Abstract
Chromium (VI) a highly toxic metal, a major constituent of industrial waste. It is continuously release in soil and water, causes environmental and health related issues, which is increasing public concern in developing countries like Pakistan. The basic aim of this study was isolation and screening of chromium resistant bacteria from industrial waste collected from Korangi and Lyari, Karachi (24˚52ʹ46.0ʺN 66˚59ʹ25.7ʺE and 24˚48ʹ37.5ʺN 67˚06ʹ52.6ʺE). Among total of 53 isolated strains, seven bacterial strains were selected through selective enrichment and identified on the basis of morphological and biochemical characteristics. These strains were designated as S11, S13, S17, S18, S30, S35 and S48, resistance was determined against varying concentrations of chromium (100-1500 mg/l). Two bacterial strains S35 and S48 showed maximum resistance to chromium (1600 mg/l). Bacterial strains S35 and S48 were identified through 16S rRNA sequence and showed 99% similarity to Bacillus paranthracis and Bacillus paramycoides. Furthermore, growth condition including temperature and pH were optimized for both bacterial strains, showed maximum growth at temperature 30ºC and at optimum pH 7.5 and 6.5 respectively. It is concluded that indigenous bacterial strains isolated from metal contaminated industrial effluent use their innate ability to transform toxic heavy metals to less or nontoxic form and can offer an effective tool for monitoring heavy metal contamination in the environment.
Keywords:
chromium resistance; industrial waste; Bacillus paramycoides, Bacillus paranthracis
Resumo
O cromo (VI), metal altamente tóxico, é um dos principais constituintes dos resíduos industriais. É liberado no solo e na água, causa problemas ambientais e de saúde de crescente preocupação pública em países em desenvolvimento como o Paquistão. O objetivo básico deste estudo foi o isolamento e a triagem de bactérias resistentes ao cromo de resíduos industriais coletados em Korangi e Lyari, Karachi (24˚52’46,0”N 66˚59’25,7”E e 24˚48’37,5”N 67˚06’52,6”E). Do total de 53 cepas isoladas, sete cepas bacterianas foram selecionadas por enriquecimento seletivo e identificadas com base em características morfológicas e bioquímicas. Essas cepas foram designadas como S11, S13, S17, S18, S30, S35 e S48, apresentaram alta resistência aos metais contra concentrações variáveis (100-1500 mg / l) de cromo. Já as cepas S35 e S48 foram identificadas por meio da sequência 16S rRNA e apresentaram 99% de similaridade com Bacillus paranthracis e Bacillus paramycoides. Além disso, as condições de crescimento incluindo temperatura e pH foram otimizadas e ambas as cepas bacterianas apresentaram crescimento máximo na temperatura de 30 ºC, enquanto seu pH ótimo foi observado em 7,5 e 6,5, respectivamente. Conclui-se que o potencial de resistência dessas bactérias resistentes ao cromo pode ser efetivamente utilizado na remoção de cromo de efluentes industriais contaminados. Técnicas de base biológica usando bactérias ajudarão a fornecer métodos mais baratos e ecológicos de remoção, recuperação e desintoxicação de cromo.
Palavras-chave:
resistência ao cromo; resíduos industriais; Bacillus paramycoides; Bacillus paranthracis
1. Introduction
In last few decades, the increase concentration of different heavy metals causes environmental contaminations and devastating effect on public health. Human expose to metals due to increase of heavy metals in various industry such as textile, painting, leather etc. Effluents releasing from these industries have profound effect on human health as well as environment (Raskin and Ensley, 2000RASKIN, I. and ENSLEY, B.D., 2000. Phytoremediation of toxic metals. Nova Jersey: John Wiley and Sons.). Accumulation of potentially toxic heavy metals i.e. Hg, Cd, Cr, Cu and Zn in a body causes mental abnormalities, neuromuscular disorders, cancer and growth abnormalities (Wuana and Okieimen, 2011WUANA, R.A. and OKIEIMEN, F.E., 2011. Heavy metals in contaminated soils: A review of sources, chem. risks and best available strategies for remediation. ISRN Ecology, vol. 2011, pp. 1-20. http://dx.doi.org/10.5402/2011/402647.
http://dx.doi.org/10.5402/2011/402647...
). Chromium is used in electroplating, tanning, textile dyeing, and metal processing industries. Industrial wastewater and effluents contain chromium, which has toxic effects on the microbial consortia of wastewater treatment systems (Stasinakis et al., 2003STASINAKIS, A.S., THOMAIDIS, N.S., MAMAIS, D., PAPANIKOLAOU, E.C., TSAKON, A. and LEKKAS, T.D., 2003. Effects of chromium (VI) addition on the activated sludge process. Water Research, vol. 37, no. 9, pp. 2140-2148. http://dx.doi.org/10.1016/S0043-1354(02)00623-1. PMid:12691900.
http://dx.doi.org/10.1016/S0043-1354(02)...
).
Naturally, Chromium exists in different oxidation states varying from Cr(II) to Cr(VI). Cr(III)is not highly toxic and has high absorbance capacity in water and soil while, Cr(VI) is the toxic form, highly soluble and not easily adsorbed in natural bodies (Kotas and Stasicka, 2000KOTAŚ, J. and STASICKA, Z., 2000. Chromium occurrence in the environment and methods of its speciation. Environmental Pollution, vol. 107, no. 3, pp. 263-283. http://dx.doi.org/10.1016/S0269-7491(99)00168-2. PMid:15092973.
http://dx.doi.org/10.1016/S0269-7491(99)...
). The permissible limit of Cr in drinking water is 0.05 mg/L according to the World Health Organization (WHO, 1993WORLD HEALTH ORGANIZATION – WHO. (1993). Guidelines for drinking-water quality. Geneva: WHO.).
To reduce the toxicity of chromium in the environment, industrial effluents must be treated before release. Many conventional methods have been adopted for removing metals from industrial effluents i.e. chemical (precipitation, oxidation or reduction, ion exchange) and physical(filtration and membrane technologies) (Ahluwalia and Goyal, 2007AHLUWALIA, S.S. and GOYAL, D., 2007. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresource Technology, vol. 98, no. 12, pp. 2243-2257. http://dx.doi.org/10.1016/j.biortech.2005.12.006. PMid:16427277.
http://dx.doi.org/10.1016/j.biortech.200...
