Abstract
Tiliroside is a glycosidic flavonoid present in many plants species including Helicteres velutina K. Schum (Malvaceae sensu lato), commonly known in Brazil as “pitó”. This molecule has been shown to have many biological activities, however no study has been carried out to investigate the toxicity of this substance. The present work aimed to evaluate the possible cellular toxicity in silico, in vitro and ex-vivo of the kaempferol-3-O-β-D-(6”-E-p-coumaroyl) glucopyranoside (tiliroside), through chemical structure analysis, toxicity assessment and predictive bioactive properties, using human samples for in vitro and ex-vivo tests. The in silico analysis suggests that tiliroside exhibited great absorption index when penetrating biological membranes. In addition, it also displayed considerable potential for cellular protection against free radicals, and anticarcinogenic, antioxidant, antineoplastic, anti-inflammatory, anti-hemorrhagic and antithrombotic activities. The assessment of the hemolytic and genotoxic effects of tiliroside showed low hemolysis rates in red blood cells and absence of cellular toxicity in the oral mucosa cells. The data obtained indicate that this molecule could be a promising therapeutic approach as a possible new drug with biotechnological potential.
Keywords:
hemolytic; genotoxicity; toxicity; Helicteres velutina
Resumo
O tilirosídeo é um flavonóide glicosídico presente em muitas espécies de plantas, incluindo Helicteres velutina K. Schum (Malvaceae sensu lato), conhecida no Brasil como “pitó”. Esta molécula mostrou ter muitas atividades biológicas, porém nenhum estudo foi realizado para investigar a toxicidade dessa substância. O presente trabalho teve como objetivo avaliar a possível toxicidade celular in silico, in vitro e ex-vivo do kaempferol-3-O-β-D- (6 ”-Ep-coumaroil) glucopiranosídeo (tilirosídeo), por meio de análises de estrutura química, toxicidade avaliação e propriedades bioativas preditivas, utilizando amostras humanas para testes in vitro e ex-vivo. A análise in silico sugere que o tilirosídeo exibe bom índice de absorção para penetrar nas membranas biológicas. Além disso, apresentou considerável potencial de proteção celular contra os radicais livres e com atividades anticarcinogênica, antioxidante, antineoplásica, antiinflamatória, anti-hemorrágica e antitrombótica. A avaliação dos efeitos hemolíticos e genotóxicos do tilirosídeo mostrou baixas taxas de hemólise nas hemácias e ausência de toxicidade em células da mucosa oral. Os dados obtidos indicam que esta molécula pode possuir uma abordagem terapêutica promissora como uma possível nova droga com potencial biotecnológico.
Palavras-chave:
hemolítico; genotoxicidade; toxicidade; Helicteres velutina
1. Introduction
The use of natural products as sources of new drugs has become a valuable resource for humankind over the years. Plants exhibit a multitude of applications and advantages that are instrumental in people healing from physical ailments and, for this reason, have raised scientific interest as targets in the investigation of their biotechnological potential (Hikal et al., 2017HIKAL, W.M., BAESHEN, R.S. and SAID-AL AHL, H.A., 2017. Botanical insecticide as simple extractives for pest control. Cogent Biology, vol. 3, no. 1, 1404274. http://dx.doi.org/10.1080/23312025.2017.1404274.
http://dx.doi.org/10.1080/23312025.2017....
).
The genus Helicteres (Malvaceae senso lato) comprises indigenous species found in the Northeast and Southeast regions of Brazil. Phytochemical and pharmacological studies have indicated that this genus is rich in constituents with diverse biological activities, including antioxidant, anticarcinogenic, antimicrobial, antiplasmodial, antinociceptive and hepatoprotective in H. isora (Kumar and Singh, 2014KUMAR, N. and SINGH, A.K., 2014. Plant profile, phytochemistry and pharmacology of Avartani (Helicteres isora Linn.): a review. Asian Pacific Journal of Tropical Biomedicine, vol. 4, pp. S22-S26. http://dx.doi.org/10.12980/APJTB.4.2014C872.
http://dx.doi.org/10.12980/APJTB.4.2014C...
); antihypertensive and antiulcerogenic in H. sacarolha (Balogun et al., 2014BALOGUN, S.O., SILVA JÚNIOR, I.F., COLODEL, E.M., OLIVEIRA, R.G., ASCÊNCIO, S.D. and DE OLIVEIRA MARTINS, D.T., 2014. Toxicological evaluation of hydroethanolic extract of Helicteres sacarolha A. St.- Hil. et al. Journal of Ethnopharmacology, vol. 157, pp. 285-291. http://dx.doi.org/10.1016/j.jep.2014.09.013. PMid:25239833.
http://dx.doi.org/10.1016/j.jep.2014.09....
), and analgesic, anti-inflammatory, antimicrobial and anticarcinogenic in H. angustifólia (Huang et al., 2013HUANG, Q., HUANG, R., WEI, L., CHEN, Y., LV, S., LIANG, C., ZHANG, X., YIN, F., LI, H., ZHUO, L. and LIN, X., 2013. Antiviral activity of methyl helicterate isolated from Helicteres angustifolia (Sterculiaceae) against hepatitis B virus. Antiviral Research, vol. 100, no. 2, pp. 373-381. http://dx.doi.org/10.1016/j.antiviral.2013.09.007. PMid:24055834.
http://dx.doi.org/10.1016/j.antiviral.20...
; Fernandes et al., 2020aFERNANDES, D., ASSIS, E., SOUZA, M., SOUZA, P. and SOUZA, M., 2020a. Helicteres, L. species (Malvaceae sensu lato) as source of new drugs: a review. Quimica Nova. In press. http://dx.doi.org/10.21577/0100-4042.20170533.
http://dx.doi.org/10.21577/0100-4042.201...
). The species Helicteres velutina K. Schum, popularly known as “Pitó”, is a plant used as an insect repellent by the Pankararé indians in Bahia state/Brazil. Recent studies have shown the larvicidal activity of flavonoids from this species against Aedes aegypti (Santos et al., 2012SANTOS, E.A.D., CARVALHO, C.M.D., COSTA, A.L., CONCEIÇÃO, A.S., MOURA, F.D.B.P. and SANTANA, A.E.G., 2012. Bioactivity evaluation of plant extracts used in indigenous medicine against the snail, Biomphalaria glabrata, and the larvae of Aedes aegypti. Evidence-Based Complementary and Alternative Medicine, vol. 2012, pp. 846583. http://dx.doi.org/10.1155/2012/846583.
http://dx.doi.org/10.1155/2012/846583...
; Fernandes et al., 2020bFERNANDES, D.A., OLIVEIRA, L.H.G., RIQUE, H.L., SOUZA, M.F.V. and NUNES, F.C., 2020b. Insights on the larvicidal mechanism of action of fractions and compounds from Aerial Parts of Helicteres velutina K. Schum against Aedes aegypti L. Molecules, vol. 25, no. 13, pp. 3015. http://dx.doi.org/10.3390/molecules25133015.
http://dx.doi.org/10.3390/molecules25133...
).
