Abstract
The lack of knowledge about the majority of fish species harvested in Amazonian small-scale fisheries, in association with impacts from hydroelectric power plants, may lead to biodiversity loss and a decrease in the protein food supply for riverine Amazonians. This study uses existing datasets on fisheries and riverine developmental projects to infer effects associated with fish losses where actual data and outcomes are not available. The targeted fish species’ status may be regarded as either threatened or there being no knowledge of their conservation requirements, biology or ecology. Among the 90 Amazonian fish species that are the most important for the diet of the riverine fishers, 78% are not assessed or their biological information is unknown, according to the IUCN Red List. Consequently, the effects created by the thoroughly disregarded trade-off between energy generation and food security in the planning of Amazonian land use have been worsened by the lack of biological and ecological information on fish species.
Keywords:
small-scale fisheries; Amazon River; caboclos; diet; food security
Resumo
A falta de conhecimento sobre a maioria das espécies alvo de comunidades pesqueiras da Amazonia, associada ao impacto das hidrelétricas pode levar ao descréscimo da biodiversidade e na disponibilidade de proteína para os ribeirinhos da Amazônia. As espécies alvo são vulneráveis ou pouco conhecidas em sua biologia ou ecologia. Dentre 90 espécies de peixes importantes na dieta dos ribeirinhos, 78% não são estudadas ou sua biologia é desconhecida, de acordo com a lista da UICN. Dessa forma, os efeitos criados pela negligenciada relação de custo e benefício entre a produção de energia e a segurança alimentar no planejamento da Amazônia tem ainda piorado a situação de desconhecimento sobre as espécies de peixes.
Palavras-chave:
comunidades pesqueiras artesanais; Rios da Amazônia; caboclos; dieta; segurança alimentar
1. Introduction
The Amazon River basin is one of the most biodiverse basins in the world for freshwater species, being, for instance, a known habitat for 2,320 fish species, including 1,488 endemic ones ( Abell et al., 2008 ABELL., THIEME, M.L., REVENGA, C., BRYER, M., KOTTELAT, M., BOGUTSKAYA, N., COAD, B., MANDRAK, N., BALDERAS, S.C., BUSSING, W., STIASSNY, M.L.J., SKELTON, P., ALLEN, G.R., UNMACK, P., NASEKA, A., NG, R., SINDORF, N., ROBERTSON, J., ARMIJO, E., HIGGINS, J.V., HEIBEL, T.J., WIKRAMANAYAKE, E., OLSON, D., LÓPEZ, H.L., REIS, R.E., LUNDBERG, J.G., PÉREZ, M.H.S. and PETRY, P., 2008. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience, vol. 58, no. 5, pp. 403-414. http://dx.doi.org/10.1641/B580507.
http://dx.doi.org/10.1641/B580507 ...
; Winemiller et al., 2016 WINEMILLER, K.O., MCINTYRE, P.B., CASTELLO, L., FLUET-CHOUINARD, E., GIARRIZZO, T., NAM, S., BAIRD, I.G., DARWALL, W., LUJAN, N.K., HARRISON, I., STIASSNY, M.L.J., SILVANO, R.A.M., FITZGERALD, D.B., PELICICE, F.M., AGOSTINHO, A.A., GOMES, L.C., ALBERT, J.S., BARAN, E., PETRERE, M., ZARFL, C., MULLIGAN, M., SULLIVAN, J.P., ARANTES, C.C., SOUSA, L.M., KONING, A.A., HOEINGHAUS, D.J., SABAJ, M., LUNDBERG, J.G., ARMBRUSTER, J., THIEME, M.L., PETRY, P., ZUANON, J., VILARA, G.T., SNOEKS, J., OU, C., RAINBOTH, W., PAVANELLI, C.S., AKAMA, A., SOESBERGEN, A. and SAENZ, L., 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, vol. 351, no. 6269, pp. 128-129. http://dx.doi.org/10.1126/science.aac7082. PMid:26744397.
http://dx.doi.org/10.1126/science.aac70...
). Historically, these rich waters have provided fish that help maintain ecological, cultural and economic aspects of Amazonian livelihoods ( Begossi and Braga, 1992 BEGOSSI, A. and BRAGA, S., 1992. Food taboos and folk medicine among fishermen from the Tocantins River (Brazil). Amazoniana, vol. 12, no. 1, pp. 101-118. ; Begossi, 2014 BEGOSSI, A., 2014. Ecological, cultural, and economic approaches to managing artisanal fisheries. Environment, Development and Sustainability, vol. 16, no. 1, pp. 5-34. http://dx.doi.org/10.1007/s10668-013-9471-z.
http://dx.doi.org/10.1007/s10668-013-94...
; Begossi et al.,2004 BEGOSSI, A., HANAZAKI, N. and RAMOS, R.M., 2004. Food chain and the reasons for fish food taboos among Amazonian and Atlantic Forest fishers (Brazil). Ecological Applications , vol. 14, no. 5, pp. 1334-1343. http://dx.doi.org/10.1890/03-5072.
http://dx.doi.org/10.1890/03-5072 ...
, 2012a BEGOSSI, A., LOPES, P. and SILVANO, R., 2012a. Co-Management of reef fisheries of the snapper-grouper complex in a human ecological context in Brazil. In: G.H. Kruse, H.I. Browman, K.L. Cochrane, D. Evans, G.S. Jamieson, P.A. Livingston, D. Woodby, and C.I. Zhang, eds. Global progress in ecosystem-based fisheries management. Fairbanks: Alaska Sea Grant, University of Alaska Fairbanks. http://dx.doi.org/10.4027/gpebfm.2012.018.
http://dx.doi.org/10.4027/gpebfm.2012.0...
,b BEGOSSI, A., SALIVONCHYK, S.V., HANAZAKI, N., MARTINS, I.V. and BUELONI, F., 2012b. Fishers (Paraty, RJ) and fish manipulation time: a variable associated to the choice for consumption and sale. Brazilian Journal of Biology = Revista Brasileira de Biologia , vol. 72, no. 4, pp. 973-975. http://dx.doi.org/10.1590/S1519-69842012000500030. PMid:23295533.
http://dx.doi.org/10.1590/S1519-6984201...
,c BEGOSSI, A., SALYVONCHYK, S.V., NORA, V., LOPES, P.F. and SILVANO, R.A.M., 2012c. The Paraty artisanal fishery (southeastern Brazilian coast): ethnoecology and management of a social-ecological system (SES). Journal of Ethnobiology and Ethnomedicine, vol. 8, no. 1, pp. 22. http://dx.doi.org/10.1186/1746-4269-8-22. PMid:22738073.
http://dx.doi.org/10.1186/1746-4269-8-2...
; Hallwass et al., 2011 HALLWASS, G., LOPES, P.F., JURAS, A.A. and SILVANO, R.A.M., 2011. Fishing effort and catch composition of urban market and rural villages in Brazilian Amazon. Environmental Management, vol. 47, no. 2, pp. 188-200. http://dx.doi.org/10.1007/s00267-010-9584-1. PMid:21153639.
http://dx.doi.org/10.1007/s00267-010-95...
; Hallwass and Silvano, 2016 HALLWASS, G. and SILVANO, R.A.M., 2016. Patterns of selectiveness in the Amazonian freshwater fisheries: implications for management. Journal of Environmental Planning and Management, vol. 59, no. 9, pp. 1537-1559. http://dx.doi.org/10.1080/09640568.2015.1081587.
http://dx.doi.org/10.1080/09640568.2015...
). Most Amazonian fishing is done by small-scale fishers, predominantly from the Caboclo culture (descendants of indigenous Brazilians, Portuguese colonizers and immigrants from the Northeastern Brazil). Many investigations have focused on the Caboclo’s anthropology and ecology, such as the classical studies by Moran (1996) MORAN, E.F., 1996. The adaptive system of the Amazonian caboclo. Man in the Amazon. 1974; 136: 139-159. Regulated Rivers: Research and Management, vol. 12, pp. 81-98. , Nugent (1993) NUGENT, S., 1993. Amazonian caboclo society: an essay on invisibility and peasant economy. Providence, Rhode Island: Berg Publishers Ltd. , and Wagley (1974) WAGLEY, C., 1974. Man in the Amazon. Bloomington: University Presses of Florida. .
Caboclo fishers’ diets rely heavily on fish protein, and their livelihoods on the supply of fish to local and regional markets ( Silva and Begossi, 2009 SILVA, A.L. and BEGOSSI, A., 2009. Biodiversity, food consumption and ecological niche dimension: a study case of the riverine populations from the Rio Negro, Amazonia, Brazil. Environment, Development and Sustainability, vol. 11, no. 3, pp. 489-507. http://dx.doi.org/10.1007/s10668-007-9126-z.
http://dx.doi.org/10.1007/s10668-007-91...
; Isaac et al., 2008 ISAAC, V.J., SILVA, C.O. and RUFFINO, M.L., 2008. The artisanal fishery fleet of the lower Amazon. Fisheries Management and Ecology, vol. 15, no. 3, pp. 179-187. http://dx.doi.org/10.1111/j.1365-2400.2008.00599.x.
http://dx.doi.org/10.1111/j.1365-2400.2...
; Sarti et al., 2015 SARTI, F.M., ADAMS, C., MORSELLO, C., VAN VLIET, N., SCHOR, T., YAGÜE, B., TELLEZ, L., QUICENO-MESA, M.P. and CRUZ, D., 2015. Beyond protein intake: bushmeat as source of micronutrients in the Amazon. Ecology and Society, vol. 20, no. 4, pp. 22. http://dx.doi.org/10.5751/ES-07934-200422.
http://dx.doi.org/10.5751/ES-07934-2004...
). Therefore, Caboclo fishers affect and are affected by the availability of freshwater resources and by fish diversity. The observed decline of fishing resources, caused by pollution, habitat destruction, overfishing and river dams for hydropower projects ( Pauly et al., 2002 PAULY, D., CHRISTENSEN, V., GUÉNETTE, S., PITCHER, T.J., SUMAILA, U.R., WALTERS, C.J., WATSON, R. and ZELLER, D., 2002. Towards sustainability in world fisheries. Nature, vol. 418, no. 6898, pp. 689-695. http://dx.doi.org/10.1038/nature01017. PMid:12167876.
http://dx.doi.org/10.1038/nature01017 ...
; Welcomme et al., 2010 WELCOMME, R.L., COWX, I.G., COATES, D., BÉNÉ, C., FUNGE-SMITH, S., HALLS, A. and LORENZEN, K., 2010. Inland capture fisheries. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, vol. 365, no. 1554, pp. 2881-2896. http://dx.doi.org/10.1098/rstb.2010.0168. PMid:20713391.
http://dx.doi.org/10.1098/rstb.2010.016...
; Hallwass et al., 2013 HALLWASS, G., LOPES, P.F., JURAS, A.A. and SILVANO, R.A.M., 2013. Fishers’ knowledge identifies environmental changes and fish abundance trends in impounded tropical rivers. Ecological Applications, vol. 23, no. 2, pp. 392-407. http://dx.doi.org/10.1890/12-0429.1. PMid:23634590.
http://dx.doi.org/10.1890/12-0429.1 ...
; Winemiller et al., 2016) WINEMILLER, K.O., MCINTYRE, P.B., CASTELLO, L., FLUET-CHOUINARD, E., GIARRIZZO, T., NAM, S., BAIRD, I.G., DARWALL, W., LUJAN, N.K., HARRISON, I., STIASSNY, M.L.J., SILVANO, R.A.M., FITZGERALD, D.B., PELICICE, F.M., AGOSTINHO, A.A., GOMES, L.C., ALBERT, J.S., BARAN, E., PETRERE, M., ZARFL, C., MULLIGAN, M., SULLIVAN, J.P., ARANTES, C.C., SOUSA, L.M., KONING, A.A., HOEINGHAUS, D.J., SABAJ, M., LUNDBERG, J.G., ARMBRUSTER, J., THIEME, M.L., PETRY, P., ZUANON, J., VILARA, G.T., SNOEKS, J., OU, C., RAINBOTH, W., PAVANELLI, C.S., AKAMA, A., SOESBERGEN, A. and SAENZ, L., 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, vol. 351, no. 6269, pp. 128-129. http://dx.doi.org/10.1126/science.aac7082. PMid:26744397.
http://dx.doi.org/10.1126/science.aac70...
, will increase food insecurity of fish dependent communities. Such a decline has already been observed in African countries ( Mcclanahan et al., 2015 MCCLANAHAN, T., ALLISON, E.H. and CINNER, J.E., 2015. Managing fisheries for human and food security. Fish and Fisheries, vol. 16, no. 1, pp. 78-103. http://dx.doi.org/10.1111/faf.12045.
http://dx.doi.org/10.1111/faf.12045 ...
).
Specifically, hydropower dam construction is rapidly expanding in major river basins around the globe, as countries with emerging economies seek to supply their energy demands without the use of fossil fuels ( Zarfl et al., 2014 ZARFL, C., LUMSDON, A.E., BERLEKAMP, J., TYDECKS, L. and TOCKNER, K., 2014. A global boom in hydropower dam construction. Aquatic Sciences, vol. 77, no. 1, pp. 161-170. http://dx.doi.org/10.1007/s00027-014-0377-0.
http://dx.doi.org/10.1007/s00027-014-03...
; Winemiller et al., 2016 WINEMILLER, K.O., MCINTYRE, P.B., CASTELLO, L., FLUET-CHOUINARD, E., GIARRIZZO, T., NAM, S., BAIRD, I.G., DARWALL, W., LUJAN, N.K., HARRISON, I., STIASSNY, M.L.J., SILVANO, R.A.M., FITZGERALD, D.B., PELICICE, F.M., AGOSTINHO, A.A., GOMES, L.C., ALBERT, J.S., BARAN, E., PETRERE, M., ZARFL, C., MULLIGAN, M., SULLIVAN, J.P., ARANTES, C.C., SOUSA, L.M., KONING, A.A., HOEINGHAUS, D.J., SABAJ, M., LUNDBERG, J.G., ARMBRUSTER, J., THIEME, M.L., PETRY, P., ZUANON, J., VILARA, G.T., SNOEKS, J., OU, C., RAINBOTH, W., PAVANELLI, C.S., AKAMA, A., SOESBERGEN, A. and SAENZ, L., 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, vol. 351, no. 6269, pp. 128-129. http://dx.doi.org/10.1126/science.aac7082. PMid:26744397.
http://dx.doi.org/10.1126/science.aac70...
). For instance, more than 450 new dams are under construction or proposed in the Amazon, Congo and Mekong river basins ( Winemiller et al., 2016 WINEMILLER, K.O., MCINTYRE, P.B., CASTELLO, L., FLUET-CHOUINARD, E., GIARRIZZO, T., NAM, S., BAIRD, I.G., DARWALL, W., LUJAN, N.K., HARRISON, I., STIASSNY, M.L.J., SILVANO, R.A.M., FITZGERALD, D.B., PELICICE, F.M., AGOSTINHO, A.A., GOMES, L.C., ALBERT, J.S., BARAN, E., PETRERE, M., ZARFL, C., MULLIGAN, M., SULLIVAN, J.P., ARANTES, C.C., SOUSA, L.M., KONING, A.A., HOEINGHAUS, D.J., SABAJ, M., LUNDBERG, J.G., ARMBRUSTER, J., THIEME, M.L., PETRY, P., ZUANON, J., VILARA, G.T., SNOEKS, J., OU, C., RAINBOTH, W., PAVANELLI, C.S., AKAMA, A., SOESBERGEN, A. and SAENZ, L., 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, vol. 351, no. 6269, pp. 128-129. http://dx.doi.org/10.1126/science.aac7082. PMid:26744397.
http://dx.doi.org/10.1126/science.aac70...
