Abstract
Visceral leishmaniosis is a neglected tropical disease. We evaluated the spatial distribution of cases of visceral leishmaniosis in the state of Alagoas, Brazil. All cases of VL, registered by the health department, were analyzed and georeferenced. Results: Between 2008 and 2017, 97.1% of the municipalities presented sporadic classification of transmission. With temporal evolution, the incidence of cases of visceral leishmaniosis was concentrated in most municipalities in the microregion of Santana do Ipanema-AL. Space-time analysis, if considered, may promote the improvement of surveillance and control actions of visceral leishmaniosis.
Keywords:
human visceral leishmaniasis; temporal analysis; spatial and spatio-temporal analysis; Brazil
Resumo
A leishmaniose visceral é uma doença tropical negligenciada. Foram avaliadas a distribuição espacial dos casos de leishmaniose visceral no estado de Alagoas. Todos os casos de LV, registrados pela secretaria de saúde, foram analisados e georreferenciados. Entre 2008 e 2017, 97,1% dos municípios apresentaram classificação esporádica de transmissão. Com a evolução temporal, a incidência de casos de leishmaniose visceral se concentrou na maioria dos municípios da microrregião de Santana do Ipanema-AL. A análise espaço-tempo, se considerada, pode promover o aprimoramento das ações de vigilância e controle da leishmaniose visceral.
Palavras-chave:
leishmaniose visceral humana; análise temporal; análise espacial e espacial; Brasil
1. Introduction
Visceral leishmaniosis (VL) known as calazar is a chronic and systemic disease, which, if not properly diagnosed and treated appropriately, may progress to death in more than 90% of cases (Brasil, 2017BRASIL. Ministério da Saúde. Secretaria de Vigilância em Saúde. Coordenação-Geral de Desenvolvimento da Epidemiologia em Serviços, 2017. Guia de vigilância em saúde. Brasília: Ministério da Saúde, vol. 3.; OPS, 2019Organización Panamericana de la Salud – OPS, 2019. Communicable diseases and environmental determinants of health. Washington, DC: OPS.). It is considered an endemic disease in 12 countries in the Americas, where 63,331 new cases were recorded from 2001 to 2018, with an average of 3,518 cases per year (Karagiannis-Voules et al., 2013KARAGIANNIS-VOULES, D., SCHOLTE, R.G.C., GUIMARÃES, L.H., UTZINGER, J. and VOUNATSOU, P., 2013. Bayesian geostatistical modeling of leishmaniasis incidence in Brazil. PLoS Neglected Tropical Diseases, vol. 7, no. 5, pp. e2213. http://dx.doi.org/10.1371/journal.pntd.0002213. PMid:23675545.
http://dx.doi.org/10.1371/journal.pntd.0...
). In 2018, of the total number of cases, 97% (3,466) were concentrated in Brazil. In addition, in Brazil between 2003 and 2018, more than 51,000 human cases were confirmed, with an average incidence of 1.7 cases/100,000 inhabitants, ranging from 1.4 to 2.1 and lethality of 7.2%, the Northeast region responsible for the largest number of cases. This expansion has been occurring in space and time, with an increase in the number of affected municipalities and the number of cases and deaths (Brasil, 2019BRASIL. Ministério da Saúde. Secretaria de Vigilância em Saúde, 2019. Vigilância em saúde no Brasil 2003/2019: da criação da Secretaria de Vigilância em Saúde aos dias atuais. Brasília: Ministério da Saúde, Boletim Epidemiológico, vol. 50, no. esp.; Lima et al., 2019LIMA, A.F., SANTOS, A.F.S., CALHEIROS, T.R.S.P., XAVIER JUNIOR, A.F.S. and OLIVEIRA, S.G., 2019. Leishmaniose visceral: perfil epidemiológico dos casos no município de Maceió, Alagoas no período de 2011 a 2016. Interfaces Científicas, vol. 7, no. 3, pp. 133-142. http://dx.doi.org/10.17564/2316-3798.2019v7n3p133-142.
http://dx.doi.org/10.17564/2316-3798.201...
).
