Medicine, vol. 43, no. s1, suppl. 1, pp. 71-73. http://dx.doi.org/10.1080/13693780400020089. PMid:16110795.
http://dx.doi.org/10.1080/1369378040002...
) may be related to conflicting results found in our study compared to others in the literature.
The high rate of false-positive results found in our study may be related to colonization of the respiratory tract by Aspergillus species Fumigati section, exposure to environmental strains or mainly to the presence of a cross-reactive antigen has not been elucidated, which is also suggested by other authors ( Le Loch et al., 2005 LE LOCH, G., DEVILLE, M., RISI, E., BRETAGNE, S. and GUILLOT, J. 2005. Evaluation of the serological test Platelia Aspergillus for the diagnosis of aspergillosis. Proceedings of The 8th European Association of Avian Veterinarians Conference and The 6th Scientific European College of Avian Medicine and Surgery Meeting, 24-30 April 2005, Arles, France. Paris: AFVAC, pp. 260-266. ). On the other hand, false negative rates in the Platelia Aspergillus EIA® are generally related to encapsulation of the infection, immunocomplex formation by anti- Aspergillus antibodies or prior exposure to antifungal agents (for prophylaxis) ( Xavier et al., 2011 XAVIER, M.O., AQUINO, V.R. and SEVERO, L.C., 2011. Galactomanana no diagnóstico de aspergilose invasiva. Revista Brasileira de Oncologia Clínica , vol. 7, no. 7, pp. 41-50. ). However, none of these hypotheses can be extrapolated to our study, whereas all penguins with aspergillosis included had lesions spread through the respiratory tract to the post-mortem examination, not characterizing frame encapsulation of the infection and were not in antifungal treatment, moreover, no significant difference in the GM results comparing animals with and without antibodies to Aspergillus sp. (PKW = 0.449). However, the interference factors in the test for this animal species (penguins) are not well been elucidated, as well as other animals ( Arca-Ruibal et al., 2006 ARCA-RUIBAL, B., WERNERY, U., ZACHARIAH, R., BAILEY, T.A., SOMMA, A., SILVANOSE, C. and MCKINNEY, P., 2006. Assessment of a commercial sandwich ELISA in the diagnosis of aspergillosis in falcons. The Veterinary Record, vol. 158, no. 13, pp. 442-444. http://dx.doi.org/10.1136/vr.158.13.442. PMid:16581995.
http://dx.doi.org/10.1136/vr.158.13.442...
; Fischer et al., 2014 FISCHER, D., VAN WAEYENBERGHE, L., CRAY, C., GROSS, M., USLEBER, E., PASMANS, F., MARTEL, A. and LIERZ, M., 2014. Comparison of diagnostic tools for the detection of aspergillosis in blood samples of experimentally infected falcons. Avian Diseases , vol. 58, no. 4, pp. 587-598. http://dx.doi.org/10.1637/10831-032714-Reg. PMid:25619004.
http://dx.doi.org/10.1637/10831-032714-...
).
In attempt to reduce the false-positive rate, authors recommend testing in at least two serum collections ( Arca-Ruibal et al., 2006 ARCA-RUIBAL, B., WERNERY, U., ZACHARIAH, R., BAILEY, T.A., SOMMA, A., SILVANOSE, C. and MCKINNEY, P., 2006. Assessment of a commercial sandwich ELISA in the diagnosis of aspergillosis in falcons. The Veterinary Record, vol. 158, no. 13, pp. 442-444. http://dx.doi.org/10.1136/vr.158.13.442. PMid:16581995.
http://dx.doi.org/10.1136/vr.158.13.442...
; Cray et al., 2009a CRAY, C., REAVILL, D., ROMAGNANO, A., VAN SANT, F., CHAMPAGNE, D., STEVENSON, R., ROLFE, V., GRIFFIN, C. and CLUBB, S., 2009a. Galactomannan assay and plasma protein electrophoresis findings in psittacine birds with aspergillosis. Journal of Avian Medicine and Surgery, vol. 23, no. 2, pp. 125-135. http://dx.doi.org/10.1647/2007-041.1. PMid:19673459.
http://dx.doi.org/10.1647/2007-041.1 ...
, b CRAY, C., WATSON, T., RODRIGUEZ, M. and ARHEART, K.L., 2009b. Application of galactomannan analysis and protein electrophoresis in the diagnosis of aspergillosis in avian species. Journal of Zoo and Wildlife Medicine, vol. 40, no. 1, pp. 64-70. http://dx.doi.org/10.1638/2007-0138.1. PMid:19368241.
http://dx.doi.org/10.1638/2007-0138.1 ...
; Verweij et al., 1995 VERWEIJ, P.E., STYNEN, D., RIJS, J., PAUW, B.E., HOOGKAMP-KORSTANJE, J.A. and MEIS, J.F., 1995. Sandwich enzyme-linked immunosorbent assay compared with Pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. Journal of Clinical Microbiology, vol. 33, no. 7, pp. 1912-1914. PMid:7665670. ). In our study, only one clinical sample per animal was included, being this a limitation, in that it was not possible to evaluate the test results when performed as serial monitoring of serum levels of GM penguins.
