Abstract
Transplanting time and genotype contribute to improving crop yield and quality of eggplant (Solanum melongena L.). A field experiment was conducted to investigate the impact of foliar applied of triacontanol (TRIA) and eggplant genotypes 25919, Nirala, 28389 and Pak-10927,transplanted on 1 March,15 March, and 1 April on exposure to high air temperature conditions. The experiment was performed according to Randomized Complete Block Design and the data was analyzed by using Tuckey,s test . The TRIA was applied at 10µM at flowering stage; distilled water was used as the control. Rate of photosynthesis and transpiration, stomatal conductance, water use efficiency, and effects on antioxidative enzymes (superoxide dismutase, catalase and peroxidase) were evaluated. The 10µM TRIA increased photosynthesis rate and water use efficiency and yield was improved in all genotypes transplanted at the different dates. Foliar application of 10µM TRIA increased antioxidative enzyme activities (SOD, POD & CAT) and improved physiological as well as biochemical attributes of eggplant genotypes exposed to high heat conditions. Highest activity of dismutase enzyme 5.41mg/1g FW was recorded in Nirala genotype in second transplantation. Whereas, lowest was noted in PAK-10927 (2.30mg/g FW). Maximum fruit yield was found in accession 25919 (1.725kg per plant) at 1st transplantation with Triacontanol, whereas accession PAK-10927 gave the lowest yield (0.285 kg per plant) at control treatment on 3rd transplantation. Genotype, transplanting date and application of TRIA improved growth, yield and quality attributes under of heat stress in eggplant.
Keywords:
triacontanol; Solanum melongena; heat stress; genotype; physiological attributes
Resumo
O tempo de transplante e o genótipo contribuem para melhorar a produtividade e a qualidade da cultura da berinjela (Solanum melongena L.). Um experimento de campo foi conduzido para investigar o impacto da aplicação foliar de triacontanol (TRIA) e genótipos de berinjela 25919, Nirala, 28389 e Pak-10927, transplantados em 1 de março, 15 de março e 1 de abril de exposição a condições de alta temperatura do ar. O experimento foi realizado de acordo com o Randomized Complete Block Design e os dados foram analisados pelo teste de Tuckey. O TRIA foi aplicado a 10 µM na fase de floração; água destilada foi utilizada como controle. Taxa de fotossíntese e transpiração, condutância estomática, eficiência do uso da água e efeitos sobre as enzimas antioxidantes (superóxido dismutase, catalase e peroxidase) foram avaliados. O TRIA 10 µM aumentou a taxa de fotossíntese e a eficiência do uso da água e o rendimento foi melhorado em todos os genótipos transplantados nas diferentes datas. A aplicação foliar de TRIA 10µM aumentou as atividades das enzimas antioxidantes (SOD, POD e CAT) e melhorou os atributos fisiológicos e bioquímicos de genótipos de berinjela expostos a condições de alto calor. A atividade mais elevada da enzima dismutase 5,41mg / 1g FW foi registrada no genótipo Nirala no segundo transplante. Considerando que o mais baixo foi observado em PAK-10927 (2,30 mg / g FW). A produtividade máxima de frutos foi encontrada no acesso 25919 (1,725 kg por planta) no 1º transplante com Triacontanol, enquanto o acesso PAK-10927 deu a menor produção (0,285 kg por planta) no tratamento de controle no 3º transplante. Genótipo, data de transplante e aplicação de TRIA, melhoramento do crescimento, rendimento e atributos de qualidade sob estresse térmico em berinjela.
Palavras-chave:
triacontanol; Solanum melongena; estresse térmico; genótipo; atributos fisiológicos
1. Introduction
Elevated temperature is an outcome of global warming as reported in the fourth assessment report of IPPC (Intergovernmental Panel on Climate Change) which is the most serious concern in achieving goals of food security (Hoegh-Guldberg and Bruno, 2010HOEGH-GULDBERG, O. and BRUNO, J.F., 2010. The impact of climate change on the world’s marine ecosystems.Science, vol. 328, no. 5985, pp. 1523-1528. http://dx.doi.org/10.1126/science.1189930. PMid:20558709.
http://dx.doi.org/10.1126/science.118993...
). Symptoms of sun burn on plant leaves and fruit appear as burnt leaves, blemished twigs, dissipated and disfigured inflorescence causing poor quality eggplant (Solanum melongena L.) (Goraya and Asthir, 2016GORAYA, G.K. and ASTHIR, B., 2016. Magnificent role of intracellular reactive oxygen species production and its scavenging encompasses downstream processes.Journal of Plant Biology, vol. 59, no. 3, pp. 215-222. http://dx.doi.org/10.1007/s12374-016-0057-9.
http://dx.doi.org/10.1007/s12374-016-005...
). High temperature is detrimental to plant cell membranes, osmotic balance, and amino acids and peptide concentrates (Zinn et al., 2010ZINN, K.E., TUNC-OZDEMIR, M. and HARPER, J.F., 2010. Temperature stress and plant sexual reproduction: uncovering the weakest links.Journal of Experimental Botany, vol. 61, no. 7, pp. 1959-1968. http://dx.doi.org/10.1093/jxb/erq053. PMid:20351019.
http://dx.doi.org/10.1093/jxb/erq053...
). The production of reactive oxygen species (ROS) as a result of heat causes damage to chlorophyll a and b, DNA (Sharma et al., 2012SHARMA, P., JHA, A.B., DUBEY, R.S. and PESSARAKLI, M., 2012. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions.Le Journal de Botanique, vol. 2012, pp. 217037. http://dx.doi.org/10.1155/2012/217037.
http://dx.doi.org/10.1155/2012/217037...
). Most plants have developed complex systems for protection against damage (Hasanuzzaman et al., 2013HASANUZZAMAN, M., NAHAR, K., ALAM, M.M., ROYCHOWDHURY, R. and FUJITA, M., 2013. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants.International Journal of Molecular Sciences, vol. 14, no. 5, pp. 9643-9684. http://dx.doi.org/10.3390/ijms14059643. PMid:23644891.
http://dx.doi.org/10.3390/ijms14059643...
). Nevertheless, some of these systems demand external stimuli, or an elicitor, to make resistant systems fully functional. These may be plant hormones, or their synthetic analogues (Hasanuzzaman et al., 2013HASANUZZAMAN, M., NAHAR, K., ALAM, M.M., ROYCHOWDHURY, R. and FUJITA, M., 2013. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants.International Journal of Molecular Sciences, vol. 14, no. 5, pp. 9643-9684. http://dx.doi.org/10.3390/ijms14059643. PMid:23644891.
http://dx.doi.org/10.3390/ijms14059643...
). (Hussain et al., 2019HUSSAIN, T., AYYUB, C.M., AHMAD, I., ALI, I., MUSTAFA, Z., ANWAR, A., AHMAD, A., LATIF, S. and IQBAL, T., 2019. Mitigation of adverse effects of heat stress in chillies by using glycine betaine.International Journal of Biosciences, vol. 15, no. 2, pp. 1-10.) worked on mitigation of hest stress effects on chillies through application of glycinebetain. Triacontanol (TRIA) has proven plant growth regulatory effects such as maize and wheat (Perveen et al., 2010PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2010. Regulation in gas exchange and quantum yield of photosystem II (PSII) in salt-stressed and non-stressed wheat plants raised from seed treated with triacontanol.Pakistan Journal of Botany, vol. 42, no. 5, pp. 3073-3081.).The TRIA has been reported to improve growth, yield, photosynthesis, protein synthesis, uptake of water and nutrients, nitrogen-fixation, enzymes activities, free amino acids, reducing sugars, and soluble protein contents (Naeem et al., 2012NAEEM, M., KHAN, M.M.A. and MOINUDDIN., 2012. Triacontanol: a potent plant growth regulator in agriculture.Journal of Plant Interactions, vol. 7, no. 2, pp. 129-142. http://dx.doi.org/10.1080/17429145.2011.619281.
http://dx.doi.org/10.1080/17429145.2011....
