Abstract
The native stands of ‘candeia’ (Eremanthus erythropappus) have been explored through management plans due to the economic potential of essential oil. The rescue of adult trees, as well as the application of silvicultural techniques that favor the restoration of the stand, can contribute to the genetic conservation of this species. This study’s objective was to assess the efficiency of propagation techniques for the rescue of 26 matrices of ‘candeia’ in a natural managed stand and discussion about the rhizogenesis. In August 2017, trees were induced to regrowth by coppice, followed by exposure and scarification of roots. The emergence of shoots and morphology were evaluated according to the origin (i.e., stump or root). After that period, 19 matrices had their sprouts collected for the preparation of apical cuttings. Indole-3-butyric acid (IBA) was applied at the base of the cuttings. Cutting survival at greenhouse exit (GE), rooting at shade house exit (SHE), morphology and root anatomy were evaluated. In 189 days, the scarification of roots promoted 76.92% of budding. The percentage of sprouted matrices, number of shoots per matrice, length, diameter, and shoot length/diameter ratio increased over time. Only 12.2% of the cuttings survived in GE, and of these, 7.9% rooted in SHE. The cutting resulted in the formation of a clonal mini-garden of ‘candeia’, with seven of the 19 matrices submitted to propagation. The anatomical analyses showed that bud formation occurs from cell redifferentiation in the phloem parenchyma, and presence of crystals on the walls of the vessel elements of the secondary xylem. The shoots induction from scarification of roots could be used as a silvicultural practice for the reestablishment of the native fragments handle.
Keywords:
‘candeia’; cloning; cutting technique; adventitious rooting
Resumo
Os povoamentos nativos de candeia (Eremanthus erythropappus) vêm sendo explorados por planos de manejo devido ao potencial econômico do óleo essencial. O resgate de árvores adultas, bem como a aplicação de técnicas silviculturais que favoreçam o restabelecimento do povoamento podem contribuir para a conservação genética dessa espécie. O objetivo desse trabalho foi avaliar a eficiência de técnicas de propagação para o resgate de 26 matrizes de candeia em um povoamento natural manejado e discutir sobre a rizogênese. Em agosto de 2017, as árvores foram induzidas à rebrota por meio da decepa, seguida da exposição e escarificação das raízes. A emissão brotações e morfologia foram avaliadas de acordo com a origem (toco ou raiz). Após esse período, 19 matrizes tiveram as brotações recolhidas para o preparo de estacas apicais, que foram tratadas com ácido indolbutírico (AIB). A sobrevivência das estacas na saída da casa de vegetação (SCV), o enraizamento na saída da casa de sombra (SCS), a morfologia e a anatomia da raiz foram avaliados. Aos 189 dias, a escarificação das raízes resultou em 76,92% de emissão de brotos. O percentual de matrizes brotadas, número de brotos por matriz, comprimento, diâmetro e relação comprimento/diâmetro dos brotos aumentaram ao longo do período avaliado. Somente 12,2% das estacas sobreviveram na SCV e 7,9% enraizaram na SCS. A estaquia resultou na formação de um minijardim clonal de candeia com sete das dezenove matrizes submetidas à propagação. As análises anatômicas mostraram a diferenciação das células na região do parênquima floemático e a presença de cristais de inulina nas paredes dos elementos de vaso do xilema secundário. A indução de brotos radiculares pode ser usada como prática silvicultural visando o restabelecimento de fragmentos nativos manejados.
Palavras-chave:
candeia; clonagem; estaquia; raiz adventícia
1. Introduction
Eremanthus erythropappus (Asteraceae) is a forest species of relevant commercial interest, considering the chemical properties present in essential oils extracted through the distillation of wood (Dutra et al., 2010DUTRA, R.C., FERRAZ, S.O., PIMENTA, D.S. and SOUSA, O.V., 2010. Caracterização morfoanatômica das folhas de Eremanthus erythropappus (DC.) MacLeisch, Asteraceae. Revista Brasileira de Farmacognosia, vol. 20, no. 6, pp. 818-824. http://dx.doi.org/10.1590/S0102-695X2011005000003.
http://dx.doi.org/10.1590/S0102-695X2011...
). The species is typically found in highland areas of the Mantiqueira Range, in the southern region of Minas Gerais (MG), forming homogeneous masses in soils characterized by low fertility and the presence of rocky outcrops (Scolforo et al., 2012SCOLFORO, J.R.S., OLIVEIRA, A.D. and DAVIDE, A.C. 2012. Caracterização da candeia. In: J. R. S. SCOLFORO, B. F. P. LOEUILLE, eds. O manejo sustentável da candeia: o caminhar de uma nova experiência em Minas Gerais. Lavras: Ed. UFLA, pp. 19-27.). Plants that have a high degree of specificity regarding reproduction, auto-ecology, morphology, and life cycles occupy these environments, needing differentiated conservation strategies (Scarano, 2007SCARANO, F.R., 2007. Rock outcrop vegetation in Brazil: a brief overview. Revista Brasileira de Botanica. Brazilian Journal of Botany, vol. 30, no. 4, pp. 561-568. http://dx.doi.org/10.1590/S0100-84042007000400002.
http://dx.doi.org/10.1590/S0100-84042007...
).
Currently, legal limitations for ‘candeia’ management have technical implications, including matters for its cultivation and management. The legislation foresees that natural stands that can be exploited should hold at least 70% of these tree species and that this predominance is reestablished through the conduction of natural regeneration in the exploited area (Belo Horizonte, 2013BELO HORIZONTE, 2013 [viewed 02 July 2020]. Resolução conjunta SEMAD/IEF nº 1905, de 12 de agosto de 2013. Dispõe sobre os processos de autorização para intervenção ambiental no âmbito do Estado de Minas Gerais e dá outras providências [online]. Publicação – Diário do Executivo – “Minas Gerais” 13/08/2013, Belo Horizonte. Available from: http://www.meioambiente.mg.gov.br/servicos-semad/1675
http://www.meioambiente.mg.gov.br/servic...
). Although seminal propagation is considered to be the main strategy adopted in the current ‘candeia’ management plans (Pérez et al., 2004PÉREZ, J.F.M., SCOLFORO, J.R.S., OLIVEIRA, A.D., MELLO, J.M., BORGES, L.R.F. and CAMOLESI, J.F., 2004 [viewed 11 November 2017]. Sistema de manejo para a candeia – Eremanthus erythropappus (DC.) MacLeish – a opção do sistema de corte seletivo. Cerne [online], vol. 10, no. 2, pp. 257-273. Available from: http://www.redalyc.org/pdf/744/74410209.pdf
http://www.redalyc.org/pdf/744/74410209....
), alternative forms of plant perpetuation may occur through periodic restriction of the environment (Appezzato-da-Glória et al., 2008APPEZZATO-DA-GLÓRIA, B., CURY, G., KASUE MISAKI SOARES, M., ROCHA, R. and HISSAE HAYASHI, A., 2008. Underground systems of Asteraceae species from Brazilian Cerrado. The Journal of the Torrey Botanical Society, vol. 135, no. 1, pp. 103-113. http://dx.doi.org/10.3159/07-RA-043.1.
http://dx.doi.org/10.3159/07-RA-043.1...
; Silva et al., 2015SILVA, T.M., VILHALVA, D.A.A., MORAES, M.G. and FIGUEIREDO-RIBEIRO, R.C.L., 2015. Anatomy and fructan distribution in vegetative organs of Dimerostemma vestitum (Asteraceae) from the campos rupestres. Anais da Academia Brasileira de Ciências, vol. 87, no. 2, pp. 797-812. http://dx.doi.org/10.1590/0001-3765201520140214. PMid:26062118.
http://dx.doi.org/10.1590/0001-376520152...
). These authors have been conducted morpho-anatomy studies in root plants of Asteraceae family with the aim to identify structures and sources able to promote the regenerative shoots in plants, after natural or anthropogenic disturbances.
