Unusual or accidental cases of ovotestis in regular gonochoric species has been observed in several sea urchin species that led to indicate that they are hermaphrodites ( Boolootian and Moore, 1956 BOOLOOTIAN, R.A. and MOORE, A.R., 1956. Hermaphroditism in echinoids. Biological Bulletin, vol. 111, no. 3, pp. 328-335. http://dx.doi.org/10.2307/1539139.
http://dx.doi.org/10.2307/1539139 ...
; Gonor, 1973 GONOR, J.J., 1973. Sex ratio and hermaphroditism in Oregon intertidal populations of the echinoid Strongylocentrotus purpuratus. Marine Biology , vol. 19, no. 4, pp. 278-280. http://dx.doi.org/10.1007/BF00348894.
http://dx.doi.org/10.1007/BF00348894 ...
; Ijiri et al., 1981 IJIRI, K.I., EJIMA, Y. and AMEMIYA, S., 1981 [viewed 30 November 2017]. Two cases of hermaphroditism in the sea urchins, Clypeaster japonicas and Hemicentrotus pulcherrimus. Zoological Magazine, vol. 90, pp. 394-397. Available from: http://dl.ndl.go.jp/view/download/digidepo_10850674_po_ART0003840093.pdf?contentNo=1&alternativeNo=
http://dl.ndl.go.jp/view/download/digid...
; Carrasco, 2007 CARRASCO, J.F., 2007. Dimorfismo sexual en Coelopleurus coronalis (Echinoidea, Eoceno). Batalleria, vol. 13, pp. 1-19. ), however, broadly some of these events are isolated and most likely there are not hermaphrodites as mode of sexuality, instead is probable it refers to sexual differentiation or isolated situation of sex change.
Classifying the sexual mode (sexuality) of an animal can be much more challenging. Particularly, the condition of having or produce of both male and female gametes by the same gonad (ovotestis or syngonic) occurs as a process (sexual differentiation), as well as a mode of sexuality (hermaphroditism) over lifespan of the invertebrates ( Ghiselin, 1969 GHISELIN, M.T., 1969. The evolution of hermaphroditism among animals. Quarterly Review of Biology, vol. 44, no. 2, pp. 189-208. http://dx.doi.org/10.1086/406066. PMid:4901396.
http://dx.doi.org/10.1086/406066 ...
; Warner, 1975 WARNER, R.R., 1975. The adaptive significance of sequential hermaphroditism in animals. American Naturalist, vol. 109, no. 965, pp. 61-82. http://dx.doi.org/10.1086/282974.
http://dx.doi.org/10.1086/282974 ...
). Unfortunately this condition is usually conceptualized without regard to size or life stage of the animal, which creates confusion between the process of ‘sexual differentiation’ that, occurs during juvenile or sub-adult stage and the mode of sexuality ‘hermaphroditism’ that is reached only in adulthood ( Strathmann and Strathmann, 1982 STRATHMANN, R.R. and STRATHMANN, M.F., 1982. The relationship between adult size and brooding in marine invertebrates. American Naturalist, vol. 119, no. 1, pp. 91-101. http://dx.doi.org/10.1086/283892.
http://dx.doi.org/10.1086/283892 ...
; Wasson and Newberry, 1997 WASSON, K. and NEWBERRY, A.T., 1997. Modular metazoans: Gonochoric, hermaphroditic, or both at once? Invertebrate Reproduction & Development, vol. 31, no. 1-3, pp. 159-175. http://dx.doi.org/10.1080/07924259.1997.9672574.
http://dx.doi.org/10.1080/07924259.1997...
).
The aim of this paper is merely give the histological description of the coexistence of male and female germ cells (gametes) in the same gonads of an adult specimen of Loxechinus albus , without conclusion about sexuality as is just one isolated phenomenon of ovotestis observed in this gonochoric echinoid, nonsignificant at a population scale; but is interesting and is important not to miss out this phenomenon, like L. albus is an important benthic herbivorous at intertidal and shallow subtidal ecosystems of Peru, Chile and Argentina covering off the southernmost tip of South America, besides to be an important commercial species due gonads are edible. We discussed possible depicts to this ovotestis.