Phlebotomines (Diptera: Psychodidae) are small insects, and the hematophagous females of some phlebotomine species are responsible for transmitting protozoan parasites belonging to the genus Leishmania. In the Americas, the genus Lutzomyia is the most important genus, with more than 400 identified species; however, little more than 50 are considered as potential vector species or involved in the transmission of the different species of Leishmania in the Region (Diniz et al., 2014DINIZ, M.M.C.S.L., OVALLOS, F.G., GOMES, C.M.C., LAVITSCHKA, C.O. and GALATI, E.A.B., 2014. Hostbiting rate and susceptibility of some suspected vectors to Leishmania braziliensis. Parasites & Vectors, vol. 7, no. 1, pp. 139. http://dx.doi.org/10.1186/1756-3305-7-139. PMid:24684943.
http://dx.doi.org/10.1186/1756-3305-7-13...
). The community composition and richness of sand flies result of ecological interactions and local geographic aspects (Okwor and Uzonna, 2016OKWOR, I. and UZONNA, J., 2016. Social and economic burden of human leishmaniasis. The American Journal of Tropical Medicine and Hygiene, vol. 94, no. 3, pp. 489-493. http://dx.doi.org/10.4269/ajtmh.15-0408. PMid:26787156.
http://dx.doi.org/10.4269/ajtmh.15-0408...
), then environmental changes affect the abundance, diversity, behaviour and habits of the different species within a community (Martins-Melo et al., 2018MARTINS-MELO, F.R., CARNEIRO, M., RAMOS JUNIOR, A.N., HEUKELBACH, J., RIBEIRO, A.L.P. and WERNECK, G.L., 2018. The burden of neglected tropical diseases in Brazil, 1990-2016: a subnational analysis from the Global Burden of Disease Study 2016. PLoS Neglected Tropical Diseases, vol. 12, no. 6, pp. e0006559. http://dx.doi.org/10.1371/journal.pntd.0006559. PMid:29864133.
http://dx.doi.org/10.1371/journal.pntd.0...
).
The clinical form of VL revolves around the reticulo endothelial system, presenting symptoms that can range from an asymptomatic infection to the appearance of chronic and systemic symptoms such as persistent fever, paleness in the ethopamucosa, weight loss, asthenia, adynamia, associated with exuberant hepatosplenomegaly (Brasil, 2015BRASIL. Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis, 2015. Manual de recomendações para diagnóstico, tratamento e acompanhamento de pacientes com a coinfecção leishmania-HIV. Brasília: Ministério da Saúde.). Clinical suspicion of VL should be raised when the patient has fever and splenomegaly associated or not with hepatomegaly (Lima et al., 2019LIMA, A.F., SANTOS, A.F.S., CALHEIROS, T.R.S.P., XAVIER JUNIOR, A.F.S. and OLIVEIRA, S.G., 2019. Leishmaniose visceral: perfil epidemiológico dos casos no município de Maceió, Alagoas no período de 2011 a 2016. Interfaces Científicas, vol. 7, no. 3, pp. 133-142. http://dx.doi.org/10.17564/2316-3798.2019v7n3p133-142.
http://dx.doi.org/10.17564/2316-3798.201...
; Aguiar and Rodrigues, 2017AGUIAR, P.F. and RODRIGUES, R.K., 2017. Leishmaniose visceral no Brasil: artigo de revisão. Rev Unimontes Cient, vol. 19, no. 1, pp. 191-204.; Queiroz et al., 2004QUEIROZ, M.J., ALVES, J.G. and CORREIA, J.B., 2004. Leishmaniose visceral: características clínico-epidemiológicas em crianças de área endêmica. Jornal de Pediatria, vol. 80, no. 2, pp. 141-146. http://dx.doi.org/10.2223/1154. PMid:15079185.
http://dx.doi.org/10.2223/1154...
). Thus, the present study aimed to describe in space and space-time the occurrence of VL in AL, between 2008 and 2017.