Vegetative propagation methods can be used for the rescue and multiplication of genotypes of interest or for the genetic conservation of the species. But, establishing efficient rescue protocols for tropical forest species is a challenge to be overcome, given the plasticity with which these species respond to conventional treatments with indole-3-butyric acid (IBA) (Santos et al., 2011SANTOS, J.P., DAVIDE, A.C., TEIXEIRA, L.A.F., MELO, A.J.S. and MELO, L.A., 2011. Enraizamento de estacas lenhosas de espécies florestais. Cerne, vol. 17, no. 3, pp. 293-301. http://dx.doi.org/10.1590/S0104-77602011000300002.
http://dx.doi.org/10.1590/S0104-77602011...
; Barbosa Filho et al., 2018BARBOSA FILHO, J., DI CARVALHO, M.A., OLIVEIRA, L.S., KONZEN, E.R. and BRONDANI, G.E., 2018. Mini-cutting technique for Khaya anthotheca: selection of suitable IBA concentration and nutrient solution for its vegetative propagation. Journal of Forestry Research, vol. 2, no. 1, pp. 73-84. http://dx.doi.org/10.1007/s11676-017-0429-0.
http://dx.doi.org/10.1007/s11676-017-042...
; Barroso et al., 2018BARROSO, D.G., OLIVEIRA, T.P.F., SIQUEIRA, D.P., LAMÔNICA, K.R. and CARVALHO, G.C.M.W., 2018. Mini-stumps productivity and rooting of Khaya ivorensis A. Chev mini-cuttings treated with IBA. Cerne, vol. 24, no. 2, pp. 114-120. http://dx.doi.org/10.1590/01047760201824022536.
http://dx.doi.org/10.1590/01047760201824...
; Araújo et al., 2019ARAÚJO, E.F., GIBSON, E.L., SANTOS, A.R., GONÇALVES, E.O., WENDLING, I., ALEXANDRE, R.S. and POLA, L.A., 2019. Mini-cutting technique for vegetative propagation of Paratecoma peroba. Cerne, vol. 25, no. 3, pp. 314-325. http://dx.doi.org/10.1590/01047760201925032647.
http://dx.doi.org/10.1590/01047760201925...
). However, once established, vegetative propagation can overcome problems such as the restricted period of the year in which perennial species produce seeds, variation in the physiological vigor of seeds, difficulties in seed collection, processing and storage, and genetic variations between progenies.
Based on the foregoing, the aim of this work was testing the efficiency of an induction method for adventitious Eremanthus erythropappus budding in a natural stand and vegetative propagating selected matrices in the field. In addition, anatomical analyses were performed to illustrate the cellular roots differentiation and the type of sources reserves presents.
2. Material and Methods
2.1. Study area
The experiment was conducted in a native stand of Eremanthus erythropappus, located in the municipality of Conceição das Pedras/MG, Brazil at the geographic coordinates 45°22’16.26”W and 22°7’35.05”, and 1,100 m of altitude. The municipality is inserted in the ‘Serra da Mantiqueira’, southern region of the MG State and has mountainous relief with high slope. The climate, according to Köppen-Geiger, is classified as altitude tropical - Cwb, with dry winters and temperate summers (Alvares et al., 2013ALVARES, C.A., STAPE, J.L., SENTELHAS, P.C., GONÇALVES, J.L.M. and SPAROVEK, G., 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift (Berlin), vol. 22, no. 6, pp. 711-728. http://dx.doi.org/10.1127/0941-2948/2013/0507.
http://dx.doi.org/10.1127/0941-2948/2013...
).
2.2. In situ rescue
In August 2017, 26 adult trees with approximately thirty years-old, were selected (E.e1 at E.e26) from native forest explored by Sustainable Management Plan, previously approved by Forest State Institute – IEF/MG, taking into account their vigor, size, and phytosanitary status. Rescue technique consisted in felling the tree (coppice) with a chainsaw at a length of 0.30-0.40 m, followed by root exposure and scarification, as proposed by Melo et al. (2012)MELO, L.A., DAVIDE, A.C. and TEIXEIRA, L.A.F., 2012. Metodologia para resgate de matrizes e enraizamento de estacas de Eremanthus erythropappus. Cerne, vol. 18, no. 4, pp. 631-638. http://dx.doi.org/10.1590/S0104-77602012000400013.
http://dx.doi.org/10.1590/S0104-77602012...
. Shoot induction was evaluated for the origin (i.e., stump or root), percentage of matrices sprouted, number of shoots per matrice, length (cm), diameter (mm) and length/diameter ratio at intervals of 30, 60, 90, 120, 157, and 189 days after induction (DAI), for each induced matrice. Control of the weed grass close to the strain was carried out, where appropriate, at each evaluation. The experimental design was completely randomized with subdivided plots in time, where each matrice was considered a replication within each plot.
During the experiment, climatological data were collected from the A-531 Automatic Station, located at the geographic coordinates 45°22’22.97”W and 22°18’52.44”S, and near 21 km of the experimental area. The highest precipitation register occurred between December 2017 and January 2018, which 258.4 mm of rainfall accumulated. In the same period, the highest average temperature was 19.4°C.
2.3. Vegetative propagation by cutting technique
At 204 DAI, shoots of 19 matrices were collected from a native forest with approximately thirty years-old. Shoots were then placed in black plastic bags, moistened and sealed with string, and were transported to the Forest Nursery of the Federal University of Lavras. The time of displacement between the field and the nursery was approximately 3 h. After cleaning the shoots in solution of hypochlorite (concentration 0.05% for 10 min), apical cuttings between 7.5 and 12.0 cm of length, were made with a 50% reduction in leaf area. Thus, 4 g L-1 IBA in liquid solution was applied in the cutting base for 10 s. The control group did not receive IBA. Tubes of 55 cm3 were used as containers, filled with substrate containing vermiculite of medium granulometry, carbonized rice husk, and sand in the ratio 4.5:4.5:1.0 (v-1 v-1 v-1), enriched of 4 kg m-3 of Osmocote® fertilizer, in the NPK 19:06:10. The cuttings were conditioned in a greenhouse with scheduled temperatures between 25 and 35°C, and relative humidity of more than 80%. At 57 days, cutting survival at greenhouse exit (GE) was evaluated. Then, the cuttings were transferred to the shade house, with 50% shade, where they remained for 27 days. The adventitious rooting at shade exit (SHE) was then evaluated.
The experimental design was completely randomized, and each matrice was considered a sampling unit, and the cuttings its replicates. The factors of variation were the levels of auxin applied on the cuttings (i.e., presence and absence). Altogether, 362 cuttings were made, 181 were submitted to plant growth regulator treatment, and 181 to the control group.
2.4. Statistical analysis
The data was submitted to Shapiro-Wilk’s normality test (p < 0.05), and homogeneity of variance by the Bartlett’s test (p < 0.05), and later were transformed by Box-Cox, by equation 1/[(x +0.5)^2], where x represents the sampled variable. Analysis of variance (ANOVA, p < 0.05), regression analysis (logistic model) to in situ rescue experiment, and Duncan’s test (p < 0.05) to vegetative propagation experiment were performed with SOC (EMBRAPA, 1990EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA – EMBRAPA, 1990. Programa SOC – software científico: versão 2.1. Campinas: Embrapa Informática Agropecuária.) and R (R Development Core Team, 2012R DEVELOPMENT CORE TEAM, 2012. An introduction to R. Notes on R: a programming environment for data analysis and graphics: version 2.15.1. Vienna: R Foundation for Statistical Computing.).