).Throughout the Amazon River Basin there are 416 hydroelectric plants operating or under construction, with another 334 proposed or planned ( Winemiller et al., 2016 WINEMILLER, K.O., MCINTYRE, P.B., CASTELLO, L., FLUET-CHOUINARD, E., GIARRIZZO, T., NAM, S., BAIRD, I.G., DARWALL, W., LUJAN, N.K., HARRISON, I., STIASSNY, M.L.J., SILVANO, R.A.M., FITZGERALD, D.B., PELICICE, F.M., AGOSTINHO, A.A., GOMES, L.C., ALBERT, J.S., BARAN, E., PETRERE, M., ZARFL, C., MULLIGAN, M., SULLIVAN, J.P., ARANTES, C.C., SOUSA, L.M., KONING, A.A., HOEINGHAUS, D.J., SABAJ, M., LUNDBERG, J.G., ARMBRUSTER, J., THIEME, M.L., PETRY, P., ZUANON, J., VILARA, G.T., SNOEKS, J., OU, C., RAINBOTH, W., PAVANELLI, C.S., AKAMA, A., SOESBERGEN, A. and SAENZ, L., 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, vol. 351, no. 6269, pp. 128-129. http://dx.doi.org/10.1126/science.aac7082. PMid:26744397.
http://dx.doi.org/10.1126/science.aac70...
). The Brazilian plan for energy production alone encompasses 58 hydroelectric plants on the largest Amazonian rivers ( Kahn et al., 2014 KAHN, J.R., FREITAS, C.E. and PETRERE, M., 2014. False shades of green: the case of Brazilian Amazonian hydropower. Energies, vol. 7, no. 9, pp. 6063-6082. http://dx.doi.org/10.3390/en7096063.
http://dx.doi.org/10.3390/en7096063 ...
).
Besides the impacts caused by the construction and functioning of hydroelectric dams themselves, the electricity transmission networks also create biodiversity impacts by increasing the development of roads, which are the key driver of deforestation in the Amazon ( Fearnside, 2015 FEARNSIDE, P.M., 2015. Highway construction as a force in destruction of the Amazon forest. In: R. VAN DER REE, D.J. SMITH and C. GRILO, eds. Handbook of road ecology . Oxford: John Wiley & Sons Publishers. pp. 414-424. http://dx.doi.org/10.1002/9781118568170.ch51.
http://dx.doi.org/10.1002/9781118568170...
). However, there is little sign that such concerns are being considered by development agencies. For example, the Brazilian government stated the intention to significantly increase electricity grid interconnections for energy distribution ( Molle et al., 2010 MOLLE, F., MOLLINGA, P.P. and WESTER, P., 2010. Hydraulic bureaucracies: flows of water, flows of power. Water Alternatives, vol. 2, pp. 328-349. ) and to provide additional 10,632 MW of installed generation capacity through future dams by 2023 ( Tolmasquim, 2007 TOLMASQUIM, M., 2007. Plano Nacional de energia 2030. Brasília: Conselho Nacional de Politica Energetica. ).
There are many well-known negative consequences of dam development, including: displacement of people; deforestation due to flooding and increased road access for other developments; changes in waterflow, nutrients and sediments impacting downstream terrestrial and aquatic ecosystems including farmlands; and loss of fish (Fearnside, 1999 FEARNSIDE, P.M., 1999. Social impacts of Brazil’s Tucuruí Dam. Environmental Management, vol. 24, no. 4, pp. 483-495. http://dx.doi.org/10.1007/s002679900248. PMid:10501861.
http://dx.doi.org/10.1007/s002679900248...
, 2004 FEARNSIDE, P.M., 2004. Greenhouse gas emissions from hydroelectric dams: controversies provide a springboard for rethinking a supposedly ‘clean’ energy source. an editorial comment. Climatic Change, vol. 66, no. 1/2, pp. 1-18. http://dx.doi.org/10.1023/B:CLIM.0000043174.02841.23.
http://dx.doi.org/10.1023/B:CLIM.000004...
, 2016 FEARNSIDE, P.M., 2016. Tropical dams: to build or not to build? Science , vol. 351, no. 6272, pp. 456-457. http://dx.doi.org/10.1126/science.351.6272.456-b. PMid:26823417.
http://dx.doi.org/10.1126/science.351.6...
; Molle et al., 2010 MOLLE, F., MOLLINGA, P.P. and WESTER, P., 2010. Hydraulic bureaucracies: flows of water, flows of power. Water Alternatives, vol. 2, pp. 328-349. ; Orr et al., 2012 ORR, S., PITTOCK, J., CHAPAGAIN, A. and DUMARESQ, D., 2012. Dams on the Mekong river: lost fish protein and the implications for land and water resources. Global Environmental Change, vol. 22, no. 4, pp. 925-932. http://dx.doi.org/10.1016/j.gloenvcha.2012.06.002.
http://dx.doi.org/10.1016/j.gloenvcha.2...
; Hallwass et al., 2013 HALLWASS, G., LOPES, P.F., JURAS, A.A. and SILVANO, R.A.M., 2013. Fishers’ knowledge identifies environmental changes and fish abundance trends in impounded tropical rivers. Ecological Applications, vol. 23, no. 2, pp. 392-407. http://dx.doi.org/10.1890/12-0429.1. PMid:23634590.
http://dx.doi.org/10.1890/12-0429.1 ...
; Tolmasquim, 2014 TOLMASQUIM, M.T., 2014. Perspectivas e planejamento do setor energético no Brasil. Estudos Avançados, vol. 26, no. 74, pp. 247-260. http://dx.doi.org/10.1590/S0103-40142012000100017. ; Benchimol and Peres, 2015 BENCHIMOL, M. and PERES, C.A., 2015. Widespread forest vertebrate extinctions induced by a mega hydroelectric dam in lowland Amazonia. PLoS One, vol. 10, no. 7, pp. e0129818. http://dx.doi.org/10.1371/journal.pone.0129818. PMid:26132139.
http://dx.doi.org/10.1371/journal.pone....
; Winemiller et al., 2016 WINEMILLER, K.O., MCINTYRE, P.B., CASTELLO, L., FLUET-CHOUINARD, E., GIARRIZZO, T., NAM, S., BAIRD, I.G., DARWALL, W., LUJAN, N.K., HARRISON, I., STIASSNY, M.L.J., SILVANO, R.A.M., FITZGERALD, D.B., PELICICE, F.M., AGOSTINHO, A.A., GOMES, L.C., ALBERT, J.S., BARAN, E., PETRERE, M., ZARFL, C., MULLIGAN, M., SULLIVAN, J.P., ARANTES, C.C., SOUSA, L.M., KONING, A.A., HOEINGHAUS, D.J., SABAJ, M., LUNDBERG, J.G., ARMBRUSTER, J., THIEME, M.L., PETRY, P., ZUANON, J., VILARA, G.T., SNOEKS, J., OU, C., RAINBOTH, W., PAVANELLI, C.S., AKAMA, A., SOESBERGEN, A. and SAENZ, L., 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, vol. 351, no. 6269, pp. 128-129. http://dx.doi.org/10.1126/science.aac7082. PMid:26744397.
http://dx.doi.org/10.1126/science.aac70...
). Dam impacts on aquatic fauna, including fish, result in the blockage of migratory routes essential for the life cycle of many fish species, fragmentation of river connectivity, alteration of physical-chemical structure of the river causing changes in fish communities, as well as local extinctions and a reduction in the abundance of fish species (Petrere Junior, 1989 PETRERE JUNIOR, M., 1989. River fisheries in Brazil: a review. Regulated Rivers: Research and Management, vol. 4, no. 1, pp. 1-6. http://dx.doi.org/10.1002/rrr.3450040102.
http://dx.doi.org/10.1002/rrr.345004010...
; Zhong and Power, 1996 ZHONG, Y. and POWER, G., 1996. Environmental impacts of hydroelectric projects on fish resources in China. Regulated Rivers: Research and Management, vol. 12, no. 1, pp. 81-98. http://dx.doi.org/10.1002/(SICI)1099-1646(199601)12:1<81::AID-RRR378>3.0.CO;2-9.
http://dx.doi.org/10.1002/(SICI)1099-16...
; Ponton and Vauchel, 1998 PONTON, D. and VAUCHEL, P., 1998. Immediate downstream effects of the Petit-Saut Dam on Young neotropical fish in a large tributary of the Sinnamary River (French Guiana, South America). Regulated Rivers: Research and Management, vol. 14, no. 3, pp. 227-243. http://dx.doi.org/10.1002/(SICI)1099-1646(199805/06)14:3<227::AID-RRR490>3.0.CO;2-6.
http://dx.doi.org/10.1002/(SICI)1099-16...
; Barthem et al., 1991; Merona et al., 2001; Gehrke et al., 2002 GEHRKE, P.C., GILLIGAN, D.M. and BARWICK, M., 2002. Changes in fish communities of the Shoalhaven River 20 years after construction of Tallowa Dam, Australia. River Research and Applications, vol. 18, no. 3, pp. 265-286. http://dx.doi.org/10.1002/rra.669.
http://dx.doi.org/10.1002/rra.669 ...
; Petesse and Petrere Junior, 2012 PETESSE, M.L. and PETRERE JUNIOR, M., 2012. As barragens e os peixes: o impacto das grandes hidrelétricas nas espécies dos rios represados. Ciência Hoje, vol. 49, pp. 30-35. ; Hallwass et al., 2013) HALLWASS, G., LOPES, P.F., JURAS, A.A. and SILVANO, R.A.M., 2013. Fishers’ knowledge identifies environmental changes and fish abundance trends in impounded tropical rivers. Ecological Applications, vol. 23, no. 2, pp. 392-407. http://dx.doi.org/10.1890/12-0429.1. PMid:23634590.
http://dx.doi.org/10.1890/12-0429.1 ...
.
Many of these impacts are not correctly identified in impact assessment reports, and if identified, decision-makers often ignore negative implications of proposed hydropower developments for reasons of corruption, culture or politics ( Fearnside, 1999 FEARNSIDE, P.M., 1999. Social impacts of Brazil’s Tucuruí Dam. Environmental Management, vol. 24, no. 4, pp. 483-495. http://dx.doi.org/10.1007/s002679900248. PMid:10501861.
http://dx.doi.org/10.1007/s002679900248...
; Solarte et al., 2008 SOLARTE, L.G., CASTAÑO, M.L.M., ARENA, A.H. and CHIOSSONE, T., 2008. Global corruption report 2008: corruption in the water sector. Cambridge: Cambridge University Press. Water Integrity Network Transparency International, Berlín, Report No. 363.61 G562 2008. ; Isaac et al., 2015 ISAAC, V.J., ALMEIDA, M.C., GIARRIZZO, T., DEUS, C.P., VALE, R., KLEIN, G. and BEGOSSI, A., 2015. Food consumption as an indicator of the conservation of natural resources in riverine communities of the Brazilian Amazon. Anais da Academia Brasileira de Ciências , vol. 87, no. 4, pp. 2229-2242. http://dx.doi.org/10.1590/0001-3765201520140250. PMid:26628023.
http://dx.doi.org/10.1590/0001-37652015...
; Winemiller et al., 2016 WINEMILLER, K.O., MCINTYRE, P.B., CASTELLO, L., FLUET-CHOUINARD, E., GIARRIZZO, T., NAM, S., BAIRD, I.G., DARWALL, W., LUJAN, N.K., HARRISON, I., STIASSNY, M.L.J., SILVANO, R.A.M., FITZGERALD, D.B., PELICICE, F.M., AGOSTINHO, A.A., GOMES, L.C., ALBERT, J.S., BARAN, E., PETRERE, M., ZARFL, C., MULLIGAN, M., SULLIVAN, J.P., ARANTES, C.C., SOUSA, L.M., KONING, A.A., HOEINGHAUS, D.J., SABAJ, M., LUNDBERG, J.G., ARMBRUSTER, J., THIEME, M.L., PETRY, P., ZUANON, J., VILARA, G.T., SNOEKS, J., OU, C., RAINBOTH, W., PAVANELLI, C.S., AKAMA, A., SOESBERGEN, A. and SAENZ, L., 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, vol. 351, no. 6269, pp. 128-129. http://dx.doi.org/10.1126/science.aac7082. PMid:26744397.
http://dx.doi.org/10.1126/science.aac70...
). Even in the relevant literature, the impacts of hydroelectric plants on riverine fishing communities are almost absent. One approach to identifying the impacts on such riverine peoples is by considering the changes to the fish fauna, the possible changes in peoples’ selective methods of fishing and on their food intake. For example, in the Araguaia-Tocantins system, a heavily impacted region since the 1970’s, the livelihoods of about 44,000 people are expected to be affected by the 27 planned dams ( Carneiro and Souza, 2009 CARNEIRO, F. and SOUZA, O.B., 2009. Atlas de pressões e ameaças às terras indígenas na Amazônia Brasileira. São Paulo: Instituto Socioambiental. 48 p. ).
Besides developmental projects, overfishing has also affected the biodiversity of various aquatic ecosystems ( Pauly et al., 2002 PAULY, D., CHRISTENSEN, V., GUÉNETTE, S., PITCHER, T.J., SUMAILA, U.R., WALTERS, C.J., WATSON, R. and ZELLER, D., 2002. Towards sustainability in world fisheries. Nature, vol. 418, no. 6898, pp. 689-695. http://dx.doi.org/10.1038/nature01017. PMid:12167876.
http://dx.doi.org/10.1038/nature01017 ...
; Jackson et al., 2001 JACKSON, J.B.C., KIRBY, M.X., BERGER, W.H., BJORNDAL, K.A., BOTSFORD, L.W., BOURQUE, B.J., BRADBURY, R.H., COOKE, R., ERLANDSON, J., ESTES, J.A., HUGHES, T.P., KIDWELL, S., LANGE, C.B., LENIHAN, H.S., PANDOLFI, J.M., PETERSON, C.H., STENECK, R.S., TEGNER, M.J. and WARNER, R.R., 2001. Historical overfishing and the recent collapse of coastal ecosystems. Science, vol. 293, no. 5530, pp. 629-637. http://dx.doi.org/10.1126/science.1059199. PMid:11474098.
http://dx.doi.org/10.1126/science.10591...
; Myers and Worm, 2003 MYERS, R.A. and WORM, B., 2003. Rapid worldwide depletion of predatory fish communities. Nature, vol. 423, no. 6937, pp. 280-283. http://dx.doi.org/10.1038/nature01610. PMid:12748640.
http://dx.doi.org/10.1038/nature01610 ...
; Worm et al., 2006 WORM, B., BARBIER, E.B., BEAUMONT, N., DUFFY, J.E., FOLKE, C., HALPERN, B.S., JACKSON, J.B.C., LOTZE, H.K., MICHELI, F., PALUMBI, S.R., SALA, E., SELKOE, K.A., STACHOWICZ, J.J. and WATSON, R., 2006. Impacts of biodiversity loss on ocean ecosystem services. Science , vol. 314, no. 5800, pp. 787-790. http://dx.doi.org/10.1126/science.1132294. PMid:17082450.
http://dx.doi.org/10.1126/science.11322...