Tiliroside (Kaempferol-3-O-β-D-(6”-E-p-coumaryl) glucopyranoside), a glycosidic flavonoid present in specific plant parts (fruits, leaves and roots), is one of the major bioactive compounds of Helicteres velutina (Grochowski et al., 2018GROCHOWSKI, D.M., LOCATELLI, M., GRANICA, S., CACCIAGRANO, F. and TOMCZYK, M., 2018. Uma revisão sobre o tilirosídeo flavonóide da dieta. Revisões Abrangentes em Ciência e Segurança Alimentar, vol. 17, no. 5, pp. 1395-1421.; Fernandes et al., 2019FERNANDES, D.A., BARROS, R.P.C., TELES, Y.C.F., OLIVEIRA, L.H.G., LIMA, J.B., SCOTTI, M.T., NUNES, F.C., CONCEIÇÃO, A.S. and SOUZA, M.D.F.V., 2019. Larvicidal compounds extracted from Helicteres velutina K. Schum (Sterculiaceae) evaluated against Aedes aegypti L. Molecules, vol. 24, no. 12, pp. 2315. http://dx.doi.org/10.3390/molecules24122315. PMid:31234501.
http://dx.doi.org/10.3390/molecules24122...
) known to exert various effects such as antibacterial and larvicidal, antithrombotic (Han et al., 2012HAN, N., GU, Y., YE, C., CAO, Y., LIU, Z. and YIN, J., 2012. Atividade antitrombótica de frações e componentes obtidos de folhas de framboesa (Rubus chingii). Química de Alimentos, vol. 132, no. 1, pp. 181-185.), anticoagulant (Gevrenova et al., 2013GEVRENOVA, R., BADJAKOV, I., NIKOLOVA, M. and DOICHINOVA, I., 2013. Phenolic derivatives in raspberry (Rubus L.) germplasm collection in Bulgaria. Biochemical Systematics and Ecology, vol. 50, pp. 419-427. http://dx.doi.org/10.1016/j.bse.2013.06.002.
http://dx.doi.org/10.1016/j.bse.2013.06....
), hepatoprotective (Goto et al., 2012aGOTO, T., HORITA, M., NAGAI, H., NAGATOMO, A., NISHIDA, N., MATSUURA, Y. and NAGAOKA, S., 2012a. Tiliroside, a glycosidic flavonoid, inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract. Molecular Nutrition & Food Research, vol. 56, no. 3, pp. 435-445. http://dx.doi.org/10.1002/mnfr.201100458. PMid:22173993.
http://dx.doi.org/10.1002/mnfr.201100458...
), anti-inflammatory (Jin et al., 2016JIN, X., SONG, S., WANG, J., ZHANG, Q., QIU, F. and ZHAO, F., 2016. Tiliroside, the major component of Agrimonia pilosa Ledeb ethanol extract, inhibits MAPK/JNK/p38-mediated inflammation in lipopolysaccharide-activated RAW 264.7 macrophages. Experimental and Therapeutic Medicine, vol. 12, no. 1, pp. 499-505. http://dx.doi.org/10.3892/etm.2016.3305. PMid:27347085.
http://dx.doi.org/10.3892/etm.2016.3305...
), antiobesity and antidiabetic (Goto et al., 2012bGOTO, T., TERAMINAMI, A., LEE, J.Y., OHYAMA, K., FUNAKOSHI, K., KIM, Y.I., HIRAIA, S., UEMURA, T., YUC, R., TAKAHASHI, N. and KAWADA, T., 2012b. Tiliroside, a glycosidic flavonoid, ameliorates obesity-induced metabolic disorders via activation of adiponectin signaling followed by enhancement of fatty acid oxidation in liver and skeletal muscle in obese–diabetic mice. The Journal of Nutritional Biochemistry, vol. 23, no. 7, pp. 768-776.), and anticancer (Lu et al., 2009LU, Y.H., CHEN, J., WEI, D.Z., WANG, Z.T. and TAO, X.Y., 2009. Tyrosinase inhibitory effect and inhibitory mechanism of tiliroside from raspberry. Journal of Enzyme Inhibition and Medicinal Chemistry, vol. 24, no. 5, pp. 1154-1160. http://dx.doi.org/10.1080/14756360802694252. PMid:19772488.
http://dx.doi.org/10.1080/14756360802694...
).
In this study, the potential toxicity and pharmacokinetic of tiliroside was investigated in silico, in vitro and ex vivo using assays with chemical structure analysis to predict bioactive properties, and with toxicological methods using anucleated (hemolysis in ABO system) and nucleated (oral mucosa toxicity) human cells.
2. Material and Methods
2.1. Plant material
The aerial parts of H. velutina were collected in February 2015, in Jeremoabo (Bahia, State, Brazil) coordinates are 09° 44’34.6” S and 38° 52’20.4” W, were identified by Prof. Adilva de Souza Conceição (Sate University of Bahia - UNEB). A voucher specimen, registered under the number 28709-1, was deposited in the Herbarium of the Federal University of Bahia (HUNEB, Paulo Afonso Collection). This study has been registered in the National System of Genetic Resource Management and Associated Traditional Knowledge (SisGen—A568B8A).
2.2. Preparation of sample
The plant material H. velutina was oven dried at 40 °C, and subsequently powdered and macerated with 95% ethanol (5 L) for 72 hours. The extract solution was dried under reduced pressure at 40 °C yielding crude ethanolic extracts (CEEs). Afterwards it was subjected to separation by liquid-liquid chromatography using hexane, chloroform (CHCl3), ethyl acetate and n-butanol, which resulted in the respective fractions in addition to the hydroalcoholic fraction.
The dichloromethane fraction was chromatographed in flash silica using petroleum ether, dichloromethane and methanol individually or in binary mixtures. The resulting polar fractions were chromatographed in Sephadex (LH-20), employing methanol and chloroform:methanol (1:1) as the mobile phase for isolation of tiliroside. Tiliroside was used in the experiments described in this study, more information on the identified, obtention procedures and structural determination is described in a previous study (Fernandes et al., 2019FERNANDES, D.A., BARROS, R.P.C., TELES, Y.C.F., OLIVEIRA, L.H.G., LIMA, J.B., SCOTTI, M.T., NUNES, F.C., CONCEIÇÃO, A.S. and SOUZA, M.D.F.V., 2019. Larvicidal compounds extracted from Helicteres velutina K. Schum (Sterculiaceae) evaluated against Aedes aegypti L. Molecules, vol. 24, no. 12, pp. 2315. http://dx.doi.org/10.3390/molecules24122315. PMid:31234501.
http://dx.doi.org/10.3390/molecules24122...
).
2.3. In silico analysis
The chemical structure of tiliroside was obtained through Pubchem® (PUBCHEM, 2004PUBCHEM SUBSTANCE AND COMPOUND DATABASES – PUBCHEM, 2004 [viewed 07 June 2020]. Chemical structure of tiliroside. [online]. Available from: https://pubchem.ncbi.nlm.nih.gov
https://pubchem.ncbi.nlm.nih.gov...
). The toxicity assessment used the AdmetSAR® software (ADMETSAR, 2012ABSORPTION, DISTRIBUTION, METABOLISM, EXCRETION, AND TOXICITY – ADMETSAR, 2012 [accessed 07 June 2020]. Toxicity of tiliroside. [software]. Available from: http://lmmd.ecust.edu.cn/admetsar1/
http://lmmd.ecust.edu.cn/admetsar1/...