2.5. Anatomical analyses
Root segments were collected from a planted forest with 13-year-old, localized in the Federal University of Lavras – UFLA, after applied the same rescue technique describe before. Then, two analyses of root were performed. To illustrate the cellular roots differentiation, the samples were fixed at FAA70 (Johansen, 1940JOHANSEN, D.A., 1940. Plant microtechnique. New York: McGraw-Hill Book.) for 150 days, and treated with a vacuum pump at 160 mm Hg pressure. They were then dehydrated in an ethanol series with concentration 80, 90 and 100% with two hours of rest and the material overnight in Leica Historesin® infiltration resin in the resin: alcohol (1:1) mixture. Then, the material was transferred to pure resin, for a further 30 days, and then blotted into historesin. Cross sections were performed on a table microtome of 6 μm thickness. The sections were dehydrated in ethyl alcohol, stained with toluidine blue pH 4.7 solution. Acrilex® assembly medium, mixed in 50% glycerin (Paiva et al., 2006PAIVA, J.G.A., FANK-DE-CARVALHO, S.M., MAGALHÃES, M.P. and GRACIANO-RIBEIRO, D., 2006. Verniz vitral incolor 500®: uma alternativa de meio de montagem economicamente viável. Acta Botanica Brasílica, vol. 20, no. 2, pp. 257-264. http://dx.doi.org/10.1590/S0102-33062006000200002.
http://dx.doi.org/10.1590/S0102-33062006...
), was used to prepare the permanent slides.
To identify the type of source reserve present in the root cellular, fresh root samples collected from the planted forest were taken to the Wood Anatomy Laboratory of UFLA and placed in 70% ethyl alcohol for crystal visualization. After harvest, the roots were cut crosswise into a 7 μm thick table microtome, and the slides visualized under polarized light. Histochemical tests with 15% ethyl thimol and concentrated sulfuric acid (Johansen, 1940JOHANSEN, D.A., 1940. Plant microtechnique. New York: McGraw-Hill Book.) were conducted to confirm the nature of the crystal. Considering the vegetative propagation experiment, one stake in the Shade House was sample and the material was fixed in FAA70 (Johansen, 1940JOHANSEN, D.A., 1940. Plant microtechnique. New York: McGraw-Hill Book.) for three days and then transferred to 70% alcohol for twelve hours. Thereafter, dehydration in 80, 90 and 100% ethyl series were made at 90-minute intervals. After 24 hours in resin: alcohol (1:1), the material rested for a further 30 days on pure resin, to then be stored in the historesin. Cross sections were made in a 10 μm thick table microtome, and sections were stained with toluidine blue - pH 4.7. Permanent slides were made using Acrilex® stained glass varnish (Paiva et al., 2006PAIVA, J.G.A., FANK-DE-CARVALHO, S.M., MAGALHÃES, M.P. and GRACIANO-RIBEIRO, D., 2006. Verniz vitral incolor 500®: uma alternativa de meio de montagem economicamente viável. Acta Botanica Brasílica, vol. 20, no. 2, pp. 257-264. http://dx.doi.org/10.1590/S0102-33062006000200002.
http://dx.doi.org/10.1590/S0102-33062006...
).
3. Results
3.1. In situ rescue
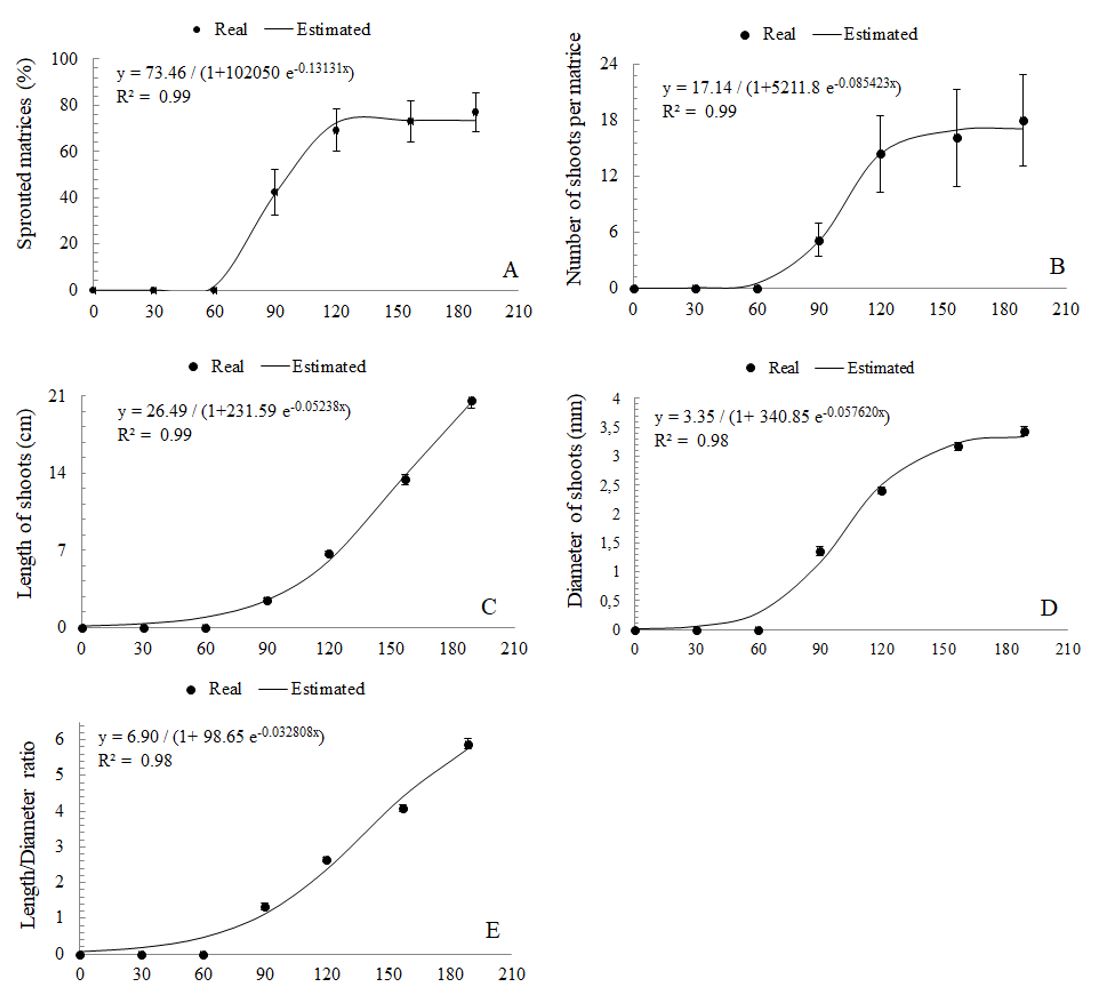
At 189 days after installation (DAI) of the experiment, four matrices did not show shoot emission and two were lost due to the fall of nearby trees on the exposed root area. The applied technique promoted the sprouting of 76.92% of the matrices (Figure 1A), which at the end of the follow-up period totaled 396 shoots, exclusively of root origin, without shoots issued from stump (coppice). The matrices Ee6, Ee8, Ee16, Ee19, and Ee20 accounted for 68.93% of the total number of shoots counted, which influenced variation coefficients for the percentage of sprouted matrices and number of shoots per matrice (Table 1). Furthermore, the emission of new shoots by E. erythropappus roots, at each evaluated interval, resulted in mean length and diameter variation (Table 2).
Eremanthus erythropappus matrices behavior for (A) percentage of sprouted matrices, (B) number of shoots, (C) length of shoots, (D) diameter, and (E) length/diameter, as a function of the time (0, 30, 60, 90, 120, 157, and 189 days after induction).
Summary of the analysis of variance for the sprouted matrices of Eremanthus erythropappus and for the number of shoots per matrices counted over time (0, 30, 60, 90, 120, 157, and 189 days after induction) and shoot origin (i.e., stump and root).
Summary of the analysis of variance for the length, diameter and length/diameter ratio of Eremanthus erythropappus shoots measured over time (0, 30, 60, 90, 120, 157, and 189 days after induction) and shoot origin (i.e., stump and root).
Shoot emission occurred from 90 DAI, and regression analyses (Figure 1AE) revealed a trend of increase in the percentage of sprouted matrices, number of shoots per matrice, length, diameter, and length/diameter during the evaluated period. These parameters showed significant interaction with time and matrices (p < 0.01). Among the evaluation intervals, new shoots coexisted with those that were more developed.
