Abstract
Magnolia biondii Pamp is an important ornamental tree species widely grown and used as a rootstock in the propagation of different Magnolia varieties. In the current studies, anatomical, physiological and endogenous hormones were studied to check the effect of IBA 750 mg/L on the adventitious rooting and to provide theoretical and technical support for the propagation of Magnolia biondii Pamp through stem cuttings. Two thousand stem cuttings were prepared and divided into two groups i.e., IBA treated cuttings and water control. For the evaluation of antioxidant enzyme activities, and endogenous hormones levels, samples were collected on the day of planting and each 5th day and further steps were carried out in the laboratory according to the protocols and proper precautions. For the anatomical observations, samples were collected on the 13th, 15th, and 17th day for IBA treated cuttings while 21st, 23rd, and 25th day for control. Collected samples were preserved in the FAA solution and further observations were carried out in the laboratory. Anatomical observations showed that it took 13 days for the differentiation of root primordia to the appearance of young adventitious roots in IBA treated cuttings, while it took 21 days to develop primordia in the control. Antioxidant enzyme activities involved in ROS were significantly higher in the IBA treated cuttings compared to control. POD showed a peak on the 13th day before the emergence of roots in IBA treated cuttings while it showed a peak on the 21st day in the control. PPO showed a peak on the 21st day in the IBA treated cuttings while it showed a peak on the 29th day in the control. SOD showed a peak on the 17th day in IBA treated cuttings, while it showed a peak on the 25th day in the control. Exogenous application of IBA enhanced the endogenous IAA and GA3 levels compared to CK, while it reduced the levels of ABA continuously at the time of rooting and then increased gradually. Inclusively, our study suggests that IBA 750 mg/L is efficient for the rooting of Magnolia biondii Pamp cuttings, as it enhanced the process of antioxidant enzyme activities, endogenous hormones levels and reduced the time of root formation which is evident from the anatomical observations.
Keywords:
Magnolia biondii Pamp; antioxidant enzyme activities; endogenous hormones; anatomical observations; adventitious rooting; stem cuttings
Resumo
Magnolia biondii Pamp é uma importante espécie de árvore ornamental muito cultivada e utilizada como porta-enxerto na propagação de diferentes variedades de Magnolia. Nos estudos atuais, hormônios anatômicos, fisiológicos e endógenos foram estudados para verificar o efeito do AIB na dose de 750 mg / L no enraizamento adventício e fornecer suporte teórico e técnico para a propagação de M. biondii Pamp por meio de estacas. Duas mil estacas foram preparadas e divididas em dois grupos, ou seja, tratadas com AIB e controle de água. Para a avaliação das atividades das enzimas antioxidantes e dos níveis de hormônios endógenos, as amostras foram coletadas no dia do plantio e a cada 5 dias, enquanto as demais etapas foram realizadas em laboratório de acordo com os protocolos e os devidos cuidados. Para as observações anatômicas, as amostras foram coletadas no 13º, 15º e 17º dias para estacas tratadas com AIB e no 21º, 23º e 25º dias para o controle. As amostras coletadas foram preservadas em solução FAA, e outras observações foram realizadas em laboratório. Observações anatômicas mostraram a necessidade de 13 dias para a diferenciação dos primórdios radiculares até o aparecimento de raízes adventícias jovens em estacas tratadas com AIB e de 21 dias para o desenvolvimento dos primórdios no controle. As atividades das enzimas antioxidantes envolvidas nas ROS foram significativamente maiores nas estacas tratadas com AIB em comparação com o controle. A POD apresentou pico no 13º dia antes da emergência das raízes nas estacas tratadas com AIB, enquanto no 21º dia apresentou pico no controle. A PPO teve pico no 21º dia nas estacas tratadas com AIB e no 29º dia no controle. A SOD apresentou pico no 17º dia nas estacas tratadas com AIB e no 25º dia no controle. A aplicação exógena de AIB aumentou os níveis endógenos de IAA e GA3 em relação ao controle, enquanto reduziu os níveis de ABA continuamente no momento do enraizamento e, em seguida, aumentou gradativamente. Inclusive, nosso estudo sugere que o AIB na dose de 750 mg / L é eficiente para o enraizamento de estacas de M. biondii Pamp, visto que potencializou o processo de atividades de enzimas antioxidantes e os níveis de hormônios endógenos, além de reduzir o tempo de formação de raízes, o que fica evidente nas observações anatômicas.
Palavras-chave:
Magnolia biondii Pamp; atividades de enzimas antioxidantes; hormônios endógenos; observações anatômicas; enraizamento adventício; estacas caulinares
1. Introduction
Adventitious root formation is a complex developmental process regulated by several endogenous and exogenous factors (Leakey, 2004LEAKEY, R.R., 2004. Physiology of vegetative reproduction. In: J. Burley, J. Evans, and J.A Youngquist, eds. Encyclopaedia of forest sciences. London: Academic Press.). Auxins perform a critical role in the early development and formation of adventitious roots by enhancing the initiation of root primordia by cell division (Fogaça and Fett-Neto, 2005FOGAÇA, C.M. and FETT-NETO, A.G., 2005. Role of auxin and its modulators in the adventitious rooting of Eucalyptus species differing in recalcitrance. Plant Growth Regulation, vol. 45, no. 1, pp. 1-10. http://dx.doi.org/10.1007/s10725-004-6547-7.
http://dx.doi.org/10.1007/s10725-004-654...
). Auxins stimulate starch hydrolysis, nutrients, and sugars to the base of the cuttings (Das et al., 1997DAS, P., BASAK, U.C. and DAS, A.B., 1997. Metabolic changes during rooting in pre-girdled stem cuttings and air-layers of Heritiera. Botanical Bulletin of Academia Sinica, vol. 38, pp. 91-95.). During the process of cell division and transport of auxins, auxins play the main role through selective proteolysis and cell wall loosening with auxin binding protein 1 (ABP-1) and receptor protein transporting inhibitor response 1 (TIR-1) (Costa et al., 2013COSTA, C.T., ALMEIDA, M.R., RUEDELL, C.M., SCHWAMBACH, J., MARASCHIN, F.D.S. and FETT-NETO, A.G., 2013. When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Frontiers in Plant Science, vol. 4, pp. 133. http://dx.doi.org/10.3389/fpls.2013.00133. PMid:23717317.
http://dx.doi.org/10.3389/fpls.2013.0013...
). However, de novo root organogenesis is the process of adventitious root formation from the wounded or detached part of the parent plant. Usually, adventitious roots originate and develop from the outside or just next to the central core of vascular tissues. The development of adventitious roots in many easy to root plant species occurs from the phloem ray parenchyma cells. De novo root organogenesis depends on the plant cell to dedifferentiate and form a new root system. The dedifferentiation process is the ability of developed differentiated cells to enhance the initiation of cell division to form a new meristematic point. With the formation of meristematic point, a new root initial formation starts and ultimately fully developed root primordia develops in the cortex and phloem. Some plant species have a more pronounced dedifferentiation process than others and need some proper conditions for regeneration. However, IBA 750 mg/L is useful for the propagation of Magnolia biondii Pamp through stem cuttings (Khan et al., 2020KHAN, M., WANG, Y., UDDIN, S., MUHAMMAD, B., BADSHAH, M., KHAN, D., MUNEER, M., MUNIR, M. and JIA, Z., 2020. Propagation of magnolia biondii pamp through stem cuttings using exogenous hormones. Applied Ecology and Environmental Research, vol. 18, no. 2, pp. 2213-2229. http://dx.doi.org/10.15666/aeer/1802_22132229.
http://dx.doi.org/10.15666/aeer/1802_221...
) but it wasn’t proved by the anatomical studies and there was no proper research on the underlying science of adventitious root formation.
The adventitious root formation in the base of stem cuttings is a key developmental process in the growth and subsistence of cuttings which involves the initiation of several new meristematic capacities in different tissues of stem cuttings (Kaur et al., 2002KAUR, S., CHEEMA, S., CHHABRA, B. and TALWAR, K., 2002. Chemical induction of physiological changes during adventitious root formation and bud break in grapevine cuttings. Plant Growth Regulation, vol. 37, no. 1, pp. 63-68. http://dx.doi.org/10.1023/A:1020355505105.
http://dx.doi.org/10.1023/A:102035550510...
). Enzymes regulating auxin metabolism represent an important factor that affects the formation of adventitious roots in almost all stages of rhizogenesis (Hartmann et al., 2002Hartmann, H., Kester, D. , Driver, F. and Geneve, R., 2002. Plant propagation: principles and practices. Upper Saddle River: Prentice Hall.). The enzymatic activities in the rooting areas of cuttings provide an easy, fast and reliable means of evaluating cellular differentiation into the roots (Husen and Pal, 2007HUSEN, A. and PAL, M., 2007. Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f.(teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatment. New Forests, vol. 33, no. 3, pp. 309-323. http://dx.doi.org/10.1007/s11056-006-9030-7.
http://dx.doi.org/10.1007/s11056-006-903...
). Plants in the early stage bear oxidative stress and lose redox balance because their nutrition and water supply get lost while propagation through stem cuttings (Papadakis and Roubelakis-Angelakis, 2002PAPADAKIS, A. and ROUBELAKIS-ANGELAKIS, K., 2002. Oxidative stress could be responsible for the recalcitrance of plant protoplast. Plant Physiology and Biochemistry, vol. 40, no. 6-8, pp. 549-559. http://dx.doi.org/10.1016/S0981-9428(02)01423-7.
http://dx.doi.org/10.1016/S0981-9428(02)...
). How to recover redox balance, increase antioxidant enzyme activities and enhance adventitious root formation are difficult for stem survival (De Klerk et al., 1999DE KLERK, G., VAN DER KRIEKEN, W. and DE JONG, J.C., 1999. Review the formation of adventitious roots: new concepts, new possibilities. In Vitro Cellular & Developmental Biology. Plant, vol. 35, no. 3, pp. 189-199. http://dx.doi.org/10.1007/s11627-999-0076-z.
http://dx.doi.org/10.1007/s11627-999-007...