), where the following parameters were analyzed: Gene Inhibition (GI), Ames Toxicity (AT), Potential Carginogens (C), Acute Oral Toxicity (AOT) and Carcinogenicity (Car). The predictive bioactive properties were determined by the software PASS online® (PASS ONLINE, 2014), which predicts a compound's activity spectrum as probable activity (Pa) or probable inactivity (Pi). The values of Pa and Pi vary from 0.000 to 1.000. When Pa is greater than Pi, the compound is thought to be experimentally active. Pa greater than 0.7 (or Pa > 0.7) and Pi less than 0.3 (or Pi < 0.3) indicate great probability of pharmacological potential, reflecting considerable experimental pharmacological effects (Rakib et al., 2019RAKIB, A., AHMED, S., ISLAM, M.A., HAYE, A., UDDIN, S.N., UDDIN, M.M.N., HOSSAIN, M.K., PAUL, A. and EMRAN, T.B., 2019. Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Science & Nutrition, vol. 8, no. 1, pp. 547-556. http://dx.doi.org/10.1002/fsn3.1339. PMid:31993178.
http://dx.doi.org/10.1002/fsn3.1339...
; Ahmad et al., 2016AHMAD, W., JANTAN, I. and BUKHARI, S.N., 2016. Tinospora crispa (L.) Hook. f. & Thomson: a review of its ethnobotanical, phytochemical, and pharmacological aspects. Frontiers in Pharmacology, vol. 7, pp. 59. http://dx.doi.org/10.3389/fphar.2016.00059. PMid:27047378.
http://dx.doi.org/10.3389/fphar.2016.000...
).
2.4. Human erythrocytes and oral mucosa cells collection
The assays were carried out according to the Ethics Code of the World Medical Association and were approved by the Ethics Committee of the University Center of Patos (protocol number: 3.621.284). The blood samples (A, B and O) and smear samples from the oral mucosa were obtained from healthy young adults in order to generate eukaryotic cell pellets. The participants comprised Biological Sciences and Dental students from the Federal University of Campina Grande (campus Patos – PB), healthy young adults of both sexes between 18 and 40 years of age.
2.5. In vitro analysis
2.5.1. Hemolytic activity
Human blood aliquots (types A, B and O) were mixed with NaCl 0.9% (1:30) and centrifuged at 2500 rpm for 5 min for separation of the red blood cells (RBC). This procedure was repeated two more times and the sediment from the last centrifuge was resuspended in NaCl 0.9% to obtain a 0.5% suspension free of white cells and platelets.
Tiliroside was added to 2 mL of the RBC suspension at different concentrations (50, 100, 500 and 1000 µg/mL) in distinct preparations to reach a final volume of 2 mL. The RBC suspension was used as a negative control (0% hemolysis) and a RBC suspension with 1%Triton X-100 was used as positive control (100% hemolysis). The samples were incubated for 1 h at 22 ± 2 ºC with slow and constant agitation (100 rpm) following centrifugation at 2.500 rpm for 5 min. Hemolysis was quantified by spectrophotometry at its absorbance maximum wavelength, 540 nm (Rangel et al., 1997RANGEL, M., MALPEZZI, E.L., SUSINI, S.M. and DE FREITAS, J., 1997. Hemolytic activity in extracts of the diatom Nitzschia. Toxicon, vol. 35, no. 2, pp. 305-309. http://dx.doi.org/10.1016/S0041-0101(96)00148-1. PMid:9080587.
http://dx.doi.org/10.1016/S0041-0101(96)...
). The tests were carried out in triplicate and the results were expressed as percentages representing the arithmetic average of three measurements.
2.6. Ex-vivo analysis
2.6.1. Evaluation of the genotoxic effects on oral mucosa cells
Epithelial cells were obtained from the buccal mucosa of both sides by cytobrush sampling. According to Kassie et al. (2001)KASSIE, F., DARROUDI, F., KUNDI, M., SCHULTE‐HERMANN, R. and KNASMÜLLER, S., 2001. Khat (Catha edulis) consumption causes genotoxic effects in humans. International Journal of Cancer, vol. 92, no. 3, pp. 329-332. http://dx.doi.org/10.1002/ijc.1195. PMid:11291066.
http://dx.doi.org/10.1002/ijc.1195...
, the cytobrush (endocervical sample/cell collector) is the most adequate tool to collect exfoliated cells of the oral mucosa. The cells were placed in a tube containing 5 mL 0.9% NaCl, which is considered a suitable cell preservation medium before the slide preparation. Control cells were divided into two groups: positive control – cells treated with hydrogen peroxide (0.0005%), and negative control – cells not exposed to any treatment.
The cell samples were washed twice in saline solution and then centrifuged for 10 min at 1.500 rpm and kept in 5 mL of saline. The supernatant was discarded. After the third wash they were exposed ex-vivo to tiliroside at different concentrations (50, 100, 500 and 1000 µg/mL) for 30 min. Next, the cells were centrifuged and the supernatant was once again removed. The cells were homogenized in a vortex mixer and placed on the slides. They were placed on three slides to each concentrations analysed, dried at room temperature and fixed in methanol: acetic acid (3:1) for 15 min (Thomas et al., 2008THOMAS, P., HARVEY, S., GRUNER, T. and FENECH, M., 2008. The buccal cytome and micronucleus frequency is substantially altered in Down’s syndrome and normal ageing compared to young healthy controls. Mutation Research. Fundamental and Molecular Mechanisms of Mutagenesis, vol. 638, no. 1-2, pp. 37-47. http://dx.doi.org/10.1016/j.mrfmmm.2007.08.012. PMid:17920640.
http://dx.doi.org/10.1016/j.mrfmmm.2007....
). After fixation, the slides were kept at room temperature for 12 h and later immersed in distilled water for 1 min and stained in 2% Giemsa for optical microscopy observation (Gabriel et al., 2006GABRIEL, H.E., CROTT, J.W., GHANDOUR, H., DALLAL, G.E., CHOI, S.W., KEYES, M.K., JANG, H., LIU, Z., NADEAU, M., JOHNSTON, A., MAGER, D. and MASON, J.B., 2006. Chronic cigarette smoking is associated with diminished folate status, altered folate form distribution, and increased genetic damage in the buccal mucosa of healthy adults. The American Journal of Clinical Nutrition, vol. 83, no. 4, pp. 835-841. http://dx.doi.org/10.1093/ajcn/83.4.835. PMid:16600936.
http://dx.doi.org/10.1093/ajcn/83.4.835...
). Cell toxicity can be assessed through the presence of cellular indicators such as micronuclei, bi-nucleation, karyolysis, karyorrhexis, and macronuclei (Sponchiado et al., 2016SPONCHIADO, G., ADAM, M.L., SILVA, C.D., SOLEY, B.S., MELLO-SAMPAYO, C., CABRINI, D.A., CORRER, C.J. and OTUKI, M.F., 2016. Quantitative genotoxicity assays for analysis of medicinal plants: A systematic review. Journal of Ethnopharmacology, vol. 178, pp. 289-296. http://dx.doi.org/10.1016/j.jep.2015.10.026. PMid:26680588.
http://dx.doi.org/10.1016/j.jep.2015.10....
). Approximately 1000 cells were analyzed per slide, and the data were expressed as percentages representing the arithmetic average of three measurements.
2.7. Statistical analysis
The experiments were carried out in triplicate and the results were expressed as percentages representing the arithmetic average of three measurements. The data were analyzed using One-way Analysis of Variance (ANOVA) and the Bonferroni post hoc test. The tests were performed with the GraphPadPrism software (version 6.0 for Windows, San Diego, CA-USA). Differences were considered significant when P ≤ 0.05.