As part of a study about the reproductive cycle of L. albus, adult sea urchins were collected monthly during 18 months covering 2000-2001 (from rocky subtidal area at II Region, Antofagasta, Chile (23°38’S, 70°24’W) at 5-15 m depth. All collected sea urchins (n=950) were above 45 mm in test diameter ‘TD’, i.e., they are adults that had already reached the size at maturity ‘TDm50%’ ( Buckle et al., 1978 BUCKLE, L.F., GUISADO, Ch., TARIFEÑO, E., ZULETA, A., CORDOBA, L. and SERRANO, C., 1978. Biological studies on the Chilean sea-urchin Loxechinus albus (Molina) (Echinodermata; Echinoidea). IV. Maturation cycle and seasonal biochemical changes in the gonad. Ciencias Marinas, vol. 5, no. 1, pp. 1-18. http://dx.doi.org/10.7773/cm.v5i1.318.
http://dx.doi.org/10.7773/cm.v5i1.318 ...
), hence in fulfillment with the reproductive cycle. Standard histological procedures were performed and stained using the routine Harris hematoxylin-eosin regressive method “HHE 2” ( Howard and Smith, 1983 HOWARD, D.W. and SMITH, C.S., 1983. NOAA Technical Memorandum NMFS-F/NEC-25: histological techniques for marine bivalve mollusks. Woods Hole: National Oceanic and Atmospheric Administration, U.S. Department of Commerce. ). Annual sex ratio (male: female) was calculated with the Chi-square.
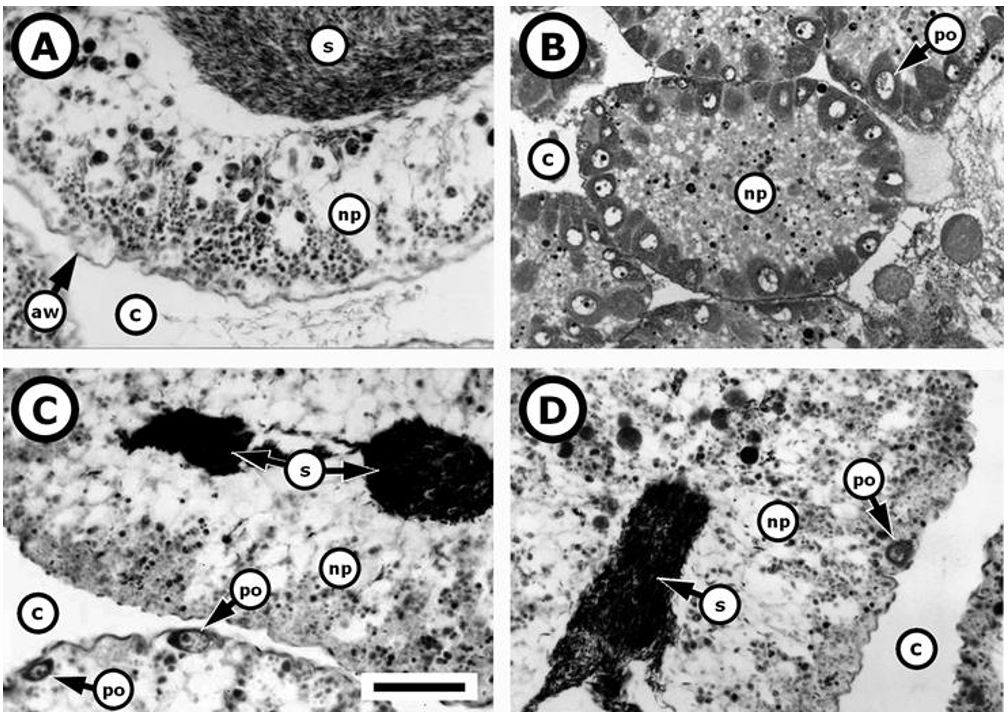
The specimen showing ovotestis (have sperm and ova in a same organ) was observed in March, 2000. It weighed 114 g in body weight, 4.3 g in gonads, measured 70 mm TD and 38.4 mm in test height. Macroscopically, the ovotestis show typical coloration yellow with released white fluid, thus, resemble to male gonad. This ovotestis was in stage of post-spawning of sperm ( Figure 1 C, D) and their histological character did not differ from those of the gonochoric individuals ( Figure 1 A, B). The acinal lumen is occupied by spermatozoa and nutritive phagocytes, but some primary oocytes appear bordering the acinal wall ( Figure 1 C, D). This sea urchin with ovotestis represented in 0.1% of all sampled specimens, for which 440 was males (46.3%) and 509 females (53.6%) representing 0.9:1 (m/f) sex ratio that not differ significantly from a sex ratio 1:1 (χ2c = 2.44, p = 0.12).