2. Material and Methods
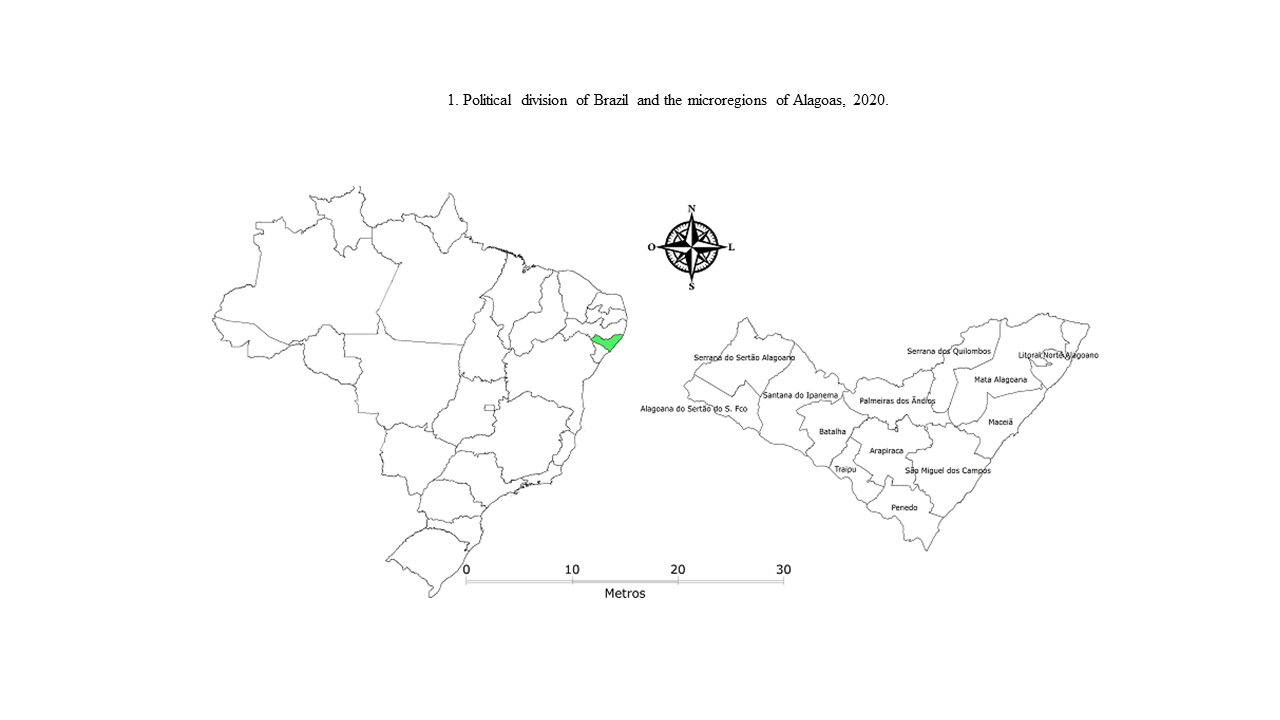
The state of Alagoas is located in the east of the northeast region and has as limits Pernambuco to the north and northwest, Sergipe to the south, Bahia to the southwest and bathed by the Atlantic Ocean to the east. It has an estimated average population of 3,337,357 inhabitants, a territorial extension of approximately 27,843,295 km2 and a population density of 112.33 inhabitants/km2, according to the IBGE census in 2010, and is organized in three mesoregions and 13 microregions, including: Serrana do Sertão Alagoano (composed of 5 municipalities), Sertão do São Francisco (3), Santana do Ipanema (10), Batalha (8), Palmeira dos Índios (11), Arapiraca (10), Traipu (3), Serra dos Quilombos (7), Mata Alagoana (16), Litoral Norte Alagoano (5), Maceió (10), São Miguel dos Campos (9) and Penedo (5), totaling 102 municipalities (Figure 1).
A descriptive, cross-sectional, retrospective and quantitative study of reported cases of AVL was conducted in Alagoas, from 2008 to 2017. Data were collected from the website of the Department of Informatics of the Unified Health System (DATASUS), through the Notifiable Diseases Information System (SINAN). To carry out this study, variables were analyzed as an incidence of the number of cases in Alagoas during the study period.
Regarding the classification of the level of transmission of VL in Brazilian municipalities according to the Ministry of Health, it considers the average number of cases in the last 5 years, resulting in the following categories: no transmission (mean = 0 cases); sporadic transmission (mean> 0 and <2.4 cases); moderate transmission (mean ≥ 2.4 and <4.4 cases) and intense transmission (mean ≥ 4.4 cases) Brasil, 2017).