). Exogenous TRIA improved plant tolerance to abiotic challenges (Perveen et al., 2012PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2012. Changes in mineral composition, uptake and use efficiency of salt stressed wheat (Triticumaestivum L.) plants raised from seed treated with triacontanol.Pakistan Journal of Botany, vol. 44, pp. 27-35.) and helped improve nutrients uptake and photosynthesis (Naeem et al., 2009NAEEM, M., KHAN, M.M.A., MOINUDDIN. and SIDDIQUI, M.H., 2009. Triacontanol stimulates nitrogen-fixation, enzyme activities, photosynthesis, crop productivity and quality of hyacinth bean (Lablab purpureusL.).Scientia Horticulturae, vol. 121, no. 4, pp. 389-396. http://dx.doi.org/10.1016/j.scienta.2009.02.030.
http://dx.doi.org/10.1016/j.scienta.2009...
). Its role in amelioration of salinity has been well documented (Perveen et al., 2012PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2012. Changes in mineral composition, uptake and use efficiency of salt stressed wheat (Triticumaestivum L.) plants raised from seed treated with triacontanol.Pakistan Journal of Botany, vol. 44, pp. 27-35.). Many scientists tried to discover the mode of action of Triacontanol, though, the key mechanism that loosen Tria-mediated tolerance to main environmental pressures remained very little explored in plants (Islam and Mohammad, 2020ISLAM, S. and MOHAMMAD, F., 2020. Triacontanol as a dynamic growth regulator for plants under diverse environmental conditions.Physiology and Molecular Biology of Plants, vol. 26, no. 5, pp. 871-883. http://dx.doi.org/10.1007/s12298-020-00815-0. PMid:32377038.
http://dx.doi.org/10.1007/s12298-020-008...
).
Eggplant is rich in anthocyanin, a possible counter oxidative agent eradicates radicals (Jing et al., 2015JING, P., QIAN, B., ZHAO, S., QI, X., YE, L., GIUSTI, M.M. and WANG, X., 2015. Effect of glycosylation patterns of Chinese eggplant anthocyanins and other derivatives on antioxidant effectiveness in human colon cell lines.Food Chemistry, vol. 172, pp. 183-189. http://dx.doi.org/10.1016/j.foodchem.2014.08.100. PMid:25442541.
http://dx.doi.org/10.1016/j.foodchem.201...
). Although eggplant is a warm season vegetable heat injuries are common in egg plant seedlings (Sękara et al., 2016SĘKARA, A., BĄCZEK-KWINTA, R., GAWĘDA, M., KALISZ, A., POKLUDA, R. and JEZDINSKÝ, A., 2016. Sequential abiotic stress applied to juvenile eggplant modifies the seedlings parameters, plant ontogeny and yield.Horticultural Science, vol. 43, no. 3, pp. 149-157. http://dx.doi.org/10.17221/162/2015-HORTSCI.
http://dx.doi.org/10.17221/162/2015-HORT...
). There is little literature available on effects of high temperature on ameliorating effect of TRIA on eggplant exposed to high heat. The study was carried to determine the effect of TRIA on eggplant genotypes having varied levels of heat tolerance.
2. Materials and Methods
The investigation was carried at the University of Agriculture, Faisalabad (31° 25' 22.26" N,73° 05' 3.30" E) during the year 2017-18. The climate is subtropical with annual rainfall of 31 inches. Average temperature is 28-39oC during the experimental period. Maximum temperature was 46.7oC in June. The soil of site was a well-drained sandy loam.
The eggplant genotypes 25919, Nirala, 28389, Pak-10927 were sourced from the Plant Genetic Resource Institute, Islamabad and Vegetable Research Institute, Faisalabad. Seed of the genotypes (25 each) were sown in plastic pots. Standard cultural practices were used during the experiment. Half-strength Hoagland’s solution was applied to the medium at seedling emergence stage.
Seedlings were transplanted at principal growth stage 1: leaf development stage, or after 50 days, 1 March, 15 March and 1 April on raised beds at spacing of 90×60 cm. After transplanting, fertilizer 42N-23P-25K kg∙ha-1 was applied at soil preparation. Irrigation was applied immediately after transplanting and weekly irrigation was provided from March to August. Eggplant seedling were treated with 10µM TRIA; untreated eggplant were taken as control (treated with water). Leaves of the TRIA treated eggplant were thoroughly wetted on both sides until run-off. The impact of TRIA on gaseous exchange, biochemical and yield related parameters of eggplant were studied.
Data was recorded for leaf gaseous exchange including rate of photosynthetic activity, transpiration rate, and stomatal conductance. Three young, healthy and full-developed leaves from 1 plant (2 plants from every replication treatment) were selected. After careful cleaning leaves were placed one after another in the square chamber of a portable Infra-Red Gas Analyzer apparatus used to determine multiple physiological parameters at a time (LCi-SD, ADC Bio-scientific, city, UK). All measurements were made at the same time at 403.3 mmol∙m-1∙s-1 molar air flow per unit leaf area, water vapor pressure in the chamber ranged from 6.0 to 8.9 mbar, Photosynthetically active radiation (PAR) at leaf surface was up to 1711pmol∙m-1∙s-1, atmospheric pressure of 99.9 kPa, ambient temperature from 22.4 to 27.9°C and ambient CO2concentration of 352pmol∙mol-1 (Moya et al., 2003MOYA, L.J., GOMEZ-CADENAS, A., PRIMO-MILLO, E. and TALON, M., 2003. Chloride absorption in salt- sensitive Carrizo citrange and salt-tolerant Cleopatra mandarin citrus rootstocks is linked to water use. Journal of Experimental Botany, vol. 54, pp. 825-833.).Water use efficiency (WUE) (pmol CO2∙mmol-1 H2O) was determined using the formula.
2.1. Chlorophyll contents
Chlorophyll contents were estimated with a portable chlorophyll meter (CCM-200 plus Bio-Scientific.
2.2. Super Oxide Dismutase (SOD) activity
The antioxidant activity of super oxide dismutase (SOD) enzyme was estimated by recording its ability to hinder photo-reduction of nitrobluetetrazolium (NBT)following the methodology of Wu et al. (2012)WU, G.Q., ZHANG, L.N. and WANG, Y.Y., 2012. Response of growth and antioxidant enzyme to osmotic stress in two different wheat (Triticumaestivum L.) cultivars seedlings.Plant, Soil and Environment, vol. 58, no. 12, pp. 534-539. http://dx.doi.org/10.17221/373/2012-PSE.
http://dx.doi.org/10.17221/373/2012-PSE...
. The reaction solution was 0.2 M buffer, 0.222 g methionine in 15 mL of deionized water, 0.015 g NBT in 17.5 mL of deionized water, 0.0375 mL Triton-X in 17.5 mL of deionized water and 0.0132 g Riboflavin in 17.5 mL deionized water. Tubes containing the reaction mix were placed under a UV lamp for 15 min then riboflavin was added and absorbance at 560 nm determined. One unit of SOD activity was the amount of enzyme inhibiting 50% of NBT.
2.3. Activities of catalase and peroxidase (CAT & POD)
Measurements of catalase (CAT) and peroxidase (POD) enzyme activities were with the methodology of Chance and Maehly (1995) with few adjustments. The reaction solution for measurement of catalase activity comprised of 50 mM phosphate buffer, pH 7, 5.9 mM hydrogen peroxide, and 0.1 mL of gum solution. Addition of the sample initiated the reaction and absorbance was read at 240 nm after 20 sec. One unit catalase enzyme activity was an absorbance variation of 0.01 units∙min-1. The reaction solution for peroxidase enzyme activity contained 50 mM phosphate buffer with pH5, 20 mM guaiacol, 40 mM hydrogen peroxide and 0.1 mL of gum solution. At every 20 sec, variations in absorbance at 470 nm were measured. One unit activity of peroxidase enzyme was described as an absorbance alteration of 0.01 units∙min-1.