3. Results and Discussion
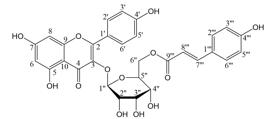
The flavonoid tiliroside, isolated from H. velutina (Figure 1), has a molar mass of 594.52 g/mol and 43 total atoms.
The in silico analysis determined the theoretical physico-chemical properties of tiliroside, indicating that the flavonoid molecule has theoretical aspect of bioavailability such as lipophilicity coefficient (LogP: 2.49), aqueous solubility coefficient (LogS: - 3.09) and the topological polar surface area (TPSA: 216.58 Å2). Tha data suggest that the molecule has notable absorption index when penetrating biological membranes (LogP: ≤ 5.00, and LogS: ≤ - 4.00), showing a polar surface area greater than the parameter adopted in the indication of good permeability in TPSA membrane, i.e. ≤ 140 Å2 (Veber et al., 2002VEBER, D.F., JOHNSON, S.R., CHENG, H.Y., SMITH, B.R., WARD, K.W. and KOPPLE, K.D., 2002. Molecular properties that influence the oral bioavailability of drug candidates. Journal of Medicinal Chemistry, vol. 45, no. 12, pp. 2615-2623. http://dx.doi.org/10.1021/jm020017n. PMid:12036371.
http://dx.doi.org/10.1021/jm020017n...
).
Recent studies suggest that flavonoids alter the organization of biomembranes, which can lead to changes in membrane protein function (Tsuchiya, 2015TSUCHIYA, H., 2015. Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules, vol. 20, no. 10, pp. 18923-18966. http://dx.doi.org/10.3390/molecules201018923. PMid:26501254.
http://dx.doi.org/10.3390/molecules20101...
; Ingólfsson et al., 2014INGÓLFSSON, H.I., THAKUR, P., HEROLD, K.F., HOBART, E.A., RAMSEY, N.B., PERIOLE, X., DE JONG, D.H., ZWAMA, M., YILMAZ, D., HALL, K., MARETZKY, T., HEMMINGS JUNIOR, H.C., BLOBEL, C., MARRINK, S.J., KOÇER, A., SACK, J.T. and ANDERSEN, O.S., 2014. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chemical Biology, vol. 9, no. 8, pp. 1788-1798. http://dx.doi.org/10.1021/cb500086e. PMid:24901212.
http://dx.doi.org/10.1021/cb500086e...
; Selvaraj et al., 2015SELVARAJ, S., KRISHNASWAMY, S., DEVASHYA, V., SETHURAMAN, S. and KRISHNAN, U.M., 2015. Influence of membrane lipid composition on flavonoid–membrane interactions: implications on their biological activity. Progress in Lipid Research, vol. 58, pp. 1-13. http://dx.doi.org/10.1016/j.plipres.2014.11.002. PMid:25479162.
http://dx.doi.org/10.1016/j.plipres.2014...
). Such information reinforces the absorption capacity and permeability of membranes described for tiliroside, expressed in LogS, LogP and TPSA values. They also indicate viability for other in vivo administration routes of this molecule, such as intramuscular, cutaneous and intravenous.
In the predictive analysis of tiliroside toxicity, it was evaluated whether the molecule would induce or inhibit mutagenicity. The gene inhibition (GI: 0.752) and AMES test (AT: 0.574) revealed that tiliroside does not inhibit gene to expressed, while the carcinogens (C: 0.965) and carcinogenicity (Car: 0.677) showed that the molecule is not carcinogenic. When the parameters of toxicity analysis is (> 0.500) indicate probability of pharmacological potential (Rakib et al., 2019RAKIB, A., AHMED, S., ISLAM, M.A., HAYE, A., UDDIN, S.N., UDDIN, M.M.N., HOSSAIN, M.K., PAUL, A. and EMRAN, T.B., 2019. Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Science & Nutrition, vol. 8, no. 1, pp. 547-556. http://dx.doi.org/10.1002/fsn3.1339. PMid:31993178.
http://dx.doi.org/10.1002/fsn3.1339...
; Ahmad et al., 2016AHMAD, W., JANTAN, I. and BUKHARI, S.N., 2016. Tinospora crispa (L.) Hook. f. & Thomson: a review of its ethnobotanical, phytochemical, and pharmacological aspects. Frontiers in Pharmacology, vol. 7, pp. 59. http://dx.doi.org/10.3389/fphar.2016.00059. PMid:27047378.
http://dx.doi.org/10.3389/fphar.2016.000...
). In the acute oral toxicity (AOT: 0.404), the molecule was classified as category III, with LD50 values higher than 500 mg/kg and lower than 5000 mg/kg (Drwal et al., 2014DRWAL, M.N., BANERJEE, P., DUNKEL, M., WETTIG, M.R. and PREISSNER, R., 2014. ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Research, vol. 42, no. W1, pp. W53-W58. http://dx.doi.org/10.1093/nar/gku401. PMid:24838562.
http://dx.doi.org/10.1093/nar/gku401...
), as showed in (Table 1).
Tiliroside's antigenotoxic activity is based on its ability to protect the cell against oxidative stress. Considering the performance indicated by the toxicity parameters (C, AOT and Car), showed that tiliroside has potential against damage in cells and DNA, also it suggested that flavonoid molecule exhibits marked cellular protection against free radicals. However, the in silico approach for predicting the antigenotoxic effect of flavonoids must be further investigated by in vitro and in vivo studies, as these molecules are subject to metabolization, involving a wide range of reactions such as deglycosylation, glucuronidation, sulfation, methylation, ring fission that modify the bioavailability of the molecule (Erlund, 2004ERLUND, I., 2004. Review of the flavonoids quercetin, hesperetin, and naringenin: dietary sources, bioactivities, bioavailability, and epidemiology. Nutrition Research, vol. 24, no. 10, pp. 851-874. http://dx.doi.org/10.1016/j.nutres.2004.07.005.
http://dx.doi.org/10.1016/j.nutres.2004....
).
The bioactive properties revealed by the predictive analysis on tiliroside showed promising results for the activation probability (Pa) in relation to the inactivation probability values (Pi). It also exhibited greater probability of having a wide range of biological activities, including anticarcinogenic (Pa: 0.986; Pi: 0.001), antioxidant (Pa: 0.910; Pi: 0.003), antineoplastic (Pa: 0.837; Pi: 0.008), anti-inflammatory (Pa: 0.759; Pi: 0.009), anti-hemorrhagic (Pa: 0.736; Pi: 0.002) and antithrombotic (Pa: 0.666; Pi: 0.010) (Table 2).
According to Luca et al. (2016)LUCA, V.S., MIRON, A. and APROTOSOAIE, A.C., 2016. The antigenotoxic potential of dietary flavonoids. Phytochemistry Reviews, vol. 15, no. 4, pp. 591-625. http://dx.doi.org/10.1007/s11101-016-9457-1.
http://dx.doi.org/10.1007/s11101-016-945...
, flavonoids are molecules with an important biological value due to their potential pharmacological activities (e.g. antioxidant, anti-inflammatory and immunological). They found a correlation between food consumption and some illnesses, since a diet rich in flavonoids has been associated with reduced risks of several chronic diseases.
The flavonoid tiliroside showed an inhibitory effect on the growth of instinct, liver and skin cancer cells on in vitro assays (Rao et al., 2007RAO, Y.K., GEETHANGILI, M., FANG, S.H. and TZENG, Y.M., 2007. Antioxidant and cytotoxic activities of naturally occurring phenolic and related compounds: a comparative study. Food and Chemical Toxicology, vol. 45, no. 9, pp. 1770-1776. http://dx.doi.org/10.1016/j.fct.2007.03.012. PMid:17475387.
http://dx.doi.org/10.1016/j.fct.2007.03....