). In marine costal areas for instance, studies based on interviews with older fishers indicate that fish catch was higher in the past ( Pauly, 1995 PAULY, D., 1995. Anecdotes and the shifting baseline syndrome in fisheries. Trends in Ecology & Evolution, vol. 10, no. 10, pp. 430. http://dx.doi.org/10.1016/S0169-5347(00)89171-5. PMid:21237093.
http://dx.doi.org/10.1016/S0169-5347(00...
; Ainsworth et al., 2008 AINSWORTH, C.H., PITCHER, T.J. and ROTINSULU, C., 2008. Evidence of fishery depletions and shifting cognitive baselines in Eastern Indonesia. Biological Conservation , vol. 141, no. 3, pp. 848-859. http://dx.doi.org/10.1016/j.biocon.2008.01.006.
http://dx.doi.org/10.1016/j.biocon.2008...
). Historical and archaeological studies have shown the occurrence of higher fish abundance in the remote past, which highlights the importance of fish to ancient populations ( Pinnegar and Engelhard, 2007 PINNEGAR, J.K. and ENGELHARD, G.H., 2007. The ‘shifting baseline’ phenomenon: a global perspective. Reviews in Fish Biology and Fisheries, vol. 18, no. 1, pp. 1-16. http://dx.doi.org/10.1007/s11160-007-9058-6.
http://dx.doi.org/10.1007/s11160-007-90...
; Prestes-Carneiro et al., 2015 PRESTES-CARNEIRO, G., BÉAREZ, P., BAILON, S., PY-DANIEL, A.R. and NEVES, E.G., 2015. Subsistence fishery at Hatahara (750-1230 CE), a pre-Columbian central Amazonian village. Journal of Archaeological Science: Reports, vol. 8, pp. 454-462. https://doi.org/10.1016/j.jasrep.2015.10.033. ). One archaeological study indicated that fish fauna accounted for more than 75% of the vertebrate species recovered in an ancient (750 to 1020 A.D.) settlement of indigenous populations in the Central Brazilian Amazon ( Prestes-Carneiro et al., 2015 PRESTES-CARNEIRO, G., BÉAREZ, P., BAILON, S., PY-DANIEL, A.R. and NEVES, E.G., 2015. Subsistence fishery at Hatahara (750-1230 CE), a pre-Columbian central Amazonian village. Journal of Archaeological Science: Reports, vol. 8, pp. 454-462. https://doi.org/10.1016/j.jasrep.2015.10.033. ). In spite of long lasting importance of fish to ensure food security of human populations (Bené et al., 2015, 2016 BÉNÉ, C., ARTHUR, R., NORBURY, H., ALLISON, E.H., BEVERIDGE, M., BUSH, S., CAMPLING, L., LESCHEN, W., LITTLE, D., SQUIRES, D., THILSTED, S.H., TROELL, M. and WILLIAMS, M., 2016. Contribution to fisheries and Aquaculture to food security and poverty reduction: assessing the current evidence. World Development, vol. 79, pp. 177-196. http://dx.doi.org/10.1016/j.worlddev.2015.11.007.
http://dx.doi.org/10.1016/j.worlddev.20...
; Mcclanahan et al., 2015 MCCLANAHAN, T., ALLISON, E.H. and CINNER, J.E., 2015. Managing fisheries for human and food security. Fish and Fisheries, vol. 16, no. 1, pp. 78-103. http://dx.doi.org/10.1111/faf.12045.
http://dx.doi.org/10.1111/faf.12045 ...
), few studies have shown a link of fish species consumption to food security. Small-scale fisheries (SSFs) ensure income for millions of people worldwide, especially in developing countries ( Andrew et al., 2007 ANDREW, N.L., BÉNÉ, C., HALL, S.J., ALLISON, E.H., HECK, S. and RATNER, B.D., 2007. Diagnosis and management of small-scale fisheries in developing countries. Fish and Fisheries, vol. 8, no. 3, pp. 227-240. http://dx.doi.org/10.1111/j.1467-2679.2007.00252.x.
http://dx.doi.org/10.1111/j.1467-2679.2...
; De Graaf et al., 2011 DE GRAAF, G.J., GRAINGER, R.J.R., WESTLUND, L., WILLMANN, R., MILLS, D., KELLEHER, K. and KORANTENG, K., 2011. The status of routine fishery data collection in Southeast Asia, central America, the South Pacific, and West Africa, with special reference to small-scale fisheries. ICES Journal of Marine Science, vol. 68, no. 8, pp. 1743-1750. http://dx.doi.org/10.1093/icesjms/fsr054.
http://dx.doi.org/10.1093/icesjms/fsr05...
). In such countries, while fishing may not guarantee large cash incomes, it helps prevent livelihoods of deprivation and poverty (Bené, 2006 BENE, C., 2006. Small-scale fisheries: assessing their contribution to rural livelihoods in developing countries. Rome: FAO. FAO Fisheries Circular, no. 2008. ). Besides, most of the fish caught by SSFs is for direct human consumption (food security) with little discard and reduced costs compared to large-scale commercial fisheries ( Pauly, 2006 PAULY, D., 2006. Major trends in small-scale marine fisheries, with emphasis on developing countries, and some implications for the social sciences. Maritime Studies , vol. 4, no. 2, pp. 7-22. ). However, SSFs remain largely underreported and underestimated ( Welcomme et al., 2010 WELCOMME, R.L., COWX, I.G., COATES, D., BÉNÉ, C., FUNGE-SMITH, S., HALLS, A. and LORENZEN, K., 2010. Inland capture fisheries. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, vol. 365, no. 1554, pp. 2881-2896. http://dx.doi.org/10.1098/rstb.2010.0168. PMid:20713391.
http://dx.doi.org/10.1098/rstb.2010.016...
; De Graaf et al., 2011 DE GRAAF, G.J., GRAINGER, R.J.R., WESTLUND, L., WILLMANN, R., MILLS, D., KELLEHER, K. and KORANTENG, K., 2011. The status of routine fishery data collection in Southeast Asia, central America, the South Pacific, and West Africa, with special reference to small-scale fisheries. ICES Journal of Marine Science, vol. 68, no. 8, pp. 1743-1750. http://dx.doi.org/10.1093/icesjms/fsr054.
http://dx.doi.org/10.1093/icesjms/fsr05...
; Bartley et al., 2015 BARTLEY, D.M., DE GRAAF, G.J., VALBO-JØRGENSEN, J. and MARMULLA, G., 2015. Inland capture fisheries: status and data issues. Fisheries Management and Ecology , vol. 22, no. 1, pp. 71-77. http://dx.doi.org/10.1111/fme.12104.
http://dx.doi.org/10.1111/fme.12104 ...
).
Freshwater fish catch is about one seventh of that of marine fish globally (2013) ( Food and Agriculture Organization, 2013a FOOD AND AGRICULTURE ORGANIZATION – FAO, 2013a. Fish, crustaceans, molluscs, etc.: world capture production. Rome: Food and Agriculture Organization of the United Nations. ). Fish provide 6.5% of global protein, although in Brazil this figure is estimated at only 2.9% (2011) ( Food and Agriculture Organization, 2011 FOOD AND AGRICULTURE ORGANIZATION – FAO, 2011. World by continent - food balance sheet of fish and fishery products in live weight and fish contribution to protein supply. Rome: Food and Agriculture Organisation of the United Nations. ). In Brazil, fisheries provide 62% (2013) of the fish supply ( Food and Agriculture Organization, 2013b FOOD AND AGRICULTURE ORGANIZATION – FAO, 2013b. World fisheries production, by capture and aquaculture, by country. Rome: Food and Agriculture Organisation of the United Nations. ), the remaining being provided by aquaculture. Yet for Amazonians fish comprise a major portion of their diet. The average quantity of fish eaten in the Amazon is 462 g.person-1. day-1 or 169 kg.person-1.year-1 ( Isaac et al., 2015 ISAAC, V.J., ALMEIDA, M.C., GIARRIZZO, T., DEUS, C.P., VALE, R., KLEIN, G. and BEGOSSI, A., 2015. Food consumption as an indicator of the conservation of natural resources in riverine communities of the Brazilian Amazon. Anais da Academia Brasileira de Ciências , vol. 87, no. 4, pp. 2229-2242. http://dx.doi.org/10.1590/0001-3765201520140250. PMid:26628023.
http://dx.doi.org/10.1590/0001-37652015...
). ( Table 1 ). Further, in places such as the Lower Amazon (n=586 records of meals), Lower Purus (n= 341), and Trombetas (n=850) while a wide variety of animal protein sources, such as cayman, canned meat, beef, game meat, chicken egg, pork, poultry, turtle and turtle egg were recorded, fish correspond to 64-76% of the weight of the animal protein intake ( Isaac et al., 2015) ISAAC, V.J., ALMEIDA, M.C., GIARRIZZO, T., DEUS, C.P., VALE, R., KLEIN, G. and BEGOSSI, A., 2015. Food consumption as an indicator of the conservation of natural resources in riverine communities of the Brazilian Amazon. Anais da Academia Brasileira de Ciências , vol. 87, no. 4, pp. 2229-2242. http://dx.doi.org/10.1590/0001-3765201520140250. PMid:26628023.
http://dx.doi.org/10.1590/0001-37652015...
.
Fish consumption at some Amazonian localities according to the literature. All data shown in the references converted to grams/per capita/day.
In the Negro River, fish account for 75% of the animal protein locally extracted or farmed and for 57%of the total animal protein consumed (in 482 meals recorded) ( Silva and Begossi, 2009 SILVA, A.L. and BEGOSSI, A., 2009. Biodiversity, food consumption and ecological niche dimension: a study case of the riverine populations from the Rio Negro, Amazonia, Brazil. Environment, Development and Sustainability, vol. 11, no. 3, pp. 489-507. http://dx.doi.org/10.1007/s10668-007-9126-z.
http://dx.doi.org/10.1007/s10668-007-91...
). However, within nutrition transitional systems, there are records of people starting to acquire and rely on processed and imported food rather than fish. Such a transition has been observed, for example, at Ituqui Island (Amazon River, Santarém, Pará State) ( Food and Agriculture Organization, 2013a FOOD AND AGRICULTURE ORGANIZATION – FAO, 2013a. Fish, crustaceans, molluscs, etc.: world capture production. Rome: Food and Agriculture Organization of the United Nations. ) and at the Solimões River ( Dugan et al., 2002 DUGAN, P.J., BARAN, E., THARME, R., PREIN, M., AHMED, R., AMERASINGHE, P., BUENO, P., BROWN, C., DEY, M., JAYASINGHE, G., NIASSE, M., NIELAND, A., SMAKHTIN, V., TINH, N., VISWANATHAN, K. and WELCOMME, R., 2002. The contribution of aquatic ecosystems and fisheries to food security and livelihoods: a research agenda. In: CGIAR. Challenge Program on Water and Food: background papers to the full proposal. Colombo: CGIAR Challenge Program on Water and Food. pp. 85-113. Background Paper, no. 3. ). Given the value of fish in the diets of local people and the risks associated to transitions to other types of food, it is important to raise the question of whether there is enough knowledge to manage Amazonian fish species sustainably.
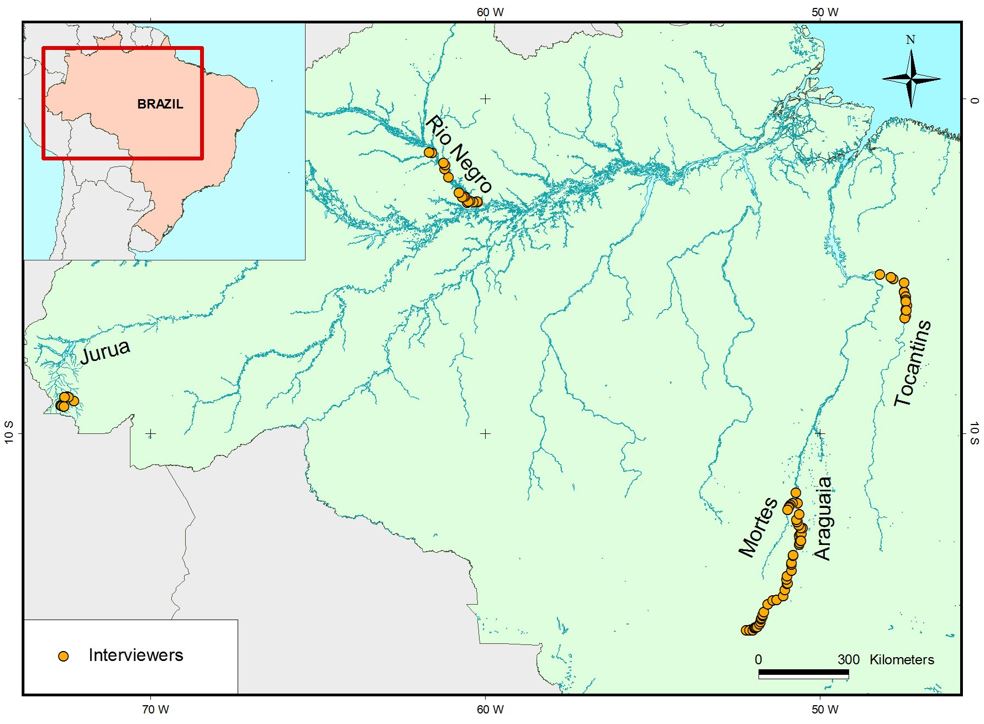
In this study, we investigate the possible impacts of current fish loss on the food security of riverine inhabitants and livelihoods of small-scale fishers in the Brazilian Amazon. We focus on the rivers Juruá (123), Negro (66), and Araguaia-Tocantins sub-system (328) ( Figure 1 , Table 1 ). Our main goal is to conduct a meta-analysis of data collected over 11 years for fish species preferred and consumed by scattered riverine small-scale inhabitants of the Amazon (n=517 interviews) ( Figure 1 ), to investigate potential relationships between the fish species consumed and those that are threatened by dam developments.
Brazilian Amazon showing the locations of interviews with riverine small-scale fishers (Caboclos).
2. Methods
This study was based on a compilation of interviews on riverine communities in the Brazilian Amazon (Araguaia, Tocantins, Negro and Jurua rivers, n=517 interviews, Figure 1 ) at different periods from 1987 to 1998. These studies were conducted in earlier projects coordinated by one of the authors (AB) and data are deposited in archives at the Fisheries and Food Institute ( Appendix A
Appendix A
Study sites, archive number of interviews deposited at the Fisheries and Food Institute (2015) , Santos, SP, at Unisanta, Santos, SP.
The grant # and funding agency is shown.
ARCHIVE NUMBER/FIFO
1
STUDY SITE
FUNDING AGENCY
PAJUR 007
ALTO JURUÁ ACRE AM
FAPESP 1998/02619-8/
Project F. MacArthur 92/21848
Coords. Brown, Almeida & Cunha (UNICAMP)
PDRPTO 008
TOCANTINS RIVER AM
R. P.: FAPESP [1994/6258-7] CNPq [400.185/95-4]
TO: 1987/ 1988: THEMAG/ELETROBRAS
PTOCETNO 008
TOCANTINS RIVER AM
TO: 1987/ 1988: THEMAG/ELETROBRAS
PTOCGOMA 008
TOCANTINS RIVER AM
TO: 1987/ 1988: THEMAG/ELETROBRAS
PDDARA 009
ARAGUAIA RIVER AM
FAPESP [1996/1036-1]
PARA 009
ARAGUAIA RIVER AM
FAPESP [1996/1036-1]
PDRNMA 018
QUEST RIO NEGRO AM
FAPESP 1998/16160-5
1
Fisheries and Food Institute.