3.2. Vegetative propagation by cutting technique
In propagation by cutting, only 12.26% survived GE, without significant difference between matrices (Table 3). Overall, the absence of IBA resulted in higher survival percentages, when compared to the 4 g L-1 IBA concentration (Table 3), and there were no significant differences with the control treatment. From the surviving cuttings, only 7.9% formed roots in SHE, considering the treatment with IBA and the control (Table 3). Variance analyses revealed no interaction between the propagated matrices and IBA concentration applied. In spite of the low percentage of survival of the cuttings, 7 of the 19 matrices were rescued (i.e., Ee3, Ee6, Ee8, Ee15, Ee18, Ee20 and Ee26).
Mean survival and rooting percentages of Eremanthus erythropappus cuttings at greenhouse exit (GE), and shade house exit (SHE), according the matrices and IBA application.
3.3. Anatomical analyses
The anatomy performed on the E. erythropappus root revealed the ability of sprouting from redistribution of phloem cells (Figure 2A). Secondary roots have uniseriate parenchyma rays that divide and organize the xylem vessel elements. Organized radially in line, vessel elements are numerous and dense (Figure 2B). Secondary phloems are characterized by bundles of fibers interspersed with parenchyma and crimped tube elements that accompany the direction of xylem rays (Figure 2C). Bud formation occurs from cell redifferentiation in the phloem parenchyma region, marked by the alteration in the cellular organization pattern and evidenced by the thickening of the fiber bundles in this area (Figure 2D).
Anatomy of Eremanthus erythropappus. (A) Anatomy of the root, with differentiation in aerial part (4x); (B) Secondary growth root (4x); (C) Secondary phloem region without gem differentiation (10x); (D) Phloem region with alteration of periclinal and anticline divisions of parenchyma cells (10x); (E) Radial segment (dashed line: cross-section); (F) Anatomical cut of the root seen under polarized light (10x). In particular, the distribution of the crystals along the walls of the vessel elements of the secondary xylem; (G) Histochemical test result with thimol 15% ethylic solution; (H) Stake used for anatomical analyses and place of embedding; (I) Distribution of vascular bundles forming a cylindrical ring around the medulla (M) (4x); (J) Vascular cylinder with interfascicular parenchyma (10x); (K) Adventitious root establishing vascular attachment with stem change (20x). Legend: P: periderm; Ph: secondary phloem; X: secondary xylem; F: fibers; Ca: cambium; V: vessels; G.r.: gemiferous region; S: sprout; R: root; E: epiderm; Co: cortex; Vc: vascular cylinder; Ic: interfascicular cambium; Ar: adventitious root; M: medula center.
Under polarized light, ‘candeia’ roots formed crystals on the walls of the vessel elements of the secondary xylem (Figure 2F). Histochemical tests were positive for inulin, indicating that the species stores type fructan inulin in the root cells (Figure 2G). The vascular bundles form a cylindrical ring around the stem, with interfascicular parenchyma in the vascular cylinder region (Figures 2HJ). The vascular connection between the adventitious root and the cambium is shown in Figures 2JL.
4. Discussion
4.1. In situ rescue
The applied rescue technique revealed that the E. erythropappus species, even under natural conditions, has great potential for the emission of shoots from the root. This characteristic is particularly important for the genetic conservation of the managed natural stands, since asexual reproduction mechanisms can promote the spontaneous vegetative propagation of the regenerants, guaranteeing the survival of a species in a certain place (Rodrigues et al., 2004RODRIGUES, R.R., TORRES, R.B., MATTHES, L.A.F. and PENHA, A.S., 2004. Tree species sprouting from root buds in a Semideciduous Forests affected by fires. Brazilian Archives of Biology and Technology, vol. 47, no. 1, pp. 127-133. http://dx.doi.org/10.1590/S1516-89132004000100017.
http://dx.doi.org/10.1590/S1516-89132004...
; Vacek et al., 2012VACEK, S., HEJCMANOVÁ, P. and HEJCMAN, M., 2012. Vegetative reproduction of Picea abies by artificial layering at the ecotone of the alpine timberline in the Giant (Krkonoše) Mountains, Czech Republic. Forest Ecology and Management, vol. 263, pp. 199-207. http://dx.doi.org/10.1016/j.foreco.2011.09.037.
http://dx.doi.org/10.1016/j.foreco.2011....
).
The emission shoots by adult trees under coppicing, have been associated to imbalance endogenous level of auxin and cytokinin able to promote the genes expression, enzymes and proteins, which aid auxin translocation between plant parts and results at differentiation of tissues (Xiong and Zhu, 2003XIONG, L. and ZHU, J.K., 2003. Regulation of abscisic acid biosynthesis. Plant Physiology, vol. 133, no. 1, pp. 29-36. http://dx.doi.org/10.1104/pp.103.025395. PMid:12970472.
http://dx.doi.org/10.1104/pp.103.025395...
). The origin of shoots, exclusively radicular, could occurred in reason of injury caused by roots scarification, which would act at physiological and cellular levels, demanding from the plant large amounts of solutes to the damage region. In other hand, the thickness of the rythidoma, typical of the E. erythropappus species, may have acted as a physical barrier to emission and development of buds.
The data recorded in this study, with matrices at thirty years-old, differ from those found by Melo et al. (2012)MELO, L.A., DAVIDE, A.C. and TEIXEIRA, L.A.F., 2012. Metodologia para resgate de matrizes e enraizamento de estacas de Eremanthus erythropappus. Cerne, vol. 18, no. 4, pp. 631-638. http://dx.doi.org/10.1590/S0104-77602012000400013.
http://dx.doi.org/10.1590/S0104-77602012...
. Using the same technique and species, they recorded 1,764 shoots in 24 matrices planted at four years of age. This difference underscores the strong influence of the genotype, the juvenile degree of the matrices, natural environmental conditions, and the nutritional reserves of the stump and root (Wendling et al., 2014aWENDLING, I., TRUEMAN, S.J. and XAVIER, A., 2014a. Maturation and related aspects in clonal forestry – Part I: concepts, regulation and consequences of phase change. New Forests, vol. 45, no. 4, pp. 449-471. http://dx.doi.org/10.1007/s11056-014-9421-0.
http://dx.doi.org/10.1007/s11056-014-942...
). It is possible that the physiological vigor and ontogenetic age of some plants have been a limiting factor, given that matrices induced by sprouting developed under natural conditions without any fertilization or silvicultural treatment that favors growth (Wendling et al., 2014bWENDLING, I., TRUEMAN, S.J. and XAVIER, A., 2014b. Maturation and related aspects in clonal forestry – Part II: reinvigoration, rejuvenation and juvenility maintenance. New Forests, vol. 45, no. 4, pp. 473-486. http://dx.doi.org/10.1007/s11056-014-9415-y.
http://dx.doi.org/10.1007/s11056-014-941...
). This fact explains why differentiated emission of shoots occurred in the Ee6, Ee8, Ee16, Ee19 and Ee20 matrices, which together accounted for 68.93% of shoots, at 189 DAI. Borges-Júnior et al. (2004)BORGES JÚNIOR, N., MARTINS-CORDER, M.P., SOBROSA, R.C. and SANTOS, E.M., 2004. Rebrota de cepas de árvores adultas de acácia-negra (Acacia mearnsii De Wild). Revista Árvore, vol. 28, no. 4, pp. 611-615. http://dx.doi.org/10.1590/S0100-67622004000400015.
http://dx.doi.org/10.1590/S0100-67622004...
, considering shoots from stump, showed seasons influence on the percentage of sprouted matrices and number of sprouts per stump in a five-year-old Acacia mearnsii De Wild cultivar, with better results in the spring, registering an average of 24 and 35 shoots per tree from stumps with length 0.30 and 0.45 m, respectively, and at 90 days. Regarding E. erythropappus, accounting only the individuals at 189 DAI, the average was 19.8 shoots per matrice, emitted from 90 DAI, when the recorded rainfall went from 135 to 258.4 mm and the average temperature was 17.9-19.4°C. New shoots and the increase growth in length and diameter over time are important guides for future clonal programs with ‘candeia’, and to establish the use of different silvicultural methods for management, like coppice method as well as proposed by Melo et al. (2012)MELO, L.A., DAVIDE, A.C. and TEIXEIRA, L.A.F., 2012. Metodologia para resgate de matrizes e enraizamento de estacas de Eremanthus erythropappus. Cerne, vol. 18, no. 4, pp. 631-638. http://dx.doi.org/10.1590/S0104-77602012000400013.
http://dx.doi.org/10.1590/S0104-77602012...