2.4. MDA contents estimation (lipid peroxidation)
Thiobarbituric acid reaction in serum was approximated by the method of Ohkawa et al. (1979)OHKAWA, H., OHISHI, N. and YAGI, K., 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction.Analytical Biochemistry, vol. 95, no. 2, pp. 351-358. http://dx.doi.org/10.1016/0003-2697(79)90738-3. PMid:36810.
http://dx.doi.org/10.1016/0003-2697(79)9...
. A 200 serum sample was placed in a test tube, 1.5 mL of 0.8% thiobarbituric acid (TBA), 200 µL of 8.1% sodium dodecyl sulfate, 1.5 mL of 20% acetic acid solution, pH3.5 and 4 mL of distilled water was added to the mix. The test tubes were kept in a water bath at 90°C for 60 min. After cooling under tap water, 1 mL of distilled water and 5 mL of n-butanol were added and the mix shaken vigorously and centrifuged for 10 min at 4000 rpm. The upper layer of butanol was taken and its absorbance measured at 532 nm.
2.5. Yield components
Yield related attributes of fruit weight and yield per plant were recorded at harvest (done on basis of fruit ripening with 6-7 days interval) in plants of each replication per treatment and yield per plant calculated on the total after all harvests.
2.6. Statistical analysis:
The experiment was arranged as a factorial in a randomized complete block design. The data were subjected to analysis of variance and means were separated with Tuckey’s test in Statistix (ver. 8.1).
3. Results
3.1. Leaf gaseous exchange characteristics
3.1.1. Rate of transpiration (mmol m-2s-1)
It is evident from the data presented in the Figure 1 that transpiration rate increased with delay in the transplanting dates, being higher in the 3rd transplanting date i.e. 1st of April. Foliar application of TRIA significantly reduced the leaf transpiration rate of eggplant genotypes/accessions under field conditions (Figure 1). Regardless of transplanting dates, both categories (high and medium resistant)of genotypes showed more transpiration rate in control plants as compared to TRIA treated plants. Minimum value for transpiration rate was observed in heat tolerant genotype 25919 under first transplantation i.e. 1stMarch (0.60) when supplemented with TRIA foliar spray, while highest value was noted in Pak-10927 during 2ndand third sowing dates respectively (Figure 1). TRIA treatment was found effective to decrease these values i.e. leaf transpiration rate. Highly significant results were found regarding transplanting date, genotype, TRIA treatment. Similarly, the interaction between transplantation date with TRIA treatment and genotypes, TRIA with genotype and TRIA ×genotype ×transplanting date were also note to be significant during the study.
Transpiration rate in response to triacontanol application at different transplanting dates. Genotype (G)= **; Triacontanol (TRIA)= **; Transplanting date (T)= *; G × TRIA= ** G × T= **; TRIA × T=** G × TRIA × T=***, ** significant at p<0.05 or p<0.01.Genotype (G)=**; Triacontanol (TRIA)= **; Transplanting date (T)= **; G x TRIA= ** G x T= NS; TRIA x T=** G x TRIA x T=**Whereas, NS: non-significant; *: significant and ** : highly significant.
3.1.2. Rate of photosynthesis (µmol m-2s-1)
A declined trend for values representing photosynthesis rate was observed with the advancements of the dates of sowing i.e. higher at in plants sown at 1st of March while lower in the plans sown during the last date of transplanting i.e. 1st of April, regardless of the genotypes and treatment (Figure 2). Maximum value for photosynthetic rate was recorded in case of genotype 25919 (11.71) when plants were treated with TRIA at first transplantation (1st March); while minimum was recorded in the untreated plants of Pak-10927(3.21) which were transplanted on 1st of April i.e. 3rd transplanting date (Figure 2). Though a declining trend for photosynthesis was observed with the transplanting dates, yet the foliar sprays of TRIA maintained significantly higher photosynthesis as compared unsprayed plants (Figure 2).
Rate of photosynthesis in response to triacontanol application under different transplanting dates. Genotype (G)=*; Triacontanol (TRIA)= **; Transplanting date (T)= *; G x TRIA= ** G x T= NS; TRIA x T=** G x TRIA x T=** Whereas, NS: non-significant; *: significant and ** : highly significant.
3.1.3. Water use efficiency (µmol CO2mmol-1 H2O)
A steady and continues declining trend was observed for water use efficiency in plants for second and third transplantation dates in all genotypes when no foliar application of TRIA was given. Water use efficiency was recorded higher at 1st and 2nd transplantation date; while lowest values were noted when plants were transplanted after 1stof April. On the other hand foliar sprays of TRIA were able to maintain significantly higher values of water use efficiency in all the genotypes as compared to control irrespective to transplanting dates. Maximum value for water use efficiency was recorded in 25919 eggplant genotype sprayed with TRIA at first transplantation (Figure 3).
Water use efficiency in response to triacontanol application under different transplanting dates. Genotype (G)=**; Triacontanol (TRIA)= *; Transplanting date (T)= **; G x TRIA= ** G x T= NS; TRIA x T=** G x TRIA x T=NS. Whereas, NS: non-significant; *: significant and ** : highly significant
3.1.4. Stomatal conductance (mmol m-2 s-1)
Higher values for stomatal conductance were maintained TRIA treated plants under first, second and third transplantation dates i.e. 1st of March, 15th of March and 1st of April. While, stomatal conductance was decreased as temperature increased from 1st to 3rd transplanting. Eggplant genotype 28389 exhibited significantly higher stomatal conductance among all genotypes; whereas, minimum stomatal conductance was recorded in Nirala genotype (0.85) after third transplantation in leaves of eggplants. Highly significant results were noted for transplanting date, TRIA application and genotype (Figure 4).
Stomatal conductance in response to triacontanol application under different transplanting dates. Genotype (G)=**; Triacontanol (TRIA)= **; Transplanting date (T)= **; G x TRIA= ** G x T= **; TRIA x T=** G x TRIA x T=NS.
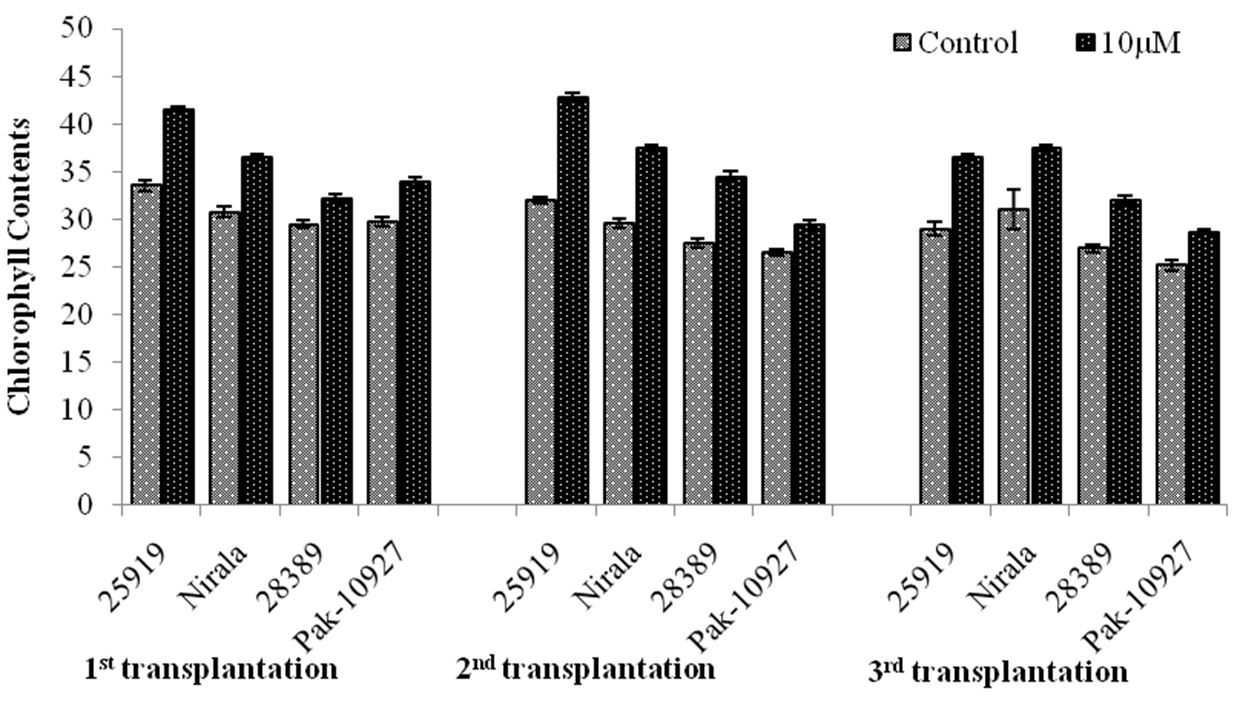
3.1.5. Chlorophyll contents (SPAD value)
It is clear from the data presented in the Figure 5 that transplanting dates and treatment of TRIA had significant effect on the leaf chlorophyll contents in all eggplant genotypes. Heat tolerant genotype 25919 gave significantly higher leaf chlorophyll contents regardless of sowing date and was at par with Nirala that is also a heat tolerant genotype of eggplant. Maximum chlorophyll contents (42.8) were observed in genotype 25919 in second transplanting date in leaves of those plants provided with foliar spray of TRIA. Minimum value for chlorophyll contents (25.2) were recorded in pak-10927 which was at par with heat sensitive genotype 28389 (26.3) in second transplantation date in leaves of control plants (Figure 5). The obtained results were highly significant regarding transplanting dates, TRIA treatment and genotypes. The interaction effects of transplanting date with TRIA treatment and genotypes, TRIA and genotype were highly significant.