; Negishi et al., 2014NEGISHI, N., NAKAHAMA, K., URATA, N., KOJIMA, M., SAKAKIBARA, H. and KAWAOKA, A., 2014. Hormone level analysis on adventitious root formation in Eucalyptus globulus. New Forests, vol. 45, no. 4, pp. 577-587. http://dx.doi.org/10.1007/s11056-014-9420-1.
http://dx.doi.org/10.1007/s11056-014-942...
). Exogenous hormones, such as auxins, contributes an important role in the formation of adventitious roots (Haissig 1974HAISSIG, B., 1974. Influences of auxins and auxin synergists on adventitious root primordium initiation and development. New Zealand Journal of Forestry Science, vol. 4, no. 2, pp. 311-323.; Bellamine et al., 1998BELLAMINE, J., PENEL, C., GREPPIN, H., GASPAR, T. and GASPAR, T., 1998. Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. Plant Growth Regulation, vol. 26, no. 3, pp. 191-194. http://dx.doi.org/10.1023/A:1006182801823.
http://dx.doi.org/10.1023/A:100618280182...
). Exogenous hormone treatment not only promotes adventitious root formation but also enhances the activity of polyphenol oxidase (PPO) and peroxidase (POD) activities and decreases the activity of IAAO. Kose et al. (2011)KOSE, C., ERDAL, S., KAYA, O. and ATICI, O., 2011. Comparative evaluation of oxidative enzyme activities during adventitious rooting in the cuttings of grapevine rootstocks. Journal of the Science of Food and Agriculture, vol. 91, no. 4, pp. 738-741. http://dx.doi.org/10.1002/jsfa.4244.
http://dx.doi.org/10.1002/jsfa.4244...
reported that several changes occur in the enzyme activities in the base of stem cuttings during the adventitious root formation and these changes are caused by auxins and enhance or inhibit adventitious root formation.
Peroxidases and polyphenols have a crucial role in the rooting of stem cuttings. Peroxidase and polyphenol activities catalyze the process of cell wall lignification and synthesize the phenoxyl radicals from the aromatic compound in apoplastic space (Fukuda and Komamine, 1982FUKUDA, H. and KOMAMINE, A., 1982. Lignin synthesis and its related enzymes as markers of tracheary-element differentiation in single cells isolated from the mesophyll of Zinnia elegans. Planta, vol. 155, no. 5, pp. 423-430. http://dx.doi.org/10.1007/BF00394471. PMid:24271974.
http://dx.doi.org/10.1007/BF00394471...
; Takahama, 1997TAKAHAMA, U., 1997. Enhancement of the peroxidase-dependent oxidation of dopa by components of Vicia leaves. Phytochemistry, vol. 46, no. 3, pp. 427-432. http://dx.doi.org/10.1016/S0031-9422(97)00336-1.
http://dx.doi.org/10.1016/S0031-9422(97)...
). Peroxidases and polyphenols activities increase continuously during the induction and initiation phases of adventitious rooting and decrease during the period of extension, while H2O2 and IAAO activity are opposite to the activity of peroxidases and polyphenols (Nag et al., 2001NAG, S., SAHA, K. and CHOUDHURI, M., 2001. Role of auxin and polyamines in adventitious root formationin relation to changes in compounds involved in rooting. Journal of Plant Growth Regulation, vol. 20, no. 2, pp. 182-194. http://dx.doi.org/10.1007/s003440010016.
http://dx.doi.org/10.1007/s003440010016...
; Rout, 2006ROUT, G.R., 2006. Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant Growth Regulation, vol. 48, no. 2, pp. 111-117. http://dx.doi.org/10.1007/s10725-005-5665-1.
http://dx.doi.org/10.1007/s10725-005-566...
). Therefore, changes in peroxidases and polyphenols are suggested to evaluate, as these changes indicate biochemical changes for different stages of adventitious root formation (Moncousin and Gaspar, 1983MONCOUSIN, C. and GASPAR, T., 1983. Peroxidase as a marker for rooting improvement of Cynara scolymus L. cultured in vitro. Biochemie und Physiologie der Pflanzen, vol. 178, no. 4, pp. 263-271. http://dx.doi.org/10.1016/S0015-3796(83)80040-7.
http://dx.doi.org/10.1016/S0015-3796(83)...
; Kose et al., 2011KOSE, C., ERDAL, S., KAYA, O. and ATICI, O., 2011. Comparative evaluation of oxidative enzyme activities during adventitious rooting in the cuttings of grapevine rootstocks. Journal of the Science of Food and Agriculture, vol. 91, no. 4, pp. 738-741. http://dx.doi.org/10.1002/jsfa.4244.
http://dx.doi.org/10.1002/jsfa.4244...
).
Moreover, the effectiveness of adventitious rooting by cuttings can also be explained by various endogenous factors in which endogenous hormones have an important role. Plant endogenous hormones such as gibberellin (GA3), auxin (IAA) and abscisic acid (ABA) have important regulatory effects on plant growth and development. Auxin produces the response of growth at a distance from its location of synthesis, transport of IAA is cell to cell, generally in the vascular cambium, while the transport of IAA to the root involves phloem (Davies, 2010DAVIES, P.J., 2010. Plant hormones: biosynthesis, signal transduction, action! Dordrecht: Springer. The plant hormones: their nature, occurrence, and functions, pp. 1-15.). Besides other functions, endogenous auxin generally stimulates the root initiation in stem cuttings and also helps in the development and differentiation of branch roots (Davies, 2010DAVIES, P.J., 2010. Plant hormones: biosynthesis, signal transduction, action! Dordrecht: Springer. The plant hormones: their nature, occurrence, and functions, pp. 1-15.). GA3 is the most commonly existing compound of the gibberellins family. Gibberellins are mostly synthesized from glyceraldehyde-3-phosphate by isopentenyl diphosphate in new developing young tissues (Davies, 2010DAVIES, P.J., 2010. Plant hormones: biosynthesis, signal transduction, action! Dordrecht: Springer. The plant hormones: their nature, occurrence, and functions, pp. 1-15.). Gibberellins enhance cell division and cell elongation. ABA is generally known as an inhibitor and it is synthesized from the glyceraldehyde-3-phosphate by isopentenyl diphosphate and carotenoids in roots and leaves (Davies, 2010DAVIES, P.J., 2010. Plant hormones: biosynthesis, signal transduction, action! Dordrecht: Springer. The plant hormones: their nature, occurrence, and functions, pp. 1-15.). ABA is transported by vascular bundles and functions in stomatal closure, prompts protein storage synthesis in seeds and inhibits shoot growth (Davies, 2010DAVIES, P.J., 2010. Plant hormones: biosynthesis, signal transduction, action! Dordrecht: Springer. The plant hormones: their nature, occurrence, and functions, pp. 1-15.).
The study aims to provide theoretical and technical support for the rooting of Magnolia biondii Pamp through stem cuttings, to get an insight into the de novo root organogenesis through anatomical observation and to evaluate the physiological changes and endogenous hormones levels during the adventitious root formation.
2. Materials and Methods
2.1. Plant material and experiment site
The plant material of Magnolia biondii Pamp cuttings was obtained from the seven-year-old donor plants located at the Silviculture test station of Beijing Forestry University at Jiufeng, Beijing in late June 2019. All the cuttings were 20-25 cm in length with two half leaves. The experiment was conducted in the greenhouse (temperature: 20oC – 30oC, relative humidity: about 80%) located near the test station. The geographical coordinates of the test station are 40.3054” N and 116.05045” E (Figure 1). This area has a temperate humid monsoon climate zone with hot, variable rainy summers and dry cold winters. The average annual temperature is 12.5oC with an accumulating temperature of 42oC, and the number of annual sunshine hours is 2662h.
Green point in the map shows the study area; Silviculture Test Station of Beijing Forestry University, Jiufeng Beijing, China.
2.2. Anatomical studies
For anatomical analysis of Magnolia biondii Pamp cuttings, the basal portion of the cuttings at about 1cm was collected on three-time points i.e., day of planting (DAP) 0 day, 15th day for the treatment and on 24th day for the control. At least 12 samples were collected for each time point. The collected samples were fixed in FAA solution (70% ethanol, 5% glacial acetic, and 5% formaldehyde) and afterward treated samples with the protocol previously described by Lodama et al. (2016LODAMA, K.E., DU TOIT, E., STEYN, J., ARAYA, H.T., PRINSLOO, G., DU PLOOY, C. and ROBBERTSE, P., 2016. Improving rooting of Lobostemon fruticosus L. cuttings with delayed auxin treatment. South African Journal of Botany, vol. 105, pp. 111-115. http://dx.doi.org/10.1016/j.sajb.2016.01.005.
http://dx.doi.org/10.1016/j.sajb.2016.01...
). Sections (8µm) were made by using g rotary microtome (Leica RM2255) and then stained with Safra-nin (1%) for 15 minutes, followed by three times rinsed in deionized water to remove the excess stains from the slides, then dipped in fast-green solution (1%) for 10 seconds and again used deionized water three times for final rinse. At least 12 cross-sections for a 1cm cutting base were selected for adventitious root primordium. All the sections were studied and photographed were taken by Olympus BX51 microscope. The software FV10-ASW 3.1 viewer (Olympus Support) was used to export photographs.
2.3. Measurement of antioxidant enzymes activities
For the evaluation of dynamic changes in the antioxidant enzyme activities (POD, PPO, SOD) during the rooting process of Magnolia biondii Pamp cuttings, about two thousand cuttings were prepared and treated with IBA 750 mg/L and water (control). Prepared cuttings were implanted in the soil beds and samples were collected randomly on the day of planting and each 5th day. Collected samples were rinsed with water and preserved at -80oC for further procedures. The experiment lasted for forty-five days and a total of 12 days of samples were collected.