.
The average shoot length and diameter were 21.09 cm and 3.52 mm, at 189 DAI, respectively (Figure 1CE). These values are similar to those reported by Stuepp et al. (2016)STUEPP, C.A., BITENCOURT, J., WENDLING, I., KOEHLER, H.S. and RIBAS, K.C.Z., 2016. Indução de brotações epicórmicas por meio de anelamento e decepa em erva-mate. Ciência Florestal, vol. 26, no. 3, pp. 1009-1022. http://dx.doi.org/10.5902/1980509824230.
http://dx.doi.org/10.5902/1980509824230...
for Ilex paraguariensis St. Hill, with mean values of 19.95 cm in length, and 3.7 mm in diameter, registered in matrices of over 17 years, to 180 DAI. Souza et al. (2016)SOUZA, F.C., REIS, G.G., REIS, M.G.F., LEITE, H.G., FARIA, R.S., CALIMAN, J.P., BARBOSA, R.A. and OLIVEIRA, C.H.R., 2016. Growth of intact plants and coppice in short rotation eucalypt plantations. New Forests, vol. 47, no. 2, pp. 195-208. http://dx.doi.org/10.1007/s11056-015-9509-1.
http://dx.doi.org/10.1007/s11056-015-950...
reported that the initial growth of sprouts in Eucalyptus contributes for carbohydrate reserves of the remaining parts of the tree. However, Klimeš and Klimešová (1999)KLIMEŠ, L. and KLIMEŠOVÁ, J., 1999. Root sprouting in Rumex acetosella L. under different nutrient levels. Plant Ecology, vol. 141, no. 1-2, pp. 33-39. http://dx.doi.org/10.1023/A:1009877923773.
http://dx.doi.org/10.1023/A:100987792377...
found no evidence of nutritional support among root-linked Rumex acetosella (L.) shoots subjected to different nutritional conditions.
During the evaluation period new shoots were emitted and coexist with older shoots, still connected with the matrices by roots. This behavior allows the use of different strategies of vegetative propagation, because the explant pattern was very broad. Thus, others alternative propagation techniques can be tested for ‘candeia’. Considering the legal obligation to conduct natural regeneration of the ‘candeia’ after the exploitation of the managed stand (Belo Horizonte, 2013BELO HORIZONTE, 2013 [viewed 02 July 2020]. Resolução conjunta SEMAD/IEF nº 1905, de 12 de agosto de 2013. Dispõe sobre os processos de autorização para intervenção ambiental no âmbito do Estado de Minas Gerais e dá outras providências [online]. Publicação – Diário do Executivo – “Minas Gerais” 13/08/2013, Belo Horizonte. Available from: http://www.meioambiente.mg.gov.br/servicos-semad/1675
http://www.meioambiente.mg.gov.br/servic...
), the restoration of the forest managed by root shoots is a viable alternative to reestablish the predominance of this species, mainly in the first year after cutting, given that the first shoots were counted at 90 DAI.
4.2. Vegetative propagation by cutting technique
The high mortality of ‘candeia’ stakes can be attributed to the oxidation of phenolic compounds in the plant (Lattuada et al., 2011LATTUADA, D.S., SPIER, M. and SOUZA, P.V.D., 2011. Pré-tratamento com água e doses de ácido indolbutírico para estaquia herbácea de pitangueiras. Ciência Rural, vol. 41, no. 12, pp. 2073-2079. http://dx.doi.org/10.1590/S0103-84782011001200006.
http://dx.doi.org/10.1590/S0103-84782011...
), verified in this work by the darkening of the apex towards the base of the stake. Leaves secrete phenolic substances responsible for the whitish coloration in this region (Dutra et al., 2010DUTRA, R.C., FERRAZ, S.O., PIMENTA, D.S. and SOUSA, O.V., 2010. Caracterização morfoanatômica das folhas de Eremanthus erythropappus (DC.) MacLeisch, Asteraceae. Revista Brasileira de Farmacognosia, vol. 20, no. 6, pp. 818-824. http://dx.doi.org/10.1590/S0102-695X2011005000003.
http://dx.doi.org/10.1590/S0102-695X2011...
), because they have a large amount of tectonic trichomes in the abaxial part, which hinders propagation via cuttings.
Melo et al. (2012)MELO, L.A., DAVIDE, A.C. and TEIXEIRA, L.A.F., 2012. Metodologia para resgate de matrizes e enraizamento de estacas de Eremanthus erythropappus. Cerne, vol. 18, no. 4, pp. 631-638. http://dx.doi.org/10.1590/S0104-77602012000400013.
http://dx.doi.org/10.1590/S0104-77602012...
, working with the same species and method of propagation, recorded 100% survival in GE, and 35.15% rooting in SHE in four-year-old matrices. According to Ikeuchi et al. (2016)IKEUCHI, M., OGAWA, Y.I., IWASE, A. and SUGIMOTO, K., 2016. Plant regeneration: cellular origins and molecular mechanisms. Development, vol. 143, no. 9, pp. 1442-1451. http://dx.doi.org/10.1242/dev.134668. PMid:27143753.
http://dx.doi.org/10.1242/dev.134668...
, the parent plant’s youth is a characteristic directly related to the potential of cell regeneration and adventitious root formation. Thus, with the advancement of maturity the tissues lose their meristematic capacity and the formation of adventitious roots is difficult. Santos et al. (2011)SANTOS, J.P., DAVIDE, A.C., TEIXEIRA, L.A.F., MELO, A.J.S. and MELO, L.A., 2011. Enraizamento de estacas lenhosas de espécies florestais. Cerne, vol. 17, no. 3, pp. 293-301. http://dx.doi.org/10.1590/S0104-77602011000300002.
http://dx.doi.org/10.1590/S0104-77602011...
tested the propagation by cutting in twenty forest species, obtaining rooting percentages that varied from 0.5% to 91.5% according to the species and the classes of diameter of the cuttings. In the same study, as a function of the concentration of IBA applied, the rooting percentage ranged from 0.5% to 88% among the species. This demonstrates that other factors besides the exogenous application of auxin are involved in the formation of adventitious roots by plants, like the ontogenetic age (Wendling et al., 2014aWENDLING, I., TRUEMAN, S.J. and XAVIER, A., 2014a. Maturation and related aspects in clonal forestry – Part I: concepts, regulation and consequences of phase change. New Forests, vol. 45, no. 4, pp. 449-471. http://dx.doi.org/10.1007/s11056-014-9421-0.
http://dx.doi.org/10.1007/s11056-014-942...
, bWENDLING, I., TRUEMAN, S.J. and XAVIER, A., 2014b. Maturation and related aspects in clonal forestry – Part II: reinvigoration, rejuvenation and juvenility maintenance. New Forests, vol. 45, no. 4, pp. 473-486. http://dx.doi.org/10.1007/s11056-014-9415-y.
http://dx.doi.org/10.1007/s11056-014-941...
; Silva et al., 2020SILVA, E.R., SIMÕES, I.M., BAPTISTA, J.O., SCHMILDT, E.R., LOPES, J.C., GONÇALVES, E.O., CALDEIRA, M.V.W. and ALEXANDRE, R.S., 2020. In vitro of Melanoxylon brauna Schott. morphogenesis: responsiveness of explants to permanent and temporary immersion growth regulators. Cerne, vol. 26, no. 1, pp. 26-36. http://dx.doi.org/10.1590/01047760202026012709.
http://dx.doi.org/10.1590/01047760202026...
).