Chlorophyll contents in response to triacontanol application under different transplanting dates.Genotype (G)=**; Triacontanol (TRIA)= **; Transplanting date (T)= **; G x TRIA= ** G x T= **; TRIA x T=** G x TRIA x T=**.
3.2. Biochemical characteristics
3.2.1. Superoxide dismutase enzyme activity (mg g-1 fwt)
It is obvious from the findings that antioxidant activity of superoxide dismutase (SOD) enzyme was significantly higher in second transplanting date as compared to first and third date of transplantation (Figure 6). From the data it is evident that the treatment of TRIA improved the levels of SOD significantly as compared to untreated plants in all genotypes of egg plants on all the three dates of transplantation. Free radical scavenging activity of SOD in heat tolerant genotype Nirala was highest in TRIA treated plants under second transplantation as compared to the rest of values for all the genotypes whether treated or untreated (Figure 6). Results with high degree of significance were observed regarding transplantation date, TRIA treatment and genotypes. However, highly significant interactions between transplanting date and TRIA treatment, transplanting date and genotype, TRIA and genotype as well as transplanting date × TRIA× Genotype were observed when the values were analyzed statistically.
Superoxide dismutase enzyme activity in response to triacontanol application under different transplanting dates.Genotype (G)=**; Triacontanol (TRIA)= **; Transplanting date (T)= *; G x TRIA= ** G x T= **; TRIA x T=** G x TRIA x T=**.
3.2.2. Peroxidase enzyme activity (mg g-1 fwt)
Likewise in the results of SOD, foliar application of TRIA significantly enhanced enzymatic activity of peroxidase (POD) activities in eggplant genotypes under second transplanting date as compared to the first and third transplanting dates. Heat tolerant genotype 25919 genotype had maximum peroxidase activity (3.23) followed by Nirala under second transplantation date (Figure 7). Highly significant results were found for transplanting dates, TRIA treatment and genotype. Interactive effects (G×TRIA, G×TD, TRIA× TD, G× TRIA×TD) were also found highly significant (Figure 7).
Peroxidase enzyme activity in response to triacontanol application under different transplanting dates.Genotype (G)=*; Triacontanol (TRIA)= **; Transplanting date (T)= *; G x TRIA= ** G x T= **; TRIA x T=** G x TRIA x T=NS.
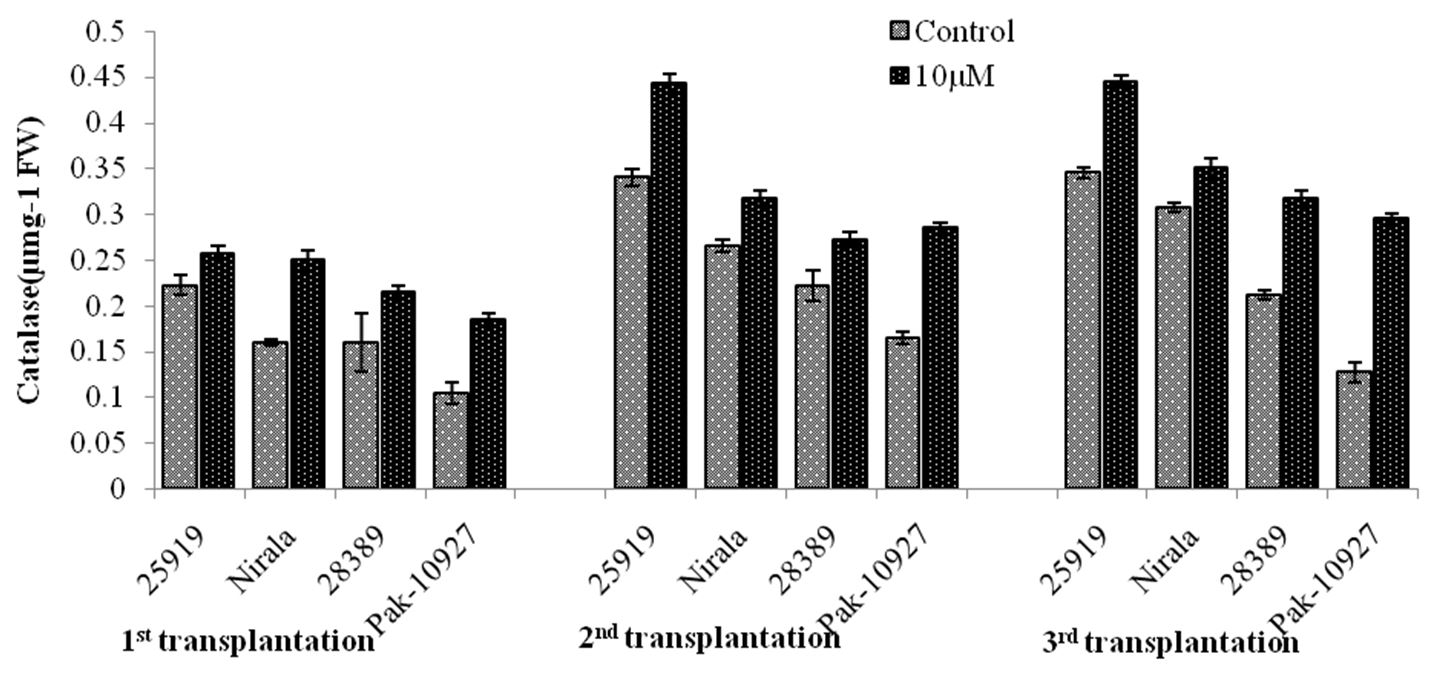
3.2.3. Catalase enzyme activity (mg g-1 fwt)
Catalase (CAT) enzyme activity was significantly increased at third transplanting i.e. 1st of April. Foliar application of TRIA significantly enhanced the enzymatic CAT activity in all eggplant genotypes at varying transplantation dates. Minimum activity of catalase enzyme in leaves of both groups (High and medium resistant) of genotypes was found in first transplantation date i.e. 1st of March. Heat tolerant genotype 25919 gave significantly higher catalase activity in leaves (0.45) of TRIA sprayed eggplants at second and third transplanting dates i.e. 15th of March and 1st of April (Figure 8).