Histological sections from testicular and ovarian acini of Loxechinus albus . (A) normal testis in post-spawning stage; (B) normal ovary in development stage; (C and D) testis in post-spawning stage that includes primary oocytes, i.e., syngonic (ovotestis) gonad. Abbreviations: aw = acinal wall; c = coelom; np = nutritive phagocytes; po = primary oocyte; s = spermatozoa. Gonad development stages according to Benitez-Villalobos et al. (2015) BENITEZ-VILLALOBOS, F., AVILA-POVEDA, O.H., DIAZ-MARTINEZ, J.P. and BRAVO-RUIZ, A.R., 2015. Gonad development stages and reproductive traits of Diadema mexicanum (Echinodermata: Echinoidea) from Oaxaca, Mexico. Invertebrate Reproduction & Development, vol. 59, no. 4, pp. 237-249. http://dx.doi.org/10.1080/07924259.2015.1108935.
http://dx.doi.org/10.1080/07924259.2015... . The scale bar across to all figures is 60 μm.
Referring to organ, an ovotestis or syngonic is becoming an increasingly common feature in the reproductive anatomy on invertebrates ( Davison, 2006 DAVISON, A., 2006. The ovotestis: an underdeveloped organ of evolution. BioEssays , vol. 28, no. 6, pp. 642-650. http://dx.doi.org/10.1002/bies.20424. PMid:16700066.
http://dx.doi.org/10.1002/bies.20424 ...
), which occurs in a wide range of taxa, but mainly to Phylum Mollusca (ubiquitous), Arthropoda (rare) and in lesser degree to Echinodermata (almost absent, even more absent to echinoids) ( Ghiselin, 1969 GHISELIN, M.T., 1969. The evolution of hermaphroditism among animals. Quarterly Review of Biology, vol. 44, no. 2, pp. 189-208. http://dx.doi.org/10.1086/406066. PMid:4901396.
http://dx.doi.org/10.1086/406066 ...
; Hoagland, 1978 HOAGLAND, K.E., 1978 [viewed 30 November 2017]. Protandry and the evolution of environmentally-mediated sex change: a study of the mollusca. Malacologia, vol. 17, pp. 365-391. Available from: https://www.biodiversitylibrary.org/item/47212#page/389/mode/1up
https://www.biodiversitylibrary.org/ite...
, 1984 HOAGLAND, K.E., 1984 [viewed 30 November 2017]. Use of the terms protandry, protogyny, and hermaphroditism in malacology. American Malacological Bulletin, vol. 3, pp. 85-88. Available from: https://www.biodiversitylibrary.org/item/172449#page/379/mode/1up
https://www.biodiversitylibrary.org/ite...
; Allsop and West, 2004 ALLSOP, D.J. and WEST, S.A., 2004. Sex-ratio evolution in sex changing animals. Evolution, vol. 58, no. 5, pp. 1019-1027. http://dx.doi.org/10.1111/j.0014-3820.2004.tb00435.x. PMid:15212382.
http://dx.doi.org/10.1111/j.0014-3820.2...
; Ford et al., 2008 FORD, A.T., SAMBLES, C. and KILLE, P., 2008. Intersexuality in crustaceans: Genetic, individual and population level effects. Marine Environmental Research, vol. 66, no. 1, pp. 146-148. http://dx.doi.org/10.1016/j.marenvres.2008.02.067. PMid:18396328.
http://dx.doi.org/10.1016/j.marenvres.2...
). Now referring to sexuality, the simultaneous presence of both male and female traits (gametes) in the same individual (gonad) of any species that is gonochoric during all their life refers to intersexuality and its variants (e.g., hermaphroditism) ( Grilo and Rosa, 2017 GRILO, T.F. and ROSA, R., 2017. Intersexuality in aquatic invertebrates: prevalence and causes. Science of the Total Environment, vol. 592, pp. 714-728. http://dx.doi.org/10.1016/j.scitotenv.2017.02.099. PMid:28325592.
http://dx.doi.org/10.1016/j.scitotenv.2...