).These technologies are effective only when high concentration is needed to be treated, ineffective, and highly expensive for treating metal concentration in wastewater below the range of 100 mg/L (Nourbakhsh et al., 1994NOURBAKHSH, M., SAG, Y., ÖZER, D., AKSU, Z., KUTSAL, T. and CAGLAR, A., 1994. A comparative study of various biosorbents for removal of chromium (VI) ions from industrial waste waters. Process Biochemistry, vol. 29, no. 1, pp. 1-5. http://dx.doi.org/10.1016/0032-9592(94)80052-9.
http://dx.doi.org/10.1016/0032-9592(94)8...
).Therefore, these technologies need to be replaced with highly effective, cheaper and eco-friendly techniques, which can effectively remove very small concentration of toxic heavy metals. A large number of microorganisms are found naturally in waters and soil receiving industrial effluents. These microorganisms have adopted and developed various strategies as a defense mechanism to protect themselves from the toxicity of heavy metals (adsorption, uptake, methylation, oxidation, and reduction). Accumulation of Cr (VI) in agriculture lands reduces soil productivity by decreasing the population of various soil microbes (Karthik et al., 2017KARTHIK, C., OVES, M., SATHYA, K., SRI RAMKUMAR, V. and ARULSELVI, P.I., 2017. Isolation and characterization of multi-potential Rhizobium strain ND2 and its plant growth-promoting activities under Cr (VI) stress. Archives of Agronomy and Soil Science, vol. 63, no. 8, pp. 1058-1069. http://dx.doi.org/10.1080/03650340.2016.1261116.
http://dx.doi.org/10.1080/03650340.2016....
; Wani et al., 2018WANI, P.A., SUNDAY, O.O., KEHINDE, A.M., OLUWASEYI, L.A., WASIU, I.A. and WAHID, S., 2018. Antioxidants and chromium reductases by Penibacillus species enhance the growth of soybean under chromium stress. International Journal of Environmental Science and Technology, vol. 15, no. 7, pp. 1531-1542. http://dx.doi.org/10.1007/s13762-017-1533-6.
http://dx.doi.org/10.1007/s13762-017-153...
). Chromium is carcinogenic and mutagenic, designated as priority pollutant or Class A pollutant by the United States environmental protection agency (USEPA, 1996UNITED STATES ENVIRONMENTAL PROTECTION AGENCY – USEPA. (1996). The metals translator: guidance for converting atotal recoverable permit limit from a dissolved criterion. USA: USEPA, vol. 823. Office of Water, EPA (96-007).).
Microbial reduction of hexavalent chromium to trivalent chromium is an important and cost effective remediation technology (Wang et al., 2013WANG, Y., YANG, Z., PENG, B., CHAI, L., WU, B. and WU, R., 2013. Biotreatment of chromite ore processing residue by Pannonibacterphragmitetus BB. Environmental Science and Pollution Research International, vol. 20, no. 8, pp. 5593-5602. http://dx.doi.org/10.1007/s11356-013-1526-z. PMid:23440437.
http://dx.doi.org/10.1007/s11356-013-152...
), necessary for glucose metabolism (Vincent, 2000VINCENT, J.B., 2000. Elucidating a biological role for chromium at a molecular level. Accounts of Chemical Research, vol. 33, no. 7, pp. 503-510. http://dx.doi.org/10.1021/ar990073r. PMid:10913239.
http://dx.doi.org/10.1021/ar990073r...
), enzymatic activation and DNA and RNA stabilization (Karuppanapandian et al., 2009KARUPPANAPANDIAN, T., SINHA, P.B., KAMARUL, H.A. and MANOHARAN, K., 2009. Chromium-induced accumulation of peroxide content, stimulation of antioxidative enzymes and lipid peroxidation in green gram (Vignaradiata L. cv. Wilczek) leaves. African Journal of Biotechnology, vol. 8, no. 3). Chromium (VI) remediation has been reported in both soil and water contaminated with metal by bacterium Pannonibacter phragmitetus BB (Wang et al., 2014WANG, Y., PENG, B., YANG, Z., TANG, C., CHEN, Y., LIAO, Q. and LIAO, Y., 2014. Treatment of Cr (VI) contaminated water with Pannonibacterphragmitetus BB. Environmental Earth Sciences, vol. 71, no. 10, pp. 4333-4339. http://dx.doi.org/10.1007/s12665-013-2827-8.
http://dx.doi.org/10.1007/s12665-013-282...
).Removal of Cr(VI) either by reduction or via biosorption can significantly reduce the risks to human health (Kamaludeen et al., 2003KAMALUDEEN, S. P. B., ARUNKUMAR, K. R., and RAMASAMY, K., 2003. Bioremediation of chromium contaminated environments. Bioremediation of Chromium Contaminated Environments, vol. 41, pp. 972-985.).The reduction of hexavalent Cr to trivalent Cr is a viable process, mediated by chromate reductase under aerobic conditions via its cytosolic form and in anaerobic respiration through its membrane-bound component (Camargo et al. 2004CAMARGO, F.A.O., BENTO, F.M., OKEKE, B.C. and FRANKENBERGER, W.T., 2004. Hexavalent chromium reduction by an actinomycete, Arthrobactercrystallopoietes ES 32. Biological Trace Element Research, vol. 97, no. 2, pp. 183-194. http://dx.doi.org/10.1385/BTER:97:2:183. PMid:14985627.
http://dx.doi.org/10.1385/BTER:97:2:183...
).
Main water pollution sources in Pakistan are the industrial waste released by different industries i.e. of textile, paints, chemical, leather and paper etc. (Waseem et al., 2014WASEEM, A., ARSHAD, J., IQBAL, F., SAJJAD, A., MEHMOOD, Z. and MURTAZA, G., 2014. Pollution status of Pakistan: a retrospective review on heavy metal contamination of water, soil, and vegetables. BioMed Research International, vol. 2014, pp. 813206. http://dx.doi.org/10.1155/2014/813206. PMid:25276818.
http://dx.doi.org/10.1155/2014/813206...
). In Pakistan, Karachi is the largest contributor for heavy metal pollution as approximately 8000 industries running in 9 industrial estates released 362 million gallons per day sewage. Total of 60% of this industrial waste released directly into Korangi and Lyari River without treatment and disposed-off into coastal belt of Karachi (World Bank, 2006WORLD BANK, 2006. Pakistan: strategic Country Environmental Assessment Report: rising to the challenges. Washington: World Bank.). Lacking the waste treatment facility in industries of billion-dollar business is responsible for ruining environment by releasing excessive chemicals in the water and soil which in turn harmfully affect the public health (Sundar et al., 2010SUNDAR, K., VIDYA, R., MUKHERJEE, A. and CHANDRASEKARAN, N., 2010. High chromium tolerant bacterial strains from Palar River Basin: impact of tannery pollution. Research Journal of Environmental and Earth Sciences, vol. 2, no. 2, pp. 112-117.). The solution to this pollution control is the installation of effective treatment plant, which is efficient, cost-effective, simple and ecofriendly.