To measure the antioxidant enzymes, samples were ground with mortar and pestle using liquid nitrogen. About 0.5g of the ground sample was measured with three replicates and 5ml of 50 µmol/L phosphate buffer (pH 7.8) was added for enzyme extraction. The homogenate was centrifuged at 6000 rpm for 30 minutes at 4oC. The supernatant was kept as the enzyme assay and pellet were wasted.
POD activity was measured by following the protocol of Lu and Li (2012)Lu, W. and Li, Y., 2012. Plant physiology course. Beijing: China Forestry., in which 0.1 ml of enzyme extract was added to 3 mL of 25 µmol/L guaiacol and 0.2 mL of 250 µmol/L 30% H2O2. Phosphate buffer was placed as blank control and absorbance was measured at 470 nm wavelength, immediately and after 3 minutes of reaction. One unit of POD was measured according to the following Equation 1.
Equation.1: Here ∆A indicates the change in the absorbance of the reaction mixture (A4703min-A4700min), V indicates the total volume of sample extract, ∆t indicates the change in enzymatic reaction time, Vs indicates the volume of the sample taken for the determination, and W indicates the fresh weight of the sample.
PPO activity was measured by following the method of Yan et al. (2014)YAN, Y., LI, J., ZHANG, X., YANG, W., WAN, Y., MA, Y., ZHU, Y., PENG, Y. and HUANG, L., 2014. Effect of naphthalene acetic acid on adventitious root development and associated physiological changes in stem cutting of Hemarthria compressa. PLoS One, vol. 9, no. 3, pp. e90700. http://dx.doi.org/10.1371/journal.pone.0090700 PMid:24595064.
http://dx.doi.org/10.1371/journal.pone.0...
with modification. The reaction mixture consisted of 3 ml in which 0.5 ml of enzyme extract, 0.5 ml of 0.01µMol/L catechol, and 2 ml of 50 µmol/L phosphate buffer (pH 7.8). The control consisted of 2.5 ml phosphate buffer and 0.5 ml of catechol. Absorbance was measured at the wavelength of 410 nm immediately and again after 2 minutes of reaction. PPO was measured according to the following Equation 2.
Equation.2: Here ∆A indicates the change in the absorbance of the reaction mixture (A4102min-A4100min), V indicates the total volume of sample extract, ∆t indicates the change in enzymatic reaction time, Vs indicates the volume of the sample taken for the determination, and W indicates the fresh weight of the sample.
SOD enzymatic activity was measured according to the method of Li et al. (2000)LI, H., SUN, Q., ZHAO, S. and ZHANG, W., 2000. Principles and techniques of plant physiological biochemical experiment. Beijing: Higher Education, pp. 195-197.. Reaction solution to determine SOD consisted of 0.05 ml the enzyme extract, 0.3 ml of 130 µmol/L methionine, 0.3 ml of 750 µmol/L nitrogen blue tetrazole (NBT), 0.3 ml of 100 µmol/L EDTA-Na2, 0.3 ml of 20 µmol/L riboflavin, 1.5 ml of 50 µmol/L phosphate buffer (pH 7.8), and 0.25 ml of distilled water. One control was also prepared without enzyme extract. The reaction solution was kept under the 4000 lx fluorescent light for 20 minutes, whereas the control was kept under the dark. The reaction solution was then placed for 5 minutes in the dark to stop the reaction. Phosphate buffer was used as the blank control. The absorbance of the reaction solutions was measured at 560 nm wavelength. SOD activity was calculated according to the following Equation 3.
Equation.3: Here Ack indicates the absorbance of the spectrometer of the control, Ae indicates the absorbance of the spectrometer of the sample tube, V indicates the total volume of the sample, W indicates the fresh weight of the sample, and Vt indicates the amount of the sample used.
2.4 Determination of endogenous hormones
Cuttings implantation, samples preparation, collection, and time of samples collection were consistent with the antioxidant enzyme activities reported in the previous section 2.3. Samples were brought to the lab and ground in liquid nitrogen for further steps. The determination of endogenous hormones was done by following the protocol developed by Xiao et al. (2020)XIAO, A., CHEN, F., JIA, Z., SANG, Z., ZHU, Z. and MA, L., 2020. Determination of 4 plant hormones in Magnolia wufengensis by gradient elution high performance liquid chromatography. Chinese Journal of Analysis Laboratory, vol. 39, pp. 249-254. with some minor changes using high-performance liquid chromatography (HPLC).
3. Results
3.1. Anatomical observations
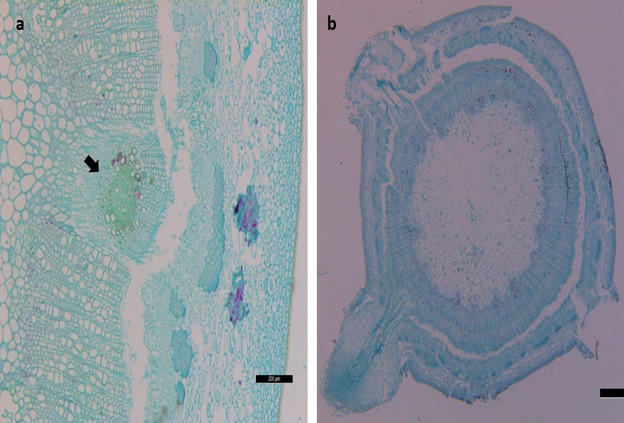
In comparison, the anatomical structure of the IBA 750 mg/L treated cuttings showed the early emergence of root primordia compared to control (CK). The stem anatomy of Magnolia biondii Pamp was based on transverse sections that were made from the basal portion of the cuttings. To observe the actual differences between the control and IBA treated cuttings, both treatments were tested for anatomical analysis and there were no cellular activities observed on the first day after planting (Figure 2a). However, it took 12 days for the initiation of primordia in IBA treated cuttings (Figure 2b), the base of the cuttings started to enlarge to the outside of the periderm near the incision thickened slightly, swelled and formed small protrusions. On the 15th day after planting, very dense adventitious roots primordia were observed in the IBA treated cuttings (Figure 2c). Finally, transparent and white young adventitious roots emerged successively in all cuttings up to 17th day (Figure 2d), and after 45 days adventitious roots grew up to 5-6 cm (Figure 2e).
Histological observation on the rooting of Magnolia biondii Pamp cuttings. (a) Cuttings without hormones treatment on the 0 day, (b) cuttings treated with IBA, primordium initiation and cells elongation has started on the 12th day, arrows in the picture indicates the dedifferentiation and formation of meristematic areas (c) Cuttings treated with IBA, the primordia extension, root elongation and growth on the 15th day, Pr indicates root primordia, Vc indicates vascular cambium, P indicates pith, X indicates xylem, Ph indicates phloem, C indicates cortex and e indicates epidermis, (d) cuttings treated with IBA after 17 days, (e) cuttings treated with IBA after 45 days.
The stem of Magnolia biondii Pamp is composed of periderm (including skin debris), cortex and secondary vascular tissues from the outside to the inside. Anatomical differences indicated that early accumulation in the cambium zone triggered up the initial cell activation in IBA 750 mg/L treated cuttings, while in control, it was studied that cell activation occurred late and as a result, late adventitious rooting occurred. It took 21 days to develop the initiation of the first adventitious root primordia in control (Figure 3a), that continuously enlarged and fully matured adventitious root primordia were observed 25th day after planting (Figure 3b).
Histological observation on the rooting of Magnolia biondii Pamp cuttings. (a) Cuttings treated with control (Ck), arrow indicates the primordium initiation and cells elongation on the 21st day, (b) control treated cuttings, the primordia extension, root elongation and growth on the 25th day.