). The results of the in silico analysis of the present study show the possible beneficial effects of tiliroside on several pathological processes, indicating that molecule has bioactivity against cancer cells and low levels of toxicity as shown by the previous findings.
The sensibility measurement of human RBC at different concentrations of tiliroside obtained from H. velutina suggests low cytotoxic effects. The percentage of hemolysis is characterized as low when between 0 and 40%, as moderate when between 40 and 80%, and as high when above 80% (Rangel et al., 1997RANGEL, M., MALPEZZI, E.L., SUSINI, S.M. and DE FREITAS, J., 1997. Hemolytic activity in extracts of the diatom Nitzschia. Toxicon, vol. 35, no. 2, pp. 305-309. http://dx.doi.org/10.1016/S0041-0101(96)00148-1. PMid:9080587.
http://dx.doi.org/10.1016/S0041-0101(96)...
). Tiliroside exhibited low levels of hemolysis in all concentrations tested. In particular, at the highest concentration (1000 µg/mL), the hemolytic rate for blood types O, B and A was < 29%, < 28%, and < 20%, respectively. Therefore, this compound possesses the rate of lysed RBC in the following order: A< B< O (Figure 2).
Cytotoxic effect of tiliroside (H. velutina) against RBC; (C-) Negative control (erythrocytes 0.5%), (C+) Positive control (1% Triton X-100). P < 0.05 (*), P < 0.01(**) and P < 0.001 (***) versus positive control.
The results obtained in the hemolysis assay showed a small difference between the tiliroside and the human RBC types (A, B and O). The system ABO is characterized by the antigenic portion, with the presence of specific monosaccharides on the RBC membrane surface: Serum type A (N-acetylgalactosamine), serum type B (D-galactose), serum type AB (possesses both antigens) and serum type O (has no antigens) (Van Ginkel and Sevanian, 1994VAN GINKEL, G. and SEVANIAN, A., 1994. [28] Lipid peroxidation-induced membrane structural alterations. Methods in Enzymology, vol. 233, pp. 273-288. http://dx.doi.org/10.1016/S0076-6879(94)33031-X. PMid:8015462.
http://dx.doi.org/10.1016/S0076-6879(94)...
). Lower hemolysis was observed after exposure to tiliroside in type A blood < 20%, suggesting a better interaction with N-acetylgalactosamine, which was responsible for the less cytotoxic effect at the highest concentration. The hemolysis in the RBC, after exposure to different concentrations of test substances that promote cellular lysis, is the ideal measurement to reveal the cytotoxic potential of the natural products tested in this cell model. High levels of cytotoxicity can be a great disadvantage for the use of products, such as phytoconstituents, as they may interfere with the biological effects (Hooijberg et al., 1997HOOIJBERG, J.H., BROXTERMAN, H.J., HEIJN, M., FLES, D.L.A., LANKELMA, J. and PINEDO, H.M., 1997. Modulation by (iso) flavonoids of the ATPase activity of the multidrug resistance protein. FEBS Letters, vol. 413, no. 2, pp. 344-348. http://dx.doi.org/10.1016/S0014-5793(97)00940-X. PMid:9280310.
http://dx.doi.org/10.1016/S0014-5793(97)...
).
Studies developed by Al Muqarrabun and Ahmat (2015)AL MUQARRABUN, L.M.R. and AHMAT, N., 2015. Usos medicinais, fitoquímica e farmacologia da família Sterculiaceae: uma revisão. Revista Européia de Química Medicinal, vol. 92, pp. 514-530. evidenced that the most common class of secondary metabolites of the genus Helicteres are flavonoids, which are characterized by low hemolysis activity (values < 50%). In addition, the results described in the in silico analysis for bioactive properties anti-hemorrhagic (Pa: 0.736; Pi: 0.002) and anti-thrombotic (Pa: 0.666; Pi: 0.010) confirms the protective influence of tiliroside on RBC. Therefore, the predictive in silico analysis and the in vitro study reaffirm the molecule performance against its bioactivity and less cytotoxic effect in human anucleated cells such as RBC, where the oxidation and lysis mechanisms are more sensitive and the molecular interaction is less complex.
Genotoxicity refers to the ability of chemical agents to damage genetic information in a cell. Cells have developed several mechanisms for repairing DNA damage, but if the lesions in the genetic material are not adjusted, they lead to genetic mutations that manifest themselves in a slew of genetic diseases that have common traits, most notably the development of cancer (Clancy, 2008CLANCY, S., 2008. DNA damage & repair: mechanisms for maintaining DNA integrity. Nature Education, vol. 1, no. 1, pp. 103.; Jackson and Bartek, 2009JACKSON, S.P. and BARTEK, J., 2009. The DNA-damage response in human biology and disease. Nature, vol. 461, no. 7267, pp. 1071-1078. http://dx.doi.org/10.1038/nature08467. PMid:19847258.
http://dx.doi.org/10.1038/nature08467...
; Swift and Golsteyn, 2014SWIFT, L.H. and GOLSTEYN, R.M., 2014. Genotoxic anti-cancer agents and their relationship to DNA damage, mitosis, and checkpoint adaptation in proliferating cancer cells. International Journal of Molecular Sciences, vol. 15, no. 3, pp. 3403-3431. http://dx.doi.org/10.3390/ijms15033403. PMid:24573252.
http://dx.doi.org/10.3390/ijms15033403...
).
Cells containing macronuclei due to toxic exposures show nuclear hyperactivity that increases the diameter of the nucleus, causing cell division, which results in binucleation (Popova et al., 2007POPOVA, L., KISHKILOVA, D., HADJIDEKOVA, V.B., HRISTOVA, R.P., ATANASOVA, P., HADJIDEKOVA, V.V., ZIYA, D. and HADJIDEKOV, V.G., 2007. Micronucleus test in buccal epithelium cells from patients subjected to panoramic radiography. Dento Maxillo Facial Radiology, vol. 36, no. 3, pp. 168-171. http://dx.doi.org/10.1259/dmfr/29193561. PMid:17463102.
http://dx.doi.org/10.1259/dmfr/29193561...
). Moreover, alterations involving karyolysis and karyorrhexis are observed when the cell is induced to undergo necrosis/apoptosis. In the karyorrhexis, the chromatin is fragmented and the nuclear membrane disappears, while in the karyolysis, there is a complete dissolution of the nucleus with loss of nuclear material. Hence, the more intense these alterations are expressed, the more severe is the pathological condition of the cells/tissues (Antonio et al., 2017ANTONIO, E.L., NASCIMENTO, A.J.D., LIMA, A.A.S.D., LEONART, M.S.S. and FERNANDES, Â., 2017. Genotoxicidade e citotoxicidade dos raios x em crianças submetidas à radiografia panorâmica. Revista Paulista de Pediatria : Orgao Oficial da Sociedade de Pediatria de Sao Paulo, vol. 35, no. 3, pp. 296-301. http://dx.doi.org/10.1590/1984-0462/;2017;35;3;00010. PMid:28977295.
http://dx.doi.org/10.1590/1984-0462/;201...