Keeping in view the toxicity of chromium, it needs to analyze industrial wastes for the isolation and screening of indigenous bacteria and to explore their role in bioremediation of toxic chromium in such industrial wastes. The objectives of this study are; isolation and screening of indigenous chromium resistant bacteria from industrial waste and optimization of growth condition for resistant strains.
2. Materials and Methods
2.1. Collection of chromium contaminated Industrial Waste Samples
Samples were collected from two different sites, River bank of Lyari (24˚52ʹ46.0ʺN 66˚59ʹ25.7ʺE) and Korangi industrial area (24˚48ʹ37.5ʺN 67˚06ʹ52.6ʺE) located in Karachi, Pakistan. Soil samples were collected in triplicate down to 10 cm depth in sterile zippered bags and water/ sludge samples were collected in sterile plastic bottles using standard sampling protocols (Carter and Gregorich, 2007CARTER, M.R., & GREGORICH, E.G., 2007.Soil sampling and methods of analysis. CRC press.). Samples were kept at 4oC and immediately transported to the Applied, Environmental and Geomicrobiology lab, Quaid-i-Azam University, Islamabad.
2.1.1. Physico-chemical and elemental analysis of samples
During sample collection temperature and pH were determined by mean of thermometer and pH meter electrode respectively. Moisture content in soil and sludge samples was monitored by dry oven method. For elemental analysis samples were prepared following standard protocol with minor modification (Cantle, 1986CANTLE, J.E. (Ed.), 1986. Atomic absorption spectrometry. Elsevier.). All soil samples (20 g) were dried over night at 60°C and grounded/sieved to small particles. Then one gram of each grounded soil samples was mixed in 15 ml of HNO3 and HCl (1:3), boiled for 30 minutes, and then left overnight. Then 5ml of HClO4 was added and boiled until the total volume was reduced to 3–5 ml. After cooling, the mixture was filtered through Whatman filter paper (No 42) and then volume was brought up to 20 ml with sterile deionized water. As a control, an ordinary garden soil sample was similarly collected and processed. A blank was processed in the same manner without the addition of any soil and its values were subtracted from the rest of the experimental values in order to remove procedural errors. All samples were processed and analyzed for presence of heavy metals concentration i.e Chromium, Copper, Nickel and cobalt by atomic absorption spectroscopy (AA-7000, Shimadzu, Japan) (Din et al., 2020DIN, G., HASSAN, A., RAFIQ, M., HASAN, F., BADSHAH, M., KHAN, S., CHEN, G., RIPP, S. and SHAH, A.A., 2020. Characterization of Organic Acid Producing Aspergillus tubingensis FMS1 and its Role in Metals Leaching from Soil. Geomicrobiology Journal, vol. 37, no. 4, pp. 336-344. http://dx.doi.org/10.1080/01490451.2019.1701585.
http://dx.doi.org/10.1080/01490451.2019....
).
2.2. Isolation and enumeration of bacteria
For isolation of bacteria the soil, water and sludge samples were ten-fold diluted ranging from 10-1 to 10-10. A 100µl of sample from each dilution was shifted aseptically with the help of micropipette to separate nutrient agar plate. Plates were then incubated for 24 h at 30°C. After incubation, the plates were observed for distinct colonies with varying colony morphology and culture characteristics.
2.3. Characterization of bacteria
All the isolated strains were examined for colony morphology in terms of shape, elevation, margin, surface and size whereas microscopic examination was carried out by mean of Gram staining. Different biochemical tests were performed for S35 and S48 strains i.e. oxidase test, catalase test, indole production, methyl red, Voges Proskauer, Urease test and citrate (Barrow and Feltham, 1993BARROW, G.I., and FELTHAM, R.K.A., 1993. Cowan and Steel’s manual for the identification of medical bacteria. Cambridge: Cambridge University Press.).
2.4. Primary screening for Chromium resistant bacteria
For screening of resistance bacteria, all the bacterial isolates were spread separately on nutrient agar plates containing 100 ppm of (K2Cr2O7) in the medium. Chromium amended nutrient agar media were prepared in distilled water; pH was adjusted to 7.5. The plates were incubated at 30°C for 24 h and growth of the bacterial colonies was observed after incubation.
2.5. Chromium resistance determination
2.5.1 Minimum inhibitory concentration (MIC)
Minimum inhibitory concentrations of chromium against the tested isolates were determined by the plate dilution method as described earlier (Alam and Malik, 2008ALAM, M.Z. and MALIK, A., 2008. Chromate resistance, transport and bioreduction by Exiguobacterium sp. ZM‐2 isolated from agricultural soil irrigated with tannery effluent. Journal of Basic Microbiology, vol. 48, no. 5, pp. 416-420. http://dx.doi.org/10.1002/jobm.200800046. PMid:18759228.
http://dx.doi.org/10.1002/jobm.200800046...
). K2Cr2O7 was used in the medium with increasing concentrations ranging from 100-1600 ppm. The bacterial strains were inoculated and incubated at 30°C for 24 h.
2.6. Optimization of growth condition
The effect of temperature and pH were determined for maximum growth of chromium resistance bacteria strains S35 and S48. The effect of temperature and pH were carried out by incubating strains S35 and S48 with 200ppm chromium concentration and 1ml inoculum size at different temperature from 10 to 50ºC and pH5.5 to 9.5 for 72 h. Growth optical density at 600 nm for both strains were carried out at after 24 hours of time intervals. Incubation time of bacterial strain S35 and S48 were studied in 250 ml flasks containing 50 ml of nutrient broth supplemented with K2Cr2O7 (200ppm) and control (without chromium). Flasks were then inoculated with 100μl of overnight culture and agitated on a rotary shaker at 150 rpm. Growth was monitored as a function of biomass by measuring the absorbance at 600 nm using spectrophotometer at different time intervals 2, 4, 6, 8, 10, 24, 48 and 72 h (Shaikh and Qureshi, 2013SHAIKH, Z. and QURESHI, P., 2013. Screening and isolation of organic acid producers from samples of diverse habitats. International Journal of Current Microbiology and Applied Sciences, vol. 2, no. 9, pp. 39-44.).
2.7. Statistical analysis
All experiments were carried out in triplicate and data were plotted in excel sheet version 2010 and mean value was determined by standard deviation.