). Parts of these data have been previously published ( Begossi and Garavello, 1990 BEGOSSI, A. and GARAVELLO, J.C., 1990. Notes on the ethnoicthyology of fishermen from the Tocantins River (Brazil). Acta Amazonica, vol. 20, no. 20, pp. 341-351. http://dx.doi.org/10.1590/1809-43921990201351.
http://dx.doi.org/10.1590/1809-43921990...
; Begossi and Braga, 1992 BEGOSSI, A. and BRAGA, S., 1992. Food taboos and folk medicine among fishermen from the Tocantins River (Brazil). Amazoniana, vol. 12, no. 1, pp. 101-118. ; Begossi and Figueiredo, 1995 BEGOSSI, A. and FIGUEIREDO, L., 1995. Ethnoichthyology of southern coastal fishermen: cases from Búzios Island and Sepetiba Bay (Brazil). Bulletin of Marine Science , vol. 56, no. 2, pp. 710-717. ; Begossi et al., 1999 BEGOSSI, A., SILVANO, R.A.M., AMARAL, B.D. and OYAKAWA, O.T., 1999. Uses of fish and game by inhabitants of an extractive reserve (Upper Juruá, Acre, Brazil). Environment, Development and Sustainability, vol. 1, no. 1, pp. 73-93. http://dx.doi.org/10.1023/A:1010075315060.
http://dx.doi.org/10.1023/A:10100753150...
, 2012a BEGOSSI, A., LOPES, P. and SILVANO, R., 2012a. Co-Management of reef fisheries of the snapper-grouper complex in a human ecological context in Brazil. In: G.H. Kruse, H.I. Browman, K.L. Cochrane, D. Evans, G.S. Jamieson, P.A. Livingston, D. Woodby, and C.I. Zhang, eds. Global progress in ecosystem-based fisheries management. Fairbanks: Alaska Sea Grant, University of Alaska Fairbanks. http://dx.doi.org/10.4027/gpebfm.2012.018.
http://dx.doi.org/10.4027/gpebfm.2012.0...
,b BEGOSSI, A., SALIVONCHYK, S.V., HANAZAKI, N., MARTINS, I.V. and BUELONI, F., 2012b. Fishers (Paraty, RJ) and fish manipulation time: a variable associated to the choice for consumption and sale. Brazilian Journal of Biology = Revista Brasileira de Biologia , vol. 72, no. 4, pp. 973-975. http://dx.doi.org/10.1590/S1519-69842012000500030. PMid:23295533.
http://dx.doi.org/10.1590/S1519-6984201...
,c BEGOSSI, A., SALYVONCHYK, S.V., NORA, V., LOPES, P.F. and SILVANO, R.A.M., 2012c. The Paraty artisanal fishery (southeastern Brazilian coast): ethnoecology and management of a social-ecological system (SES). Journal of Ethnobiology and Ethnomedicine, vol. 8, no. 1, pp. 22. http://dx.doi.org/10.1186/1746-4269-8-22. PMid:22738073.
http://dx.doi.org/10.1186/1746-4269-8-2...
; Silva and Begossi, 2009 SILVA, A.L. and BEGOSSI, A., 2009. Biodiversity, food consumption and ecological niche dimension: a study case of the riverine populations from the Rio Negro, Amazonia, Brazil. Environment, Development and Sustainability, vol. 11, no. 3, pp. 489-507. http://dx.doi.org/10.1007/s10668-007-9126-z.
http://dx.doi.org/10.1007/s10668-007-91...
).
For this study, we compiled data about the first two items cited by fishers when they were asked about the fish species they consumed the most, referred herein respectively as “F1 (fish most consumed) and F2 (second most consumed fish, Table 2 ). Details of the methods and of fish taxonomic identification, along with other information of the areas and of the communities studied can be found in previous studies ( Begossi and Garavello, 1990 BEGOSSI, A. and GARAVELLO, J.C., 1990. Notes on the ethnoicthyology of fishermen from the Tocantins River (Brazil). Acta Amazonica, vol. 20, no. 20, pp. 341-351. http://dx.doi.org/10.1590/1809-43921990201351.
http://dx.doi.org/10.1590/1809-43921990...
; Begossi and Braga, 1992 BEGOSSI, A. and BRAGA, S., 1992. Food taboos and folk medicine among fishermen from the Tocantins River (Brazil). Amazoniana, vol. 12, no. 1, pp. 101-118. ; Begossi and Figueiredo, 1995 BEGOSSI, A. and FIGUEIREDO, L., 1995. Ethnoichthyology of southern coastal fishermen: cases from Búzios Island and Sepetiba Bay (Brazil). Bulletin of Marine Science , vol. 56, no. 2, pp. 710-717. ; Begossi et al., 1999 BEGOSSI, A., SILVANO, R.A.M., AMARAL, B.D. and OYAKAWA, O.T., 1999. Uses of fish and game by inhabitants of an extractive reserve (Upper Juruá, Acre, Brazil). Environment, Development and Sustainability, vol. 1, no. 1, pp. 73-93. http://dx.doi.org/10.1023/A:1010075315060.
http://dx.doi.org/10.1023/A:10100753150...
, 2012a BEGOSSI, A., LOPES, P. and SILVANO, R., 2012a. Co-Management of reef fisheries of the snapper-grouper complex in a human ecological context in Brazil. In: G.H. Kruse, H.I. Browman, K.L. Cochrane, D. Evans, G.S. Jamieson, P.A. Livingston, D. Woodby, and C.I. Zhang, eds. Global progress in ecosystem-based fisheries management. Fairbanks: Alaska Sea Grant, University of Alaska Fairbanks. http://dx.doi.org/10.4027/gpebfm.2012.018.
http://dx.doi.org/10.4027/gpebfm.2012.0...
,b BEGOSSI, A., SALIVONCHYK, S.V., HANAZAKI, N., MARTINS, I.V. and BUELONI, F., 2012b. Fishers (Paraty, RJ) and fish manipulation time: a variable associated to the choice for consumption and sale. Brazilian Journal of Biology = Revista Brasileira de Biologia , vol. 72, no. 4, pp. 973-975. http://dx.doi.org/10.1590/S1519-69842012000500030. PMid:23295533.
http://dx.doi.org/10.1590/S1519-6984201...
,c BEGOSSI, A., SALYVONCHYK, S.V., NORA, V., LOPES, P.F. and SILVANO, R.A.M., 2012c. The Paraty artisanal fishery (southeastern Brazilian coast): ethnoecology and management of a social-ecological system (SES). Journal of Ethnobiology and Ethnomedicine, vol. 8, no. 1, pp. 22. http://dx.doi.org/10.1186/1746-4269-8-22. PMid:22738073.
http://dx.doi.org/10.1186/1746-4269-8-2...
, 2017 BEGOSSI, A., SALIVONCHYK, S., HALLWASS, G., HANAZAKI, N., LOPES, P.F.M. and SILVANO, R.A.M., 2017. Threatened fish and fishers along the Brazilian Atlantic Forest Coast. Ambio, vol. 46, no. 8, pp. 907-914. https://doi.org/10.1007/s13280-017-0931-9. PMid:28710567
https://doi.org/10.1007/s13280-017-0931...
; Silva and Begossi, 2009 SILVA, A.L. and BEGOSSI, A., 2009. Biodiversity, food consumption and ecological niche dimension: a study case of the riverine populations from the Rio Negro, Amazonia, Brazil. Environment, Development and Sustainability, vol. 11, no. 3, pp. 489-507. http://dx.doi.org/10.1007/s10668-007-9126-z.
http://dx.doi.org/10.1007/s10668-007-91...
). We assessed the current conservation status of the most cited fish at the IUCN website and at the latest Brazilian Red List, published in November 2014 ( IUCN, 2014 INTERNATIONAL UNION FOR CONSERVATION OF NATURE – IUCN, 2014 [viewed 10 July 2015]. IUCN red list [online]. Cambridge: IUCN UK Office. Available from: http://www.iucnredlist.org/
http://www.iucnredlist.org/ ...
).
The most important fish cited as consumed by Amazonian riverine families. Results from interviews with Amazon small-scale riverine fishers (see Table 3 for scientific names of fish species) (10% citations or more); n= number of interviews; F1=fish cited first in interviews; F2= fish cited second in interviews.
3. Results
From the 517 interviews, the fish most cited as consumed in the Amazonian rivers Araguaia and Mortes, Tocantins, Jurua and Negro were Curimata (Prochilodus nigricans ) and Mandi (Pimelodella, Pimelodus, among other spp.). Tucunaré (Cichla spp.) was important in Araguaia, Mortes and Negro, Jaraqui (Semaprochilodus spp.) was important at the Negro River, and Bode (species of Loricariidade) was important at the Jurua river and its tributaries ( Table 2 ). In another set of interviews in 1987-1988 at the Tocantins river banks (n=233 interviews) 65 fishers mentioned that Pacu-manteiga (Mylossoma duriventre), an important local fish consumed, decreased in abundance due to the Tucuruí dam.
The IUCN Red List includes 90 species of the 95 species that were cited as the most important by the riverine fishers. Most of them (78%) have not been assessed and 10% are of least concern (NA) ( Table 3 ). Therefore, we have almost no knowledge on the conservation status of the most important fish for the food security of Amazonian fishers.
Conservation status of fish consumed in coastal (Atlantic Forest) and inland (Amazon Forest) areas of Brazil [partial information published (Part of this material previously published ( Winemiller et al., 2016 WINEMILLER, K.O., MCINTYRE, P.B., CASTELLO, L., FLUET-CHOUINARD, E., GIARRIZZO, T., NAM, S., BAIRD, I.G., DARWALL, W., LUJAN, N.K., HARRISON, I., STIASSNY, M.L.J., SILVANO, R.A.M., FITZGERALD, D.B., PELICICE, F.M., AGOSTINHO, A.A., GOMES, L.C., ALBERT, J.S., BARAN, E., PETRERE, M., ZARFL, C., MULLIGAN, M., SULLIVAN, J.P., ARANTES, C.C., SOUSA, L.M., KONING, A.A., HOEINGHAUS, D.J., SABAJ, M., LUNDBERG, J.G., ARMBRUSTER, J., THIEME, M.L., PETRY, P., ZUANON, J., VILARA, G.T., SNOEKS, J., OU, C., RAINBOTH, W., PAVANELLI, C.S., AKAMA, A., SOESBERGEN, A. and SAENZ, L., 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, vol. 351, no. 6269, pp. 128-129. http://dx.doi.org/10.1126/science.aac7082. PMid:26744397.
http://dx.doi.org/10.1126/science.aac70... ; Begossi, 2014 BEGOSSI, A., 2014. Ecological, cultural, and economic approaches to managing artisanal fisheries. Environment, Development and Sustainability, vol. 16, no. 1, pp. 5-34. http://dx.doi.org/10.1007/s10668-013-9471-z.
http://dx.doi.org/10.1007/s10668-013-94... ; Hallwass et al., 2011 HALLWASS, G., LOPES, P.F., JURAS, A.A. and SILVANO, R.A.M., 2011. Fishing effort and catch composition of urban market and rural villages in Brazilian Amazon. Environmental Management, vol. 47, no. 2, pp. 188-200. http://dx.doi.org/10.1007/s00267-010-9584-1. PMid:21153639.
http://dx.doi.org/10.1007/s00267-010-95... ; Pinnegar and Engelhard, 2007 PINNEGAR, J.K. and ENGELHARD, G.H., 2007. The ‘shifting baseline’ phenomenon: a global perspective. Reviews in Fish Biology and Fisheries, vol. 18, no. 1, pp. 1-16. http://dx.doi.org/10.1007/s11160-007-9058-6.
http://dx.doi.org/10.1007/s11160-007-90... ; Prestes-Carneiro et al., 2015 PRESTES-CARNEIRO, G., BÉAREZ, P., BAILON, S., PY-DANIEL, A.R. and NEVES, E.G., 2015. Subsistence fishery at Hatahara (750-1230 CE), a pre-Columbian central Amazonian village. Journal of Archaeological Science: Reports, vol. 8, pp. 454-462. https://doi.org/10.1016/j.jasrep.2015.10.033. ; Béné et al., 2015 BÉNÉ, C., BARANGE, M., SUBASINGHE, R., PINSTRUP-ANDERSEN, P., MERINO, G., HEMRE, G. and WILLIAMS, M., 2015. Feeding 9 billion by 2050 – Putting fish back on the menu. Food Security, vol. 7, no. 2, pp. 261-274. http://dx.doi.org/10.1007/s12571-015-0427-z.
http://dx.doi.org/10.1007/s12571-015-04... ).
4. Discussion
Our results highlighted the almost complete lack of knowledge on freshwater fish important for the food security of Amazonian people. This knowledge gap about the vulnerability of fish can negatively affect and compromise the food security of those Amazonian human populations who highly depend on fish as a source of protein ( Batista et al., 1998 BATISTA, V.S., INHAMUNS, A.J., FREITAS, C.D.C. and FREIRE‐BRASIL, D., 1998. Characterization of the fishery in river communities in the low‐solimões/high‐amazon region. Fisheries Management and Ecology, vol. 5, no. 5, pp. 419-435. http://dx.doi.org/10.1046/j.1365-2400.1998.550419.x.
http://dx.doi.org/10.1046/j.1365-2400.1...
; Isaac and Almeida, 2011 ISAAC, V.J. and ALMEIDA, M.C., 2011. El consumo de pescado en la amazonía brasileña. Rome: Organización de las Naciones Unidas para la Agricultura y la Alimentación. No. CIDAB-SH1-F62c-13. ; Begossi et al., 2012b BEGOSSI, A., SALIVONCHYK, S.V., HANAZAKI, N., MARTINS, I.V. and BUELONI, F., 2012b. Fishers (Paraty, RJ) and fish manipulation time: a variable associated to the choice for consumption and sale. Brazilian Journal of Biology = Revista Brasileira de Biologia , vol. 72, no. 4, pp. 973-975. http://dx.doi.org/10.1590/S1519-69842012000500030. PMid:23295533.
http://dx.doi.org/10.1590/S1519-6984201...
; Lopes et al., 2015 LOPES, P.F., PACHECO, S., CLAUZET, M., SILVANO, R.A.M. and BEGOSSI, A., 2015. Fisheries, tourism, and marine protected areas: conflicting or synergistic interactions? Ecosystem Services, vol. 16, pp. 333-340. http://dx.doi.org/10.1016/j.ecoser.2014.12.003.
http://dx.doi.org/10.1016/j.ecoser.2014...
). Capture fisheries improve nutrition and food security, besides reducing poverty, in tropical developing countries (Bené et al., 2016). A decrease in the supply of fish as a food source may have indirect consequences for these populations, their livelihoods and their biodiversity rich ecosystems. In Brazil, the food habits of riverine people can change, by including more processed and industrialized items, such as chicken nuggets, pasta, canned meat, among others. Such food items may be nutritionally poor and create a dependence on external food sources ( Silva and Begossi, 2009 SILVA, A.L. and BEGOSSI, A., 2009. Biodiversity, food consumption and ecological niche dimension: a study case of the riverine populations from the Rio Negro, Amazonia, Brazil. Environment, Development and Sustainability, vol. 11, no. 3, pp. 489-507. http://dx.doi.org/10.1007/s10668-007-9126-z.
http://dx.doi.org/10.1007/s10668-007-91...