; Obeng, 2021OBENG, E., 2021. Apoptosis (programmed cell death) and its signals: a review. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 81, no. 4, pp. 1133-1143. http://dx.doi.org/10.1590/1519-6984.228437.
http://dx.doi.org/10.1590/1519-6984.2284...
).
The ex vivo method for detecting genotoxicity in mucosa oral evaluates changes in DNA caused by test substances that shows cellular indicators such as: micronucleus, binucleation, karyolysis, karyorrhexis and macronucleus, were found in the evaluated slides (Figure 3).
Photomicrography of exfoliated oral mucosa cells with: (A) karyorrhexis; (B) karyolysis; (C) micronucleus; (D) binucleation; and (E) macronucleus. Magnification X1000.
In the genotoxicity test of oral mucosa cells, the occurrence of cell changes is consistent with toxic damage. These findings were more typically present in the positive control group exposed to the H2O2 solution. The groups treated with different concentrations of tiliroside exhibited few cellular alterations when compared to the positive control, but showed similar results to those of the negative control group that does not exhibit genotoxic effects (Table 3).
The data reveal that tiliroside exerted low toxicity effects at the highest concentrations (500 and 1000 µg / mL), with > 80% of normal cells different from the results in the positive control group (H2O2), < 90% of normal cells similar to the reported in negative control group. The exposure to 1000 µg/mL of tiliroside had the lowest alteration potential, showing >90% of normal cells, although some cellular changes were detected such as binucleation, macronucleus, karyolysis, and karyorrhexis. This set of cellular variations was lower than that described in the positive control (H2O2), suggesting a safe threshold of low toxicity with respect to concentration and time of exposure to the test substance.
The cellular changes mentioned in the genotoxicity assay reflect the beginning of induction of the physiological cell reactivity when exposed to 1000 µg/mL of tiliroside. These results corroborate the findings described in the in silico analysis that shows the tiliroside as predictive potential inducing apoptosis (Pa: 0.790; Pi: 0.009). This was attested by a lesser description of the inhibitory activity of apoptosis (Pa: 0.340; Pi: 0.015).
Studies developed by Jain et al. (2014)JAIN, A., RANADE, R., PRITAM, P., JOSHI, N., VAVILALA, S.L. and JAIN, A., 2014. A comparative study of antioxidant activity, total phenolic and flavonoid contents in different parts of Helicteres isora L. American Journal of Life Sciences, vol. 2, no. 5, pp. 292-302. http://dx.doi.org/10.11648/j.ajls.20140205.17.
http://dx.doi.org/10.11648/j.ajls.201402...
described that phenolic compounds extracted from H. isora dried fruits displayed high antioxidant and antitumor effects, demonstrating the importance of plants as a considerable source of natural phytoconstituents and antioxidant supplements. This information corroborates the results of this study, since the genus Helicteres is rich in phenolic compounds and flavonoids, exhibit low toxicity, antioxidant, anti-inflammatory and antitumor activities. However, specific data about the effects of tiliroside on genotoxicity in human oral mucosa have not been found in the literature.
4. Conclusion
The in silico analysis suggests that tiliroside isolated from H. velutina has a significant absorption index when penetrating biological membranes. In addition, it also reveals potential anticarcinogenic, antioxidant, antineoplastic, anti-inflammatory, anti-hemorrhagic and antithrombotic activities. The in vitro and ex vivo assessment of the hemolytic and genotoxic effects showed low hemolysis rates in RBC and absence of cellular toxicity in the oral mucosa cells. The reduced cytotoxic activity is indicative of the safety of the concentrations used and demonstrates different ways of interaction of the tested substances with the analyzed cells. Therefore, the data obtained in the present study suggest that tiliroside could be a promising therapeutic approach for the development of a new drug, with potential biotechnological applications.
Ethical disclosures
Protection of human and animal subjects. The authors declare that the study was performed according to the Ethics Code of the World Medical Association and approved by the Ethics Committee of the University Center of Patos (Patos, Paraíba state, Brazil) (protocol number: 3.621.284).
Acknowledgements
The authors would like to thank CAPES for financial support and PhD scholarship.
References
- ABSORPTION, DISTRIBUTION, METABOLISM, EXCRETION, AND TOXICITY – ADMETSAR, 2012 [accessed 07 June 2020]. Toxicity of tiliroside. [software]. Available from: http://lmmd.ecust.edu.cn/admetsar1/
» http://lmmd.ecust.edu.cn/admetsar1/ - AHMAD, W., JANTAN, I. and BUKHARI, S.N., 2016. Tinospora crispa (L.) Hook. f. & Thomson: a review of its ethnobotanical, phytochemical, and pharmacological aspects. Frontiers in Pharmacology, vol. 7, pp. 59. http://dx.doi.org/10.3389/fphar.2016.00059 PMid:27047378.
» http://dx.doi.org/10.3389/fphar.2016.00059 - AL MUQARRABUN, L.M.R. and AHMAT, N., 2015. Usos medicinais, fitoquímica e farmacologia da família Sterculiaceae: uma revisão. Revista Européia de Química Medicinal, vol. 92, pp. 514-530.
- ANTONIO, E.L., NASCIMENTO, A.J.D., LIMA, A.A.S.D., LEONART, M.S.S. and FERNANDES, Â., 2017. Genotoxicidade e citotoxicidade dos raios x em crianças submetidas à radiografia panorâmica. Revista Paulista de Pediatria : Orgao Oficial da Sociedade de Pediatria de Sao Paulo, vol. 35, no. 3, pp. 296-301. http://dx.doi.org/10.1590/1984-0462/;2017;35;3;00010 PMid:28977295.
» http://dx.doi.org/10.1590/1984-0462/;2017;35;3;00010 - BALOGUN, S.O., SILVA JÚNIOR, I.F., COLODEL, E.M., OLIVEIRA, R.G., ASCÊNCIO, S.D. and DE OLIVEIRA MARTINS, D.T., 2014. Toxicological evaluation of hydroethanolic extract of Helicteres sacarolha A. St.- Hil. et al. Journal of Ethnopharmacology, vol. 157, pp. 285-291. http://dx.doi.org/10.1016/j.jep.2014.09.013 PMid:25239833.
» http://dx.doi.org/10.1016/j.jep.2014.09.013 - CLANCY, S., 2008. DNA damage & repair: mechanisms for maintaining DNA integrity. Nature Education, vol. 1, no. 1, pp. 103.
- DRWAL, M.N., BANERJEE, P., DUNKEL, M., WETTIG, M.R. and PREISSNER, R., 2014. ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Research, vol. 42, no. W1, pp. W53-W58. http://dx.doi.org/10.1093/nar/gku401 PMid:24838562.
» http://dx.doi.org/10.1093/nar/gku401 - ERLUND, I., 2004. Review of the flavonoids quercetin, hesperetin, and naringenin: dietary sources, bioactivities, bioavailability, and epidemiology. Nutrition Research, vol. 24, no. 10, pp. 851-874. http://dx.doi.org/10.1016/j.nutres.2004.07.005
» http://dx.doi.org/10.1016/j.nutres.2004.07.005 - FERNANDES, D., ASSIS, E., SOUZA, M., SOUZA, P. and SOUZA, M., 2020a. Helicteres, L. species (Malvaceae sensu lato) as source of new drugs: a review. Quimica Nova In press. http://dx.doi.org/10.21577/0100-4042.20170533
» http://dx.doi.org/10.21577/0100-4042.20170533 - FERNANDES, D.A., BARROS, R.P.C., TELES, Y.C.F., OLIVEIRA, L.H.G., LIMA, J.B., SCOTTI, M.T., NUNES, F.C., CONCEIÇÃO, A.S. and SOUZA, M.D.F.V., 2019. Larvicidal compounds extracted from Helicteres velutina K. Schum (Sterculiaceae) evaluated against Aedes aegypti L. Molecules, vol. 24, no. 12, pp. 2315. http://dx.doi.org/10.3390/molecules24122315 PMid:31234501.