). Sexuality can be linked to both invertebrate systematics and to environment; thus some invertebrate taxa appear to be exclusively or predominantly hermaphroditic, e.g. Porifera, Ctenophora, Platyhelminthes, Opisthobranchia and Pulmonata of the Mollusca; Clitellata of the Annelida; Bryozoa ( Hodgson, 2009 HODGSON, A.N., 2009 [viewed 30 November 2017]. Reproduction and sex in invertebrates. In: A. PIRES DA SILVA, ed. Reproduction and development biology: Encyclopedia of Life Support System (EoLSS) [online]. Paris: UNESCO-EOLSS Publishers, pp. 1-27. Available from: https://www.eolss.net/ViewChapter.aspx?CategoryId=3
https://www.eolss.net/ViewChapter.aspx?...
).
Following previous descriptions and knowing that echinoids (sea urchins) are gonochoric, we argue that existence of ovotestis in Loxechinus albus corresponds to an isolated phenomenon that perhaps depicts a sequential hermaphroditism detected on this occasion first as male, then female. In the same way functional hermaphroditism was observed in Strongylocentrotus purpuratus with gonads predominantly female and areas of each gonad containing some tubules with stages of spermatogenesis and mature sperm as well as mature ova ( Gonor, 1973 GONOR, J.J., 1973. Sex ratio and hermaphroditism in Oregon intertidal populations of the echinoid Strongylocentrotus purpuratus. Marine Biology , vol. 19, no. 4, pp. 278-280. http://dx.doi.org/10.1007/BF00348894.
http://dx.doi.org/10.1007/BF00348894 ...
). Also in Clypeaster japonicas and Hemicentrotus pulcherrimus has been reported tubules filled with both sperm and oocytes, showing a typical example of an ovotestis and categorized as hermaphroditism, since self-fertilized and development of embryos was practically normal ( Ijiri et al., 1981 IJIRI, K.I., EJIMA, Y. and AMEMIYA, S., 1981 [viewed 30 November 2017]. Two cases of hermaphroditism in the sea urchins, Clypeaster japonicas and Hemicentrotus pulcherrimus. Zoological Magazine, vol. 90, pp. 394-397. Available from: http://dl.ndl.go.jp/view/download/digidepo_10850674_po_ART0003840093.pdf?contentNo=1&alternativeNo=
http://dl.ndl.go.jp/view/download/digid...
).
Although a sequential hermaphroditism is not indicated by these authors its histological examinations revealed that C. japonicas have four gonads as ovaries and the fifth one ovotestis, whiles H. pulcherrimus four gonads were testes and one was an ovotestis, which suggested a sequential hermaphroditism with functional ovotestis. The flexibility in the sexual expression of a species and the evolution of sequential hermaphroditism in previously gonochoristic populations can be simulated by setting the initial frequencies of each sex and the predominance of lifetime males and females, with which can occurs four flexibility strategies ( Warner, 1975 WARNER, R.R., 1975. The adaptive significance of sequential hermaphroditism in animals. American Naturalist, vol. 109, no. 965, pp. 61-82. http://dx.doi.org/10.1086/282974.
http://dx.doi.org/10.1086/282974 ...
): (1) change from a male to female (protandry); (2) change from female to male (protogyny); (3) change from female to hermaphrodite (protogynous hermaphroditism); (4) change from male to hermaphrodite (protandrous hermaphroditism).
In many hermaphroditic animals, a single organ (the ovotestis) produces both ova and sperm; since self-renewing cells in the ovotestis may give rise to both cell types throughout life, ova in hermaphrodites could in theory have undergone as many cell divisions as sperm ( Davison, 2006 DAVISON, A., 2006. The ovotestis: an underdeveloped organ of evolution. BioEssays , vol. 28, no. 6, pp. 642-650. http://dx.doi.org/10.1002/bies.20424. PMid:16700066.
http://dx.doi.org/10.1002/bies.20424 ...
). Since the origin of an ovotestis in Loxechinus albus could consider have any number of possibilities, such as a simultaneous hermaphrodite with unsynchronized gametogenic cycles or the ova had been spawned out leaving residual sperm. Thus, a single gonad showing ovotestis from a population do not totally warranted a condition of hermaphrodite.