). A decrease in the supply of fish as a food source may increase the use of land for agriculture and hence the rate of deforestation ( Orr et al., 2012 ORR, S., PITTOCK, J., CHAPAGAIN, A. and DUMARESQ, D., 2012. Dams on the Mekong river: lost fish protein and the implications for land and water resources. Global Environmental Change, vol. 22, no. 4, pp. 925-932. http://dx.doi.org/10.1016/j.gloenvcha.2012.06.002.
http://dx.doi.org/10.1016/j.gloenvcha.2...
). This would not only threaten the terrestrial biodiversity but could also exacerbate the current conflicts between fishers and managers of protected tropical forests ( Lopes et al., 2013 LOPES, P.F., ROSA, E.M., SALYVONCHYK, S.V., NORA, V. and BEGOSSI, A., 2013. Suggestions for fixing top-down coastal fisheries management through participatory approaches. Marine Policy, vol. 40, pp. 100-110. http://dx.doi.org/10.1016/j.marpol.2012.12.033.
http://dx.doi.org/10.1016/j.marpol.2012...
; Begossi et al., 2011 BEGOSSI, A., MAY, P.H., LOPES, P.F., OLIVEIRA, L.E., VINHA, V. and SILVANO, R.A.M., 2011. Compensation for environmental services from artisanal fisheries in SE Brazil: Policy and technical strategies. Ecological Economics, vol. 1, pp. 25-32. http://dx.doi.org/10.1016/j.ecolecon.2011.09.008.
http://dx.doi.org/10.1016/j.ecolecon.20...
). Of particularly interest here are the contrasting cases of the Negro and Araguaia rivers. In the Negro River (52 meals), the reported consumption of animal protein showed that locally caught fish responded for 75% of their diet, whereas in Araguaia fish responded for only 10% of all animal protein consumed ( Begossi et al., 2000 BEGOSSI, A., HANAZAKI, N. and PERONI, N., 2000. Knowledge and use of biodiversity in Brazilian hot spots. Environment, Development and Sustainability, vol. 2, no. 3-4, pp. 177-193. http://dx.doi.org/10.1023/A:1011409923520.
http://dx.doi.org/10.1023/A:10114099235...
).
The increased exploitation of large, slow-growing and more vulnerable fish species has caused drastic reduction in the populations or even local extinction of these fish, and they tend to be replaced by less valuable and smaller fish in multi-species fisheries ( Welcomme, 1999 WELCOMME, R.L., 1999. A review of a model for qualitative evaluation of exploitation levels in multi-species fisheries. Fisheries Management and Ecology, vol. 6, no. 1, pp. 1-19. http://dx.doi.org/10.1046/j.1365-2400.1999.00137.x.
http://dx.doi.org/10.1046/j.1365-2400.1...
). This pattern of sequential overfishing in tropical fisheries, which is usually driven by an increase demand for fish, have been described as the ‘fishing down’ process in freshwaters ( Welcomme, 1999 WELCOMME, R.L., 1999. A review of a model for qualitative evaluation of exploitation levels in multi-species fisheries. Fisheries Management and Ecology, vol. 6, no. 1, pp. 1-19. http://dx.doi.org/10.1046/j.1365-2400.1999.00137.x.
http://dx.doi.org/10.1046/j.1365-2400.1...
; Welcomme et al., 2010) WELCOMME, R.L., COWX, I.G., COATES, D., BÉNÉ, C., FUNGE-SMITH, S., HALLS, A. and LORENZEN, K., 2010. Inland capture fisheries. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, vol. 365, no. 1554, pp. 2881-2896. http://dx.doi.org/10.1098/rstb.2010.0168. PMid:20713391.
http://dx.doi.org/10.1098/rstb.2010.016...
, and as ‘fishing down the food web’ in marine ecosystems ( Pauly et al., 1998 PAULY, D., CHRISTENSEN, V., DALSGAARD, J., FROESE, R. and TORRES JUNIOR, F., 1998. Fishing down marine food webs. Science, vol. 279, no. 5352, pp. 860-863. http://dx.doi.org/10.1126/science.279.5352.860. PMid:9452385.
http://dx.doi.org/10.1126/science.279.5...
). ‘Fishing down’ implies a major reduction in the size of the fish caught (either smaller species or smaller individuals of the same species) ( Welcomme et al., 2010 WELCOMME, R.L., COWX, I.G., COATES, D., BÉNÉ, C., FUNGE-SMITH, S., HALLS, A. and LORENZEN, K., 2010. Inland capture fisheries. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, vol. 365, no. 1554, pp. 2881-2896. http://dx.doi.org/10.1098/rstb.2010.0168. PMid:20713391.
http://dx.doi.org/10.1098/rstb.2010.016...
). The ‘fishing down the food web’ implies an overall decrease in the mean trophic level of exploited fish, as larger piscivorous are depleted ( Pauly et al., 1998 PAULY, D., CHRISTENSEN, V., DALSGAARD, J., FROESE, R. and TORRES JUNIOR, F., 1998. Fishing down marine food webs. Science, vol. 279, no. 5352, pp. 860-863. http://dx.doi.org/10.1126/science.279.5352.860. PMid:9452385.
http://dx.doi.org/10.1126/science.279.5...
, 2002 PAULY, D., CHRISTENSEN, V., GUÉNETTE, S., PITCHER, T.J., SUMAILA, U.R., WALTERS, C.J., WATSON, R. and ZELLER, D., 2002. Towards sustainability in world fisheries. Nature, vol. 418, no. 6898, pp. 689-695. http://dx.doi.org/10.1038/nature01017. PMid:12167876.
http://dx.doi.org/10.1038/nature01017 ...
), although recent evidence has indicated that the sequential overfishing can be more strongly related to the net value of fishing resources than the trophic level of captured species ( Sethi et al., 2010 SETHI, S.A., BRANCH, T.A. and WATSON, R., 2010. Global fishery development patterns are driven by profit but not trophic level. Proceedings of the National Academy of Sciences of the United States of America, vol. 107, no. 27, pp. 12163-12167. http://dx.doi.org/10.1073/pnas.1003236107. PMid:20566867.
http://dx.doi.org/10.1073/pnas.10032361...
).
Ancient Amazonian indigenous people consumed at least 37 taxa of fish species. The larger fish species consumed by these ancient populations (Pirarucu, Arapaima gigas , and Tambaqui, Colossoma macropomum) ( Prestes-Carneiro et al., 2015 PRESTES-CARNEIRO, G., BÉAREZ, P., BAILON, S., PY-DANIEL, A.R. and NEVES, E.G., 2015. Subsistence fishery at Hatahara (750-1230 CE), a pre-Columbian central Amazonian village. Journal of Archaeological Science: Reports, vol. 8, pp. 454-462. https://doi.org/10.1016/j.jasrep.2015.10.033. ) are currently considered overfished in some regions of the Amazon ( Smith, 1985 SMITH, N.J.H., 1985. The impact of cultural and ecological change on Amazonian fisheries. Biological Conservation, vol. 32, no. 4, pp. 355-373. http://dx.doi.org/10.1016/0006-3207(85)90023-0.
http://dx.doi.org/10.1016/0006-3207(85)...
; Garcia et al., 2009 GARCIA, A., TELLO, S., VARGAS, G. and DUPONCHELLE, F., 2009. Patterns of Commercial Fish Landings in the Loreto Region (Peruvian Amazon) Between 1984 and 2006. Fish Physiology and Biochemistry, vol. 35, no. 1, pp. 53-67. http://dx.doi.org/10.1007/s10695-008-9212-7. PMid:19189235.
http://dx.doi.org/10.1007/s10695-008-92...
; Castello et al., 2013 CASTELLO, L., MCGRATH, D.G., HESS, L.L., COE, M.T., LEFEBVRE, P.A., PETRY, P., MACEDO, M.N., RENÓ, V.F. and ARANTES, C.C., 2013. The vulnerability of Amazon freshwater ecosystems. Conservation Letters, vol. 6, no. 4, pp. 217-229. http://dx.doi.org/10.1111/conl.12008.
http://dx.doi.org/10.1111/conl.12008 ...
). On the other hand, small fish, such as Mandi, Acari (or Bodes, Loricariidae), Jaraqui, Curimatá, Piau and Tucunaré, were consumed in lower frequencies by the ancient Amazonians ( Prestes-Carneiro et al., 2015 PRESTES-CARNEIRO, G., BÉAREZ, P., BAILON, S., PY-DANIEL, A.R. and NEVES, E.G., 2015. Subsistence fishery at Hatahara (750-1230 CE), a pre-Columbian central Amazonian village. Journal of Archaeological Science: Reports, vol. 8, pp. 454-462. https://doi.org/10.1016/j.jasrep.2015.10.033. ) ( Table 1 ). The change in the main fish species consumed over time indicates a reduction in the availability and abundance of the large and preferred fish species, which can lead to vulnerability in the food security of riverine peoples who depend on fish for protein intake. The larger fish usually provide more assimilated energy (more biomass) and comparatively demand less processing time to be consumed (fewer or larger spines), tending to be thus more valuable and preferred by fishers ( Begossi et al., 2012b BEGOSSI, A., SALIVONCHYK, S.V., HANAZAKI, N., MARTINS, I.V. and BUELONI, F., 2012b. Fishers (Paraty, RJ) and fish manipulation time: a variable associated to the choice for consumption and sale. Brazilian Journal of Biology = Revista Brasileira de Biologia , vol. 72, no. 4, pp. 973-975. http://dx.doi.org/10.1590/S1519-69842012000500030. PMid:23295533.
http://dx.doi.org/10.1590/S1519-6984201...
).
Governmental plans assigned to protected areas are often in conflict with fish biology researchers, fishery participants and fishers. Furthermore, most of the existing protected areas in Brazil in general ( Lopes et al., 2013 LOPES, P.F., ROSA, E.M., SALYVONCHYK, S.V., NORA, V. and BEGOSSI, A., 2013. Suggestions for fixing top-down coastal fisheries management through participatory approaches. Marine Policy, vol. 40, pp. 100-110. http://dx.doi.org/10.1016/j.marpol.2012.12.033.
http://dx.doi.org/10.1016/j.marpol.2012...
) or in the Brazilian Amazon ( Castello et al., 2013 CASTELLO, L., MCGRATH, D.G., HESS, L.L., COE, M.T., LEFEBVRE, P.A., PETRY, P., MACEDO, M.N., RENÓ, V.F. and ARANTES, C.C., 2013. The vulnerability of Amazon freshwater ecosystems. Conservation Letters, vol. 6, no. 4, pp. 217-229. http://dx.doi.org/10.1111/conl.12008.
http://dx.doi.org/10.1111/conl.12008 ...
, Junk et al., 2007 JUNK, W.J., SOARES, M.G.M. and BAYLEY, P.B., 2007. Freshwater fishes of the Amazon River basin: their biodiversity, fisheries, and habitats. Aquatic Ecosystem Health & Management , vol. 10, no. 2, pp. 153-173. http://dx.doi.org/10.1080/14634980701351023.
http://dx.doi.org/10.1080/1463498070135...
) have been planned for the protection of terrestrial ecosystems, and have not properly considered for the protection of aquatic biodiversity, fishing resources and local fishing communities. Besides, recent political initiatives by the Brazilian Government (Brazilian Red List and spawning closed seasons) ( Pinheiro et al., 2015 PINHEIRO, H.T., DARIO, F., GERHARDINGER, L.C., MELO, M.R., MOURA, R.L., REIS, R.E., VIEIRA, F., ZUANON, J. and ROCHA, L.A., 2015. Brazilian aquatic biodiversity in peril. Science , vol. 350, no. 6264, pp. 1043-1044. http://dx.doi.org/10.1126/science.350.6264.1043-a. PMid:26612943.
http://dx.doi.org/10.1126/science.350.6...
), might affect threatened species and species where knowledge is almost absent ( Table 3 ). In Brazil, the migratory fish such as Jaraqui (Semaprochilodus spp.), Matrinchã (Brycon sp.), Dourada (Brachyplatystoma rousseauxii ), and Filhote (Brachyplatystoma filamentosum) may be increasingly threatened by the construction of dams in the upstream reaches of large rivers ( Barthem et al., 1991 BARTHEM, R.B., RIBEIRO, M.C.L.B. and PETRERE JUNIOR, M., 1991. Life strategies of some long-distance migratory catfish in relation to hydroelectric dams in the Amazon Basin. Biological Conservation, vol. 55, no. 3, pp. 339-345. http://dx.doi.org/10.1016/0006-3207(91)90037-A.
http://dx.doi.org/10.1016/0006-3207(91)...
; Hallwass et al., 2013 HALLWASS, G., LOPES, P.F., JURAS, A.A. and SILVANO, R.A.M., 2013. Fishers’ knowledge identifies environmental changes and fish abundance trends in impounded tropical rivers. Ecological Applications, vol. 23, no. 2, pp. 392-407. http://dx.doi.org/10.1890/12-0429.1. PMid:23634590.
http://dx.doi.org/10.1890/12-0429.1 ...
; Hallwass and Silvano, 2016 HALLWASS, G. and SILVANO, R.A.M., 2016. Patterns of selectiveness in the Amazonian freshwater fisheries: implications for management. Journal of Environmental Planning and Management, vol. 59, no. 9, pp. 1537-1559. http://dx.doi.org/10.1080/09640568.2015.1081587.
http://dx.doi.org/10.1080/09640568.2015...
; Winemiller et al., 2016 WINEMILLER, K.O., MCINTYRE, P.B., CASTELLO, L., FLUET-CHOUINARD, E., GIARRIZZO, T., NAM, S., BAIRD, I.G., DARWALL, W., LUJAN, N.K., HARRISON, I., STIASSNY, M.L.J., SILVANO, R.A.M., FITZGERALD, D.B., PELICICE, F.M., AGOSTINHO, A.A., GOMES, L.C., ALBERT, J.S., BARAN, E., PETRERE, M., ZARFL, C., MULLIGAN, M., SULLIVAN, J.P., ARANTES, C.C., SOUSA, L.M., KONING, A.A., HOEINGHAUS, D.J., SABAJ, M., LUNDBERG, J.G., ARMBRUSTER, J., THIEME, M.L., PETRY, P., ZUANON, J., VILARA, G.T., SNOEKS, J., OU, C., RAINBOTH, W., PAVANELLI, C.S., AKAMA, A., SOESBERGEN, A. and SAENZ, L., 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, vol. 351, no. 6269, pp. 128-129. http://dx.doi.org/10.1126/science.aac7082. PMid:26744397.
http://dx.doi.org/10.1126/science.aac70...
).
To reverse the observed trend of increasing threats to fish and fishers, we suggest that more attention be devoted to fisheries management and conservation agendas to protect and manage those often neglected fish that are an important food source. This may be achieved by working together and cooperating with the fishers themselves, who have detailed knowledge about fish biology ( Silvano et al., 2006 SILVANO, R.A.M., MACCORD, P.F., LIMA, R.V. and BEGOSSI, A., 2006. When does this fish spawn? Fishermen’s local knowledge of migration and reproduction of Brazilian coastal fishes. Environmental Biology of Fishes, vol. 76, no. 2-4, pp. 371-386. http://dx.doi.org/10.1007/s10641-006-9043-2.
http://dx.doi.org/10.1007/s10641-006-90...