Catalase enzyme activity in response to triacontanol application under different transplanting dates.Genotype (G)=**; Triacontanol (TRIA)= **; Transplanting date (T)= **; G x TRIA= NS; G x T= *; TRIA x T=*; G x TRIA x T=NS.
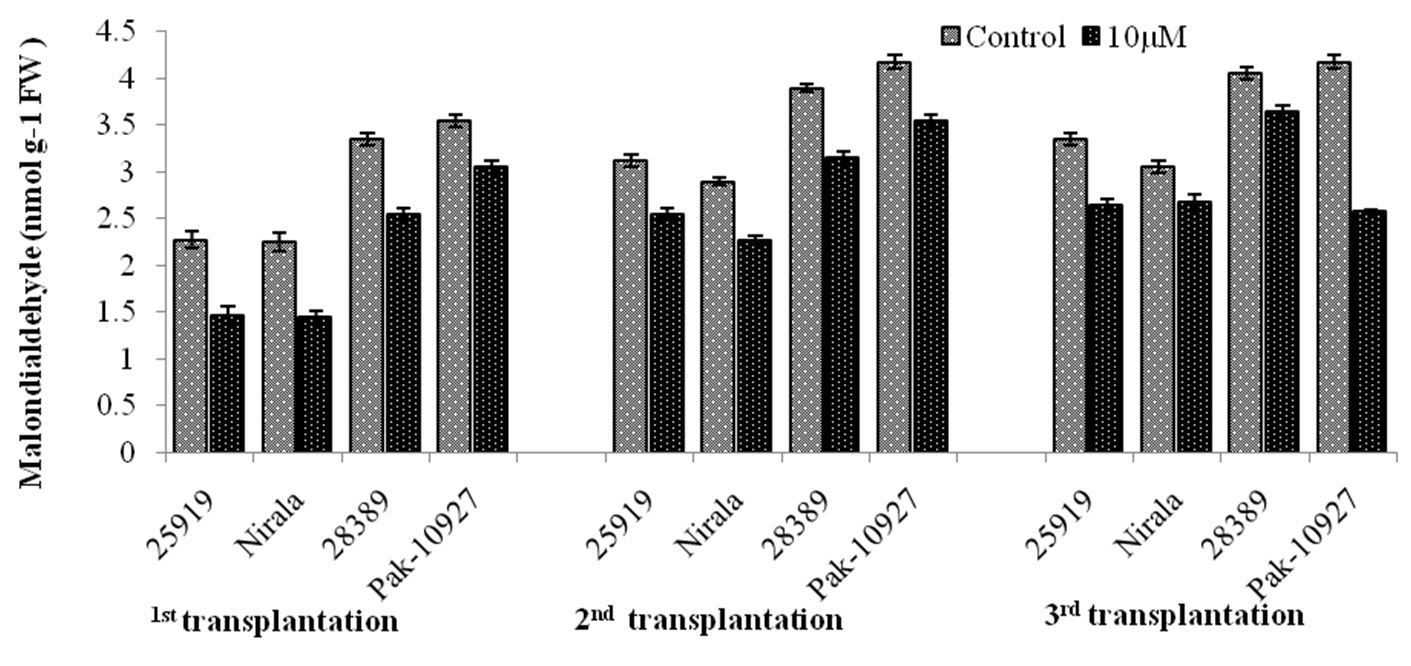
3.2.4. Malondialdehyde contents(nmol g-1fw)
Melondialdehyde (MDA) values showed a progressive increase with transplanting dates being higher in the last transplanting dates regardless of genotypes and treatments except that of Pak-10927 which showed decrease in the MDA contents during last transplanting date when received treatment of TRIA (Figure 9). Foliar application of TRIA significantly reduced MDA contents of eggplant genotypes under field conditions. Heat sensitive genotypepak-10927 gave significantly higher MDA contents in second and third transplanting dates and was at par with 28389 that is also a heat sensitive genotype of eggplant. Whilst foliar application of showed a marked reduction in MDA contents in all transplanting dates (Figure 9). Statistical analysis of collected data showed highly significant results for genotypes, TRIA treatment and transplanting dates. Interactive effect (G×TRIA) was found non-significant. However, interactions between G×TD, TRIA ×TD, G× TD×TRIA were found significant on this ground (Figure 9).
Malondialdehyde contents in response to triacontanol application under different transplanting dates.Genotype (G)=**; Triacontanol (TRIA)= **; Transplanting date (T)= **; G x TRIA= ** G x T= **; TRIA x T=** G x TRIA x T=NS.
3.3. Yield components
3.3.1. Fruit weight(g)
Foliar application of TRIA enhanced the fruit weight of eggplant genotypes under field conditions (Figure 10). Maximum fruit weight was obtained from genotype 25919 irrespective of transplanting date while minimum fruit weight was found in case of Pak-10927 genotype without TRIA spray (Figure 10). Statistical analysis of data showed highly significant results for genotypes, TRIA treatment and transplanting dates. Significant interactions (G×TRIA, G × TD, TRIA× TD) were noted for this variable while non-significant interaction was found between genotype, TRIA and transplanting date (Figure 10).
Fruit weight in response to triacontanol application under different transplanting dates .Genotype (G)=**; Triacontanol (TRIA)= **; Transplanting date (T)= **; G x TRIA= ** G x T= **; TRIA x T=NS G x TRIA x T=NS.
3.3.2. Yield per plant (kg)
Foliar application of TRIA significantly enhanced the fruit yield (kg plant-1) of eggplant genotypes under field conditions except that for genotype 25919 which showed non-significant increase in yield on 1st and last date of transplanting when compared to untreated plants of the same genotype. Fruit yield was higher in all genotypes under first transplanting date as compared to the late two transplanting. Heat tolerant genotype 25919 gave significantly higher fruit yield per plant regardless of sowing date. Minimum yield of fruits was seen in genotype Pak-10927in third transplantation when no foliar feeding of TRIA was given to plants (Figure 11). Statistical analysis of data showed highly significant results for genotypes, TRIA treatment and transplanting dates. Non- significant interaction was depicted between G×TRIA × TD. Moreover, significant interaction was found in genotype with transplanting date and TRIA application.
4. Discussion
As expected from the study stress (heat) tolerant genotypes of eggplants produced better results under stressed conditions as compared to the medium or low tolerant cultivars. Furthermore, it is clear from the result section that application of TRIA further improved their performance during stress by improving antioxidant defense system of genotypes hence improving yield and quality under stress. Numerous research studies have reported the plant growth stimulating role of TRIA, after its discovery as a constituent of plant epicuticular wax (Naeem et al., 2012NAEEM, M., KHAN, M.M.A. and MOINUDDIN., 2012. Triacontanol: a potent plant growth regulator in agriculture.Journal of Plant Interactions, vol. 7, no. 2, pp. 129-142. http://dx.doi.org/10.1080/17429145.2011.619281.
http://dx.doi.org/10.1080/17429145.2011....
). TRIA growth regulatory effect has been documented in numerous plants from many plant genera like runner bean, Brazilian palm, blueberry and several legumes like white clover and alfalfa. The salvaging role of TRIA under abiotic stressors has been particularly emphasized on its synergistic contact with phyto-hormones and orientation of 9-b-L (þ) adenosine that is analogous to cytokinin structure (Naeem et al., 2012NAEEM, M., KHAN, M.M.A. and MOINUDDIN., 2012. Triacontanol: a potent plant growth regulator in agriculture.Journal of Plant Interactions, vol. 7, no. 2, pp. 129-142. http://dx.doi.org/10.1080/17429145.2011.619281.
http://dx.doi.org/10.1080/17429145.2011....