Although causes and prevalence to intersexuality are unknown, some multifaceted aspects emerge mostly linked with environmental contamination by estrogenic and organotin endocrine disrupting chemicals (EDCs), parasitism, and genetic/environmental sex determination abnormalities ( Grilo and Rosa, 2017 GRILO, T.F. and ROSA, R., 2017. Intersexuality in aquatic invertebrates: prevalence and causes. Science of the Total Environment, vol. 592, pp. 714-728. http://dx.doi.org/10.1016/j.scitotenv.2017.02.099. PMid:28325592.
http://dx.doi.org/10.1016/j.scitotenv.2...
).
A number of theories/models have been proposed to try to explain the occurrence of hermaphroditism (e.g., sex allocation, reproductive value) that suggests that sex change is favored when the reproductive value of an individual (i.e., their genetic contributions to all future generations) varies with age or size, and when the relationship (sex ratio) is different for males and females ( Fisher, 1930 FISHER, R.A., 1930. The genetical theory of natural selection. Oxford: Oxford University Press. http://dx.doi.org/10.5962/bhl.title.27468.
http://dx.doi.org/10.5962/bhl.title.274...
; Charnov, 1982 CHARNOV, E.L., 1982. The theory of sex allocation. Princeton: Princeton University Press. ; West, 2009 WEST, S.A., 2009. Sex allocation. Princeton: Princeton University Press. http://dx.doi.org/10.1515/9781400832019.
http://dx.doi.org/10.1515/9781400832019...
). If males and females require an equal cost to their producing, the sex ratio of a population should evolve to equality 1:1 ( West, 2009 WEST, S.A., 2009. Sex allocation. Princeton: Princeton University Press. http://dx.doi.org/10.1515/9781400832019.
http://dx.doi.org/10.1515/9781400832019...
). In gonochoristic invertebrates, the sex ratio (male:female) is usually very close to 1:1 ( Olive, 2002 OLIVE, P.J.W., 2002. Reproduction and life cycles in invertebrates: Encyclopedia of Life Sciences (ELS). Chichester: John Wiley & Sons. ), as in echinoids, similar as observed here in for L. albus (0.9:1) from Antofagasta, Chile (23°38’S, 70°24’W), which would be ratifying that there is no predominance of males on females, then sex change corresponds to an isolated situation detected. However, to other L. albus population more to the south at Valparaiso, Chile (33°02’S, 71°36’W) with 516 males (50%), 652 females (39.7%) and 132 undifferentiated (10.1%) representing a sex ratio 0.8:1 (m/f) that differ significantly from a sex ratio 1:1 (χ2c = 7.80, p = 0.005) ( Buckle et al., 1978 BUCKLE, L.F., GUISADO, Ch., TARIFEÑO, E., ZULETA, A., CORDOBA, L. and SERRANO, C., 1978. Biological studies on the Chilean sea-urchin Loxechinus albus (Molina) (Echinodermata; Echinoidea). IV. Maturation cycle and seasonal biochemical changes in the gonad. Ciencias Marinas, vol. 5, no. 1, pp. 1-18. http://dx.doi.org/10.7773/cm.v5i1.318.
http://dx.doi.org/10.7773/cm.v5i1.318 ...
). Marked deviations from this 1:1 ratio suggest some modification of the normal breeding pattern ( Olive, 2002 OLIVE, P.J.W., 2002. Reproduction and life cycles in invertebrates: Encyclopedia of Life Sciences (ELS). Chichester: John Wiley & Sons. ).
Recently were evaluated functional of genes involved in the regulation of gonadal development and gamete production of Loxechinus albus through testis transcriptome, showing a participation around 68 to 134 genes involved per each reproductive processes such as: gamete generation, reproduction, reproductive behaviour, reproductive process ( Gaitan-Espitia et al., 2016 GAITÁN-ESPITIA, J.D., SÁNCHEZ, R., BRUNING, P. and CÁRDENAS, L., 2016. Functional insights into the testis transcriptome of the edible sea urchin Loxechinus albus. Scientific Reports, vol. 6, no. 1, e36516. http://dx.doi.org/10.1038/srep36516. PMid:27805042.
http://dx.doi.org/10.1038/srep36516 ...