Our results show that serum GM detection by Platelia Aspergillus EIA® does not seem to be useful for the diagnosis of aspergillosis in naturally infected penguins, with high rates of false-positive results with cut-off 0.5 (indicated by the manufacturer) and false negatives in high cut-off.
-
(With 2 figures)
References
- ARCA-RUIBAL, B., WERNERY, U., ZACHARIAH, R., BAILEY, T.A., SOMMA, A., SILVANOSE, C. and MCKINNEY, P., 2006. Assessment of a commercial sandwich ELISA in the diagnosis of aspergillosis in falcons. The Veterinary Record, vol. 158, no. 13, pp. 442-444. http://dx.doi.org/10.1136/vr.158.13.442. PMid:16581995.
» http://dx.doi.org/10.1136/vr.158.13.442 - BILLEN, F., PEETERS, D., PETERS, I.R., HELPS, C.R., HUYNEN, P., MOL, P., MASSART, L., DAY, M.J. and CLERCX, C., 2009. Comparison of the value of measurement of serum galactomannan and Aspergillus-specific antibodies in the diagnosis of canine sino-nasal aspergilosis. Veterinary Microbiology, vol. 133, no. 4, pp. 358-365. http://dx.doi.org/10.1016/j.vetmic.2008.07.018. PMid:18768268.
» http://dx.doi.org/10.1016/j.vetmic.2008.07.018 - CABANA, A.L. 2013. Monitoramento sorológico para diagnóstico precoce da aspergilose em pinguins em cativeiro Pelotas: Programa de Pós Graduação em Veterinária, Universidade Federal de Pelotas, 110 p. Dissertação de Mestrado.
- CABANA, Â.L., XAVIER, M.O., POESTER, V., KLAFKE, G.B., BRUNO FILHO, P.L., MARTINS, A., SILVA FILHO, R.P. and MEIRELES, M.C.A., 2015. Serological monitoring of antibodies for an early diagnosis ofaspergillosis in captive penguins. Pesquisa Veterinária Brasileira, vol. 35, no. 6, pp. 573-578. http://dx.doi.org/10.1590/S0100-736X2015000600015.
» http://dx.doi.org/10.1590/S0100-736X2015000600015 - CRAY, C., REAVILL, D., ROMAGNANO, A., VAN SANT, F., CHAMPAGNE, D., STEVENSON, R., ROLFE, V., GRIFFIN, C. and CLUBB, S., 2009a. Galactomannan assay and plasma protein electrophoresis findings in psittacine birds with aspergillosis. Journal of Avian Medicine and Surgery, vol. 23, no. 2, pp. 125-135. http://dx.doi.org/10.1647/2007-041.1. PMid:19673459.
» http://dx.doi.org/10.1647/2007-041.1 - CRAY, C., WATSON, T., RODRIGUEZ, M. and ARHEART, K.L., 2009b. Application of galactomannan analysis and protein electrophoresis in the diagnosis of aspergillosis in avian species. Journal of Zoo and Wildlife Medicine, vol. 40, no. 1, pp. 64-70. http://dx.doi.org/10.1638/2007-0138.1. PMid:19368241.
» http://dx.doi.org/10.1638/2007-0138.1 - DEEM, S.L., 2003. Fungal diseases of birds of prey. The Veterinary Clinics of North America. Exotic animal practice, vol. 6, no. 2, pp. 363-376. http://dx.doi.org/10.1016/S1094-9194(03)00004-5. PMid:12827728.
» http://dx.doi.org/10.1016/S1094-9194(03)00004-5 - DHAMA, K., CHAKRABORTY, S., VERMA, A.K., TIWARI, R., BARATHIDASAN, R., KUMAR, A. and SINGH, S.D., 2013. Fungal/mycotic diseases of poultry-diagnosis, treatment and control: a review. Pakistan Journal of Biological Sciences: PJBS, vol. 16, no. 23, pp. 1626-1640. PMid:24506030.
- FISCHER, D., VAN WAEYENBERGHE, L., CRAY, C., GROSS, M., USLEBER, E., PASMANS, F., MARTEL, A. and LIERZ, M., 2014. Comparison of diagnostic tools for the detection of aspergillosis in blood samples of experimentally infected falcons. Avian Diseases , vol. 58, no. 4, pp. 587-598. http://dx.doi.org/10.1637/10831-032714-Reg. PMid:25619004.
» http://dx.doi.org/10.1637/10831-032714-Reg - FRANÇA, M., CRAY, C. and SHIVAPRASAD, H.L., 2012. Serologic testing for aspergillosis in commercial broiler chickens and turkeys. Avian Diseases, vol. 56, no. 1, pp. 160-164. http://dx.doi.org/10.1637/9836-061911-Reg.1. PMid:22545542.