It is possible that the time elapsed between shoot collection in the field and the entrance of the cuttings in the greenhouse could have caused a biological stress at the cellular level, resulting in the loss of solutes and metabolites that contributed to the death of the cuttings. Rezende (2007)REZENDE, A.A., 2007. Enraizamento de estacas de candeia Eremanthus erytropappus (DC.) MacLeish. Lavras: Universidade Federal de Lavras. 75 p. Dissertação de Mestrado em Florestas de Produção. verified the presence of anatomical barriers in stem cuttings collected from trees at different stages of development. The author observed a difference in the sclerenchyma deposition around the stem cells, so that this substance was thicker and more continuous in adult trees compared to new trees, and minimal in mini-cuttings.
In spite of low cutting survival, seven of the 19 propagated matrices rooted and were placed in a clonal mini-garden (Ee3, Ee6, Ee8, Ee15, Ee18, Ee20 and Ee26) (Table 3). Thus, cuttings may be an option for the formation of a clonal mini-garden, which can be achieved by means of serial mini-sequencing (Wendling et al., 2014aWENDLING, I., TRUEMAN, S.J. and XAVIER, A., 2014a. Maturation and related aspects in clonal forestry – Part I: concepts, regulation and consequences of phase change. New Forests, vol. 45, no. 4, pp. 449-471. http://dx.doi.org/10.1007/s11056-014-9421-0.
http://dx.doi.org/10.1007/s11056-014-942...
, bWENDLING, I., TRUEMAN, S.J. and XAVIER, A., 2014b. Maturation and related aspects in clonal forestry – Part II: reinvigoration, rejuvenation and juvenility maintenance. New Forests, vol. 45, no. 4, pp. 473-486. http://dx.doi.org/10.1007/s11056-014-9415-y.
http://dx.doi.org/10.1007/s11056-014-941...
). This hypothesis needs to be tested.
4.3. Anatomical analyses
Anatomical studies performed on ‘candeia’ roots confirmed the great capacity of root blossom emission in species belonging to the family Asteraceae (Appezzato-da-Glória et al., 2008APPEZZATO-DA-GLÓRIA, B., CURY, G., KASUE MISAKI SOARES, M., ROCHA, R. and HISSAE HAYASHI, A., 2008. Underground systems of Asteraceae species from Brazilian Cerrado. The Journal of the Torrey Botanical Society, vol. 135, no. 1, pp. 103-113. http://dx.doi.org/10.3159/07-RA-043.1.
http://dx.doi.org/10.3159/07-RA-043.1...
). Several authors have attributed this regrowth ability to the presence of fructan-type inulin crystals in the root cells (Assega and Carvalho, 2004ASSEGA, A.F. and CARVALHO, M.A.M., 2004. Fructan metabolising enzymes in rhizophores of Vernonia herbacea upon excision of aerial organs. Plant Physiology and Biochemistry, vol. 42, no. 4, pp. 313-319. http://dx.doi.org/10.1016/j.plaphy.2004.02.005. PMid:15120116.
http://dx.doi.org/10.1016/j.plaphy.2004....
; Assega et al., 2011ASSEGA, A.F., NASCIMENTO, J.R.O. and CARVALHO, M.A.M., 2011. Increased expression. of fructan 1-exohydrolase in rhizophores of Vernonia herbacea during sprouting and exposure to low temperature. Journal of Plant Physiology, vol. 168, no. 6, pp. 558-565. http://dx.doi.org/10.1016/j.jplph.2010.09.002. PMid:20950891.
http://dx.doi.org/10.1016/j.jplph.2010.0...
; Santos et al., 2016SANTOS, V.S., SOUZA, V.P., VILHALVA, D.A.A., FERREIRA, F.P.S., PAULA, J.R. and REZENDE, M.H., 2016. Morpho-anatomy and ontogeny of the underground system of Chrysolaena simplex (Less.) Dematt. (Asteraceae). Anais da Academia Brasileira de Ciências, vol. 88, no. 1, pp. 269-280. http://dx.doi.org/10.1590/0001-3765201620140676. PMid:26871494.
http://dx.doi.org/10.1590/0001-376520162...
; Silva et al., 2015SILVA, T.M., VILHALVA, D.A.A., MORAES, M.G. and FIGUEIREDO-RIBEIRO, R.C.L., 2015. Anatomy and fructan distribution in vegetative organs of Dimerostemma vestitum (Asteraceae) from the campos rupestres. Anais da Academia Brasileira de Ciências, vol. 87, no. 2, pp. 797-812. http://dx.doi.org/10.1590/0001-3765201520140214. PMid:26062118.
http://dx.doi.org/10.1590/0001-376520152...
). The occurrence of crystals on the walls of the vessel elements of the secondary xylem (Figure 2FG) is in accordance with what Tertuliano and Figueiredo‐Ribeiro (1993)TERTULIANO, M.F. and FIGUEIREDO‐RIBEIRO, R.C.L., 1993. Distribution of fructose polymers in herbaceous species of Asteraceae from the Cerrado. The New Phytologist, vol. 123, no. 4, pp. 741-749. http://dx.doi.org/10.1111/j.1469-8137.1993.tb03785.x.
http://dx.doi.org/10.1111/j.1469-8137.19...
described for other species of the Asteraceae family.
The fructan helps in shoot emission, yet it is still not clear how. Valluru and Van den Ende (2008)VALLURU, R. and VAN DEN ENDE, W., 2008. Plant fructans in stress environments: emerging concepts and future prospects. Journal of Experimental Botany, vol. 59, no. 11, pp. 2905-2916. http://dx.doi.org/10.1093/jxb/ern164. PMid:18603617.
http://dx.doi.org/10.1093/jxb/ern164...
stated that this substance is important to supply the plant’s energy demand between periods of availability and scarcity of resources. Morvan-Bertrand et al. (2001)MORVAN-BERTRAND, A., BOUCAUD, J., LE SAOS, J. and PRUD’HOMME, M.P., 2001. Roles of the fructans from leaf sheaths and from the elongating leaf bases in the regrowth following defoliation of Lolium perenne L. Planta, vol. 213, no. 1, pp. 109-120. reported a decline in the content of this carbohydrate in the leaf base of Lolium perene L. in the process of elongation, which was accompanied by a peak in the activity of the fructan enzyme: exohydroxylase in the growth zone, shortly after defoliation, suggesting the fructan as the source of carbon responsible for sustaining growth resumption. Santos et al. (2016)SANTOS, V.S., SOUZA, V.P., VILHALVA, D.A.A., FERREIRA, F.P.S., PAULA, J.R. and REZENDE, M.H., 2016. Morpho-anatomy and ontogeny of the underground system of Chrysolaena simplex (Less.) Dematt. (Asteraceae). Anais da Academia Brasileira de Ciências, vol. 88, no. 1, pp. 269-280. http://dx.doi.org/10.1590/0001-3765201620140676. PMid:26871494.
http://dx.doi.org/10.1590/0001-376520162...
reported seasonal variation in the amount and location of crystal accumulation in Chrysolaena simplex (Less.) Dematt. The crystals fill the cellular lumen and occur in larger amounts in the driest seasons of the year. On the other hand, the crystals were visualized near the cell wall and in smaller amounts in the rainy season, which was related to the regrowth of the vegetative structures in this period. Rigui et al. (2015)RIGUI, A.P., GASPAR, M., OLIVEIRA, V.F., PURGATTO, E. and CARVALHO, M.A.M., 2015. Endogenous hormone concentrations correlate with fructan metabolism throughout the phenological cycle in Chrysolaena obovata. Annals of Botany, vol. 115, no. 7, pp. 1163-1175. http://dx.doi.org/10.1093/aob/mcv053. PMid:25921788.
http://dx.doi.org/10.1093/aob/mcv053...
working with Chrysolaena obovata (Less.) Dematt, observed a great quantity of this carbohydrate and low concentration of abscisic acid (ABA) in the beginning of the spring, favoring shoot formation. By the end of this season, the authors reported a lower amount of fructan, accompanied by an increase in the indole-3-acetic acid (IAA) concentration, promoting the cellular stretching of shoots. More detailed anatomical and molecular studies can identify a possible seasonal variation of fructan in the radicular ‘candeia’ cells and also, to relate the amount of this carbohydrate with the production of hormones by the plant. Such advances would help in the development of more efficient protocols of rescue and vegetative propagation of this species, and may be tested in other native tropical species.