3. Results and Discussion
3.1. Physico-chemical analysis of soil
Four different parameters (temperature, pH, moisture content, and metal concentration) were examined through physical and chemical analysis of soil samples. Temperature and pH of different sampling sites of Korangi was recorded as 30°C and pH-8.0 for water and sludge samples, whereas soil sample from Lyari, was recorded as 45oC and pH 7.0 and its moisture content was calculated as 3.4% (Table 1). The chromium concentration of samples was recorded by mean of atomic absorption spectroscopy (AA-7000, Shimadzu, Japan). The chromium concentration in Lyari sample was 2317.235 mg/kg, while in Korangi sample it was found 2648.644 mg/kg. Similar study was conducted in which high concentration of chromium and other metals in the industrial waste was studied (Wani and Khan, 2010WANI, P.A. and KHAN, M.S., 2010. Bacillus species enhance growth parameters of chickpea (Cicerarietinum L.) in chromium stressed soils. Food and Chemical Toxicology, vol. 48, no. 11, pp. 3262-3267. http://dx.doi.org/10.1016/j.fct.2010.08.035. PMid:20813149.
http://dx.doi.org/10.1016/j.fct.2010.08....
). Zahoor and Rehman (2009)ZAHOOR, A. and REHMAN, A., 2009. Isolation of Cr (VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. Journal of Environmental Sciences (China), vol. 21, no. 6, pp. 814-820. http://dx.doi.org/10.1016/S1001-0742(08)62346-3. PMid:19803088.
http://dx.doi.org/10.1016/S1001-0742(08)...
reported Cr (VI) in the waste water to be in the range 0.70-1.84 g/ml. Wang et al. (2014)WANG, Y., PENG, B., YANG, Z., TANG, C., CHEN, Y., LIAO, Q. and LIAO, Y., 2014. Treatment of Cr (VI) contaminated water with Pannonibacterphragmitetus BB. Environmental Earth Sciences, vol. 71, no. 10, pp. 4333-4339. http://dx.doi.org/10.1007/s12665-013-2827-8.
http://dx.doi.org/10.1007/s12665-013-282...
also found high concentration of metals including Cr (VI) in the industrial wastewater which was found to be 534 mg/l. Korangi sample was slightly basic in nature. Generally, microorganisms are found unable to tolerate pH levels above 9.5 or lower than 4.0 (Sardrood et al., 2013SARDROOD, B.P., GOLTAPEH, E.M. and VARMA, A., 2013. An introduction to bioremediation. In: E. GOLTAPEH, Y. DANESH and A. VARMA, eds. Fungi as bioremediators (pp. 3-27). Berlin, Heidelberg: Springer. http://dx.doi.org/10.1007/978-3-642-33811-3_1.
http://dx.doi.org/10.1007/978-3-642-3381...
). Normally, the optimum pH for bacterial growth is between 6.5 and 7.5. Similar results reported by other researchers (Norzatulakma, 2010NORZATULAKMA, M.K., 2010. Treatment of industrial wastewater at Gebeng area using Eichornia Crassipes sp. (Water Hyacinth), Pistia Stratiotessp. (Water Lettuce) and Salvinia Molesta sp. (Giant Salvinia). Pahang, Malaysia: Universiti Malaysia Pahang.; Sobahan et al., 2013SOBAHAN, M.A., MIR, S.I., ZAKARIA, I. and HOSSAIN, M. (2013, May). Surface water contamination due to industrial activities in gebeng area, Kuantan, Malaysia. In: Proceedings of the International Conference on Civil and Architecture Engineering, May 2013, Barcelona, Spain. ICCEA, pp. 6-7.; Sujaul et al., 2013SUJAUL, I.M., HOSSAIN, M.A., NASLY, M.A. and SOBAHAN, M.A., 2013. Effect of industrial pollution on the spatial variation of surface water quality. American Journal of Environmental Sciences, vol. 9, no. 2, pp. 120-129. http://dx.doi.org/10.3844/ajessp.2013.120.129.
http://dx.doi.org/10.3844/ajessp.2013.12...
).
3.2. Isolation and enumeration of bacteria
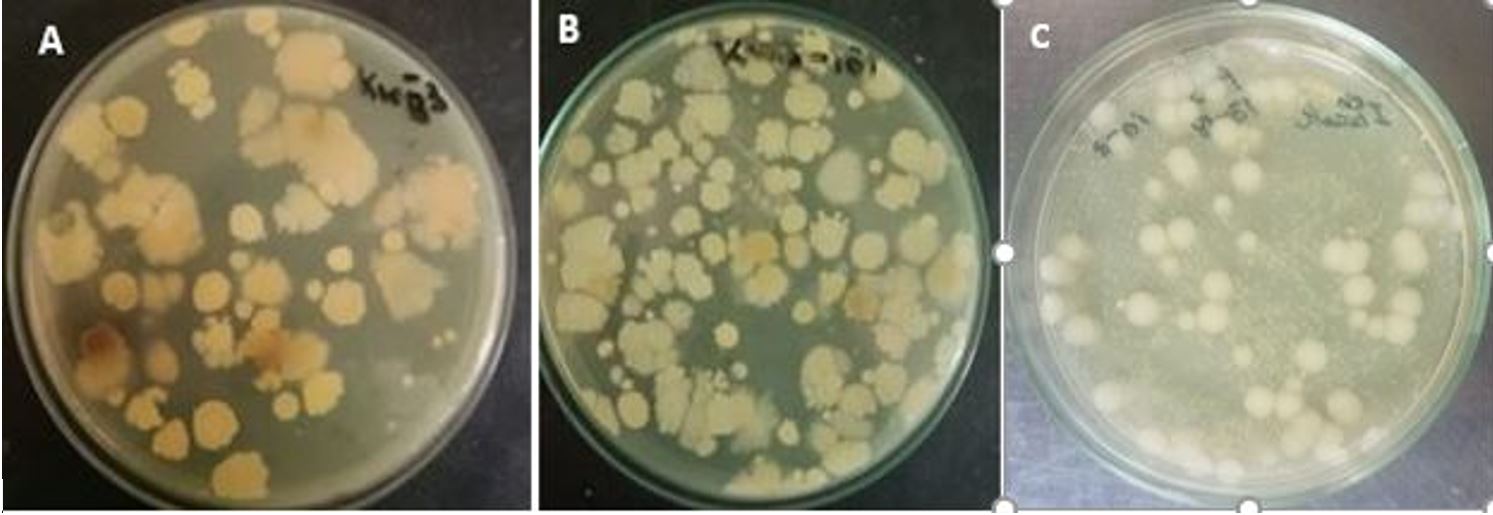
In this study, the bacterial population present in the samples was range from 250 – 350 colonies/ml. A total of seven bacterial colonies were selected in which 5 strains were from Korangi sample (water and sludge), 2 strains from Lyari sample (Figure 1). Colonies forming unit/ml were calculated as 2×10-6 for Korangi sludge and 3×10-5 Korangi water while 5×10-4 for Lyaris soil ample and used for enumeration of bacterial population in industrial waste. Similarly, CFU for industrial effluent was calculated as 1.68 × 10-4 CFU/ml from 10-1 dilution (Sanjay et al., 2018SANJAY, M. S., SUDARSANAM, D., RAJ, G. A., and BASKAR, K., 2018. Isolation and identification of chromium reducing bacteria from tannery effluent. Journal of King Saud University-Science, vol. 32, no. 1, pp. 265-271. https://doi.org/10.1016/j.jksus.2018.05.001.
https://doi.org/10.1016/j.jksus.2018.05....