; 2008 SILVANO, R.A.M., SILVA, A.L., CERONI, M. and BEGOSSI, A., 2008. Contributions of ethnobiology to the conservation of tropical rivers and streams. Aquatic Conservation , vol. 18, no. 3, pp. 241-260. http://dx.doi.org/10.1002/aqc.825.
http://dx.doi.org/10.1002/aqc.825 ...
; Silvano and Begossi, 2012 SILVANO, R.A.M. and BEGOSSI, A., 2012. Fishermen’s local ecological knowledge on Southeastern Brazilian coastal fishes: contributions to research, conservation, and management. Neotropical Ichthyology, vol. 10, no. 1, pp. 133-147. http://dx.doi.org/10.1590/S1679-62252012000100013.
http://dx.doi.org/10.1590/S1679-6225201...
; Leite and Gasalla, 2013 LEITE, M.C. and GASALLA, M.A., 2013. A method for assessing fishers’ ecological knowledge as a practical tool for ecosystem-based fisheries management: Seeking consensus in Southeastern Brazil. Fisheries Research, vol. 145, pp. 43-53. http://dx.doi.org/10.1016/j.fishres.2013.02.013.
http://dx.doi.org/10.1016/j.fishres.201...
), and can help to protect fish stocks through co-management systems ( Defeo and Castilla, 2005 DEFEO, O. and CASTILLA, J.C., 2005. More than one bag for the world fishery crisis and keys for co-management successes in selected artisanal Latin American shellfisheries. Reviews in Fish Biology and Fisheries, vol. 15, no. 3, pp. 265-283. http://dx.doi.org/10.1007/s11160-005-4865-0.
http://dx.doi.org/10.1007/s11160-005-48...
; Gelcich et al., 2008 GELCICH, S., KAISER, M.J., CASTILLA, J.C. and EDWARDS-JONES, G., 2008. Engagement in co-management of marine benthic resources influences environmental perceptions of artisanal fishers. Environmental Conservation, vol. 35, no. 1, pp. 36-45. http://dx.doi.org/10.1017/S0376892908004475.
http://dx.doi.org/10.1017/S037689290800...
; Lopes et al., 2011 LOPES, P.F.M., SILVANO, R.A.M. and BEGOSSI, A., 2011. Extractive and Sustainable Development Reserves in Brazil: resilient alternatives to fisheries? Journal of Environmental Planning and Management, vol. 54, no. 4, pp. 421-443. http://dx.doi.org/10.1080/09640568.2010.508687.
http://dx.doi.org/10.1080/09640568.2010...
; Silvano et al., 2014 SILVANO, R.A.M., HALLWASS, G., LOPES, P.F., RIBEIRO, A.R., LIMA, R.P., HASENACK, H., JURAS, A.A. and BEGOSSI, A., 2014. Co-management and spatial features contribute to secure fish abundance and fishing yields in tropical floodplain lakes. Ecosystems (New York, N.Y.), vol. 17, no. 2, pp. 271-285. http://dx.doi.org/10.1007/s10021-013-9722-8.
http://dx.doi.org/10.1007/s10021-013-97...
). A first step would be to reveal those fish most important to sustain livelihoods and food security; to identify those most threatened; and then devise policies to maintain these fish species before they are gone. For several years, Brazilian researchers have been stressing the impacts that hydroelectric power plants have on fish. Most important are those that cause a sharp decrease in the species richness (for example, in Tucuruí Reservoir about 50% of the fish species have been lost), and those that have a drastic impact on migratory species (Petrere Junior, 1989 PETRERE JUNIOR, M., 1989. River fisheries in Brazil: a review. Regulated Rivers: Research and Management, vol. 4, no. 1, pp. 1-6. http://dx.doi.org/10.1002/rrr.3450040102.
http://dx.doi.org/10.1002/rrr.345004010...
, 1996 PETRERE JUNIOR, M., 1996. Fisheries in large tropical reservoirs in South America. Lakes and Reservoirs: Research and Management, vol. 2, no. 1-2, pp. 111-133. http://dx.doi.org/10.1111/j.1440-1770.1996.tb00054.x.
http://dx.doi.org/10.1111/j.1440-1770.1...
; Barthem et al., 1991 BARTHEM, R.B., RIBEIRO, M.C.L.B. and PETRERE JUNIOR, M., 1991. Life strategies of some long-distance migratory catfish in relation to hydroelectric dams in the Amazon Basin. Biological Conservation, vol. 55, no. 3, pp. 339-345. http://dx.doi.org/10.1016/0006-3207(91)90037-A.
http://dx.doi.org/10.1016/0006-3207(91)...
; Kahn et al., 2014 KAHN, J.R., FREITAS, C.E. and PETRERE, M., 2014. False shades of green: the case of Brazilian Amazonian hydropower. Energies, vol. 7, no. 9, pp. 6063-6082. http://dx.doi.org/10.3390/en7096063.
http://dx.doi.org/10.3390/en7096063 ...
). More recently, a study has shown that the environmental impact assessments are very limited in Brazil, with poor methodologies and imprecise requirements, preventing the identification of impacts and possible mitigating actions (one example approached the Belo Monte Dam) ( Ritter et al., 2017 RITTER, C.D., MCCRATE, G., NILSSON, R.K., FEARNSIDE, P.M., PALME, U. and ANTONELLI, A., 2017. Environmental impact assessment in Brazilian Amazonia: challenges and prospects to assess biodiversity. Biological Conservation, vol. 206, pp. 161-168. http://dx.doi.org/10.1016/j.biocon.2016.12.031.
http://dx.doi.org/10.1016/j.biocon.2016...
). However, more than 10 years ago researchers have claimed on the impacts hydroelectric could cause ( Tundisi, 2007 TUNDISI, J.G., 2007. Explosão do potencial hidrelétrico da Amazônia. Estudos Avançados, vol. 59, no. 59, pp. 109-117. http://dx.doi.org/10.1590/S0103-40142007000100009.
http://dx.doi.org/10.1590/S0103-4014200...
). Giving the scale and timing of change happening in Amazonian rivers, action is needed now to ensure that our rivers can continue to feed riverine people.
5. Conclusions
Our concluding remarks summarize the evidence and observations on the association between biodiversity loss and increased food insecurity, as follows:
-
1
Hydropower development in the Amazon could negatively affect river fisheries.
-
2
While the national diet in Brazil has only a small portion of freshwater fish (1.5%), the protein diet of the riverine Amazonian Caboclos small-scale fishers (based on research in communities of 773,000 estimated people in total/IBGE) is substantially dependant on riverine fish for protein (around 70% of protein in their diet).
-
3
The Caboclos target particular species when fishing.
-
4
The majority (78%) of the targeted fish species are either regarded as ‘not known’ or threatened. This is a very important. consideration, since it is likely that some species could to disappear before we will have appropriate knowledge for their conservation.
-
5
The targeted fish species might be vulnerable as a result of many individual and combined impacts, such as overfishing and multiple hydropower dam development.
-
6
In this review we reinforce suggestions that hydropower development (along with other development pressures) in the Amazon may threaten the food security of the Caboclos in Brazil. The data of the main fish species consumed by the Caboclos show that impact from dams could potentially affect the main species consumed and therefore the food security of riverine Caboclos.
Appendix A Study sites, archive number of interviews deposited at the Fisheries and Food Institute (2015) FISHERIES AND FOOD INSTITUTE [online], 2015 [viewed 10 July 2015]. Available from: www.fisheriesandfood.com , Santos, SP, at Unisanta, Santos, SP.
The grant # and funding agency is shown.| ARCHIVE NUMBER/FIFO 1 1 Fisheries and Food Institute. | STUDY SITE | FUNDING AGENCY |
|---|---|---|
| PAJUR 007 | ALTO JURUÁ ACRE AM | FAPESP 1998/02619-8/ |
| Project F. MacArthur 92/21848 | ||
| Coords. Brown, Almeida & Cunha (UNICAMP) | ||
| PDRPTO 008 | TOCANTINS RIVER AM | R. P.: FAPESP [1994/6258-7] CNPq [400.185/95-4] |
| TO: 1987/ 1988: THEMAG/ELETROBRAS | ||
| PTOCETNO 008 | TOCANTINS RIVER AM | TO: 1987/ 1988: THEMAG/ELETROBRAS |
| PTOCGOMA 008 | TOCANTINS RIVER AM | TO: 1987/ 1988: THEMAG/ELETROBRAS |
| PDDARA 009 | ARAGUAIA RIVER AM | FAPESP [1996/1036-1] |
| PARA 009 | ARAGUAIA RIVER AM | FAPESP [1996/1036-1] |
| PDRNMA 018 | QUEST RIO NEGRO AM | FAPESP 1998/16160-5 |
Acknowledgements
We are very grateful to FAPESP grants (14/24994-8 and in the Appendix) as well as to the collaborative project (authors AB, DD, JP): “Managing links between hydropower development, fish and foods” funded by ANU’s Brazilian Regional Reference Group Collaboration Scheme (Australia). We thank CNPq for productivity scholarships to A.B., N.H. and R.A.M.S.
-
(With 1 figure)
-
Erratum
Erratum In the article “Fish consumption on the Amazon: a review of biodiversity, hydropower and food security issues”, DOI: https://doi.org/10.1590/1519-6984.186572 , published ahead of print on Oct 29, 2018 in Brazilian Journal of Biology, in the Acknowledgements the article:Where it reads:AcknowledgementsWe are very grateful to FAPESP grants (14/24994-8 and in the Appendix) as well as to the collaborative project (authors AB, DD, JP): “Managing links between hydropower development, fish and foods” funded by ANU’s Brazilian Regional Reference Group Collaboration Scheme (Australia). We thank CNPq for productivity scholarships to A.B., N.H. and R.A.M.S.It should be read:AcknowledgementsWe are very grateful to FAPESP grants (14/24994-8 and in the Appendix) as well as to the collaborative project (authors AB, DD, JP): “Managing links between hydropower development, fish and foods” funded by ANU’s Brazilian Regional Reference Group Collaboration Scheme (Australia). We thank CNPq for productivity scholarships to A.B., N.H. and R.A.M.S.In memoriam Benedito D. do Amaral.
References
- ABELL., THIEME, M.L., REVENGA, C., BRYER, M., KOTTELAT, M., BOGUTSKAYA, N., COAD, B., MANDRAK, N., BALDERAS, S.C., BUSSING, W., STIASSNY, M.L.J., SKELTON, P., ALLEN, G.R., UNMACK, P., NASEKA, A., NG, R., SINDORF, N., ROBERTSON, J., ARMIJO, E., HIGGINS, J.V., HEIBEL, T.J., WIKRAMANAYAKE, E., OLSON, D., LÓPEZ, H.L., REIS, R.E., LUNDBERG, J.G., PÉREZ, M.H.S. and PETRY, P., 2008. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience, vol. 58, no. 5, pp. 403-414. http://dx.doi.org/10.1641/B580507.
» http://dx.doi.org/10.1641/B580507 - AINSWORTH, C.H., PITCHER, T.J. and ROTINSULU, C., 2008. Evidence of fishery depletions and shifting cognitive baselines in Eastern Indonesia. Biological Conservation , vol. 141, no. 3, pp. 848-859. http://dx.doi.org/10.1016/j.biocon.2008.01.006.
» http://dx.doi.org/10.1016/j.biocon.2008.01.006 - ANDREW, N.L., BÉNÉ, C., HALL, S.J., ALLISON, E.H., HECK, S. and RATNER, B.D., 2007. Diagnosis and management of small-scale fisheries in developing countries. Fish and Fisheries, vol. 8, no. 3, pp. 227-240. http://dx.doi.org/10.1111/j.1467-2679.2007.00252.x.
» http://dx.doi.org/10.1111/j.1467-2679.2007.00252.x - BARTHEM, R.B., RIBEIRO, M.C.L.B. and PETRERE JUNIOR, M., 1991. Life strategies of some long-distance migratory catfish in relation to hydroelectric dams in the Amazon Basin. Biological Conservation, vol. 55, no. 3, pp. 339-345. http://dx.doi.org/10.1016/0006-3207(91)90037-A.
» http://dx.doi.org/10.1016/0006-3207(91)90037-A - BARTLEY, D.M., DE GRAAF, G.J., VALBO-JØRGENSEN, J. and MARMULLA, G., 2015. Inland capture fisheries: status and data issues. Fisheries Management and Ecology , vol. 22, no. 1, pp. 71-77. http://dx.doi.org/10.1111/fme.12104.
» http://dx.doi.org/10.1111/fme.12104 - BATISTA, V.S., INHAMUNS, A.J., FREITAS, C.D.C. and FREIRE‐BRASIL, D., 1998. Characterization of the fishery in river communities in the low‐solimões/high‐amazon region. Fisheries Management and Ecology, vol. 5, no. 5, pp. 419-435. http://dx.doi.org/10.1046/j.1365-2400.1998.550419.x.
» http://dx.doi.org/10.1046/j.1365-2400.1998.550419.x - BEGOSSI, A. and BRAGA, S., 1992. Food taboos and folk medicine among fishermen from the Tocantins River (Brazil). Amazoniana, vol. 12, no. 1, pp. 101-118.
- BEGOSSI, A. and FIGUEIREDO, L., 1995. Ethnoichthyology of southern coastal fishermen: cases from Búzios Island and Sepetiba Bay (Brazil). Bulletin of Marine Science , vol. 56, no. 2, pp. 710-717.
- BEGOSSI, A. and GARAVELLO, J.C., 1990. Notes on the ethnoicthyology of fishermen from the Tocantins River (Brazil). Acta Amazonica, vol. 20, no. 20, pp. 341-351. http://dx.doi.org/10.1590/1809-43921990201351.
» http://dx.doi.org/10.1590/1809-43921990201351 - BEGOSSI, A., 2014. Ecological, cultural, and economic approaches to managing artisanal fisheries. Environment, Development and Sustainability, vol. 16, no. 1, pp. 5-34. http://dx.doi.org/10.1007/s10668-013-9471-z.
» http://dx.doi.org/10.1007/s10668-013-9471-z - BEGOSSI, A., HANAZAKI, N. and PERONI, N., 2000. Knowledge and use of biodiversity in Brazilian hot spots. Environment, Development and Sustainability, vol. 2, no. 3-4, pp. 177-193. http://dx.doi.org/10.1023/A:1011409923520.
» http://dx.doi.org/10.1023/A:1011409923520 - BEGOSSI, A., HANAZAKI, N. and RAMOS, R.M., 2004. Food chain and the reasons for fish food taboos among Amazonian and Atlantic Forest fishers (Brazil). Ecological Applications , vol. 14, no. 5, pp. 1334-1343. http://dx.doi.org/10.1890/03-5072.
» http://dx.doi.org/10.1890/03-5072 - BEGOSSI, A., LOPES, P. and SILVANO, R., 2012a. Co-Management of reef fisheries of the snapper-grouper complex in a human ecological context in Brazil. In: G.H. Kruse, H.I. Browman, K.L. Cochrane, D. Evans, G.S. Jamieson, P.A. Livingston, D. Woodby, and C.I. Zhang, eds. Global progress in ecosystem-based fisheries management. Fairbanks: Alaska Sea Grant, University of Alaska Fairbanks. http://dx.doi.org/10.4027/gpebfm.2012.018.