). The induction of 9-b-L (þ) adenosine by TRIA is thought to be one of the main reasons for the increased dry matter as it turns on a swift cascade of metabolic events all over the plant within a minute. For the similar cause, Perveen et al. (2014)PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2014. Triacontanol-induced changes in growth, yield, leaf water relations, oxidative defense system, minerals, and some key osmoprotectants in Triticumaestivumunder saline conditions.Turkish Journal of Botany, vol. 38, no. 5, pp. 896-913. http://dx.doi.org/10.3906/bot-1401-19.
http://dx.doi.org/10.3906/bot-1401-19...
examined the effect of TRIA on maize and wheat plants under salinity stress for shorter and longer time periods separately. Present results of heat stress tolerance in eggplant through TRIA are in accordance with the findings of Perveen et al. (2014)PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2014. Triacontanol-induced changes in growth, yield, leaf water relations, oxidative defense system, minerals, and some key osmoprotectants in Triticumaestivumunder saline conditions.Turkish Journal of Botany, vol. 38, no. 5, pp. 896-913. http://dx.doi.org/10.3906/bot-1401-19.
http://dx.doi.org/10.3906/bot-1401-19...
who reported the salvaging effect of TRIA on salt stressed wheat plants.
Regarding gaseous exchange related attributes, maximum photosynthesis rate was recorded in 1st transplantation supposed to be the normal temperature condition (Figure 1). Transpiration rate was increased with the increase of temperature i.e. in 2nd and 3rd transplantations (Figure2).Tolerant genotypes showed the highest water use efficiency in1st transplantation i.e. 1stof March (Figure 3). Similarly these stress tolerant genotypes in 1sttransplantation showed maximum chlorophyll contents (Figure 5). The results were in line with the findings published by (Wahid et al., 2007WAHID, A., GELANI, S., ASHRAF, M. and FOOLAD, M., 2007. Heat tolerance in plants: an overview.Environmental and Experimental Botany, vol. 61, no. 3, pp. 199-223. http://dx.doi.org/10.1016/j.envexpbot.2007.05.011.
http://dx.doi.org/10.1016/j.envexpbot.20...
). It has been proved earlier that leaf area, chlorophyll contents, leaf temperature are main factors for carbohydrate synthesis during photosynthesis. Genotypes with higher number of leaves displayed higher photosynthetic rate. This might be due to more leaf area captured more sun light for photosynthesis; consequently increase in carbon assimilates formation for enhanced growth and development (Asadipour and Madani, 2014ASADIPOUR, A. and MADANI, H., 2014. The effects of irrigation and sowing date on the quantitative traits of okra (Abelmoschusesculentus L.).International Journal of Farming and Allied Sciences, vol. 3, no. 5, pp. 497-501.). In one of study it was seen that genotypes with less leaf surface temperature and more chlorophyll contents revealed better performance in photosynthetic rate. This is reinforced by the statements of Shaheen et al. (2016)SHAHEEN, M.R., AYYUB, C.M., AMJAD, M. and WARAICH, E.A., 2016. Morpho-physiological evaluation of tomato genotypes under high temperature stress conditions.Journal of the Science of Food and Agriculture, vol. 96, no. 8, pp. 2698-2704. http://dx.doi.org/10.1002/jsfa.7388. PMid:26304011.
http://dx.doi.org/10.1002/jsfa.7388...
who observed that if a genotype has less leaf surface temperature and higher leaf chlorophyll contents would have more photosynthetic rate. During present study, it was observed that heat tolerant genotypes have high chlorophyll contents (SPAD value) than susceptible genotypes in all transplanting, which is also confirmed by Hussain et al. (2016)HUSSAIN, R., AYYUB, C.M., AMJAD, M., WARAICH, E.A., SHAHEEN, M.R., MUSTAFA, Z., SHAH, S.Z.H., SHAHID, A., HAIDER, M.W., FAIZ, H. and RAZA, A., 2016. Evaluation of heat tolerance potential of okra genotypes in field conditions at different sowing times.Transylvanian Review, vol. 12, pp. 3416-3427., might be due to alterations in microscopic structures of okra genotypes when observed in different sowing times. Such microscopic alterations have more influence in susceptible varieties might be due to ability of tolerant genotypes to resist such transformations during heat stress (Balouchi, 2010BALOUCHI, H.R., 2010. Screening wheat parents of mapping population for heat and drought tolerance, detection of wheat genetic variation.International Journal of Biology and Life Sciences, vol. 6, no. 1, pp. 56-66.). Statements of Reda and Mandoura (2011)REDA, F. and MANDOURA, H.M.H., 2011. Response of enzymes activities, photosynthetic pigments, proline to low or high temperature stressed wheatplant (TriticumaestivumL.) in the presence or absence of exogenous proline or cysteine.International Journal of Academic Research, vol. 3, no. 4, pp. 108-115. revealed that high-temperature decreases synthesis of mesophyll apparatus in crop plants. In current research study, chlorophyll contents decreased with rise in temperature via late transplantation and TRIA foliar spray enhanced the chlorophyll in leaves, this observation were in accordance to various reports where TRIA was found significant in improving chlorophyll contents (Sarwar, 2017SARWAR, M., 2017. Alleviation of salt stress in cucumber (Cucumis sativus) through seed priming with triacontanol.International Journal of Agriculture and Biology, vol. 19, no. 4, pp. 771-778. http://dx.doi.org/10.17957/IJAB/15.0356.
http://dx.doi.org/10.17957/IJAB/15.0356...
). Physiological processes in plants are suddenly influenced by a slight increase in temperature. Present research revealed that high transpiration rate which can reduce turgor and osmotic potential of leaves. More loss of water in susceptible genotypes due to high transpiration rate through stomata cause low photosynthetic rate under heat stress (Zhang et al., 2006ZHANG, Y., MIAN, M.A.R. and BOUTON, J.H., 2006. Recent molecular and genomic studies on stress tolerance of forage and turf grasses.Crop Science, vol. 46, no. 2, pp. 497-511. http://dx.doi.org/10.2135/cropsci2004.0572.
http://dx.doi.org/10.2135/cropsci2004.05...
).In this research heat susceptible genotypes, decrease in photosynthetic rate during heat stress was probably because of increased stomatal conductance. At high-temperature stress enhanced CO2 assimilation rate in heat tolerant genotypes than susceptible genotypes were due to efficient photosynthetic apparatus in tolerant genotypes were also found in previous researches. Higher stomatal conductance of water and carbon dioxide was observed in susceptible genotypes (Perveen et al., 2014PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2014. Triacontanol-induced changes in growth, yield, leaf water relations, oxidative defense system, minerals, and some key osmoprotectants in Triticumaestivumunder saline conditions.Turkish Journal of Botany, vol. 38, no. 5, pp. 896-913. http://dx.doi.org/10.3906/bot-1401-19.
http://dx.doi.org/10.3906/bot-1401-19...
).
It was observed that both heat tolerant and heat susceptible genotypes exhibited improved antioxidant activities of superoxide dismutase, peroxidase and catalase enzymes irrespective of transplanting dates with the foliar application of TRIA when compared with control. The results of current research work were in line with those of a previous studies (Perveen et al., 2014PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2014. Triacontanol-induced changes in growth, yield, leaf water relations, oxidative defense system, minerals, and some key osmoprotectants in Triticumaestivumunder saline conditions.Turkish Journal of Botany, vol. 38, no. 5, pp. 896-913. http://dx.doi.org/10.3906/bot-1401-19.
http://dx.doi.org/10.3906/bot-1401-19...
) who reported improved peroxidase activity under salinity stress but not significant variations in superoxide dismutase activity were reported in that study. MDA contents were increased in second and third transplantation dates in present study. Similar findings were also reported in lettuce crop, where enhanced total soluble sugars and MDA were seen at high temperatures (42/37o C) compared to normal growing temperature (Han et al., 2013HAN, Y., FAN, S., ZHANG, Q. and WANG, Y., 2013. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings.Agricultural Sciences, vol. 4, no. 5, pp. 112-115. http://dx.doi.org/10.4236/as.2013.45B021.
http://dx.doi.org/10.4236/as.2013.45B021...