» http://dx.doi.org/10.3390/molecules24122315 - FERNANDES, D.A., OLIVEIRA, L.H.G., RIQUE, H.L., SOUZA, M.F.V. and NUNES, F.C., 2020b. Insights on the larvicidal mechanism of action of fractions and compounds from Aerial Parts of Helicteres velutina K. Schum against Aedes aegypti L. Molecules, vol. 25, no. 13, pp. 3015. http://dx.doi.org/10.3390/molecules25133015
» http://dx.doi.org/10.3390/molecules25133015 - GABRIEL, H.E., CROTT, J.W., GHANDOUR, H., DALLAL, G.E., CHOI, S.W., KEYES, M.K., JANG, H., LIU, Z., NADEAU, M., JOHNSTON, A., MAGER, D. and MASON, J.B., 2006. Chronic cigarette smoking is associated with diminished folate status, altered folate form distribution, and increased genetic damage in the buccal mucosa of healthy adults. The American Journal of Clinical Nutrition, vol. 83, no. 4, pp. 835-841. http://dx.doi.org/10.1093/ajcn/83.4.835 PMid:16600936.
» http://dx.doi.org/10.1093/ajcn/83.4.835 - GEVRENOVA, R., BADJAKOV, I., NIKOLOVA, M. and DOICHINOVA, I., 2013. Phenolic derivatives in raspberry (Rubus L.) germplasm collection in Bulgaria. Biochemical Systematics and Ecology, vol. 50, pp. 419-427. http://dx.doi.org/10.1016/j.bse.2013.06.002
» http://dx.doi.org/10.1016/j.bse.2013.06.002 - GOTO, T., HORITA, M., NAGAI, H., NAGATOMO, A., NISHIDA, N., MATSUURA, Y. and NAGAOKA, S., 2012a. Tiliroside, a glycosidic flavonoid, inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract. Molecular Nutrition & Food Research, vol. 56, no. 3, pp. 435-445. http://dx.doi.org/10.1002/mnfr.201100458 PMid:22173993.
» http://dx.doi.org/10.1002/mnfr.201100458 - GOTO, T., TERAMINAMI, A., LEE, J.Y., OHYAMA, K., FUNAKOSHI, K., KIM, Y.I., HIRAIA, S., UEMURA, T., YUC, R., TAKAHASHI, N. and KAWADA, T., 2012b. Tiliroside, a glycosidic flavonoid, ameliorates obesity-induced metabolic disorders via activation of adiponectin signaling followed by enhancement of fatty acid oxidation in liver and skeletal muscle in obese–diabetic mice. The Journal of Nutritional Biochemistry, vol. 23, no. 7, pp. 768-776.
- GROCHOWSKI, D.M., LOCATELLI, M., GRANICA, S., CACCIAGRANO, F. and TOMCZYK, M., 2018. Uma revisão sobre o tilirosídeo flavonóide da dieta. Revisões Abrangentes em Ciência e Segurança Alimentar, vol. 17, no. 5, pp. 1395-1421.
- HAN, N., GU, Y., YE, C., CAO, Y., LIU, Z. and YIN, J., 2012. Atividade antitrombótica de frações e componentes obtidos de folhas de framboesa (Rubus chingii). Química de Alimentos, vol. 132, no. 1, pp. 181-185.
- HIKAL, W.M., BAESHEN, R.S. and SAID-AL AHL, H.A., 2017. Botanical insecticide as simple extractives for pest control. Cogent Biology, vol. 3, no. 1, 1404274. http://dx.doi.org/10.1080/23312025.2017.1404274
» http://dx.doi.org/10.1080/23312025.2017.1404274 - HOOIJBERG, J.H., BROXTERMAN, H.J., HEIJN, M., FLES, D.L.A., LANKELMA, J. and PINEDO, H.M., 1997. Modulation by (iso) flavonoids of the ATPase activity of the multidrug resistance protein. FEBS Letters, vol. 413, no. 2, pp. 344-348. http://dx.doi.org/10.1016/S0014-5793(97)00940-X PMid:9280310.
» http://dx.doi.org/10.1016/S0014-5793(97)00940-X - HUANG, Q., HUANG, R., WEI, L., CHEN, Y., LV, S., LIANG, C., ZHANG, X., YIN, F., LI, H., ZHUO, L. and LIN, X., 2013. Antiviral activity of methyl helicterate isolated from Helicteres angustifolia (Sterculiaceae) against hepatitis B virus. Antiviral Research, vol. 100, no. 2, pp. 373-381. http://dx.doi.org/10.1016/j.antiviral.2013.09.007 PMid:24055834.
» http://dx.doi.org/10.1016/j.antiviral.2013.09.007 - INGÓLFSSON, H.I., THAKUR, P., HEROLD, K.F., HOBART, E.A., RAMSEY, N.B., PERIOLE, X., DE JONG, D.H., ZWAMA, M., YILMAZ, D., HALL, K., MARETZKY, T., HEMMINGS JUNIOR, H.C., BLOBEL, C., MARRINK, S.J., KOÇER, A., SACK, J.T. and ANDERSEN, O.S., 2014. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chemical Biology, vol. 9, no. 8, pp. 1788-1798. http://dx.doi.org/10.1021/cb500086e PMid:24901212.
» http://dx.doi.org/10.1021/cb500086e - JACKSON, S.P. and BARTEK, J., 2009. The DNA-damage response in human biology and disease. Nature, vol. 461, no. 7267, pp. 1071-1078. http://dx.doi.org/10.1038/nature08467 PMid:19847258.
» http://dx.doi.org/10.1038/nature08467 - JAIN, A., RANADE, R., PRITAM, P., JOSHI, N., VAVILALA, S.L. and JAIN, A., 2014. A comparative study of antioxidant activity, total phenolic and flavonoid contents in different parts of Helicteres isora L. American Journal of Life Sciences, vol. 2, no. 5, pp. 292-302. http://dx.doi.org/10.11648/j.ajls.20140205.17
» http://dx.doi.org/10.11648/j.ajls.20140205.17 - JIN, X., SONG, S., WANG, J., ZHANG, Q., QIU, F. and ZHAO, F., 2016. Tiliroside, the major component of Agrimonia pilosa Ledeb ethanol extract, inhibits MAPK/JNK/p38-mediated inflammation in lipopolysaccharide-activated RAW 264.7 macrophages. Experimental and Therapeutic Medicine, vol. 12, no. 1, pp. 499-505. http://dx.doi.org/10.3892/etm.2016.3305 PMid:27347085.
» http://dx.doi.org/10.3892/etm.2016.3305 - KASSIE, F., DARROUDI, F., KUNDI, M., SCHULTE‐HERMANN, R. and KNASMÜLLER, S., 2001. Khat (Catha edulis) consumption causes genotoxic effects in humans. International Journal of Cancer, vol. 92, no. 3, pp. 329-332. http://dx.doi.org/10.1002/ijc.1195 PMid:11291066.