3.2. Antioxidant enzyme activities
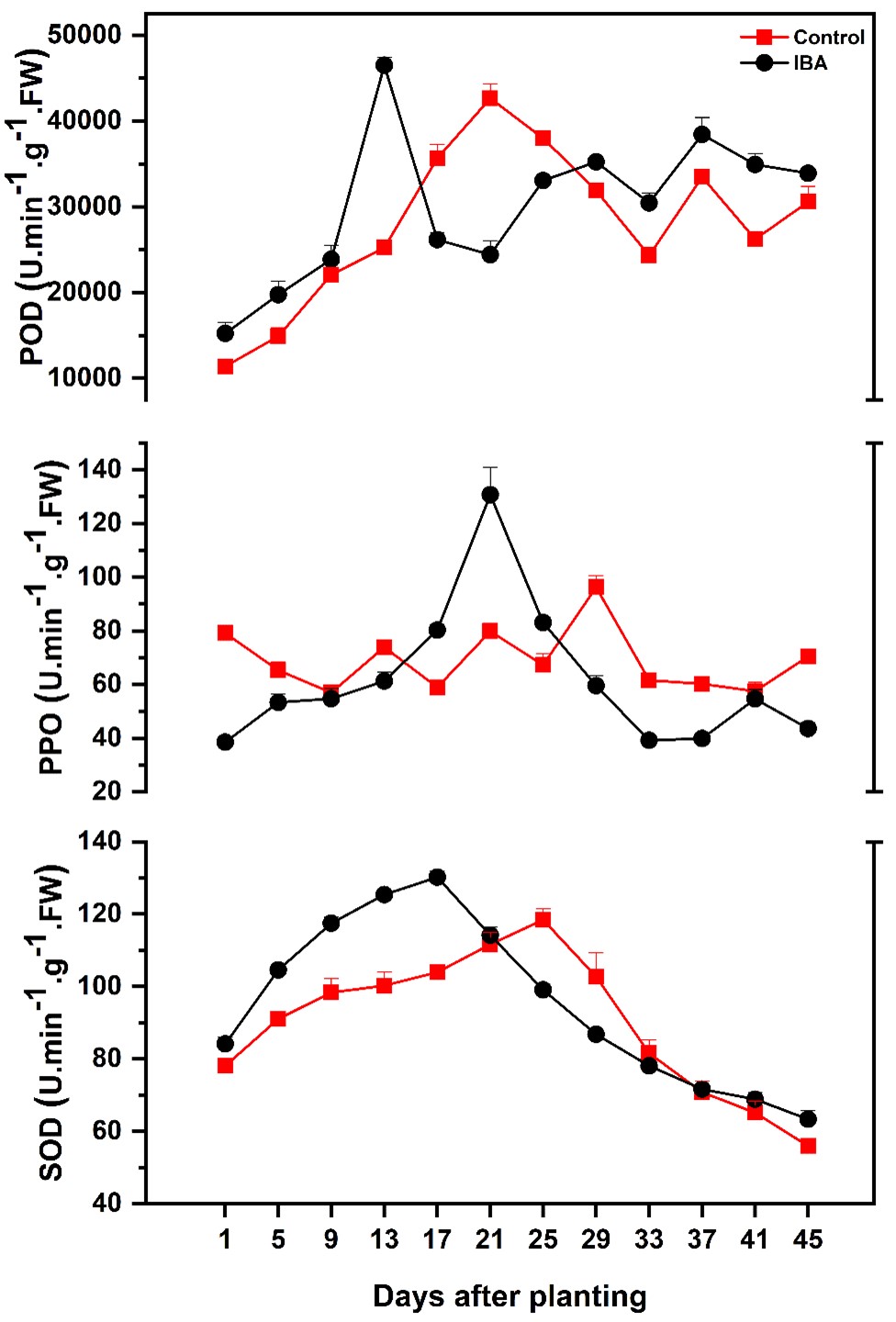
During the rooting process of Magnolia bindii Pamp, POD activity in IBA treated cuttings showed significant results (p≤0.05) and continuously increased and reached the peak value on the 13th day before the emergence of adventitious roots, which was significantly higher than the control-treated cuttings on the same day. After reaching a peak, it showed a zigzag trend. POD in water treated (CK) cuttings also showed significant results (p≤0.05). It also increased continuously and reached the peak value on the 21st day, which was also significantly higher than the IBA treated cuttings. Generally, IBA treated cuttings exhibited higher peroxidase activity values than CK treated cuttings (Figure 4).
Changes in antioxidant enzyme activities during the rhizogenesis in stem cuttings of Magnolia biondii Pamp. The data are expressed as the mean ±SD.
Analysis of variance showed significant differences (p≤0.05) during the PPO enzymatic activity of IBA treated cuttings. It continuously increased and showed the peak value on the 21st day after the emergence of adventitious roots. After reaching its peak value it gradually decreased and showed up and downtrend. It was revealed that there were also significant differences in PPO enzymatic activity in water-treated cuttings. It peaked on the 29th day after the emergence of adventitious roots (Figure 4).
SOD activity in IBA treated cuttings significantly (p≤0.05) increased and peaked at 17th day on the day of root emergence and then decreased gradually. Control also showed the same trend and peaked on the 25th day and then decreased. However, SOD enzymatic activity in IBA treated cuttings showed significantly higher results than control (Figure 4).
3.3. Determination of endogenous hormones
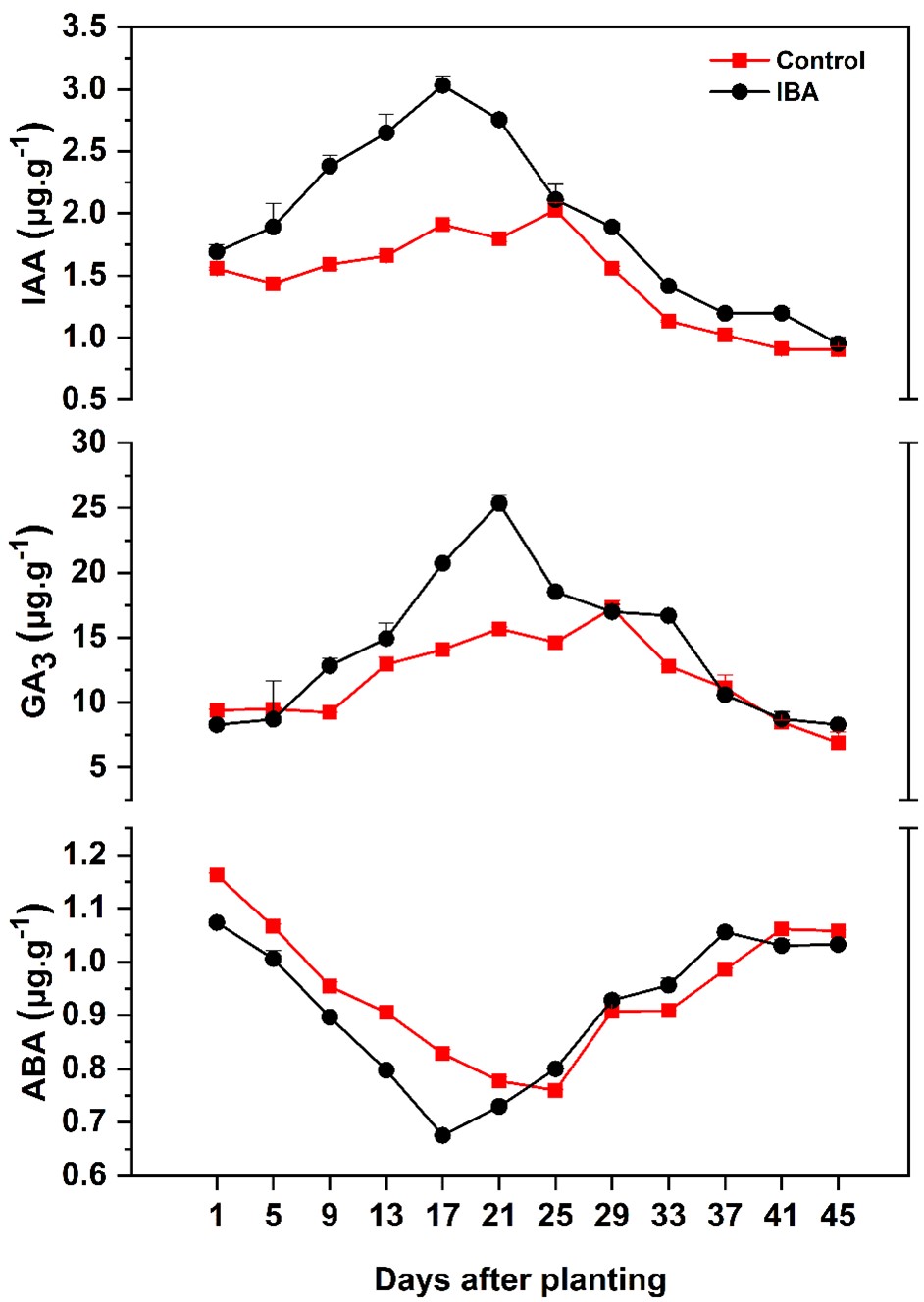
During the root formation in the cuttings, different levels of endogenous hormones were studied on different days. Endogenous IAA levels were induced in the cuttings treated with exogenous IBA. IAA continuously increased and reached the peak on the 17th day after planting on the day of root emergence and thereafter it continuously decreased up to the control level on the last day after planting. The endogenous IAA level in the control was comparatively lower than the IBA treated cuttings. During the whole process of rooting in control, IAA showed a peak on the 25th day after planting (Figure 5).
Changes in endogenous hormones during rhizogenesis in stem cuttings of Magnolia biondii Pamp. The data are expressed as the mean ±SD.
Endogenous ABA levels in IBA treated cuttings significantly (p≤0.05) decreased and showed the low peak value on the 17th day after planting on the day of root emergence and then recovered up to the control level. A similar trend was found in control in which a low peak was observed on the 25th day after planting and then it continuously raised (Figure 5).
During the rooting of Magnolia biondii Pamp, IBA continuously induced the GA3 level to the 21st day after planting after the emergence of adventitious roots, and then afterward it gradually decreased. In the water-treated cuttings, GA3 remained almost stable up to the 9th day after planting and then it gradually increased and reached its peak on the 29th day after planting and afterward showed the continuous declined trend (Figure 5).
4. Discussion
Adventitious root formation is a complex process including morphological, biochemical and physiological changes (Kevers et al., 1997KEVERS, C., HAUSMAN, J., FAIVRE-RAMPANT, O., EVERS, D. and GASPAR, T., 1997. Hormonal control of adventitious rooting: progress and questions. Journal of Applied Botany, vol. 71, no. 3-4, pp. 71-79.; Geiss et al., 2018GEISS, G., GUTIERREZ, L. and BELLINI, C., 2018. Adventitious root formation: new insights and perspectives. Annual Plant Reviews, vol. 37, no. 1, pp. 127-156. http://dx.doi.org/10.1002/9781119312994.apr0400.
http://dx.doi.org/10.1002/9781119312994....
). Exogenous application of plant growth regulators, especially auxins plays an essential role in the formation of adventitious root formation (Graves, 2002GRAVES, W.R., 2002. IBA, juvenility, and position on ortets influence propagation of Carolina buckthorn from softwood cuttings. Journal of Environmental Horticulture, vol. 20, no. 1, pp. 57-61. http://dx.doi.org/10.24266/0738-2898-20.1.57.
http://dx.doi.org/10.24266/0738-2898-20....