).
Growth increasing role of TRIA have been extensively documented to be related partially with enhanced uptake of nutrients from the growing medium (Misra and Srivastava, 1991MISRA, A. and SRIVASTAVA, N.K., 1991. Effects of the triacontanol formulations “Miraculan” on photosynthesis, growth, nutrient uptake, and essential oil yield of lemongrass (Cymbopogonflexuosus) Steud, Watts.Plant Growth Regulation, vol. 10, no. 1, pp. 57-63. http://dx.doi.org/10.1007/BF00035131.
http://dx.doi.org/10.1007/BF00035131...
). Current experimental results revealed significantly higher yields in TRIA supplemented plants as compared to those not given TRIA, irrespective of transplanting date and genetic differences. The probable reason of this yield increase may be enhanced antioxidant activity of stress related enzymes in response of foliar spray. The results are in line to the findings of (Perveen et al., 2014PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2014. Triacontanol-induced changes in growth, yield, leaf water relations, oxidative defense system, minerals, and some key osmoprotectants in Triticumaestivumunder saline conditions.Turkish Journal of Botany, vol. 38, no. 5, pp. 896-913. http://dx.doi.org/10.3906/bot-1401-19.
http://dx.doi.org/10.3906/bot-1401-19...
) who reported improved yield of salt stressed wheat plants in response to exogenous application of TRIA on various stages of plant growth. Extensive reports have been documented previously illustrating the similar yield enhancing effect of TRIA which might be due to improvement in nutrient uptake improved photosynthetic rate (as observed in the present study) and nitrogen fixation rate, enhanced translocation of secondary metabolites as well as photosynthates (Khan et al., 2006KHAN, M.M.A., MUJIBUR-RAHMAN, M., NAEEM, M., MOHAMMAD, F., SIDDIQUI, M.H. and KHAN, M.N., 2006. Triacontanol induced changes in the growth, yield and quality of tomato (Lycopersiconesculentum Mill.). Electronic Journal of Environmental.Agricultural and Food Chemistry, vol. 5, pp. 1492-1499.). In another research study, TRIA was found effective in improving the overall crop yield and quality (Aftab et al., 2010AFTAB, T., KHAN, M.M.A., IDREES, M., NAEEM, M., SINGH, M. and RAM, M., 2010. Stimulation of crop productivity, photosynthesis and artemisinin production in Artemesiaannua L. by triacontanol and gibberelic acid application.Journal of Plant Interactions, vol. 5, no. 4, pp. 273-281. http://dx.doi.org/10.1080/17429141003647137.
http://dx.doi.org/10.1080/17429141003647...
).Improvement in water and solutes uptake, cell division and elongation, membranous permeability, inflorescence number, fruit quality and ultimately increased yields were observed in various agronomic and horticultural crops as a result of TRIA application (Khan et al., 2006KHAN, M.M.A., MUJIBUR-RAHMAN, M., NAEEM, M., MOHAMMAD, F., SIDDIQUI, M.H. and KHAN, M.N., 2006. Triacontanol induced changes in the growth, yield and quality of tomato (Lycopersiconesculentum Mill.). Electronic Journal of Environmental.Agricultural and Food Chemistry, vol. 5, pp. 1492-1499., 2009KHAN, N., NAZAR, R. and ANJUM, N.A., 2009. Growth, photosynthesis and antioxidant metabolism in mustard (Brasicajuncia L.) cultivars differing in ATP- sulfurylase activity under salinity stress.Scientia Horticulturae, vol. 122, no. 3, pp. 455-460. http://dx.doi.org/10.1016/j.scienta.2009.05.020.
http://dx.doi.org/10.1016/j.scienta.2009...
). Similar results were observed when an experimental investigation was carried out with TRIA foliar application to investigate its effect on plant growth, yield and biochemical variables of summer squash crop grown in sandy soils. During a comprehensive study in China carried out in three years period, various vegetables (eggplant, tomato, winged beans and cabbage) positively responded to TRIA application.
5. Conclusion
From the current study, it may be deduce that foliar application of TRIA can be used to enhance yield of eggplant under normal conditions as well as to cope with drastic effects of high temperature stress. TRIA improved heat tolerance in eggplant by increasing enzymatic activities of superoxide dismutase, peroxidase and catalase. Leaf gaseous exchange attributes also altered by heat stress and improved by foliar spray of TRIA. Further, Triacontanol is effective in mitigation of adverse high temperature impacts.
References
- AFTAB, T., KHAN, M.M.A., IDREES, M., NAEEM, M., SINGH, M. and RAM, M., 2010. Stimulation of crop productivity, photosynthesis and artemisinin production in Artemesiaannua L. by triacontanol and gibberelic acid application.Journal of Plant Interactions, vol. 5, no. 4, pp. 273-281. http://dx.doi.org/10.1080/17429141003647137
» http://dx.doi.org/10.1080/17429141003647137 - ASADIPOUR, A. and MADANI, H., 2014. The effects of irrigation and sowing date on the quantitative traits of okra (Abelmoschusesculentus L.).International Journal of Farming and Allied Sciences, vol. 3, no. 5, pp. 497-501.
- BALOUCHI, H.R., 2010. Screening wheat parents of mapping population for heat and drought tolerance, detection of wheat genetic variation.International Journal of Biology and Life Sciences, vol. 6, no. 1, pp. 56-66.
- CHANCE, B. and MAEHLY, A.C., 1955. Assay of catalase and peroxidase.Methods in Enzymology, vol. 2, pp. 764-775. http://dx.doi.org/10.1016/S0076-6879(55)02300-8
» http://dx.doi.org/10.1016/S0076-6879(55)02300-8 - GORAYA, G.K. and ASTHIR, B., 2016. Magnificent role of intracellular reactive oxygen species production and its scavenging encompasses downstream processes.Journal of Plant Biology, vol. 59, no. 3, pp. 215-222. http://dx.doi.org/10.1007/s12374-016-0057-9
» http://dx.doi.org/10.1007/s12374-016-0057-9 - HAN, Y., FAN, S., ZHANG, Q. and WANG, Y., 2013. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings.Agricultural Sciences, vol. 4, no. 5, pp. 112-115. http://dx.doi.org/10.4236/as.2013.45B021
» http://dx.doi.org/10.4236/as.2013.45B021 - HASANUZZAMAN, M., NAHAR, K., ALAM, M.M., ROYCHOWDHURY, R. and FUJITA, M., 2013. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants.International Journal of Molecular Sciences, vol. 14, no. 5, pp. 9643-9684. http://dx.doi.org/10.3390/ijms14059643 PMid:23644891.
» http://dx.doi.org/10.3390/ijms14059643 - HOEGH-GULDBERG, O. and BRUNO, J.F., 2010. The impact of climate change on the world’s marine ecosystems.Science, vol. 328, no. 5985, pp. 1523-1528. http://dx.doi.org/10.1126/science.1189930 PMid:20558709.
» http://dx.doi.org/10.1126/science.1189930 - HUSSAIN, R., AYYUB, C.M., AMJAD, M., WARAICH, E.A., SHAHEEN, M.R., MUSTAFA, Z., SHAH, S.Z.H., SHAHID, A., HAIDER, M.W., FAIZ, H. and RAZA, A., 2016. Evaluation of heat tolerance potential of okra genotypes in field conditions at different sowing times.Transylvanian Review, vol. 12, pp. 3416-3427.
- HUSSAIN, T., AYYUB, C.M., AHMAD, I., ALI, I., MUSTAFA, Z., ANWAR, A., AHMAD, A., LATIF, S. and IQBAL, T., 2019. Mitigation of adverse effects of heat stress in chillies by using glycine betaine.International Journal of Biosciences, vol. 15, no. 2, pp. 1-10.