» http://dx.doi.org/10.1002/ijc.1195 - KUMAR, N. and SINGH, A.K., 2014. Plant profile, phytochemistry and pharmacology of Avartani (Helicteres isora Linn.): a review. Asian Pacific Journal of Tropical Biomedicine, vol. 4, pp. S22-S26. http://dx.doi.org/10.12980/APJTB.4.2014C872
» http://dx.doi.org/10.12980/APJTB.4.2014C872 - LU, Y.H., CHEN, J., WEI, D.Z., WANG, Z.T. and TAO, X.Y., 2009. Tyrosinase inhibitory effect and inhibitory mechanism of tiliroside from raspberry. Journal of Enzyme Inhibition and Medicinal Chemistry, vol. 24, no. 5, pp. 1154-1160. http://dx.doi.org/10.1080/14756360802694252 PMid:19772488.
» http://dx.doi.org/10.1080/14756360802694252 - LUCA, V.S., MIRON, A. and APROTOSOAIE, A.C., 2016. The antigenotoxic potential of dietary flavonoids. Phytochemistry Reviews, vol. 15, no. 4, pp. 591-625. http://dx.doi.org/10.1007/s11101-016-9457-1
» http://dx.doi.org/10.1007/s11101-016-9457-1 - OBENG, E., 2021. Apoptosis (programmed cell death) and its signals: a review. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 81, no. 4, pp. 1133-1143. http://dx.doi.org/10.1590/1519-6984.228437
» http://dx.doi.org/10.1590/1519-6984.228437 - PASS ONLINE, 2014 [accessed 07 June 2020]. Biological Activity of tiliroside. [software]. Available from: www.pharmaexpert.ru/passonline
- POPOVA, L., KISHKILOVA, D., HADJIDEKOVA, V.B., HRISTOVA, R.P., ATANASOVA, P., HADJIDEKOVA, V.V., ZIYA, D. and HADJIDEKOV, V.G., 2007. Micronucleus test in buccal epithelium cells from patients subjected to panoramic radiography. Dento Maxillo Facial Radiology, vol. 36, no. 3, pp. 168-171. http://dx.doi.org/10.1259/dmfr/29193561 PMid:17463102.
» http://dx.doi.org/10.1259/dmfr/29193561 - PUBCHEM SUBSTANCE AND COMPOUND DATABASES – PUBCHEM, 2004 [viewed 07 June 2020]. Chemical structure of tiliroside. [online]. Available from: https://pubchem.ncbi.nlm.nih.gov
» https://pubchem.ncbi.nlm.nih.gov - RAKIB, A., AHMED, S., ISLAM, M.A., HAYE, A., UDDIN, S.N., UDDIN, M.M.N., HOSSAIN, M.K., PAUL, A. and EMRAN, T.B., 2019. Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Science & Nutrition, vol. 8, no. 1, pp. 547-556. http://dx.doi.org/10.1002/fsn3.1339 PMid:31993178.
» http://dx.doi.org/10.1002/fsn3.1339 - RANGEL, M., MALPEZZI, E.L., SUSINI, S.M. and DE FREITAS, J., 1997. Hemolytic activity in extracts of the diatom Nitzschia. Toxicon, vol. 35, no. 2, pp. 305-309. http://dx.doi.org/10.1016/S0041-0101(96)00148-1 PMid:9080587.
» http://dx.doi.org/10.1016/S0041-0101(96)00148-1 - RAO, Y.K., GEETHANGILI, M., FANG, S.H. and TZENG, Y.M., 2007. Antioxidant and cytotoxic activities of naturally occurring phenolic and related compounds: a comparative study. Food and Chemical Toxicology, vol. 45, no. 9, pp. 1770-1776. http://dx.doi.org/10.1016/j.fct.2007.03.012 PMid:17475387.
» http://dx.doi.org/10.1016/j.fct.2007.03.012 - SANTOS, E.A.D., CARVALHO, C.M.D., COSTA, A.L., CONCEIÇÃO, A.S., MOURA, F.D.B.P. and SANTANA, A.E.G., 2012. Bioactivity evaluation of plant extracts used in indigenous medicine against the snail, Biomphalaria glabrata, and the larvae of Aedes aegypti. Evidence-Based Complementary and Alternative Medicine, vol. 2012, pp. 846583. http://dx.doi.org/10.1155/2012/846583
» http://dx.doi.org/10.1155/2012/846583 - SELVARAJ, S., KRISHNASWAMY, S., DEVASHYA, V., SETHURAMAN, S. and KRISHNAN, U.M., 2015. Influence of membrane lipid composition on flavonoid–membrane interactions: implications on their biological activity. Progress in Lipid Research, vol. 58, pp. 1-13. http://dx.doi.org/10.1016/j.plipres.2014.11.002 PMid:25479162.
» http://dx.doi.org/10.1016/j.plipres.2014.11.002 - SPONCHIADO, G., ADAM, M.L., SILVA, C.D., SOLEY, B.S., MELLO-SAMPAYO, C., CABRINI, D.A., CORRER, C.J. and OTUKI, M.F., 2016. Quantitative genotoxicity assays for analysis of medicinal plants: A systematic review. Journal of Ethnopharmacology, vol. 178, pp. 289-296. http://dx.doi.org/10.1016/j.jep.2015.10.026 PMid:26680588.
» http://dx.doi.org/10.1016/j.jep.2015.10.026 - SWIFT, L.H. and GOLSTEYN, R.M., 2014. Genotoxic anti-cancer agents and their relationship to DNA damage, mitosis, and checkpoint adaptation in proliferating cancer cells. International Journal of Molecular Sciences, vol. 15, no. 3, pp. 3403-3431. http://dx.doi.org/10.3390/ijms15033403 PMid:24573252.
» http://dx.doi.org/10.3390/ijms15033403 - THOMAS, P., HARVEY, S., GRUNER, T. and FENECH, M., 2008. The buccal cytome and micronucleus frequency is substantially altered in Down’s syndrome and normal ageing compared to young healthy controls. Mutation Research. Fundamental and Molecular Mechanisms of Mutagenesis, vol. 638, no. 1-2, pp. 37-47. http://dx.doi.org/10.1016/j.mrfmmm.2007.08.012 PMid:17920640.
» http://dx.doi.org/10.1016/j.mrfmmm.2007.08.012 - TSUCHIYA, H., 2015. Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules, vol. 20, no. 10, pp. 18923-18966. http://dx.doi.org/10.3390/molecules201018923 PMid:26501254.
» http://dx.doi.org/10.3390/molecules201018923 - VAN GINKEL, G. and SEVANIAN, A., 1994. [28] Lipid peroxidation-induced membrane structural alterations. Methods in Enzymology, vol. 233, pp. 273-288. http://dx.doi.org/10.1016/S0076-6879(94)33031-X PMid:8015462.
» http://dx.doi.org/10.1016/S0076-6879(94)33031-X - VEBER, D.F., JOHNSON, S.R., CHENG, H.Y., SMITH, B.R., WARD, K.W. and KOPPLE, K.D., 2002. Molecular properties that influence the oral bioavailability of drug candidates. Journal of Medicinal Chemistry, vol. 45, no. 12, pp. 2615-2623. http://dx.doi.org/10.1021/jm020017n PMid:12036371.
» http://dx.doi.org/10.1021/jm020017n
Publication Dates
-
Publication in this collection
21 June 2021 -
Date of issue
2023
History
-
Received
30 Sept 2020 -
Accepted
30 Dec 2020