; Keeley et al., 2004KEELEY, K., PREECE, J.E., TAYLOR, B.H. and DAMI, I.E., 2004. Effects of high auxin concentrations, cold storage, and cane position on improved rooting of Vitis aestivalis michx. Norton cuttings. American Journal of Enology and Viticulture, vol. 55, no. 3, pp. 265-268.). The science underlying the formation of adventitious root formation by the application of exogenous hormones has been widely investigated. The formation of the adventitious root at the basal portion of stem cuttings is an important developmental phenomenon in the survival and growth of cuttings which consists of the initiation of several new meristematic areas in different tissues of stem cuttings (Kaur et al., 2002KAUR, S., CHEEMA, S., CHHABRA, B. and TALWAR, K., 2002. Chemical induction of physiological changes during adventitious root formation and bud break in grapevine cuttings. Plant Growth Regulation, vol. 37, no. 1, pp. 63-68. http://dx.doi.org/10.1023/A:1020355505105.
http://dx.doi.org/10.1023/A:102035550510...
). During the formation phase of root primordia, the first developed young root meristems become visible between the phloem and cortex on the 12th day after planting in the IBA treated cuttings while the same phenomena occurred in the control on the 21st day after planting, consequently, it is an evident that IBA 750mg/L is useful for the propagation of Magnolia biondii Pamp cuttings. The mature root primordia had developed on the 15th day in the IBA treated with a domed shaped differentiated root cap. Emergence of meristem and root primordium in 12 days is an evident to the similar findings of Hybrid aspin cuttings (Yan et al., 2017YAN, S.P., YANG, R.H., WANG, F., SUN, L.N. and SONG, X.S., 2017. Effect of auxins and associated metabolic changes on cuttings of hybrid aspen. Forests, vol. 8, no. 4, pp. 117. http://dx.doi.org/10.3390/f8040117.
http://dx.doi.org/10.3390/f8040117...
) and Eucalypts hybrid cuttings (Kilkenny et al., 2012KILKENNY, A.J., WALLACE, H.M., WALTON, D.A., ADKINS, M.F. and TRUEMAN, S.J., 2012. Improved root formation in eucalypt cuttings following combined auxin and anti-ethylene treatments. Journal of Plant Sciences, vol. 7, pp. 138-153. http://dx.doi.org/10.3923/jps.2012.138.153.
http://dx.doi.org/10.3923/jps.2012.138.1...
) in which the primordia and root emergence were occurred up to 12 days.
IBA 750 mg/L treated cuttings showed a high number of enzymatic activities (POD, PPO, and SOD) compared to control which indicates the position of plant’s survival of both treated and control under the harsh conditions. Once the stems are cut from the parent plant, its nutrient and water supply are cut off. How to improve its stress resistance and reducing the time needed for root formation are critical for the survival of the cuttings. Antioxidant enzymes not only play an important role in a plant’s antioxidant defense (Almeselmani et al., 2006ALMESELMANI, M., DESHMUKH, P., SAIRAM, R., KUSHWAHA, S. and SINGH, T., 2006. Protective role of antioxidant enzymes under high temperature stress. Plant Science, vol. 171, no. 3, pp. 382-388. http://dx.doi.org/10.1016/j.plantsci.2006.04.009. PMid:22980208.
http://dx.doi.org/10.1016/j.plantsci.200...
; Mayer, 2006MAYER, A.M., 2006. Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry, vol. 67, no. 21, pp. 2318-2331. http://dx.doi.org/10.1016/j.phytochem.2006.08.006 PMid:16973188.
http://dx.doi.org/10.1016/j.phytochem.20...
) but also affect the formation and development of adventitious roots (Sato et al., 1993SATO, Y., SUGIYAMA, M., GÓRECKI, R.J., FUKUDA, H. and KOMAMINE, A., 1993. Interrelationship between lignin deposition and the activities of peroxidase isoenzymes in differentiating tracheary elements of Zinnia. Planta, vol. 189, no. 4, pp. 584-589. http://dx.doi.org/10.1007/BF00198223.
http://dx.doi.org/10.1007/BF00198223...
; Naija et al., 2008NAIJA, S., ELLOUMI, N., JBIR, N., AMMAR, S. and KEVERS, C., 2008. Anatomical and biochemical changes during adventitious rooting of apple rootstocks MM 106 cultured in vitro. Comptes Rendus Biologies, vol. 331, no. 7, pp. 518-525. http://dx.doi.org/10.1016/j.crvi.2008.04.002 PMid:18558375.
http://dx.doi.org/10.1016/j.crvi.2008.04...
; Geiss et al., 2009GEISS, G., GUTIERREZ, L. and BELLINI, C., 2009. Adventitious root formation. New Insights and Perspectives., vol. 37, pp. 127-156.). In the present study, we found that the synthesis of these three enzymes varied among the stages of the adventitious root formation process. The peak activity of POD was observed at 13 days and 21st day for IBA treated and control cuttings respectively before the emergence of adventitious roots and then decreased gradually. The peak values at different days on both IBA and control might indicate that auxins and antioxidant enzyme activities have an important role in adventitious root formation.
The cell wall is an important defense barrier against pathogen invasion and expansion (Lewis and Yamamoto, 1990LEWIS, N.G. and YAMAMOTO, E., 1990. Lignin: occurrence, biogenesis and biodegradation. Annual Review of Plant Physiology and Plant Molecular Biology, vol. 41, no. 1, pp. 455-496. http://dx.doi.org/10.1146/annurev.pp.41.060190.002323 PMid:11543592.
http://dx.doi.org/10.1146/annurev.pp.41....
). Increased lignin synthesis and extension accumulation facilitate cell wall formation and improve its strength (Jackson et al., 2001JACKSON, P.A., GALINHA, C.I., PEREIRA, C.S., FORTUNATO, A., SOARES, N.C., AMÂNCIO, S.B. and RICARDO, C.P.P., 2001. Rapid deposition of extensin during the elicitation of grapevine callus cultures is specifically catalyzed by a 40-kilodalton peroxidase. Plant Physiology, vol. 127, no. 3, pp. 1065-1076. http://dx.doi.org/10.1104/pp.010192. PMid:11706187.
http://dx.doi.org/10.1104/pp.010192...
). Increased POD activity promotes the biosynthesis of lignin and phellem layer and promotes the production of iso-2-tyrosin in hydroxyproline-rich glycoproteins (HRGP; extension) (Lewis and Yamamoto, 1990LEWIS, N.G. and YAMAMOTO, E., 1990. Lignin: occurrence, biogenesis and biodegradation. Annual Review of Plant Physiology and Plant Molecular Biology, vol. 41, no. 1, pp. 455-496. http://dx.doi.org/10.1146/annurev.pp.41.060190.002323 PMid:11543592.
http://dx.doi.org/10.1146/annurev.pp.41....
; Passardi et al., 2005PASSARDI, F., COSIO, C., PENEL, C. and DUNAND, C., 2005. Peroxidases have more functions than a Swiss army knife. Plant Cell Reports, vol. 24, no. 5, pp. 255-265. http://dx.doi.org/10.1007/s00299-005-0972-6 PMid:15856234.
http://dx.doi.org/10.1007/s00299-005-097...
; Rout 2006ROUT, G.R., 2006. Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant Growth Regulation, vol. 48, no. 2, pp. 111-117. http://dx.doi.org/10.1007/s10725-005-5665-1.
http://dx.doi.org/10.1007/s10725-005-566...
). Therefore, a moderate reduction in POD-type enzyme activity could decrease cell wall strength and consequently promote cell division, expansion, and plant growth, whereas an increase in POD-type enzyme activity increases the resistance of cells to stress (Jackson et al., 2001JACKSON, P.A., GALINHA, C.I., PEREIRA, C.S., FORTUNATO, A., SOARES, N.C., AMÂNCIO, S.B. and RICARDO, C.P.P., 2001. Rapid deposition of extensin during the elicitation of grapevine callus cultures is specifically catalyzed by a 40-kilodalton peroxidase. Plant Physiology, vol. 127, no. 3, pp. 1065-1076. http://dx.doi.org/10.1104/pp.010192. PMid:11706187.
http://dx.doi.org/10.1104/pp.010192...
; Nag et al., 2001NAG, S., SAHA, K. and CHOUDHURI, M., 2001. Role of auxin and polyamines in adventitious root formationin relation to changes in compounds involved in rooting. Journal of Plant Growth Regulation, vol. 20, no. 2, pp. 182-194. http://dx.doi.org/10.1007/s003440010016.
http://dx.doi.org/10.1007/s003440010016...
; Syros et al.; 2004SYROS, T., YUPSANIS, T., ZAFIRIADIS, H. and ECONOMOU, A., 2004. Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. Journal of Plant Physiology, vol. 161, no. 1, pp. 69-77. http://dx.doi.org/10.1078/0176-1617-00938 PMid:15002666.
http://dx.doi.org/10.1078/0176-1617-0093...
). Our study determines that the POD activity steadily and gradually increased during the early stage of root formation. The increase in POD activity at the early stage facilitates scavenging for H2O2 molecules, increases cell wall strength, and subsequently increases resistance to stress. At a later stage, POD activity decreases, which in turn facilitates cell expansion and growth.
PPO promotes cell division, differentiation, as well as root primordia formation and development (Yilmaz et al., 2003YILMAZ, H., TAŞKIN, T. and OTLUDIL, B., 2003. Polyphenol oxidase activity during rooting in cuttings of grape (Vitis vinifera L.) varieties. Turkish Journal of Botany, vol. 27, no. 6, pp. 495-498.). PPO also accelerates the formation of IAA-phenolic compounds and consequently promotes adventitious root formation (Balakrishnamurthy and Rao, 1988BALAKRISHNAMURTHY, G. and RAO, V.M., 1988. Changes in phenols during rhizogenesis in rose (Rosa bourboniana Desp). Current Science, vol. 57, no. 17, pp. 960-962.; Nag et al., 2001NAG, S., SAHA, K. and CHOUDHURI, M., 2001. Role of auxin and polyamines in adventitious root formationin relation to changes in compounds involved in rooting. Journal of Plant Growth Regulation, vol. 20, no. 2, pp. 182-194. http://dx.doi.org/10.1007/s003440010016.
http://dx.doi.org/10.1007/s003440010016...