). Probably, a hermaphrodite sexuality implies changes in the genetic regulation, generated as a consequence of an endogenous regulatory factor or some of the multiple environmental disrupting factors, in turn, changes to reproductive processes; a question to be researched. For example, Gonor (1973) GONOR, J.J., 1973. Sex ratio and hermaphroditism in Oregon intertidal populations of the echinoid Strongylocentrotus purpuratus. Marine Biology , vol. 19, no. 4, pp. 278-280. http://dx.doi.org/10.1007/BF00348894.
http://dx.doi.org/10.1007/BF00348894 ...
hypothesized and proposed that S. purpuratus (that showed ovotestis and hermaphroditism) is a labile gonochorist, with a multiple, autosomal, sex-determining mechanism whose expression may be influenced by the environment.
Acknowledgements
Avila-Poveda OH is commissioned as CONACYT Research Fellow/UAS-FACIMAR (project No. 2137) and participated as a member of the Academic group “Manejo de Recursos Pesqueros UAS-CA-132, UAS-FACIMAR”.
-
(With 1 figure)
References
- ALLSOP, D.J. and WEST, S.A., 2004. Sex-ratio evolution in sex changing animals. Evolution, vol. 58, no. 5, pp. 1019-1027. http://dx.doi.org/10.1111/j.0014-3820.2004.tb00435.x. PMid:15212382.
» http://dx.doi.org/10.1111/j.0014-3820.2004.tb00435.x - BENITEZ-VILLALOBOS, F., AVILA-POVEDA, O.H., DIAZ-MARTINEZ, J.P. and BRAVO-RUIZ, A.R., 2015. Gonad development stages and reproductive traits of Diadema mexicanum (Echinodermata: Echinoidea) from Oaxaca, Mexico. Invertebrate Reproduction & Development, vol. 59, no. 4, pp. 237-249. http://dx.doi.org/10.1080/07924259.2015.1108935.
» http://dx.doi.org/10.1080/07924259.2015.1108935 - BOOLOOTIAN, R.A. and MOORE, A.R., 1956. Hermaphroditism in echinoids. Biological Bulletin, vol. 111, no. 3, pp. 328-335. http://dx.doi.org/10.2307/1539139.
» http://dx.doi.org/10.2307/1539139 - BUCKLE, L.F., GUISADO, Ch., TARIFEÑO, E., ZULETA, A., CORDOBA, L. and SERRANO, C., 1978. Biological studies on the Chilean sea-urchin Loxechinus albus (Molina) (Echinodermata; Echinoidea). IV. Maturation cycle and seasonal biochemical changes in the gonad. Ciencias Marinas, vol. 5, no. 1, pp. 1-18. http://dx.doi.org/10.7773/cm.v5i1.318.
» http://dx.doi.org/10.7773/cm.v5i1.318 - CARRASCO, J.F., 2007. Dimorfismo sexual en Coelopleurus coronalis (Echinoidea, Eoceno). Batalleria, vol. 13, pp. 1-19.
- CHARNOV, E.L., 1982. The theory of sex allocation Princeton: Princeton University Press.
- DAVISON, A., 2006. The ovotestis: an underdeveloped organ of evolution. BioEssays , vol. 28, no. 6, pp. 642-650. http://dx.doi.org/10.1002/bies.20424. PMid:16700066.
» http://dx.doi.org/10.1002/bies.20424 - FISHER, R.A., 1930. The genetical theory of natural selection Oxford: Oxford University Press. http://dx.doi.org/10.5962/bhl.title.27468.
» http://dx.doi.org/10.5962/bhl.title.27468 - FORD, A.T., SAMBLES, C. and KILLE, P., 2008. Intersexuality in crustaceans: Genetic, individual and population level effects. Marine Environmental Research, vol. 66, no. 1, pp. 146-148. http://dx.doi.org/10.1016/j.marenvres.2008.02.067. PMid:18396328.
» http://dx.doi.org/10.1016/j.marenvres.2008.02.067 - GAITÁN-ESPITIA, J.D., SÁNCHEZ, R., BRUNING, P. and CÁRDENAS, L., 2016. Functional insights into the testis transcriptome of the edible sea urchin Loxechinus albus. Scientific Reports, vol. 6, no. 1, e36516. http://dx.doi.org/10.1038/srep36516. PMid:27805042.
» http://dx.doi.org/10.1038/srep36516 - GHISELIN, M.T., 1969. The evolution of hermaphroditism among animals. Quarterly Review of Biology, vol. 44, no. 2, pp. 189-208. http://dx.doi.org/10.1086/406066. PMid:4901396.