). Bacterial population in industrial effluents were present approximately 200 – 300 colonies per 100 ml at different sample location (Mustapha and Halimoon, 2015MUSTAPHA, M.U. and HALIMOON, N., 2015. Screening and isolation of heavy metal tolerant bacteria in industrial effluent. Procedia Environmental Sciences, vol. 30, pp. 33-37. http://dx.doi.org/10.1016/j.proenv.2015.10.006.
http://dx.doi.org/10.1016/j.proenv.2015....
). The industrial effluent contains chromium and protein, which makes it an ideal medium for many bacterial species to grow (Saranraj et al., 2013SARANRAJ, P., SIVASAKTHIVELAN, P. and SAKTHI, S.S., 2013. Prevalence and production of plant growth promoting substance by Pseudomonas fluorescens isolated from paddy rhizosphere soil of Cuddalore district, Tamil Nadu, India. African Journal of Basic and Applied Sciences, vol. 5, no. 2, pp. 95-101.).
Number of colonies present in (A) Lyari soil sample (B) Korangi sludge (C) Korangi water sample.
3.3. Primary screening for Chromium resistant bacteria
For screening of chromium resistance all seven isolates were grown on media amended with 100ppm of chromium. All isolates were found resistant to chromium at concentration of 100ppm (Figure 2). A similar study was conducted in 2015, in which six isolates were identified that were tolerant to chromium at a concentration of 40ppm (Mustapha and Halimoon, 2015MUSTAPHA, M.U. and HALIMOON, N., 2015. Screening and isolation of heavy metal tolerant bacteria in industrial effluent. Procedia Environmental Sciences, vol. 30, pp. 33-37. http://dx.doi.org/10.1016/j.proenv.2015.10.006.
http://dx.doi.org/10.1016/j.proenv.2015....
). Many researchers have reported metal resistance in bacteria isolated from wastewater and soil polluted with different heavy metals (Alam and Malik, 2008ALAM, M.Z. and MALIK, A., 2008. Chromate resistance, transport and bioreduction by Exiguobacterium sp. ZM‐2 isolated from agricultural soil irrigated with tannery effluent. Journal of Basic Microbiology, vol. 48, no. 5, pp. 416-420. http://dx.doi.org/10.1002/jobm.200800046. PMid:18759228.
http://dx.doi.org/10.1002/jobm.200800046...
; Abou-Shanab et al., 2007ABOU-SHANAB, R.A.I., VAN BERKUM, P. and ANGLE, J.S., 2007. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere, vol. 68, no. 2, pp. 360-367. http://dx.doi.org/10.1016/j.chemosphere.2006.12.051. PMid:17276484.
http://dx.doi.org/10.1016/j.chemosphere....
). Shakoori and Muneer (2002)SHAKOORI, A.R. and MUNEER, B., 2002. Copper-resistant bacteria from industrial effluents and their role in remediation of heavy metals in wastewater. Folia Microbiologica, vol. 47, no. 1, pp. 43-50. http://dx.doi.org/10.1007/BF02818564. PMid:11980269.
http://dx.doi.org/10.1007/BF02818564...
isolated bacteria from waste water and found that all isolates were resistant to Cr6+, Cd2+, Hg2+, Zn2+ etc. with varying degree of MIC values. In a study, 46 bacterial isolates from rhizospheric soil were tested for their ability to tolerate cadmium, chromium, zinc, mercury, lead, cobalt, copper and nickel. All the 46 isolates were found to be resistant to Ni, Pb, and Zn whereas 53% and 42% bacteria were resistant to Cr6+ and Cd2+, respectively (Abou-Shanab et al., 2007ABOU-SHANAB, R.A.I., VAN BERKUM, P. and ANGLE, J.S., 2007. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere, vol. 68, no. 2, pp. 360-367. http://dx.doi.org/10.1016/j.chemosphere.2006.12.051. PMid:17276484.
http://dx.doi.org/10.1016/j.chemosphere....
).
3.4. Characterization and identification of bacteria
The morphological characteristics of chromium resistant bacterial strains shown in (Table 2, Figure 3). Biochemical tests of strain S35 showed positive result for catalase, Voges Proskauer urease and citrate while negative for oxidase, indole and methyl red, similarly bacterial strain S48 showed positive results for catalase, indole and methyl red, while negative for oxidase, Voges Proskauer, urease and citrate. Identification of bacterial strain S35 and S48 were analyzed according to Bergey’s Manual of Determinative Bacteriology (Barrow and Feltham, 1993BARROW, G.I., and FELTHAM, R.K.A., 1993. Cowan and Steel’s manual for the identification of medical bacteria. Cambridge: Cambridge University Press.; Bergey et al., 1974BERGEY, D.H., BUCHANAN, R.E. and GIBBONS, N.E. (1974). Bergey’s Manual of Determinative Bacteriology. Baltimore: Williams and Wilkins Co., pp 1246.) and identified as Bacillus paranthracis and Bacillus paramycoides respectively.
3.5. Chromium resistance determination
3.5.1. Minimum inhibitory concentration (MIC)
All seven strains were selected based on their ability of resistance against 100ppm of chromium and their minimal inhibitory concentration was determined by increasing concentrations of chromium in media ranging at 100-1600ppm (Table 3). All Seven strains were selected based on their strong resistance capability at 1500ppm. Only S35 (Bacillus paranthracis) and S48 (Bacillus paramycoides) showed growth at 1600ppm (Figure 3). In a study Minimum inhibitory concentration (MIC) for heavy metal i.e. Cr, Pb and Cd were examined ranging from 50 to 1900 µg/ml. It was found that all isolates exhibited resistance to heavy metals (Marzan et al., 2017MARZAN, L.W., HOSSAIN, M., MINA, S.A., AKTER, Y. and CHOWDHURY, A.M.A., 2017. Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: bioremediation viewpoint. The Egyptian Journal of Aquatic Research, vol. 43, no. 1, pp. 65-74. http://dx.doi.org/10.1016/j.ejar.2016.11.002.
http://dx.doi.org/10.1016/j.ejar.2016.11...