; Rout, 2006ROUT, G.R., 2006. Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant Growth Regulation, vol. 48, no. 2, pp. 111-117. http://dx.doi.org/10.1007/s10725-005-5665-1.
http://dx.doi.org/10.1007/s10725-005-566...
). The present study shows that PPO activity in IBA treated cuttings continuously increased and showed a peak on the 21st day, after the emergence of adventitious roots. Control treated cuttings also showed the peak value after the emergence of ARF on the 29th day, but the trend was not obvious and there was up and down during the whole process. The results indicate that auxins promote PPO activity and a continuous increase in PPO activity promotes ARF.
SOD catalyzes the dismutation of excess O2- into O2 and H2O2, which can be further catalyzed by POD and other enzymes to form H2O and O2, and therefore prevent the cells from compositional, structural, as well as functional damages caused by free oxygen radicals (Bowler et al., 1992BOWLER, C., MONTAGU, M. and INZE, D., 1992. Superoxide dismutase and stress tolerance. Annual Review of Plant Biology, vol. 43, no. 1, pp. 83-116. http://dx.doi.org/10.1146/annurev.pp.43.060192.000503.
http://dx.doi.org/10.1146/annurev.pp.43....
; Ueda et al., 2013UEDA, Y., UEHARA, N., SASAKI, H., KOBAYASHI, K. and YAMAKAWA, T., 2013. Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiology and Biochemistry, vol. 70, pp. 396-402. http://dx.doi.org/10.1016/j.plaphy.2013.06.009 PMid:23831949.
http://dx.doi.org/10.1016/j.plaphy.2013....
). Increased SOD activity can improve the defense of the cuttings against stress by scavenging reactive oxygen (Zhao et al., 2013ZHAO, Y., CHEN, X. and LI, C., 2013. Dynamic of physiology and biochemistry during wild Rhododendron scabrifolium cutting propagation. Linye Kexue, vol. 49, no. 6, pp. 45-51.). Our studies show that the SOD activity of the cuttings treated with IBA 750 mg/L was significantly higher than control, indicating that exogenous hormone treatment increased the SOD activity of the cuttings. SOD activity in both control and IBA treated cuttings peaked after the emergence of adventitious roots and then decreased gradually. Before root formation, cuttings are exposed to stress, and SOD activity continuously increases, which in turn enhances the resistance of cuttings against stress (Zhang et al., 2017ZHANG, W., FAN, J., TAN, Q., ZHAO, M., ZHOU, T. and CAO, F., 2017. The effects of exogenous hormones on rooting process and the activities of key enzymes of Malus hupehensis stem cuttings. PLoS One, vol. 12, no. 2, pp. e0172320. http://dx.doi.org/10.1371/journal.pone.0172320 PMid:28231330.
http://dx.doi.org/10.1371/journal.pone.0...
). After adventitious root emergence, the absorption function of the roots is recovered, thereby relieving the plant from stress, whereas SOD activity starts to decrease (Zhang et al., 2017ZHANG, W., FAN, J., TAN, Q., ZHAO, M., ZHOU, T. and CAO, F., 2017. The effects of exogenous hormones on rooting process and the activities of key enzymes of Malus hupehensis stem cuttings. PLoS One, vol. 12, no. 2, pp. e0172320. http://dx.doi.org/10.1371/journal.pone.0172320 PMid:28231330.
http://dx.doi.org/10.1371/journal.pone.0...
).
Endogenous hormones produced from the axillary buds are transported basipetal down and are important in subsequent adventitious root formation at the base of stem cuttings (Hartmann et al., 2011HARTMANN, H., KESTER, D., DAVIES JUNIOR, F. and GENEVE, R., 2011. Plant propagation: principles and practices. 8th ed. Upper Saddle River: Prentice Hall. Chapter 10: techniques of propagating by cuttings, pp. 344-414.). Adventitious root formation is closely controlled by the metabolism of endogenous hormones. IAA promotes the root formation while ABA inhibits the adventitious root formation (Zhao et al., 2013ZHAO, Y., CHEN, X. and LI, C., 2013. Dynamic of physiology and biochemistry during wild Rhododendron scabrifolium cutting propagation. Linye Kexue, vol. 49, no. 6, pp. 45-51.; Negishi et al., 2014NEGISHI, N., NAKAHAMA, K., URATA, N., KOJIMA, M., SAKAKIBARA, H. and KAWAOKA, A., 2014. Hormone level analysis on adventitious root formation in Eucalyptus globulus. New Forests, vol. 45, no. 4, pp. 577-587. http://dx.doi.org/10.1007/s11056-014-9420-1.
http://dx.doi.org/10.1007/s11056-014-942...
). IAA is observed in the induction of new cambial regions and the initiation of cell division, loosening of cell wall through receptor protein transport inhibitor response 1, and auxin binding protein 1 (Haissig, 1970HAISSIG, B.E., 1970. Influence of indole-3-acetic acid on adventitious root primordia of brittle willow. Planta, vol. 95, no. 1, pp. 27-35. http://dx.doi.org/10.1007/BF00431118. PMid:24497018.
http://dx.doi.org/10.1007/BF00431118...
; Fogaça and Fett-Neto, 2005FOGAÇA, C.M. and FETT-NETO, A.G., 2005. Role of auxin and its modulators in the adventitious rooting of Eucalyptus species differing in recalcitrance. Plant Growth Regulation, vol. 45, no. 1, pp. 1-10. http://dx.doi.org/10.1007/s10725-004-6547-7.
http://dx.doi.org/10.1007/s10725-004-654...
; Costa et al., 2013COSTA, C.T., ALMEIDA, M.R., RUEDELL, C.M., SCHWAMBACH, J., MARASCHIN, F.D.S. and FETT-NETO, A.G., 2013. When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Frontiers in Plant Science, vol. 4, pp. 133. http://dx.doi.org/10.3389/fpls.2013.00133. PMid:23717317.
http://dx.doi.org/10.3389/fpls.2013.0013...
). The function of GA3 in adventitious root formation is still under discussion (Coleman and Greyson, 1976COLEMAN, W.K. and GREYSON, R., 1976. Root regeneration from leaf cuttings of Lycopersicon esculentum Mill.: application of the leaf plastochron index and responses to exogenous gibberellic acid. Journal of Experimental Botany, vol. 27, no. 6, pp. 1339-1351. http://dx.doi.org/10.1093/jxb/27.6.1339.
http://dx.doi.org/10.1093/jxb/27.6.1339...
; Guanli et al., 2001GUANLI, X., QINGHUI, Y., FUSHENG, L. and SHENGCHAO, Y., 2001. Study on the ralationship between endogenous hormones and the rooting rate of plantlet of sugarcane in the course of subculture. Yunnan Nong ye da xue xue bao= Journal of Yunnan Agricultural University, vol. 16, no. 4, pp. 271-273.; Claeys et al., 2014CLAEYS, H., BODT, S. and INZÉ, D., 2014. Gibberellins and DELLAs: central nodes in growth regulatory networks. Trends in Plant Science, vol. 19, no. 4, pp. 231-239. http://dx.doi.org/10.1016/j.tplants.2013.10.001. PMid:24182663.
http://dx.doi.org/10.1016/j.tplants.2013...
). Synthesis of auxin inhibitors and polar transport inhibitors inhibits the process of adventitious rooting (Koukourikou-Petridou and Bangerth, 1997KOUKOURIKOU-PETRIDOU, M. and BANGERTH, F., 1997. Effect of changing the endogenous concentration of auxins and cytokinins and the production of ethylene in pea stem cuttings on adventitious root formation. Plant Growth Regulation, vol. 22, no. 2, pp. 101-108. http://dx.doi.org/10.1023/A:1005833311839.
http://dx.doi.org/10.1023/A:100583331183...
; Ford et al., 2002FORD, Y., BONHAM, E., CAMERON, R., BLAKE, P., JUDD, H. and HARRISON-MURRAY, R., 2002. Adventitious rooting: examining the role of auxin in an easy-and a difficult-to-root plant. Plant Growth Regulation, vol. 36, no. 2, pp. 149-159. http://dx.doi.org/10.1023/A:1015013025513.
http://dx.doi.org/10.1023/A:101501302551...
; Negishi et al., 2014NEGISHI, N., NAKAHAMA, K., URATA, N., KOJIMA, M., SAKAKIBARA, H. and KAWAOKA, A., 2014. Hormone level analysis on adventitious root formation in Eucalyptus globulus. New Forests, vol. 45, no. 4, pp. 577-587. http://dx.doi.org/10.1007/s11056-014-9420-1.
http://dx.doi.org/10.1007/s11056-014-942...
). ABA is not only involved in the inhabitation of synthesis and polar transportation of IAA but also stops the IAA release from the bound to free state and subsequently becomes the reason to inhibit the adventitious root formation (Pilet, 1975PILET, P., 1975. Abscisic acid as a root growth inhibitor: physiological analyses. Planta, vol. 122, no. 3, pp. 299-302. http://dx.doi.org/10.1007/BF00385279 PMid:24435998.
http://dx.doi.org/10.1007/BF00385279...
; Xu and Chen, 1989XU, J. and CHEN, S., 1989. The effect of the changes of the endogenous hormone’s contents (ABA and IAA) in hardwood cuttings of peach to rooting. Yuan Yi Xue Bao, vol. 16, pp. 275-278.). Our study determines the different levels and trends of endogenous hormones in both IBA 750 mg/L and control. In the present study, almost similar trends were found in the endogenous hormones levels during the rooting of Magnolia biondii Pamp. Application of IBA exogenously promoted the endogenous IAA, GA3 levels and enhanced the catabolism level of ABA during the early stage of root formation. Inhibition of ABA and increase in the IAA level in the early days to root emergence helped the root formation and reduced the days of root formation compared to the control although similar trend was found in water treated cuttings (CK). Moreover, the peak values of endogenous IAA and GA3 were significantly higher in IBA treated cuttings than control. The results of our study demonstrate that the application of IBA 750 mg/L to the stem cuttings of Magnolia biondii Pamp not only reduced the time of root formation but also enhanced the level of endogenous hormones and accelerated the catabolism of ABA which consequently helped in root formation.