In this study, the rescue methodology applied was efficient to induce sprouting in adult trees from a natural stand, since 76.92% of the selected matrices were able to sprout at 189 DAI. In the E. erythropappus, sprouting occurs primarily at the root, therefore, an important tool for the genetic conservation of explored stands and an alternative for the reestablishment of the ‘candeia’ predominance in managed areas. The IBA concentration applied at the base of the stakes was not efficient for the formation of adventitious roots, not differing from the control treatment.
Considering that length, diameter and length/diameter ratio measured in shoots still attached to the matrice, the data recorded in this study can be considered pioneering work regarding the E. erythropappus species, as it is useful for comparing plant growth produced in nurseries and under natural conditions.
Acknowledgements
We thank the National Council for Scientific and Technological Development, Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq), Coordination for Improvement of Higher Education Personnel, Brazil (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES), and Foundation for Research of the State of Minas Gerais, Brazil (Fundação de Amparo a Pesquisa do Estado de Minas Gerais – FAPEMIG) for their financial support and scholarships for the students.
-
(With 2 figures)
References
- ALVARES, C.A., STAPE, J.L., SENTELHAS, P.C., GONÇALVES, J.L.M. and SPAROVEK, G., 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift (Berlin), vol. 22, no. 6, pp. 711-728. http://dx.doi.org/10.1127/0941-2948/2013/0507
» http://dx.doi.org/10.1127/0941-2948/2013/0507 - APPEZZATO-DA-GLÓRIA, B., CURY, G., KASUE MISAKI SOARES, M., ROCHA, R. and HISSAE HAYASHI, A., 2008. Underground systems of Asteraceae species from Brazilian Cerrado. The Journal of the Torrey Botanical Society, vol. 135, no. 1, pp. 103-113. http://dx.doi.org/10.3159/07-RA-043.1
» http://dx.doi.org/10.3159/07-RA-043.1 - ARAÚJO, E.F., GIBSON, E.L., SANTOS, A.R., GONÇALVES, E.O., WENDLING, I., ALEXANDRE, R.S. and POLA, L.A., 2019. Mini-cutting technique for vegetative propagation of Paratecoma peroba. Cerne, vol. 25, no. 3, pp. 314-325. http://dx.doi.org/10.1590/01047760201925032647
» http://dx.doi.org/10.1590/01047760201925032647 - ASSEGA, A.F. and CARVALHO, M.A.M., 2004. Fructan metabolising enzymes in rhizophores of Vernonia herbacea upon excision of aerial organs. Plant Physiology and Biochemistry, vol. 42, no. 4, pp. 313-319. http://dx.doi.org/10.1016/j.plaphy.2004.02.005 PMid:15120116.
» http://dx.doi.org/10.1016/j.plaphy.2004.02.005 - ASSEGA, A.F., NASCIMENTO, J.R.O. and CARVALHO, M.A.M., 2011. Increased expression. of fructan 1-exohydrolase in rhizophores of Vernonia herbacea during sprouting and exposure to low temperature. Journal of Plant Physiology, vol. 168, no. 6, pp. 558-565. http://dx.doi.org/10.1016/j.jplph.2010.09.002 PMid:20950891.
» http://dx.doi.org/10.1016/j.jplph.2010.09.002 - BARBOSA FILHO, J., DI CARVALHO, M.A., OLIVEIRA, L.S., KONZEN, E.R. and BRONDANI, G.E., 2018. Mini-cutting technique for Khaya anthotheca: selection of suitable IBA concentration and nutrient solution for its vegetative propagation. Journal of Forestry Research, vol. 2, no. 1, pp. 73-84. http://dx.doi.org/10.1007/s11676-017-0429-0
» http://dx.doi.org/10.1007/s11676-017-0429-0 - BARROSO, D.G., OLIVEIRA, T.P.F., SIQUEIRA, D.P., LAMÔNICA, K.R. and CARVALHO, G.C.M.W., 2018. Mini-stumps productivity and rooting of Khaya ivorensis A. Chev mini-cuttings treated with IBA. Cerne, vol. 24, no. 2, pp. 114-120. http://dx.doi.org/10.1590/01047760201824022536
» http://dx.doi.org/10.1590/01047760201824022536 - BELO HORIZONTE, 2013 [viewed 02 July 2020]. Resolução conjunta SEMAD/IEF nº 1905, de 12 de agosto de 2013. Dispõe sobre os processos de autorização para intervenção ambiental no âmbito do Estado de Minas Gerais e dá outras providências [online]. Publicação – Diário do Executivo – “Minas Gerais” 13/08/2013, Belo Horizonte. Available from: http://www.meioambiente.mg.gov.br/servicos-semad/1675
» http://www.meioambiente.mg.gov.br/servicos-semad/1675 - BORGES JÚNIOR, N., MARTINS-CORDER, M.P., SOBROSA, R.C. and SANTOS, E.M., 2004. Rebrota de cepas de árvores adultas de acácia-negra (Acacia mearnsii De Wild). Revista Árvore, vol. 28, no. 4, pp. 611-615. http://dx.doi.org/10.1590/S0100-67622004000400015
» http://dx.doi.org/10.1590/S0100-67622004000400015 - DUTRA, R.C., FERRAZ, S.O., PIMENTA, D.S. and SOUSA, O.V., 2010. Caracterização morfoanatômica das folhas de Eremanthus erythropappus (DC.) MacLeisch, Asteraceae. Revista Brasileira de Farmacognosia, vol. 20, no. 6, pp. 818-824. http://dx.doi.org/10.1590/S0102-695X2011005000003
» http://dx.doi.org/10.1590/S0102-695X2011005000003 - EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA – EMBRAPA, 1990. Programa SOC – software científico: versão 2.1 Campinas: Embrapa Informática Agropecuária.
- IKEUCHI, M., OGAWA, Y.I., IWASE, A. and SUGIMOTO, K., 2016. Plant regeneration: cellular origins and molecular mechanisms. Development, vol. 143, no. 9, pp. 1442-1451. http://dx.doi.org/10.1242/dev.134668 PMid:27143753.
» http://dx.doi.org/10.1242/dev.134668 - JOHANSEN, D.A., 1940. Plant microtechnique New York: McGraw-Hill Book.
- KLIMEŠ, L. and KLIMEŠOVÁ, J., 1999. Root sprouting in Rumex acetosella L. under different nutrient levels. Plant Ecology, vol. 141, no. 1-2, pp. 33-39. http://dx.doi.org/10.1023/A:1009877923773
» http://dx.doi.org/10.1023/A:1009877923773 - LATTUADA, D.S., SPIER, M. and SOUZA, P.V.D., 2011. Pré-tratamento com água e doses de ácido indolbutírico para estaquia herbácea de pitangueiras. Ciência Rural, vol. 41, no. 12, pp. 2073-2079. http://dx.doi.org/10.1590/S0103-84782011001200006
» http://dx.doi.org/10.1590/S0103-84782011001200006 - MELO, L.A., DAVIDE, A.C. and TEIXEIRA, L.A.F., 2012. Metodologia para resgate de matrizes e enraizamento de estacas de Eremanthus erythropappus. Cerne, vol. 18, no. 4, pp. 631-638. http://dx.doi.org/10.1590/S0104-77602012000400013
» http://dx.doi.org/10.1590/S0104-77602012000400013 - MORVAN-BERTRAND, A., BOUCAUD, J., LE SAOS, J. and PRUD’HOMME, M.P., 2001. Roles of the fructans from leaf sheaths and from the elongating leaf bases in the regrowth following defoliation of Lolium perenne L. Planta, vol. 213, no. 1, pp. 109-120.