» http://dx.doi.org/10.1637/9836-061911-Reg.1 - GARCIA, M.E., CABALLERO, J., ALVAREZ-PEREZ, S. and BLANCO, J.L., 2008. Seroprevalence of Aspergillus fumigatus antibodies in bovine herds with a history of reprodutive disorders. Veterinary Medicine, vol. 53, no. 3, pp. 117-123. http://dx.doi.org/10.17221/1939-VETMED.
» http://dx.doi.org/10.17221/1939-VETMED - GARCIA, M.E., CABALLERO, M., CRUZADO, M., ANDRINO, M., GONZALEZ-CABO, J.F. and BLANCO, J.L., 2001. The value of the determination of anti-AspergillusIgG in the serodiagnosis of canine aspergillosis: Comparison with galactomannan detection. Journal of Veterinary Medicine, vol. 48, no. 10, pp. 743-750. http://dx.doi.org/10.1046/j.1439-0450.2001.00504.x. PMid:11846019.
» http://dx.doi.org/10.1046/j.1439-0450.2001.00504.x - GUILLOT, J., SARFATI, J., BARROS, M., CADORÉ, J.L., JENSEN, H.E. and CHERMETTE, R., 1999. Comparative study of serological tests for the diagnosis of equine aspergillosis. The Veterinary Record, vol. 145, no. 12, pp. 348-349. http://dx.doi.org/10.1136/vr.145.12.348. PMid:10530885.
» http://dx.doi.org/10.1136/vr.145.12.348 - JONES, M.P. and OROSZ, S.E., 2000. The diagnosis of aspergillosis in birds. Journal of Exotic Pet Medicine, vol. 9, no. 2, pp. 52-58. http://dx.doi.org/10.1053/AX.2000.4619.
» http://dx.doi.org/10.1053/AX.2000.4619 - LE LOCH, G., DEVILLE, M., RISI, E., BRETAGNE, S. and GUILLOT, J. 2005. Evaluation of the serological test Platelia Aspergillus for the diagnosis of aspergillosis. Proceedings of The 8th European Association of Avian Veterinarians Conference and The 6th Scientific European College of Avian Medicine and Surgery Meeting, 24-30 April 2005, Arles, France. Paris: AFVAC, pp. 260-266.
- MAERTENS, J.A., KLONT, R., MASSON, C., THEUNISSEN, K., MEERSSEMAN, W., LAGROU, K., HEINEN, C., CRÉPIN, B., VAN ELDERE, J., TABOURET, M., DONNELLY, J.P. and VERWEIJ, P.E., 2007. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clinical Infectious Diseases, vol. 44, no. 10, pp. 1329-1336. http://dx.doi.org/10.1086/514349. PMid:17443470.
» http://dx.doi.org/10.1086/514349 - MENNINK-KERSTEN, M.A., DONNELLY, J.P. and VERWEIJ, P.E., 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. The Lancet Infectious Diseases, vol. 4, no. 6, pp. 349-357. http://dx.doi.org/10.1016/S1473-3099(04)01045-X. PMid:15172343.
» http://dx.doi.org/10.1016/S1473-3099(04)01045-X - NUCCI, M., COLOMBO, A.L., 2012. Quando utilizar terapia empírica em doenças fúngicas invasivas? Rev. Panam. Infectol., vol. 14, no. 1, pp. 32-44.
- TELL, L.A., 2005. Aspergillosis in mammals and birds: impact on veterinary medicine. Journal of Exotic Pet Medicine, vol. 43, no. s1, suppl. 1, pp. 71-73. http://dx.doi.org/10.1080/13693780400020089. PMid:16110795.
» http://dx.doi.org/10.1080/13693780400020089 - VERWEIJ, P.E., STYNEN, D., RIJS, J., PAUW, B.E., HOOGKAMP-KORSTANJE, J.A. and MEIS, J.F., 1995. Sandwich enzyme-linked immunosorbent assay compared with Pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. Journal of Clinical Microbiology, vol. 33, no. 7, pp. 1912-1914. PMid:7665670.
- XAVIER, M.O., AQUINO, V.R. and SEVERO, L.C., 2011. Galactomanana no diagnóstico de aspergilose invasiva. Revista Brasileira de Oncologia Clínica , vol. 7, no. 7, pp. 41-50.
- XAVIER, M.O., PASQUALOTTO, A.C., SOARES, M.P., et al 2008. Aspergillosis in penguins: gross lesions in 15 cases 3rd ed. Miami: Advances Against Aspergillosis, 132 p.
- XAVIER, M.O., SOARES, M.P., MEINERZ, A.R.M., NOBRE, M.O., OSÓRIO, L.G., SILVA FILHO, R.P. and MEIRELES, M.C.A., 2007. Aspergillosis: a limiting factor during recovery of captive Magellanic penguins. Brazilian Journal of Microbiology, vol. 38, no. 3, pp. 480-484. http://dx.doi.org/10.1590/S1517-83822007000300018.
» http://dx.doi.org/10.1590/S1517-83822007000300018
Publication Dates
-
Publication in this collection
20 Aug 2018 -
Date of issue
Apr-Jun 2019
History
-
Received
26 Oct 2016 -
Accepted
13 Nov 2017