5. Conclusion
Our study elucidates the application of IBA 750mg/L best for its propagation through stem cuttings. The response of rooting due to the application of IBA 750 mg/L was clearly revealed in the anatomical changes. Exogenous application of IBA 750 mg/L enhanced antioxidant enzyme activities and endogenous IAA and GA3 levels, while reduced the level of ABA and consequently reduced the time of rooting.
Acknowledgements
We are thankful to Wufeng Bo Ling Magnolia wufengensis Technology Development CO., Ltd for providing Magnolia biondii stock plants.
-
Funding
This research was financially supported by Special Fund for Forest Scientific Research in the Public Welfare under grant no. 201504704 and the Transformation and application of forestry intellectual property project under grant no. Intellectual property transformation 2017-11.
References
- ALMESELMANI, M., DESHMUKH, P., SAIRAM, R., KUSHWAHA, S. and SINGH, T., 2006. Protective role of antioxidant enzymes under high temperature stress. Plant Science, vol. 171, no. 3, pp. 382-388. http://dx.doi.org/10.1016/j.plantsci.2006.04.009 PMid:22980208.
» http://dx.doi.org/10.1016/j.plantsci.2006.04.009 - BALAKRISHNAMURTHY, G. and RAO, V.M., 1988. Changes in phenols during rhizogenesis in rose (Rosa bourboniana Desp). Current Science, vol. 57, no. 17, pp. 960-962.
- BELLAMINE, J., PENEL, C., GREPPIN, H., GASPAR, T. and GASPAR, T., 1998. Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. Plant Growth Regulation, vol. 26, no. 3, pp. 191-194. http://dx.doi.org/10.1023/A:1006182801823
» http://dx.doi.org/10.1023/A:1006182801823 - BOWLER, C., MONTAGU, M. and INZE, D., 1992. Superoxide dismutase and stress tolerance. Annual Review of Plant Biology, vol. 43, no. 1, pp. 83-116. http://dx.doi.org/10.1146/annurev.pp.43.060192.000503
» http://dx.doi.org/10.1146/annurev.pp.43.060192.000503 - CLAEYS, H., BODT, S. and INZÉ, D., 2014. Gibberellins and DELLAs: central nodes in growth regulatory networks. Trends in Plant Science, vol. 19, no. 4, pp. 231-239. http://dx.doi.org/10.1016/j.tplants.2013.10.001 PMid:24182663.
» http://dx.doi.org/10.1016/j.tplants.2013.10.001 - COLEMAN, W.K. and GREYSON, R., 1976. Root regeneration from leaf cuttings of Lycopersicon esculentum Mill.: application of the leaf plastochron index and responses to exogenous gibberellic acid. Journal of Experimental Botany, vol. 27, no. 6, pp. 1339-1351. http://dx.doi.org/10.1093/jxb/27.6.1339
» http://dx.doi.org/10.1093/jxb/27.6.1339 - COSTA, C.T., ALMEIDA, M.R., RUEDELL, C.M., SCHWAMBACH, J., MARASCHIN, F.D.S. and FETT-NETO, A.G., 2013. When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Frontiers in Plant Science, vol. 4, pp. 133. http://dx.doi.org/10.3389/fpls.2013.00133 PMid:23717317.
» http://dx.doi.org/10.3389/fpls.2013.00133 - DAS, P., BASAK, U.C. and DAS, A.B., 1997. Metabolic changes during rooting in pre-girdled stem cuttings and air-layers of Heritiera. Botanical Bulletin of Academia Sinica, vol. 38, pp. 91-95.
- DAVIES, P.J., 2010. Plant hormones: biosynthesis, signal transduction, action! Dordrecht: Springer. The plant hormones: their nature, occurrence, and functions, pp. 1-15.
- DE KLERK, G., VAN DER KRIEKEN, W. and DE JONG, J.C., 1999. Review the formation of adventitious roots: new concepts, new possibilities. In Vitro Cellular & Developmental Biology. Plant, vol. 35, no. 3, pp. 189-199. http://dx.doi.org/10.1007/s11627-999-0076-z
» http://dx.doi.org/10.1007/s11627-999-0076-z - FOGAÇA, C.M. and FETT-NETO, A.G., 2005. Role of auxin and its modulators in the adventitious rooting of Eucalyptus species differing in recalcitrance. Plant Growth Regulation, vol. 45, no. 1, pp. 1-10. http://dx.doi.org/10.1007/s10725-004-6547-7
» http://dx.doi.org/10.1007/s10725-004-6547-7 - FORD, Y., BONHAM, E., CAMERON, R., BLAKE, P., JUDD, H. and HARRISON-MURRAY, R., 2002. Adventitious rooting: examining the role of auxin in an easy-and a difficult-to-root plant. Plant Growth Regulation, vol. 36, no. 2, pp. 149-159. http://dx.doi.org/10.1023/A:1015013025513
» http://dx.doi.org/10.1023/A:1015013025513 - FUKUDA, H. and KOMAMINE, A., 1982. Lignin synthesis and its related enzymes as markers of tracheary-element differentiation in single cells isolated from the mesophyll of Zinnia elegans. Planta, vol. 155, no. 5, pp. 423-430. http://dx.doi.org/10.1007/BF00394471 PMid:24271974.
» http://dx.doi.org/10.1007/BF00394471 - GEISS, G., GUTIERREZ, L. and BELLINI, C., 2009. Adventitious root formation. New Insights and Perspectives., vol. 37, pp. 127-156.
- GEISS, G., GUTIERREZ, L. and BELLINI, C., 2018. Adventitious root formation: new insights and perspectives. Annual Plant Reviews, vol. 37, no. 1, pp. 127-156. http://dx.doi.org/10.1002/9781119312994.apr0400
» http://dx.doi.org/10.1002/9781119312994.apr0400 - GRAVES, W.R., 2002. IBA, juvenility, and position on ortets influence propagation of Carolina buckthorn from softwood cuttings. Journal of Environmental Horticulture, vol. 20, no. 1, pp. 57-61. http://dx.doi.org/10.24266/0738-2898-20.1.57
» http://dx.doi.org/10.24266/0738-2898-20.1.57 - GUANLI, X., QINGHUI, Y., FUSHENG, L. and SHENGCHAO, Y., 2001. Study on the ralationship between endogenous hormones and the rooting rate of plantlet of sugarcane in the course of subculture. Yunnan Nong ye da xue xue bao= Journal of Yunnan Agricultural University, vol. 16, no. 4, pp. 271-273.
- HAISSIG, B., 1974. Influences of auxins and auxin synergists on adventitious root primordium initiation and development. New Zealand Journal of Forestry Science, vol. 4, no. 2, pp. 311-323.
- HAISSIG, B.E., 1970. Influence of indole-3-acetic acid on adventitious root primordia of brittle willow. Planta, vol. 95, no. 1, pp. 27-35. http://dx.doi.org/10.1007/BF00431118 PMid:24497018.
» http://dx.doi.org/10.1007/BF00431118 - Hartmann, H., Kester, D. , Driver, F. and Geneve, R., 2002. Plant propagation: principles and practices Upper Saddle River: Prentice Hall.
- HARTMANN, H., KESTER, D., DAVIES JUNIOR, F. and GENEVE, R., 2011. Plant propagation: principles and practices 8th ed. Upper Saddle River: Prentice Hall. Chapter 10: techniques of propagating by cuttings, pp. 344-414.
- HUSEN, A. and PAL, M., 2007. Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f.(teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatment. New Forests, vol. 33, no. 3, pp. 309-323. http://dx.doi.org/10.1007/s11056-006-9030-7
» http://dx.doi.org/10.1007/s11056-006-9030-7 - JACKSON, P.A., GALINHA, C.I., PEREIRA, C.S., FORTUNATO, A., SOARES, N.C., AMÂNCIO, S.B. and RICARDO, C.P.P., 2001. Rapid deposition of extensin during the elicitation of grapevine callus cultures is specifically catalyzed by a 40-kilodalton peroxidase. Plant Physiology, vol. 127, no. 3, pp. 1065-1076. http://dx.doi.org/10.1104/pp.010192 PMid:11706187.
» http://dx.doi.org/10.1104/pp.010192 - KAUR, S., CHEEMA, S., CHHABRA, B. and TALWAR, K., 2002. Chemical induction of physiological changes during adventitious root formation and bud break in grapevine cuttings. Plant Growth Regulation, vol. 37, no. 1, pp. 63-68. http://dx.doi.org/10.1023/A:1020355505105
» http://dx.doi.org/10.1023/A:1020355505105 - KEELEY, K., PREECE, J.E., TAYLOR, B.H. and DAMI, I.E., 2004. Effects of high auxin concentrations, cold storage, and cane position on improved rooting of Vitis aestivalis michx. Norton cuttings. American Journal of Enology and Viticulture, vol. 55, no. 3, pp. 265-268.
- KEVERS, C., HAUSMAN, J., FAIVRE-RAMPANT, O., EVERS, D. and GASPAR, T., 1997. Hormonal control of adventitious rooting: progress and questions. Journal of Applied Botany, vol. 71, no. 3-4, pp. 71-79.