- ISLAM, S. and MOHAMMAD, F., 2020. Triacontanol as a dynamic growth regulator for plants under diverse environmental conditions.Physiology and Molecular Biology of Plants, vol. 26, no. 5, pp. 871-883. http://dx.doi.org/10.1007/s12298-020-00815-0 PMid:32377038.
» http://dx.doi.org/10.1007/s12298-020-00815-0 - JING, P., QIAN, B., ZHAO, S., QI, X., YE, L., GIUSTI, M.M. and WANG, X., 2015. Effect of glycosylation patterns of Chinese eggplant anthocyanins and other derivatives on antioxidant effectiveness in human colon cell lines.Food Chemistry, vol. 172, pp. 183-189. http://dx.doi.org/10.1016/j.foodchem.2014.08.100 PMid:25442541.
» http://dx.doi.org/10.1016/j.foodchem.2014.08.100 - KHAN, M.M.A., MUJIBUR-RAHMAN, M., NAEEM, M., MOHAMMAD, F., SIDDIQUI, M.H. and KHAN, M.N., 2006. Triacontanol induced changes in the growth, yield and quality of tomato (Lycopersiconesculentum Mill.). Electronic Journal of Environmental.Agricultural and Food Chemistry, vol. 5, pp. 1492-1499.
- KHAN, N., NAZAR, R. and ANJUM, N.A., 2009. Growth, photosynthesis and antioxidant metabolism in mustard (Brasicajuncia L.) cultivars differing in ATP- sulfurylase activity under salinity stress.Scientia Horticulturae, vol. 122, no. 3, pp. 455-460. http://dx.doi.org/10.1016/j.scienta.2009.05.020
» http://dx.doi.org/10.1016/j.scienta.2009.05.020 - MISRA, A. and SRIVASTAVA, N.K., 1991. Effects of the triacontanol formulations “Miraculan” on photosynthesis, growth, nutrient uptake, and essential oil yield of lemongrass (Cymbopogonflexuosus) Steud, Watts.Plant Growth Regulation, vol. 10, no. 1, pp. 57-63. http://dx.doi.org/10.1007/BF00035131
» http://dx.doi.org/10.1007/BF00035131 - MOYA, L.J., GOMEZ-CADENAS, A., PRIMO-MILLO, E. and TALON, M., 2003. Chloride absorption in salt- sensitive Carrizo citrange and salt-tolerant Cleopatra mandarin citrus rootstocks is linked to water use. Journal of Experimental Botany, vol. 54, pp. 825-833.
- NAEEM, M., KHAN, M.M.A. and MOINUDDIN., 2012. Triacontanol: a potent plant growth regulator in agriculture.Journal of Plant Interactions, vol. 7, no. 2, pp. 129-142. http://dx.doi.org/10.1080/17429145.2011.619281
» http://dx.doi.org/10.1080/17429145.2011.619281 - NAEEM, M., KHAN, M.M.A., MOINUDDIN. and SIDDIQUI, M.H., 2009. Triacontanol stimulates nitrogen-fixation, enzyme activities, photosynthesis, crop productivity and quality of hyacinth bean (Lablab purpureusL.).Scientia Horticulturae, vol. 121, no. 4, pp. 389-396. http://dx.doi.org/10.1016/j.scienta.2009.02.030
» http://dx.doi.org/10.1016/j.scienta.2009.02.030 - OHKAWA, H., OHISHI, N. and YAGI, K., 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction.Analytical Biochemistry, vol. 95, no. 2, pp. 351-358. http://dx.doi.org/10.1016/0003-2697(79)90738-3 PMid:36810.
» http://dx.doi.org/10.1016/0003-2697(79)90738-3 - PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2010. Regulation in gas exchange and quantum yield of photosystem II (PSII) in salt-stressed and non-stressed wheat plants raised from seed treated with triacontanol.Pakistan Journal of Botany, vol. 42, no. 5, pp. 3073-3081.
- PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2012. Changes in mineral composition, uptake and use efficiency of salt stressed wheat (Triticumaestivum L.) plants raised from seed treated with triacontanol.Pakistan Journal of Botany, vol. 44, pp. 27-35.
- PERVEEN, S., SHAHBAZ, M. and ASHRAF, M., 2014. Triacontanol-induced changes in growth, yield, leaf water relations, oxidative defense system, minerals, and some key osmoprotectants in Triticumaestivumunder saline conditions.Turkish Journal of Botany, vol. 38, no. 5, pp. 896-913. http://dx.doi.org/10.3906/bot-1401-19
» http://dx.doi.org/10.3906/bot-1401-19 - REDA, F. and MANDOURA, H.M.H., 2011. Response of enzymes activities, photosynthetic pigments, proline to low or high temperature stressed wheatplant (TriticumaestivumL.) in the presence or absence of exogenous proline or cysteine.International Journal of Academic Research, vol. 3, no. 4, pp. 108-115.
- SARWAR, M., 2017. Alleviation of salt stress in cucumber (Cucumis sativus) through seed priming with triacontanol.International Journal of Agriculture and Biology, vol. 19, no. 4, pp. 771-778. http://dx.doi.org/10.17957/IJAB/15.0356
» http://dx.doi.org/10.17957/IJAB/15.0356 - SĘKARA, A., BĄCZEK-KWINTA, R., GAWĘDA, M., KALISZ, A., POKLUDA, R. and JEZDINSKÝ, A., 2016. Sequential abiotic stress applied to juvenile eggplant modifies the seedlings parameters, plant ontogeny and yield.Horticultural Science, vol. 43, no. 3, pp. 149-157. http://dx.doi.org/10.17221/162/2015-HORTSCI
» http://dx.doi.org/10.17221/162/2015-HORTSCI - SHAHEEN, M.R., AYYUB, C.M., AMJAD, M. and WARAICH, E.A., 2016. Morpho-physiological evaluation of tomato genotypes under high temperature stress conditions.Journal of the Science of Food and Agriculture, vol. 96, no. 8, pp. 2698-2704. http://dx.doi.org/10.1002/jsfa.7388 PMid:26304011.
» http://dx.doi.org/10.1002/jsfa.7388 - SHARMA, P., JHA, A.B., DUBEY, R.S. and PESSARAKLI, M., 2012. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions.Le Journal de Botanique, vol. 2012, pp. 217037. http://dx.doi.org/10.1155/2012/217037
» http://dx.doi.org/10.1155/2012/217037 - WAHID, A., GELANI, S., ASHRAF, M. and FOOLAD, M., 2007. Heat tolerance in plants: an overview.Environmental and Experimental Botany, vol. 61, no. 3, pp. 199-223. http://dx.doi.org/10.1016/j.envexpbot.2007.05.011
» http://dx.doi.org/10.1016/j.envexpbot.2007.05.011 - WU, G.Q., ZHANG, L.N. and WANG, Y.Y., 2012. Response of growth and antioxidant enzyme to osmotic stress in two different wheat (Triticumaestivum L.) cultivars seedlings.Plant, Soil and Environment, vol. 58, no. 12, pp. 534-539. http://dx.doi.org/10.17221/373/2012-PSE
» http://dx.doi.org/10.17221/373/2012-PSE - ZHANG, Y., MIAN, M.A.R. and BOUTON, J.H., 2006. Recent molecular and genomic studies on stress tolerance of forage and turf grasses.Crop Science, vol. 46, no. 2, pp. 497-511. http://dx.doi.org/10.2135/cropsci2004.0572
» http://dx.doi.org/10.2135/cropsci2004.0572 - ZINN, K.E., TUNC-OZDEMIR, M. and HARPER, J.F., 2010. Temperature stress and plant sexual reproduction: uncovering the weakest links.Journal of Experimental Botany, vol. 61, no. 7, pp. 1959-1968. http://dx.doi.org/10.1093/jxb/erq053 PMid:20351019.
» http://dx.doi.org/10.1093/jxb/erq053
Publication Dates
-
Publication in this collection
12 Jan 2022 -
Date of issue
2024
History
-
Received
28 June 2021 -
Accepted
31 Aug 2021