» http://dx.doi.org/10.4027/gpebfm.2012.018 - BEGOSSI, A., MAY, P.H., LOPES, P.F., OLIVEIRA, L.E., VINHA, V. and SILVANO, R.A.M., 2011. Compensation for environmental services from artisanal fisheries in SE Brazil: Policy and technical strategies. Ecological Economics, vol. 1, pp. 25-32. http://dx.doi.org/10.1016/j.ecolecon.2011.09.008.
» http://dx.doi.org/10.1016/j.ecolecon.2011.09.008 - BEGOSSI, A., SALIVONCHYK, S.V., HANAZAKI, N., MARTINS, I.V. and BUELONI, F., 2012b. Fishers (Paraty, RJ) and fish manipulation time: a variable associated to the choice for consumption and sale. Brazilian Journal of Biology = Revista Brasileira de Biologia , vol. 72, no. 4, pp. 973-975. http://dx.doi.org/10.1590/S1519-69842012000500030. PMid:23295533.
» http://dx.doi.org/10.1590/S1519-69842012000500030 - BEGOSSI, A., SALYVONCHYK, S.V., NORA, V., LOPES, P.F. and SILVANO, R.A.M., 2012c. The Paraty artisanal fishery (southeastern Brazilian coast): ethnoecology and management of a social-ecological system (SES). Journal of Ethnobiology and Ethnomedicine, vol. 8, no. 1, pp. 22. http://dx.doi.org/10.1186/1746-4269-8-22. PMid:22738073.
» http://dx.doi.org/10.1186/1746-4269-8-22 - BEGOSSI, A., SILVANO, R.A.M., AMARAL, B.D. and OYAKAWA, O.T., 1999. Uses of fish and game by inhabitants of an extractive reserve (Upper Juruá, Acre, Brazil). Environment, Development and Sustainability, vol. 1, no. 1, pp. 73-93. http://dx.doi.org/10.1023/A:1010075315060.
» http://dx.doi.org/10.1023/A:1010075315060 - BEGOSSI, A., SALIVONCHYK, S., HALLWASS, G., HANAZAKI, N., LOPES, P.F.M. and SILVANO, R.A.M., 2017. Threatened fish and fishers along the Brazilian Atlantic Forest Coast. Ambio, vol. 46, no. 8, pp. 907-914. https://doi.org/10.1007/s13280-017-0931-9. PMid:28710567
» https://doi.org/10.1007/s13280-017-0931-9 - BENCHIMOL, M. and PERES, C.A., 2015. Widespread forest vertebrate extinctions induced by a mega hydroelectric dam in lowland Amazonia. PLoS One, vol. 10, no. 7, pp. e0129818. http://dx.doi.org/10.1371/journal.pone.0129818. PMid:26132139.
» http://dx.doi.org/10.1371/journal.pone.0129818 - BENE, C., 2006. Small-scale fisheries: assessing their contribution to rural livelihoods in developing countries Rome: FAO. FAO Fisheries Circular, no. 2008.
- BÉNÉ, C., ARTHUR, R., NORBURY, H., ALLISON, E.H., BEVERIDGE, M., BUSH, S., CAMPLING, L., LESCHEN, W., LITTLE, D., SQUIRES, D., THILSTED, S.H., TROELL, M. and WILLIAMS, M., 2016. Contribution to fisheries and Aquaculture to food security and poverty reduction: assessing the current evidence. World Development, vol. 79, pp. 177-196. http://dx.doi.org/10.1016/j.worlddev.2015.11.007.
» http://dx.doi.org/10.1016/j.worlddev.2015.11.007 - BÉNÉ, C., BARANGE, M., SUBASINGHE, R., PINSTRUP-ANDERSEN, P., MERINO, G., HEMRE, G. and WILLIAMS, M., 2015. Feeding 9 billion by 2050 – Putting fish back on the menu. Food Security, vol. 7, no. 2, pp. 261-274. http://dx.doi.org/10.1007/s12571-015-0427-z.
» http://dx.doi.org/10.1007/s12571-015-0427-z - BOISCHIO, A.A.P. and HENSHEL, P., 2000. Fish consumption, fish lore and mercury pollution: risk communication for the Madeira River People. Environmental Research Section A, vol. 84, no. 2, pp. 108-126. http://dx.doi.org/10.1006/enrs.2000.4035. PMid:11068924.
» http://dx.doi.org/10.1006/enrs.2000.4035 - CARNEIRO, F. and SOUZA, O.B., 2009. Atlas de pressões e ameaças às terras indígenas na Amazônia Brasileira São Paulo: Instituto Socioambiental. 48 p.
- CASTELLO, L., MCGRATH, D.G., HESS, L.L., COE, M.T., LEFEBVRE, P.A., PETRY, P., MACEDO, M.N., RENÓ, V.F. and ARANTES, C.C., 2013. The vulnerability of Amazon freshwater ecosystems. Conservation Letters, vol. 6, no. 4, pp. 217-229. http://dx.doi.org/10.1111/conl.12008.
» http://dx.doi.org/10.1111/conl.12008 - CERDEIRA, R.G.P., RUFFINO, M.L. and ISAAC, V.J., 1997. Consumo de pescado e outros alimentos pela população ribeirinha do Lago Grande de Monte Alegre, PA - Brasil. Acta Amazonica, vol. 27, no. 3, pp. 213-227. http://dx.doi.org/10.1590/1809-43921997273228.
» http://dx.doi.org/10.1590/1809-43921997273228 - DE GRAAF, G.J., GRAINGER, R.J.R., WESTLUND, L., WILLMANN, R., MILLS, D., KELLEHER, K. and KORANTENG, K., 2011. The status of routine fishery data collection in Southeast Asia, central America, the South Pacific, and West Africa, with special reference to small-scale fisheries. ICES Journal of Marine Science, vol. 68, no. 8, pp. 1743-1750. http://dx.doi.org/10.1093/icesjms/fsr054.
» http://dx.doi.org/10.1093/icesjms/fsr054 - DEFEO, O. and CASTILLA, J.C., 2005. More than one bag for the world fishery crisis and keys for co-management successes in selected artisanal Latin American shellfisheries. Reviews in Fish Biology and Fisheries, vol. 15, no. 3, pp. 265-283. http://dx.doi.org/10.1007/s11160-005-4865-0.
» http://dx.doi.org/10.1007/s11160-005-4865-0 - DUGAN, P.J., BARAN, E., THARME, R., PREIN, M., AHMED, R., AMERASINGHE, P., BUENO, P., BROWN, C., DEY, M., JAYASINGHE, G., NIASSE, M., NIELAND, A., SMAKHTIN, V., TINH, N., VISWANATHAN, K. and WELCOMME, R., 2002. The contribution of aquatic ecosystems and fisheries to food security and livelihoods: a research agenda. In: CGIAR. Challenge Program on Water and Food: background papers to the full proposal Colombo: CGIAR Challenge Program on Water and Food. pp. 85-113. Background Paper, no. 3.
- FEARNSIDE, P.M., 1999. Social impacts of Brazil’s Tucuruí Dam. Environmental Management, vol. 24, no. 4, pp. 483-495. http://dx.doi.org/10.1007/s002679900248. PMid:10501861.
» http://dx.doi.org/10.1007/s002679900248 - FEARNSIDE, P.M., 2004. Greenhouse gas emissions from hydroelectric dams: controversies provide a springboard for rethinking a supposedly ‘clean’ energy source. an editorial comment. Climatic Change, vol. 66, no. 1/2, pp. 1-18. http://dx.doi.org/10.1023/B:CLIM.0000043174.02841.23.
» http://dx.doi.org/10.1023/B:CLIM.0000043174.02841.23 - FEARNSIDE, P.M., 2015. Highway construction as a force in destruction of the Amazon forest. In: R. VAN DER REE, D.J. SMITH and C. GRILO, eds. Handbook of road ecology . Oxford: John Wiley & Sons Publishers. pp. 414-424. http://dx.doi.org/10.1002/9781118568170.ch51.
» http://dx.doi.org/10.1002/9781118568170.ch51 - FEARNSIDE, P.M., 2016. Tropical dams: to build or not to build? Science , vol. 351, no. 6272, pp. 456-457. http://dx.doi.org/10.1126/science.351.6272.456-b. PMid:26823417.
» http://dx.doi.org/10.1126/science.351.6272.456-b - FISHERIES AND FOOD INSTITUTE [online], 2015 [viewed 10 July 2015]. Available from: www.fisheriesandfood.com
- FOOD AND AGRICULTURE ORGANIZATION – FAO, 2011. World by continent - food balance sheet of fish and fishery products in live weight and fish contribution to protein supply Rome: Food and Agriculture Organisation of the United Nations.
- FOOD AND AGRICULTURE ORGANIZATION – FAO, 2013a. Fish, crustaceans, molluscs, etc.: world capture production Rome: Food and Agriculture Organization of the United Nations.
- FOOD AND AGRICULTURE ORGANIZATION – FAO, 2013b. World fisheries production, by capture and aquaculture, by country Rome: Food and Agriculture Organisation of the United Nations.
- GARCIA, A., TELLO, S., VARGAS, G. and DUPONCHELLE, F., 2009. Patterns of Commercial Fish Landings in the Loreto Region (Peruvian Amazon) Between 1984 and 2006. Fish Physiology and Biochemistry, vol. 35, no. 1, pp. 53-67. http://dx.doi.org/10.1007/s10695-008-9212-7. PMid:19189235.
» http://dx.doi.org/10.1007/s10695-008-9212-7 - GEHRKE, P.C., GILLIGAN, D.M. and BARWICK, M., 2002. Changes in fish communities of the Shoalhaven River 20 years after construction of Tallowa Dam, Australia. River Research and Applications, vol. 18, no. 3, pp. 265-286. http://dx.doi.org/10.1002/rra.669.
» http://dx.doi.org/10.1002/rra.669 - GELCICH, S., KAISER, M.J., CASTILLA, J.C. and EDWARDS-JONES, G., 2008. Engagement in co-management of marine benthic resources influences environmental perceptions of artisanal fishers. Environmental Conservation, vol. 35, no. 1, pp. 36-45. http://dx.doi.org/10.1017/S0376892908004475.
» http://dx.doi.org/10.1017/S0376892908004475 - HALLWASS, G. and SILVANO, R.A.M., 2016. Patterns of selectiveness in the Amazonian freshwater fisheries: implications for management. Journal of Environmental Planning and Management, vol. 59, no. 9, pp. 1537-1559. http://dx.doi.org/10.1080/09640568.2015.1081587.
» http://dx.doi.org/10.1080/09640568.2015.1081587 - HALLWASS, G., LOPES, P.F., JURAS, A.A. and SILVANO, R.A.M., 2011. Fishing effort and catch composition of urban market and rural villages in Brazilian Amazon. Environmental Management, vol. 47, no. 2, pp. 188-200. http://dx.doi.org/10.1007/s00267-010-9584-1. PMid:21153639.
» http://dx.doi.org/10.1007/s00267-010-9584-1 - HALLWASS, G., LOPES, P.F., JURAS, A.A. and SILVANO, R.A.M., 2013. Fishers’ knowledge identifies environmental changes and fish abundance trends in impounded tropical rivers. Ecological Applications, vol. 23, no. 2, pp. 392-407. http://dx.doi.org/10.1890/12-0429.1. PMid:23634590.
» http://dx.doi.org/10.1890/12-0429.1 - INTERNATIONAL UNION FOR CONSERVATION OF NATURE – IUCN, 2014 [viewed 10 July 2015]. IUCN red list [online]. Cambridge: IUCN UK Office. Available from: http://www.iucnredlist.org/
» http://www.iucnredlist.org/ - ISAAC, V.J. and ALMEIDA, M.C., 2011. El consumo de pescado en la amazonía brasileña Rome: Organización de las Naciones Unidas para la Agricultura y la Alimentación. No. CIDAB-SH1-F62c-13.
- ISAAC, V.J., ALMEIDA, M.C., GIARRIZZO, T., DEUS, C.P., VALE, R., KLEIN, G. and BEGOSSI, A., 2015. Food consumption as an indicator of the conservation of natural resources in riverine communities of the Brazilian Amazon. Anais da Academia Brasileira de Ciências , vol. 87, no. 4, pp. 2229-2242. http://dx.doi.org/10.1590/0001-3765201520140250. PMid:26628023.
» http://dx.doi.org/10.1590/0001-3765201520140250 - ISAAC, V.J., SILVA, C.O. and RUFFINO, M.L., 2008. The artisanal fishery fleet of the lower Amazon. Fisheries Management and Ecology, vol. 15, no. 3, pp. 179-187. http://dx.doi.org/10.1111/j.1365-2400.2008.00599.x.
» http://dx.doi.org/10.1111/j.1365-2400.2008.00599.x - JACKSON, J.B.C., KIRBY, M.X., BERGER, W.H., BJORNDAL, K.A., BOTSFORD, L.W., BOURQUE, B.J., BRADBURY, R.H., COOKE, R., ERLANDSON, J., ESTES, J.A., HUGHES, T.P., KIDWELL, S., LANGE, C.B., LENIHAN, H.S., PANDOLFI, J.M., PETERSON, C.H., STENECK, R.S., TEGNER, M.J. and WARNER, R.R., 2001. Historical overfishing and the recent collapse of coastal ecosystems. Science, vol. 293, no. 5530, pp. 629-637. http://dx.doi.org/10.1126/science.1059199. PMid:11474098.
» http://dx.doi.org/10.1126/science.1059199 - JUNK, W.J., SOARES, M.G.M. and BAYLEY, P.B., 2007. Freshwater fishes of the Amazon River basin: their biodiversity, fisheries, and habitats. Aquatic Ecosystem Health & Management , vol. 10, no. 2, pp. 153-173. http://dx.doi.org/10.1080/14634980701351023.
» http://dx.doi.org/10.1080/14634980701351023 - KAHN, J.R., FREITAS, C.E. and PETRERE, M., 2014. False shades of green: the case of Brazilian Amazonian hydropower. Energies, vol. 7, no. 9, pp. 6063-6082. http://dx.doi.org/10.3390/en7096063.
» http://dx.doi.org/10.3390/en7096063 - LEITE, M.C. and GASALLA, M.A., 2013. A method for assessing fishers’ ecological knowledge as a practical tool for ecosystem-based fisheries management: Seeking consensus in Southeastern Brazil. Fisheries Research, vol. 145, pp. 43-53. http://dx.doi.org/10.1016/j.fishres.2013.02.013.
» http://dx.doi.org/10.1016/j.fishres.2013.02.013 - LOPES, P.F., PACHECO, S., CLAUZET, M., SILVANO, R.A.M. and BEGOSSI, A., 2015. Fisheries, tourism, and marine protected areas: conflicting or synergistic interactions? Ecosystem Services, vol. 16, pp. 333-340. http://dx.doi.org/10.1016/j.ecoser.2014.12.003.
» http://dx.doi.org/10.1016/j.ecoser.2014.12.003 - LOPES, P.F., ROSA, E.M., SALYVONCHYK, S.V., NORA, V. and BEGOSSI, A., 2013. Suggestions for fixing top-down coastal fisheries management through participatory approaches. Marine Policy, vol. 40, pp. 100-110. http://dx.doi.org/10.1016/j.marpol.2012.12.033.