).
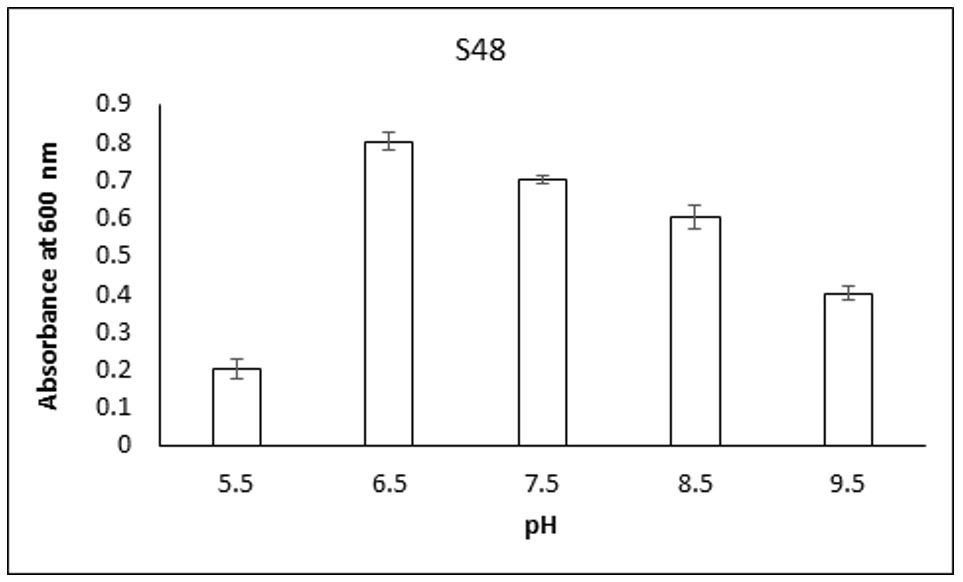
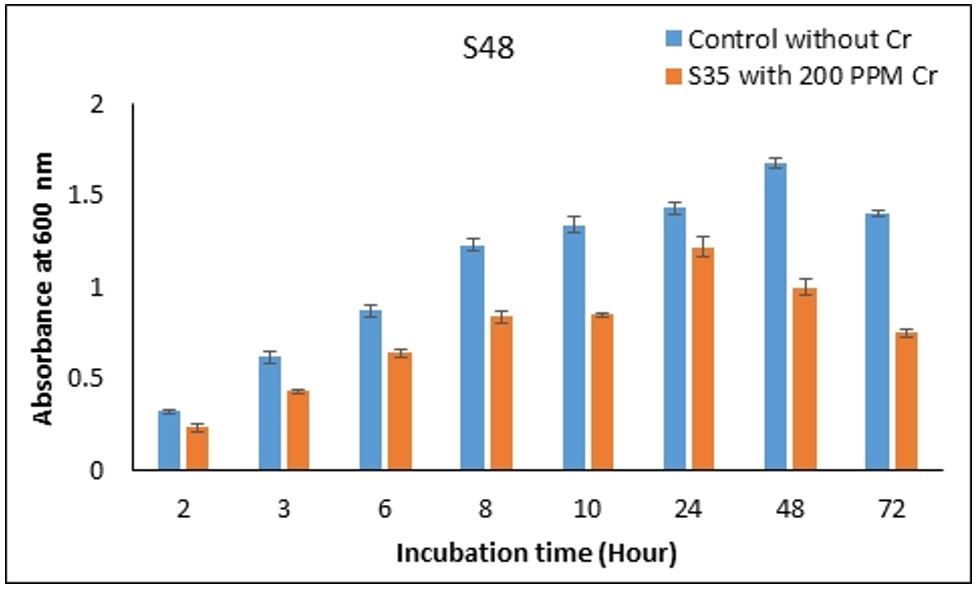
3.6. Optimization of growth condition
Growth condition in respective to temperature and pH were optimized for S35 and S48 strain which showed maximum resistance at 1600ppm. The most suitable temperature for Cr-resistant strains S35 and S48 were observed at 30°C (Figures 4, 5). Whereas strain S35 showed maximum growth at pH 7.5 and S48 showed maximum growth at pH 6.5 (Figures 6, 7). Growth curve pattern help to use bacteria for biomass production and bioremediation purposes. Both S35 and S48 strains were grown in presence of K2Cr207 (200ppm) and compared with the control culture (without Cr). The growth pattern of both strains were not significantly different from control growth (Figures 8, 9). Optimization of growth parameters such as pH, temperature and incubation time have profound effect on metal resistance capacity of bacterial strains (Shiva et al., 2014). Cr(VI) reduction (direct or indirect) is affected by mean of various parameters such as pH, temperature, chromium concentration, incubation time and Inoculum size (Soni et al., 2013SONI, S.K., SINGH, R., AWASTHI, A., SINGH, M. and KALRA, A., 2013. In vitro Cr (VI) reduction by cell-free extracts of chromate-reducing bacteria isolated from tannery effluent irrigated soil. Environmental Science and Pollution Research International, vol. 20, no. 3, pp. 1661-1674. http://dx.doi.org/10.1007/s11356-012-1178-4. PMid:22983604.
http://dx.doi.org/10.1007/s11356-012-117...
).
4. Conclusion
The strains S35 and S48 were found resistant to 1600ppm of chromium concentration. The indigenous bacterial strains isolated from metal contaminated industrial effluent use their innate ability to transform toxic heavy metals to less or nontoxic form. The potential ability of metallo-resistant bacteria against different heavy metals may offer an effective tool for monitoring heavy metal contamination in the environment.
References
- ABOU-SHANAB, R.A.I., VAN BERKUM, P. and ANGLE, J.S., 2007. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere, vol. 68, no. 2, pp. 360-367. http://dx.doi.org/10.1016/j.chemosphere.2006.12.051 PMid:17276484.
» http://dx.doi.org/10.1016/j.chemosphere.2006.12.051 - AHLUWALIA, S.S. and GOYAL, D., 2007. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresource Technology, vol. 98, no. 12, pp. 2243-2257. http://dx.doi.org/10.1016/j.biortech.2005.12.006 PMid:16427277.