- PAIVA, J.G.A., FANK-DE-CARVALHO, S.M., MAGALHÃES, M.P. and GRACIANO-RIBEIRO, D., 2006. Verniz vitral incolor 500®: uma alternativa de meio de montagem economicamente viável. Acta Botanica Brasílica, vol. 20, no. 2, pp. 257-264. http://dx.doi.org/10.1590/S0102-33062006000200002
» http://dx.doi.org/10.1590/S0102-33062006000200002 - PÉREZ, J.F.M., SCOLFORO, J.R.S., OLIVEIRA, A.D., MELLO, J.M., BORGES, L.R.F. and CAMOLESI, J.F., 2004 [viewed 11 November 2017]. Sistema de manejo para a candeia – Eremanthus erythropappus (DC.) MacLeish – a opção do sistema de corte seletivo. Cerne [online], vol. 10, no. 2, pp. 257-273. Available from: http://www.redalyc.org/pdf/744/74410209.pdf
» http://www.redalyc.org/pdf/744/74410209.pdf - R DEVELOPMENT CORE TEAM, 2012. An introduction to R. Notes on R: a programming environment for data analysis and graphics: version 2.15.1 Vienna: R Foundation for Statistical Computing.
- REZENDE, A.A., 2007. Enraizamento de estacas de candeia Eremanthus erytropappus (DC.) MacLeish Lavras: Universidade Federal de Lavras. 75 p. Dissertação de Mestrado em Florestas de Produção.
- RIGUI, A.P., GASPAR, M., OLIVEIRA, V.F., PURGATTO, E. and CARVALHO, M.A.M., 2015. Endogenous hormone concentrations correlate with fructan metabolism throughout the phenological cycle in Chrysolaena obovata. Annals of Botany, vol. 115, no. 7, pp. 1163-1175. http://dx.doi.org/10.1093/aob/mcv053 PMid:25921788.
» http://dx.doi.org/10.1093/aob/mcv053 - RODRIGUES, R.R., TORRES, R.B., MATTHES, L.A.F. and PENHA, A.S., 2004. Tree species sprouting from root buds in a Semideciduous Forests affected by fires. Brazilian Archives of Biology and Technology, vol. 47, no. 1, pp. 127-133. http://dx.doi.org/10.1590/S1516-89132004000100017
» http://dx.doi.org/10.1590/S1516-89132004000100017 - SANTOS, J.P., DAVIDE, A.C., TEIXEIRA, L.A.F., MELO, A.J.S. and MELO, L.A., 2011. Enraizamento de estacas lenhosas de espécies florestais. Cerne, vol. 17, no. 3, pp. 293-301. http://dx.doi.org/10.1590/S0104-77602011000300002
» http://dx.doi.org/10.1590/S0104-77602011000300002 - SANTOS, V.S., SOUZA, V.P., VILHALVA, D.A.A., FERREIRA, F.P.S., PAULA, J.R. and REZENDE, M.H., 2016. Morpho-anatomy and ontogeny of the underground system of Chrysolaena simplex (Less.) Dematt. (Asteraceae). Anais da Academia Brasileira de Ciências, vol. 88, no. 1, pp. 269-280. http://dx.doi.org/10.1590/0001-3765201620140676 PMid:26871494.
» http://dx.doi.org/10.1590/0001-3765201620140676 - SILVA, E.R., SIMÕES, I.M., BAPTISTA, J.O., SCHMILDT, E.R., LOPES, J.C., GONÇALVES, E.O., CALDEIRA, M.V.W. and ALEXANDRE, R.S., 2020. In vitro of Melanoxylon brauna Schott. morphogenesis: responsiveness of explants to permanent and temporary immersion growth regulators. Cerne, vol. 26, no. 1, pp. 26-36. http://dx.doi.org/10.1590/01047760202026012709
» http://dx.doi.org/10.1590/01047760202026012709 - SCARANO, F.R., 2007. Rock outcrop vegetation in Brazil: a brief overview. Revista Brasileira de Botanica. Brazilian Journal of Botany, vol. 30, no. 4, pp. 561-568. http://dx.doi.org/10.1590/S0100-84042007000400002
» http://dx.doi.org/10.1590/S0100-84042007000400002 - SCOLFORO, J.R.S., OLIVEIRA, A.D. and DAVIDE, A.C. 2012. Caracterização da candeia. In: J. R. S. SCOLFORO, B. F. P. LOEUILLE, eds. O manejo sustentável da candeia: o caminhar de uma nova experiência em Minas Gerais Lavras: Ed. UFLA, pp. 19-27.
- SILVA, T.M., VILHALVA, D.A.A., MORAES, M.G. and FIGUEIREDO-RIBEIRO, R.C.L., 2015. Anatomy and fructan distribution in vegetative organs of Dimerostemma vestitum (Asteraceae) from the campos rupestres. Anais da Academia Brasileira de Ciências, vol. 87, no. 2, pp. 797-812. http://dx.doi.org/10.1590/0001-3765201520140214 PMid:26062118.
» http://dx.doi.org/10.1590/0001-3765201520140214 - SOUZA, F.C., REIS, G.G., REIS, M.G.F., LEITE, H.G., FARIA, R.S., CALIMAN, J.P., BARBOSA, R.A. and OLIVEIRA, C.H.R., 2016. Growth of intact plants and coppice in short rotation eucalypt plantations. New Forests, vol. 47, no. 2, pp. 195-208. http://dx.doi.org/10.1007/s11056-015-9509-1
» http://dx.doi.org/10.1007/s11056-015-9509-1 - STUEPP, C.A., BITENCOURT, J., WENDLING, I., KOEHLER, H.S. and RIBAS, K.C.Z., 2016. Indução de brotações epicórmicas por meio de anelamento e decepa em erva-mate. Ciência Florestal, vol. 26, no. 3, pp. 1009-1022. http://dx.doi.org/10.5902/1980509824230
» http://dx.doi.org/10.5902/1980509824230 - TERTULIANO, M.F. and FIGUEIREDO‐RIBEIRO, R.C.L., 1993. Distribution of fructose polymers in herbaceous species of Asteraceae from the Cerrado. The New Phytologist, vol. 123, no. 4, pp. 741-749. http://dx.doi.org/10.1111/j.1469-8137.1993.tb03785.x
» http://dx.doi.org/10.1111/j.1469-8137.1993.tb03785.x - VACEK, S., HEJCMANOVÁ, P. and HEJCMAN, M., 2012. Vegetative reproduction of Picea abies by artificial layering at the ecotone of the alpine timberline in the Giant (Krkonoše) Mountains, Czech Republic. Forest Ecology and Management, vol. 263, pp. 199-207. http://dx.doi.org/10.1016/j.foreco.2011.09.037
» http://dx.doi.org/10.1016/j.foreco.2011.09.037 - VALLURU, R. and VAN DEN ENDE, W., 2008. Plant fructans in stress environments: emerging concepts and future prospects. Journal of Experimental Botany, vol. 59, no. 11, pp. 2905-2916. http://dx.doi.org/10.1093/jxb/ern164 PMid:18603617.
» http://dx.doi.org/10.1093/jxb/ern164 - WENDLING, I., TRUEMAN, S.J. and XAVIER, A., 2014a. Maturation and related aspects in clonal forestry – Part I: concepts, regulation and consequences of phase change. New Forests, vol. 45, no. 4, pp. 449-471. http://dx.doi.org/10.1007/s11056-014-9421-0
» http://dx.doi.org/10.1007/s11056-014-9421-0 - WENDLING, I., TRUEMAN, S.J. and XAVIER, A., 2014b. Maturation and related aspects in clonal forestry – Part II: reinvigoration, rejuvenation and juvenility maintenance. New Forests, vol. 45, no. 4, pp. 473-486. http://dx.doi.org/10.1007/s11056-014-9415-y
» http://dx.doi.org/10.1007/s11056-014-9415-y - XIONG, L. and ZHU, J.K., 2003. Regulation of abscisic acid biosynthesis. Plant Physiology, vol. 133, no. 1, pp. 29-36. http://dx.doi.org/10.1104/pp.103.025395 PMid:12970472.
» http://dx.doi.org/10.1104/pp.103.025395
Publication Dates
-
Publication in this collection
14 Aug 2020 -
Date of issue
Jul-Sep 2021
History
-
Received
11 June 2019 -
Accepted
14 Feb 2020