» http://dx.doi.org/10.1016/j.marpol.2012.12.033 - LOPES, P.F.M., SILVANO, R.A.M. and BEGOSSI, A., 2011. Extractive and Sustainable Development Reserves in Brazil: resilient alternatives to fisheries? Journal of Environmental Planning and Management, vol. 54, no. 4, pp. 421-443. http://dx.doi.org/10.1080/09640568.2010.508687.
» http://dx.doi.org/10.1080/09640568.2010.508687 - MCCLANAHAN, T., ALLISON, E.H. and CINNER, J.E., 2015. Managing fisheries for human and food security. Fish and Fisheries, vol. 16, no. 1, pp. 78-103. http://dx.doi.org/10.1111/faf.12045.
» http://dx.doi.org/10.1111/faf.12045 - MÉRONA, B., JURAS, A. F., SANTOS, G.M., CINTRA, I.H.A., 2010. Os peixes e a pesca no baixo Rio Tocantins: vinte anos depois da UHE Tucuruí Brasília: Centrais Elétricas do Nort e do Brasil S.A; Eletrobras-Eletronorte; Ministério de Minas e Energia.
- MÉRONA, B., SANTOS, G.M. and ALMEIDA, R.G., 2001. Short term effects of Tucuruí Dam (Amazonia, Brazil) on the trophic organization of fish communities. Environmental Biology of Fishes, vol. 60, no. 4, pp. 375-392. http://dx.doi.org/10.1023/A:1011033025706.
» http://dx.doi.org/10.1023/A:1011033025706 - MOLLE, F., MOLLINGA, P.P. and WESTER, P., 2010. Hydraulic bureaucracies: flows of water, flows of power. Water Alternatives, vol. 2, pp. 328-349.
- MORAN, E.F., 1996. The adaptive system of the Amazonian caboclo. Man in the Amazon. 1974; 136: 139-159. Regulated Rivers: Research and Management, vol. 12, pp. 81-98.
- MYERS, R.A. and WORM, B., 2003. Rapid worldwide depletion of predatory fish communities. Nature, vol. 423, no. 6937, pp. 280-283. http://dx.doi.org/10.1038/nature01610. PMid:12748640.
» http://dx.doi.org/10.1038/nature01610 - NUGENT, S., 1993. Amazonian caboclo society: an essay on invisibility and peasant economy Providence, Rhode Island: Berg Publishers Ltd.
- ORR, S., PITTOCK, J., CHAPAGAIN, A. and DUMARESQ, D., 2012. Dams on the Mekong river: lost fish protein and the implications for land and water resources. Global Environmental Change, vol. 22, no. 4, pp. 925-932. http://dx.doi.org/10.1016/j.gloenvcha.2012.06.002.
» http://dx.doi.org/10.1016/j.gloenvcha.2012.06.002 - PAULY, D., 1995. Anecdotes and the shifting baseline syndrome in fisheries. Trends in Ecology & Evolution, vol. 10, no. 10, pp. 430. http://dx.doi.org/10.1016/S0169-5347(00)89171-5. PMid:21237093.
» http://dx.doi.org/10.1016/S0169-5347(00)89171-5 - PAULY, D., 2006. Major trends in small-scale marine fisheries, with emphasis on developing countries, and some implications for the social sciences. Maritime Studies , vol. 4, no. 2, pp. 7-22.
- PAULY, D., CHRISTENSEN, V., DALSGAARD, J., FROESE, R. and TORRES JUNIOR, F., 1998. Fishing down marine food webs. Science, vol. 279, no. 5352, pp. 860-863. http://dx.doi.org/10.1126/science.279.5352.860. PMid:9452385.
» http://dx.doi.org/10.1126/science.279.5352.860 - PAULY, D., CHRISTENSEN, V., GUÉNETTE, S., PITCHER, T.J., SUMAILA, U.R., WALTERS, C.J., WATSON, R. and ZELLER, D., 2002. Towards sustainability in world fisheries. Nature, vol. 418, no. 6898, pp. 689-695. http://dx.doi.org/10.1038/nature01017. PMid:12167876.
» http://dx.doi.org/10.1038/nature01017 - PETESSE, M.L. and PETRERE JUNIOR, M., 2012. As barragens e os peixes: o impacto das grandes hidrelétricas nas espécies dos rios represados. Ciência Hoje, vol. 49, pp. 30-35.
- PETRERE JUNIOR, M., 1989. River fisheries in Brazil: a review. Regulated Rivers: Research and Management, vol. 4, no. 1, pp. 1-6. http://dx.doi.org/10.1002/rrr.3450040102.
» http://dx.doi.org/10.1002/rrr.3450040102 - PETRERE JUNIOR, M., 1996. Fisheries in large tropical reservoirs in South America. Lakes and Reservoirs: Research and Management, vol. 2, no. 1-2, pp. 111-133. http://dx.doi.org/10.1111/j.1440-1770.1996.tb00054.x.
» http://dx.doi.org/10.1111/j.1440-1770.1996.tb00054.x - PINHEIRO, H.T., DARIO, F., GERHARDINGER, L.C., MELO, M.R., MOURA, R.L., REIS, R.E., VIEIRA, F., ZUANON, J. and ROCHA, L.A., 2015. Brazilian aquatic biodiversity in peril. Science , vol. 350, no. 6264, pp. 1043-1044. http://dx.doi.org/10.1126/science.350.6264.1043-a. PMid:26612943.
» http://dx.doi.org/10.1126/science.350.6264.1043-a - PINNEGAR, J.K. and ENGELHARD, G.H., 2007. The ‘shifting baseline’ phenomenon: a global perspective. Reviews in Fish Biology and Fisheries, vol. 18, no. 1, pp. 1-16. http://dx.doi.org/10.1007/s11160-007-9058-6.
» http://dx.doi.org/10.1007/s11160-007-9058-6 - PONTON, D. and VAUCHEL, P., 1998. Immediate downstream effects of the Petit-Saut Dam on Young neotropical fish in a large tributary of the Sinnamary River (French Guiana, South America). Regulated Rivers: Research and Management, vol. 14, no. 3, pp. 227-243. http://dx.doi.org/10.1002/(SICI)1099-1646(199805/06)14:3<227::AID-RRR490>3.0.CO;2-6.
» http://dx.doi.org/10.1002/(SICI)1099-1646(199805/06)14:3<227::AID-RRR490>3.0.CO;2-6 - PRESTES-CARNEIRO, G., BÉAREZ, P., BAILON, S., PY-DANIEL, A.R. and NEVES, E.G., 2015. Subsistence fishery at Hatahara (750-1230 CE), a pre-Columbian central Amazonian village. Journal of Archaeological Science: Reports, vol. 8, pp. 454-462. https://doi.org/10.1016/j.jasrep.2015.10.033.
- RITTER, C.D., MCCRATE, G., NILSSON, R.K., FEARNSIDE, P.M., PALME, U. and ANTONELLI, A., 2017. Environmental impact assessment in Brazilian Amazonia: challenges and prospects to assess biodiversity. Biological Conservation, vol. 206, pp. 161-168. http://dx.doi.org/10.1016/j.biocon.2016.12.031.
» http://dx.doi.org/10.1016/j.biocon.2016.12.031 - SARTI, F.M., ADAMS, C., MORSELLO, C., VAN VLIET, N., SCHOR, T., YAGÜE, B., TELLEZ, L., QUICENO-MESA, M.P. and CRUZ, D., 2015. Beyond protein intake: bushmeat as source of micronutrients in the Amazon. Ecology and Society, vol. 20, no. 4, pp. 22. http://dx.doi.org/10.5751/ES-07934-200422.
» http://dx.doi.org/10.5751/ES-07934-200422 - SETHI, S.A., BRANCH, T.A. and WATSON, R., 2010. Global fishery development patterns are driven by profit but not trophic level. Proceedings of the National Academy of Sciences of the United States of America, vol. 107, no. 27, pp. 12163-12167. http://dx.doi.org/10.1073/pnas.1003236107. PMid:20566867.
» http://dx.doi.org/10.1073/pnas.1003236107 - SHRIMPTON, R. and GIUGLIANO, R., 1979. Consumo de alimentos e alguns nutrients em Manaus, Amazonas. 1973-74. Acta Amazonica, vol. 9, no. 1, pp. 117-141. http://dx.doi.org/10.1590/1809-43921979091117.
» http://dx.doi.org/10.1590/1809-43921979091117 - SILVA, A.L. and BEGOSSI, A., 2009. Biodiversity, food consumption and ecological niche dimension: a study case of the riverine populations from the Rio Negro, Amazonia, Brazil. Environment, Development and Sustainability, vol. 11, no. 3, pp. 489-507. http://dx.doi.org/10.1007/s10668-007-9126-z.
» http://dx.doi.org/10.1007/s10668-007-9126-z - SILVANO, R.A.M. and BEGOSSI, A., 2012. Fishermen’s local ecological knowledge on Southeastern Brazilian coastal fishes: contributions to research, conservation, and management. Neotropical Ichthyology, vol. 10, no. 1, pp. 133-147. http://dx.doi.org/10.1590/S1679-62252012000100013.
» http://dx.doi.org/10.1590/S1679-62252012000100013 - SILVANO, R.A.M., HALLWASS, G., LOPES, P.F., RIBEIRO, A.R., LIMA, R.P., HASENACK, H., JURAS, A.A. and BEGOSSI, A., 2014. Co-management and spatial features contribute to secure fish abundance and fishing yields in tropical floodplain lakes. Ecosystems (New York, N.Y.), vol. 17, no. 2, pp. 271-285. http://dx.doi.org/10.1007/s10021-013-9722-8.
» http://dx.doi.org/10.1007/s10021-013-9722-8 - SILVANO, R.A.M., MACCORD, P.F., LIMA, R.V. and BEGOSSI, A., 2006. When does this fish spawn? Fishermen’s local knowledge of migration and reproduction of Brazilian coastal fishes. Environmental Biology of Fishes, vol. 76, no. 2-4, pp. 371-386. http://dx.doi.org/10.1007/s10641-006-9043-2.
» http://dx.doi.org/10.1007/s10641-006-9043-2 - SILVANO, R.A.M., SILVA, A.L., CERONI, M. and BEGOSSI, A., 2008. Contributions of ethnobiology to the conservation of tropical rivers and streams. Aquatic Conservation , vol. 18, no. 3, pp. 241-260. http://dx.doi.org/10.1002/aqc.825.
» http://dx.doi.org/10.1002/aqc.825 - SMITH, N.J.H., 1985. The impact of cultural and ecological change on Amazonian fisheries. Biological Conservation, vol. 32, no. 4, pp. 355-373. http://dx.doi.org/10.1016/0006-3207(85)90023-0.
» http://dx.doi.org/10.1016/0006-3207(85)90023-0 - SOLARTE, L.G., CASTAÑO, M.L.M., ARENA, A.H. and CHIOSSONE, T., 2008. Global corruption report 2008: corruption in the water sector Cambridge: Cambridge University Press. Water Integrity Network Transparency International, Berlín, Report No. 363.61 G562 2008.
- TOLMASQUIM, M., 2007. Plano Nacional de energia 2030 Brasília: Conselho Nacional de Politica Energetica.
- TOLMASQUIM, M.T., 2014. Perspectivas e planejamento do setor energético no Brasil. Estudos Avançados, vol. 26, no. 74, pp. 247-260. http://dx.doi.org/10.1590/S0103-40142012000100017.
- TUNDISI, J.G., 2007. Explosão do potencial hidrelétrico da Amazônia. Estudos Avançados, vol. 59, no. 59, pp. 109-117. http://dx.doi.org/10.1590/S0103-40142007000100009.
» http://dx.doi.org/10.1590/S0103-40142007000100009 - WAGLEY, C., 1974. Man in the Amazon Bloomington: University Presses of Florida.
- WELCOMME, R.L., 1999. A review of a model for qualitative evaluation of exploitation levels in multi-species fisheries. Fisheries Management and Ecology, vol. 6, no. 1, pp. 1-19. http://dx.doi.org/10.1046/j.1365-2400.1999.00137.x.
» http://dx.doi.org/10.1046/j.1365-2400.1999.00137.x - WELCOMME, R.L., COWX, I.G., COATES, D., BÉNÉ, C., FUNGE-SMITH, S., HALLS, A. and LORENZEN, K., 2010. Inland capture fisheries. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, vol. 365, no. 1554, pp. 2881-2896. http://dx.doi.org/10.1098/rstb.2010.0168. PMid:20713391.
» http://dx.doi.org/10.1098/rstb.2010.0168 - WINEMILLER, K.O., MCINTYRE, P.B., CASTELLO, L., FLUET-CHOUINARD, E., GIARRIZZO, T., NAM, S., BAIRD, I.G., DARWALL, W., LUJAN, N.K., HARRISON, I., STIASSNY, M.L.J., SILVANO, R.A.M., FITZGERALD, D.B., PELICICE, F.M., AGOSTINHO, A.A., GOMES, L.C., ALBERT, J.S., BARAN, E., PETRERE, M., ZARFL, C., MULLIGAN, M., SULLIVAN, J.P., ARANTES, C.C., SOUSA, L.M., KONING, A.A., HOEINGHAUS, D.J., SABAJ, M., LUNDBERG, J.G., ARMBRUSTER, J., THIEME, M.L., PETRY, P., ZUANON, J., VILARA, G.T., SNOEKS, J., OU, C., RAINBOTH, W., PAVANELLI, C.S., AKAMA, A., SOESBERGEN, A. and SAENZ, L., 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, vol. 351, no. 6269, pp. 128-129. http://dx.doi.org/10.1126/science.aac7082. PMid:26744397.
» http://dx.doi.org/10.1126/science.aac7082 - WORM, B., BARBIER, E.B., BEAUMONT, N., DUFFY, J.E., FOLKE, C., HALPERN, B.S., JACKSON, J.B.C., LOTZE, H.K., MICHELI, F., PALUMBI, S.R., SALA, E., SELKOE, K.A., STACHOWICZ, J.J. and WATSON, R., 2006. Impacts of biodiversity loss on ocean ecosystem services. Science , vol. 314, no. 5800, pp. 787-790. http://dx.doi.org/10.1126/science.1132294. PMid:17082450.
» http://dx.doi.org/10.1126/science.1132294 - ZARFL, C., LUMSDON, A.E., BERLEKAMP, J., TYDECKS, L. and TOCKNER, K., 2014. A global boom in hydropower dam construction. Aquatic Sciences, vol. 77, no. 1, pp. 161-170. http://dx.doi.org/10.1007/s00027-014-0377-0.
» http://dx.doi.org/10.1007/s00027-014-0377-0 - ZHONG, Y. and POWER, G., 1996. Environmental impacts of hydroelectric projects on fish resources in China. Regulated Rivers: Research and Management, vol. 12, no. 1, pp. 81-98. http://dx.doi.org/10.1002/(SICI)1099-1646(199601)12:1<81::AID-RRR378>3.0.CO;2-9.
» http://dx.doi.org/10.1002/(SICI)1099-1646(199601)12:1<81::AID-RRR378>3.0.CO;2-9
Publication Dates
-
Publication in this collection
29 Oct 2018 -
Date of issue
Apr-Jun 2019
History
-
Received
16 Oct 2017 -
Accepted
22 Nov 2017