» http://dx.doi.org/10.1016/j.biortech.2005.12.006 - ALAM, M.Z. and MALIK, A., 2008. Chromate resistance, transport and bioreduction by Exiguobacterium sp. ZM‐2 isolated from agricultural soil irrigated with tannery effluent. Journal of Basic Microbiology, vol. 48, no. 5, pp. 416-420. http://dx.doi.org/10.1002/jobm.200800046 PMid:18759228.
» http://dx.doi.org/10.1002/jobm.200800046 - BARROW, G.I., and FELTHAM, R.K.A., 1993. Cowan and Steel’s manual for the identification of medical bacteria Cambridge: Cambridge University Press.
- BERGEY, D.H., BUCHANAN, R.E. and GIBBONS, N.E. (1974). Bergey’s Manual of Determinative Bacteriology Baltimore: Williams and Wilkins Co., pp 1246.
- CAMARGO, F.A.O., BENTO, F.M., OKEKE, B.C. and FRANKENBERGER, W.T., 2004. Hexavalent chromium reduction by an actinomycete, Arthrobactercrystallopoietes ES 32. Biological Trace Element Research, vol. 97, no. 2, pp. 183-194. http://dx.doi.org/10.1385/BTER:97:2:183 PMid:14985627.
» http://dx.doi.org/10.1385/BTER:97:2:183 - CANTLE, J.E. (Ed.), 1986. Atomic absorption spectrometry Elsevier.
- CARTER, M.R., & GREGORICH, E.G., 2007.Soil sampling and methods of analysis CRC press.
- DIN, G., HASSAN, A., RAFIQ, M., HASAN, F., BADSHAH, M., KHAN, S., CHEN, G., RIPP, S. and SHAH, A.A., 2020. Characterization of Organic Acid Producing Aspergillus tubingensis FMS1 and its Role in Metals Leaching from Soil. Geomicrobiology Journal, vol. 37, no. 4, pp. 336-344. http://dx.doi.org/10.1080/01490451.2019.1701585
» http://dx.doi.org/10.1080/01490451.2019.1701585 - KAMALUDEEN, S. P. B., ARUNKUMAR, K. R., and RAMASAMY, K., 2003. Bioremediation of chromium contaminated environments. Bioremediation of Chromium Contaminated Environments, vol. 41, pp. 972-985.
- KARTHIK, C., OVES, M., SATHYA, K., SRI RAMKUMAR, V. and ARULSELVI, P.I., 2017. Isolation and characterization of multi-potential Rhizobium strain ND2 and its plant growth-promoting activities under Cr (VI) stress. Archives of Agronomy and Soil Science, vol. 63, no. 8, pp. 1058-1069. http://dx.doi.org/10.1080/03650340.2016.1261116
» http://dx.doi.org/10.1080/03650340.2016.1261116 - KARUPPANAPANDIAN, T., SINHA, P.B., KAMARUL, H.A. and MANOHARAN, K., 2009. Chromium-induced accumulation of peroxide content, stimulation of antioxidative enzymes and lipid peroxidation in green gram (Vignaradiata L. cv. Wilczek) leaves. African Journal of Biotechnology, vol. 8, no. 3
- KOTAŚ, J. and STASICKA, Z., 2000. Chromium occurrence in the environment and methods of its speciation. Environmental Pollution, vol. 107, no. 3, pp. 263-283. http://dx.doi.org/10.1016/S0269-7491(99)00168-2 PMid:15092973.
» http://dx.doi.org/10.1016/S0269-7491(99)00168-2 - MARZAN, L.W., HOSSAIN, M., MINA, S.A., AKTER, Y. and CHOWDHURY, A.M.A., 2017. Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: bioremediation viewpoint. The Egyptian Journal of Aquatic Research, vol. 43, no. 1, pp. 65-74. http://dx.doi.org/10.1016/j.ejar.2016.11.002
» http://dx.doi.org/10.1016/j.ejar.2016.11.002 - MUSTAPHA, M.U. and HALIMOON, N., 2015. Screening and isolation of heavy metal tolerant bacteria in industrial effluent. Procedia Environmental Sciences, vol. 30, pp. 33-37. http://dx.doi.org/10.1016/j.proenv.2015.10.006
» http://dx.doi.org/10.1016/j.proenv.2015.10.006 - NORZATULAKMA, M.K., 2010. Treatment of industrial wastewater at Gebeng area using Eichornia Crassipes sp. (Water Hyacinth), Pistia Stratiotessp. (Water Lettuce) and Salvinia Molesta sp. (Giant Salvinia) Pahang, Malaysia: Universiti Malaysia Pahang.
- NOURBAKHSH, M., SAG, Y., ÖZER, D., AKSU, Z., KUTSAL, T. and CAGLAR, A., 1994. A comparative study of various biosorbents for removal of chromium (VI) ions from industrial waste waters. Process Biochemistry, vol. 29, no. 1, pp. 1-5. http://dx.doi.org/10.1016/0032-9592(94)80052-9
» http://dx.doi.org/10.1016/0032-9592(94)80052-9 - RASKIN, I. and ENSLEY, B.D., 2000. Phytoremediation of toxic metals Nova Jersey: John Wiley and Sons.
- SANJAY, M. S., SUDARSANAM, D., RAJ, G. A., and BASKAR, K., 2018. Isolation and identification of chromium reducing bacteria from tannery effluent. Journal of King Saud University-Science, vol. 32, no. 1, pp. 265-271. https://doi.org/10.1016/j.jksus.2018.05.001
» https://doi.org/10.1016/j.jksus.2018.05.001 - SARANRAJ, P., SIVASAKTHIVELAN, P. and SAKTHI, S.S., 2013. Prevalence and production of plant growth promoting substance by Pseudomonas fluorescens isolated from paddy rhizosphere soil of Cuddalore district, Tamil Nadu, India. African Journal of Basic and Applied Sciences, vol. 5, no. 2, pp. 95-101.
- SARDROOD, B.P., GOLTAPEH, E.M. and VARMA, A., 2013. An introduction to bioremediation. In: E. GOLTAPEH, Y. DANESH and A. VARMA, eds. Fungi as bioremediators (pp. 3-27). Berlin, Heidelberg: Springer. http://dx.doi.org/10.1007/978-3-642-33811-3_1
» http://dx.doi.org/10.1007/978-3-642-33811-3_1 - SHAIKH, Z. and QURESHI, P., 2013. Screening and isolation of organic acid producers from samples of diverse habitats. International Journal of Current Microbiology and Applied Sciences, vol. 2, no. 9, pp. 39-44.