- KHAN, M., WANG, Y., UDDIN, S., MUHAMMAD, B., BADSHAH, M., KHAN, D., MUNEER, M., MUNIR, M. and JIA, Z., 2020. Propagation of magnolia biondii pamp through stem cuttings using exogenous hormones. Applied Ecology and Environmental Research, vol. 18, no. 2, pp. 2213-2229. http://dx.doi.org/10.15666/aeer/1802_22132229
» http://dx.doi.org/10.15666/aeer/1802_22132229 - KILKENNY, A.J., WALLACE, H.M., WALTON, D.A., ADKINS, M.F. and TRUEMAN, S.J., 2012. Improved root formation in eucalypt cuttings following combined auxin and anti-ethylene treatments. Journal of Plant Sciences, vol. 7, pp. 138-153. http://dx.doi.org/10.3923/jps.2012.138.153
» http://dx.doi.org/10.3923/jps.2012.138.153 - KOSE, C., ERDAL, S., KAYA, O. and ATICI, O., 2011. Comparative evaluation of oxidative enzyme activities during adventitious rooting in the cuttings of grapevine rootstocks. Journal of the Science of Food and Agriculture, vol. 91, no. 4, pp. 738-741. http://dx.doi.org/10.1002/jsfa.4244
» http://dx.doi.org/10.1002/jsfa.4244 - KOUKOURIKOU-PETRIDOU, M. and BANGERTH, F., 1997. Effect of changing the endogenous concentration of auxins and cytokinins and the production of ethylene in pea stem cuttings on adventitious root formation. Plant Growth Regulation, vol. 22, no. 2, pp. 101-108. http://dx.doi.org/10.1023/A:1005833311839
» http://dx.doi.org/10.1023/A:1005833311839 - LEAKEY, R.R., 2004. Physiology of vegetative reproduction. In: J. Burley, J. Evans, and J.A Youngquist, eds. Encyclopaedia of forest sciences London: Academic Press.
- LEWIS, N.G. and YAMAMOTO, E., 1990. Lignin: occurrence, biogenesis and biodegradation. Annual Review of Plant Physiology and Plant Molecular Biology, vol. 41, no. 1, pp. 455-496. http://dx.doi.org/10.1146/annurev.pp.41.060190.002323 PMid:11543592.
» http://dx.doi.org/10.1146/annurev.pp.41.060190.002323 - LI, H., SUN, Q., ZHAO, S. and ZHANG, W., 2000. Principles and techniques of plant physiological biochemical experiment Beijing: Higher Education, pp. 195-197.
- LODAMA, K.E., DU TOIT, E., STEYN, J., ARAYA, H.T., PRINSLOO, G., DU PLOOY, C. and ROBBERTSE, P., 2016. Improving rooting of Lobostemon fruticosus L. cuttings with delayed auxin treatment. South African Journal of Botany, vol. 105, pp. 111-115. http://dx.doi.org/10.1016/j.sajb.2016.01.005
» http://dx.doi.org/10.1016/j.sajb.2016.01.005 - Lu, W. and Li, Y., 2012. Plant physiology course Beijing: China Forestry.
- MAYER, A.M., 2006. Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry, vol. 67, no. 21, pp. 2318-2331. http://dx.doi.org/10.1016/j.phytochem.2006.08.006 PMid:16973188.
» http://dx.doi.org/10.1016/j.phytochem.2006.08.006 - MONCOUSIN, C. and GASPAR, T., 1983. Peroxidase as a marker for rooting improvement of Cynara scolymus L. cultured in vitro. Biochemie und Physiologie der Pflanzen, vol. 178, no. 4, pp. 263-271. http://dx.doi.org/10.1016/S0015-3796(83)80040-7
» http://dx.doi.org/10.1016/S0015-3796(83)80040-7 - NAG, S., SAHA, K. and CHOUDHURI, M., 2001. Role of auxin and polyamines in adventitious root formationin relation to changes in compounds involved in rooting. Journal of Plant Growth Regulation, vol. 20, no. 2, pp. 182-194. http://dx.doi.org/10.1007/s003440010016
» http://dx.doi.org/10.1007/s003440010016 - NAIJA, S., ELLOUMI, N., JBIR, N., AMMAR, S. and KEVERS, C., 2008. Anatomical and biochemical changes during adventitious rooting of apple rootstocks MM 106 cultured in vitro. Comptes Rendus Biologies, vol. 331, no. 7, pp. 518-525. http://dx.doi.org/10.1016/j.crvi.2008.04.002 PMid:18558375.
» http://dx.doi.org/10.1016/j.crvi.2008.04.002 - NEGISHI, N., NAKAHAMA, K., URATA, N., KOJIMA, M., SAKAKIBARA, H. and KAWAOKA, A., 2014. Hormone level analysis on adventitious root formation in Eucalyptus globulus. New Forests, vol. 45, no. 4, pp. 577-587. http://dx.doi.org/10.1007/s11056-014-9420-1
» http://dx.doi.org/10.1007/s11056-014-9420-1 - PAPADAKIS, A. and ROUBELAKIS-ANGELAKIS, K., 2002. Oxidative stress could be responsible for the recalcitrance of plant protoplast. Plant Physiology and Biochemistry, vol. 40, no. 6-8, pp. 549-559. http://dx.doi.org/10.1016/S0981-9428(02)01423-7
» http://dx.doi.org/10.1016/S0981-9428(02)01423-7 - PASSARDI, F., COSIO, C., PENEL, C. and DUNAND, C., 2005. Peroxidases have more functions than a Swiss army knife. Plant Cell Reports, vol. 24, no. 5, pp. 255-265. http://dx.doi.org/10.1007/s00299-005-0972-6 PMid:15856234.
» http://dx.doi.org/10.1007/s00299-005-0972-6 - PILET, P., 1975. Abscisic acid as a root growth inhibitor: physiological analyses. Planta, vol. 122, no. 3, pp. 299-302. http://dx.doi.org/10.1007/BF00385279 PMid:24435998.
» http://dx.doi.org/10.1007/BF00385279 - ROUT, G.R., 2006. Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant Growth Regulation, vol. 48, no. 2, pp. 111-117. http://dx.doi.org/10.1007/s10725-005-5665-1
» http://dx.doi.org/10.1007/s10725-005-5665-1 - SATO, Y., SUGIYAMA, M., GÓRECKI, R.J., FUKUDA, H. and KOMAMINE, A., 1993. Interrelationship between lignin deposition and the activities of peroxidase isoenzymes in differentiating tracheary elements of Zinnia. Planta, vol. 189, no. 4, pp. 584-589. http://dx.doi.org/10.1007/BF00198223
» http://dx.doi.org/10.1007/BF00198223 - SYROS, T., YUPSANIS, T., ZAFIRIADIS, H. and ECONOMOU, A., 2004. Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. Journal of Plant Physiology, vol. 161, no. 1, pp. 69-77. http://dx.doi.org/10.1078/0176-1617-00938 PMid:15002666.
» http://dx.doi.org/10.1078/0176-1617-00938 - TAKAHAMA, U., 1997. Enhancement of the peroxidase-dependent oxidation of dopa by components of Vicia leaves. Phytochemistry, vol. 46, no. 3, pp. 427-432. http://dx.doi.org/10.1016/S0031-9422(97)00336-1
» http://dx.doi.org/10.1016/S0031-9422(97)00336-1 - UEDA, Y., UEHARA, N., SASAKI, H., KOBAYASHI, K. and YAMAKAWA, T., 2013. Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiology and Biochemistry, vol. 70, pp. 396-402. http://dx.doi.org/10.1016/j.plaphy.2013.06.009 PMid:23831949.
» http://dx.doi.org/10.1016/j.plaphy.2013.06.009 - XIAO, A., CHEN, F., JIA, Z., SANG, Z., ZHU, Z. and MA, L., 2020. Determination of 4 plant hormones in Magnolia wufengensis by gradient elution high performance liquid chromatography. Chinese Journal of Analysis Laboratory, vol. 39, pp. 249-254.
- XU, J. and CHEN, S., 1989. The effect of the changes of the endogenous hormone’s contents (ABA and IAA) in hardwood cuttings of peach to rooting. Yuan Yi Xue Bao, vol. 16, pp. 275-278.
- YAN, S.P., YANG, R.H., WANG, F., SUN, L.N. and SONG, X.S., 2017. Effect of auxins and associated metabolic changes on cuttings of hybrid aspen. Forests, vol. 8, no. 4, pp. 117. http://dx.doi.org/10.3390/f8040117
» http://dx.doi.org/10.3390/f8040117 - YAN, Y., LI, J., ZHANG, X., YANG, W., WAN, Y., MA, Y., ZHU, Y., PENG, Y. and HUANG, L., 2014. Effect of naphthalene acetic acid on adventitious root development and associated physiological changes in stem cutting of Hemarthria compressa. PLoS One, vol. 9, no. 3, pp. e90700. http://dx.doi.org/10.1371/journal.pone.0090700 PMid:24595064.
» http://dx.doi.org/10.1371/journal.pone.0090700 - YILMAZ, H., TAŞKIN, T. and OTLUDIL, B., 2003. Polyphenol oxidase activity during rooting in cuttings of grape (Vitis vinifera L.) varieties. Turkish Journal of Botany, vol. 27, no. 6, pp. 495-498.
- ZHANG, W., FAN, J., TAN, Q., ZHAO, M., ZHOU, T. and CAO, F., 2017. The effects of exogenous hormones on rooting process and the activities of key enzymes of Malus hupehensis stem cuttings. PLoS One, vol. 12, no. 2, pp. e0172320. http://dx.doi.org/10.1371/journal.pone.0172320 PMid:28231330.
» http://dx.doi.org/10.1371/journal.pone.0172320 - ZHAO, Y., CHEN, X. and LI, C., 2013. Dynamic of physiology and biochemistry during wild Rhododendron scabrifolium cutting propagation. Linye Kexue, vol. 49, no. 6, pp. 45-51.
Publication Dates
-
Publication in this collection
11 Feb 2022 -
Date of issue
2024
History
-
Received
24 Aug 2021 -
Accepted
16 Nov 2021