» http://dx.doi.org/10.1086/406066 - GONOR, J.J., 1973. Sex ratio and hermaphroditism in Oregon intertidal populations of the echinoid Strongylocentrotus purpuratus. Marine Biology , vol. 19, no. 4, pp. 278-280. http://dx.doi.org/10.1007/BF00348894.
» http://dx.doi.org/10.1007/BF00348894 - GRILO, T.F. and ROSA, R., 2017. Intersexuality in aquatic invertebrates: prevalence and causes. Science of the Total Environment, vol. 592, pp. 714-728. http://dx.doi.org/10.1016/j.scitotenv.2017.02.099. PMid:28325592.
» http://dx.doi.org/10.1016/j.scitotenv.2017.02.099 - HOAGLAND, K.E., 1978 [viewed 30 November 2017]. Protandry and the evolution of environmentally-mediated sex change: a study of the mollusca. Malacologia, vol. 17, pp. 365-391. Available from: https://www.biodiversitylibrary.org/item/47212#page/389/mode/1up
» https://www.biodiversitylibrary.org/item/47212#page/389/mode/1up - HOAGLAND, K.E., 1984 [viewed 30 November 2017]. Use of the terms protandry, protogyny, and hermaphroditism in malacology. American Malacological Bulletin, vol. 3, pp. 85-88. Available from: https://www.biodiversitylibrary.org/item/172449#page/379/mode/1up
» https://www.biodiversitylibrary.org/item/172449#page/379/mode/1up - HODGSON, A.N., 2009 [viewed 30 November 2017]. Reproduction and sex in invertebrates. In: A. PIRES DA SILVA, ed. Reproduction and development biology: Encyclopedia of Life Support System (EoLSS) [online]. Paris: UNESCO-EOLSS Publishers, pp. 1-27. Available from: https://www.eolss.net/ViewChapter.aspx?CategoryId=3
» https://www.eolss.net/ViewChapter.aspx?CategoryId=3 - HOWARD, D.W. and SMITH, C.S., 1983. NOAA Technical Memorandum NMFS-F/NEC-25: histological techniques for marine bivalve mollusks Woods Hole: National Oceanic and Atmospheric Administration, U.S. Department of Commerce.
- IJIRI, K.I., EJIMA, Y. and AMEMIYA, S., 1981 [viewed 30 November 2017]. Two cases of hermaphroditism in the sea urchins, Clypeaster japonicas and Hemicentrotus pulcherrimus. Zoological Magazine, vol. 90, pp. 394-397. Available from: http://dl.ndl.go.jp/view/download/digidepo_10850674_po_ART0003840093.pdf?contentNo=1&alternativeNo=
» http://dl.ndl.go.jp/view/download/digidepo_10850674_po_ART0003840093.pdf?contentNo=1&alternativeNo= - OLIVE, P.J.W., 2002. Reproduction and life cycles in invertebrates: Encyclopedia of Life Sciences (ELS) Chichester: John Wiley & Sons.
- STRATHMANN, R.R. and STRATHMANN, M.F., 1982. The relationship between adult size and brooding in marine invertebrates. American Naturalist, vol. 119, no. 1, pp. 91-101. http://dx.doi.org/10.1086/283892.
» http://dx.doi.org/10.1086/283892 - WARNER, R.R., 1975. The adaptive significance of sequential hermaphroditism in animals. American Naturalist, vol. 109, no. 965, pp. 61-82. http://dx.doi.org/10.1086/282974.
» http://dx.doi.org/10.1086/282974 - WASSON, K. and NEWBERRY, A.T., 1997. Modular metazoans: Gonochoric, hermaphroditic, or both at once? Invertebrate Reproduction & Development, vol. 31, no. 1-3, pp. 159-175. http://dx.doi.org/10.1080/07924259.1997.9672574.
» http://dx.doi.org/10.1080/07924259.1997.9672574 - WEST, S.A., 2009. Sex allocation Princeton: Princeton University Press. http://dx.doi.org/10.1515/9781400832019.
» http://dx.doi.org/10.1515/9781400832019
Publication Dates
-
Publication in this collection
08 Oct 2018 -
Date of issue
Jul-Sep 2019
History
-
Received
30 Nov 2017 -
Accepted
23 Feb 2018