- SHAKOORI, A.R. and MUNEER, B., 2002. Copper-resistant bacteria from industrial effluents and their role in remediation of heavy metals in wastewater. Folia Microbiologica, vol. 47, no. 1, pp. 43-50. http://dx.doi.org/10.1007/BF02818564 PMid:11980269.
» http://dx.doi.org/10.1007/BF02818564 - SHIVAKUMAR, C.K., THIPPESWAMY, B. and KRISHNAPPA, M., 2014. Optimization of heavy metals bioaccumulation in Aspergillusniger and Aspergillusflavus. International Journal of Environmental Biology, vol. 4, no. 2, pp. 188-195.
- SOBAHAN, M.A., MIR, S.I., ZAKARIA, I. and HOSSAIN, M. (2013, May). Surface water contamination due to industrial activities in gebeng area, Kuantan, Malaysia. In: Proceedings of the International Conference on Civil and Architecture Engineering, May 2013, Barcelona, Spain. ICCEA, pp. 6-7.
- SONI, S.K., SINGH, R., AWASTHI, A., SINGH, M. and KALRA, A., 2013. In vitro Cr (VI) reduction by cell-free extracts of chromate-reducing bacteria isolated from tannery effluent irrigated soil. Environmental Science and Pollution Research International, vol. 20, no. 3, pp. 1661-1674. http://dx.doi.org/10.1007/s11356-012-1178-4 PMid:22983604.
» http://dx.doi.org/10.1007/s11356-012-1178-4 - STASINAKIS, A.S., THOMAIDIS, N.S., MAMAIS, D., PAPANIKOLAOU, E.C., TSAKON, A. and LEKKAS, T.D., 2003. Effects of chromium (VI) addition on the activated sludge process. Water Research, vol. 37, no. 9, pp. 2140-2148. http://dx.doi.org/10.1016/S0043-1354(02)00623-1 PMid:12691900.
» http://dx.doi.org/10.1016/S0043-1354(02)00623-1 - SUJAUL, I.M., HOSSAIN, M.A., NASLY, M.A. and SOBAHAN, M.A., 2013. Effect of industrial pollution on the spatial variation of surface water quality. American Journal of Environmental Sciences, vol. 9, no. 2, pp. 120-129. http://dx.doi.org/10.3844/ajessp.2013.120.129
» http://dx.doi.org/10.3844/ajessp.2013.120.129 - SUNDAR, K., VIDYA, R., MUKHERJEE, A. and CHANDRASEKARAN, N., 2010. High chromium tolerant bacterial strains from Palar River Basin: impact of tannery pollution. Research Journal of Environmental and Earth Sciences, vol. 2, no. 2, pp. 112-117.
- UNITED STATES ENVIRONMENTAL PROTECTION AGENCY – USEPA. (1996). The metals translator: guidance for converting atotal recoverable permit limit from a dissolved criterion USA: USEPA, vol. 823. Office of Water, EPA (96-007).
- VINCENT, J.B., 2000. Elucidating a biological role for chromium at a molecular level. Accounts of Chemical Research, vol. 33, no. 7, pp. 503-510. http://dx.doi.org/10.1021/ar990073r PMid:10913239.
» http://dx.doi.org/10.1021/ar990073r - WANG, Y., PENG, B., YANG, Z., TANG, C., CHEN, Y., LIAO, Q. and LIAO, Y., 2014. Treatment of Cr (VI) contaminated water with Pannonibacterphragmitetus BB. Environmental Earth Sciences, vol. 71, no. 10, pp. 4333-4339. http://dx.doi.org/10.1007/s12665-013-2827-8
» http://dx.doi.org/10.1007/s12665-013-2827-8 - WANG, Y., YANG, Z., PENG, B., CHAI, L., WU, B. and WU, R., 2013. Biotreatment of chromite ore processing residue by Pannonibacterphragmitetus BB. Environmental Science and Pollution Research International, vol. 20, no. 8, pp. 5593-5602. http://dx.doi.org/10.1007/s11356-013-1526-z PMid:23440437.
» http://dx.doi.org/10.1007/s11356-013-1526-z - WANI, P.A. and KHAN, M.S., 2010. Bacillus species enhance growth parameters of chickpea (Cicerarietinum L.) in chromium stressed soils. Food and Chemical Toxicology, vol. 48, no. 11, pp. 3262-3267. http://dx.doi.org/10.1016/j.fct.2010.08.035 PMid:20813149.
» http://dx.doi.org/10.1016/j.fct.2010.08.035 - WANI, P.A., SUNDAY, O.O., KEHINDE, A.M., OLUWASEYI, L.A., WASIU, I.A. and WAHID, S., 2018. Antioxidants and chromium reductases by Penibacillus species enhance the growth of soybean under chromium stress. International Journal of Environmental Science and Technology, vol. 15, no. 7, pp. 1531-1542. http://dx.doi.org/10.1007/s13762-017-1533-6
» http://dx.doi.org/10.1007/s13762-017-1533-6 - WASEEM, A., ARSHAD, J., IQBAL, F., SAJJAD, A., MEHMOOD, Z. and MURTAZA, G., 2014. Pollution status of Pakistan: a retrospective review on heavy metal contamination of water, soil, and vegetables. BioMed Research International, vol. 2014, pp. 813206. http://dx.doi.org/10.1155/2014/813206 PMid:25276818.
» http://dx.doi.org/10.1155/2014/813206 - WORLD BANK, 2006. Pakistan: strategic Country Environmental Assessment Report: rising to the challenges Washington: World Bank.
- WORLD HEALTH ORGANIZATION – WHO. (1993). Guidelines for drinking-water quality. Geneva: WHO.
- WUANA, R.A. and OKIEIMEN, F.E., 2011. Heavy metals in contaminated soils: A review of sources, chem. risks and best available strategies for remediation. ISRN Ecology, vol. 2011, pp. 1-20. http://dx.doi.org/10.5402/2011/402647
» http://dx.doi.org/10.5402/2011/402647 - ZAHOOR, A. and REHMAN, A., 2009. Isolation of Cr (VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. Journal of Environmental Sciences (China), vol. 21, no. 6, pp. 814-820. http://dx.doi.org/10.1016/S1001-0742(08)62346-3 PMid:19803088.
» http://dx.doi.org/10.1016/S1001-0742(08)62346-3
Publication Dates
-
Publication in this collection
03 Sept 2021 -
Date of issue
2023
History
-
Received
21 Aug 2020 -
Accepted
21 Jan 2021