Data tabulation was performed through the Microsoft Office Excel program®, with conference or consistency of the collected data. After the construction of the database with the annual VL values, a descriptive analysis was made using absolute values and valid percentages, using tables and/or graph, data processing was performed on the Stata 12.0® platform.
Terraview 4.2.2 softwares (DPI, 2020DIVISÃO DE PROCESSAMENTO DE IMAGENS – DPI. Instituto Nacional de Pesquisas Espaciais - INPE, 2020 [viewed 20 February 2022]. Divisão de Processamento de Imagens/Instituto Nacional de Pesquisas Espaciais. Portal da Vigilância em Saúde [online]. Available from: http://vigilancia.saude.mg.gov.br/index.php/download/instalador-terraview-windows-v-4-2-2/
http://vigilancia.saude.mg.gov.br/index....
) was used for georeferencing the incidence rates of VL. In this analysis it was possible to verify the spatial autocorrelation of the disease under study, presenting it in thematic maps. The maps were colored according to the value of the incidence rates in five categories, based on equal step intervals, the darker the map area, the higher the incidence rate of the study period.
To know the global spatial autocorrelation, the values of the global Moran statistic were used. Moran's coefficient was used to measure the strength of the association that will allow the distinction between spatial association in the distribution of incidence rates, self-correlating with their close neighbors. The nullhypothesis is to verify whether this effect is a purely random variation in space, that is, neighboring states tend to have a more similar pattern than distant states forming possible clusters in similar areas whose characteristics are close to the event studied in space. Moran's coefficient is calculated by Equation 1:
Where: Yié the incidence or mortality rate in state i and the wij are weights belonging to the proximity matrix W. The closer the States Ai and Aj, the greater the weight wij. A Moran coefficient with a small value, that is, a value close to zero, together with significance above 0.05, provides evidence that one should not reject the hypothesis that there is no spatial autocorrelation.
Thematic maps were constructed from the Moran Map, fixing 99 permutations with a statistical significance of 95%. The results were presented in cluster clusters, through the effect of the significant spatial correlation of the dependent variable of the high-high type (Q+/+), low-low (Q-/-), high-low (Q+/-) and low-high (Q-/+). Secondary data available on official websites of the Brazilian Ministry of Health were used, without identification of subjects, and were exempted from consideration in a research ethics committee, in accordance with Resolution 466-2012 of the National Health Council.
3. Results and Discussion
In the analyzed period, cases have been reported in all three mesoregions, in the 13 microregions and in 66 of the 102 municipalities. A total of 352 cases were reported, with an average of 35.2 cases per year, with an average incidence of 1,083 cases/100,000 inhabitants, falling below the national average of 1,903 cases/100,000 inhabitants. In addition, Alagoas has always maintained a lower incidence rate than the rates in the Northeast and Brazil, representing only 0.93% of the national cases and 1.76% of the cases in the northeast, which corresponds to more than half of the cases in Brazil (53.04%). It is also noted that Alagoas had a higher incidence in 2017 with 1.42 cases/100,000 inhabitants and lower incidence in 2016 with 0.71 cases/100,000 inhabitants (Graphic 1).
Several factors are associated with the occurrence of VL such as low socioeconomic status, deforestation and poor sanitation8. The creation of population clusters without the slightest sanitary infrastructure and the destruction of the natural ecotops of the vector causes people to be exposed to the risk factors responsible for the dissemination of VL. Between 2008 and 2010, 97.1% of the municipalities had a sporadic classification and only two of them classified themselves as moderate and one as intense. Between 2011 and 2017, about 99% of cities were classified as a level of sporadic transmission and only one with a moderate level.
Surveillance and control in municipalities with moderate and intense transmission involve additional actions by health surveillance. The municipalities with transmission (with cases in the period) of VL were classified according to the criteria of the PCLV. Thus, medium and large municipalities, using the same indicator, can stratify areas or sectors within the municipality itself, thus allowing the specific surveillance and control actions for each situation to work (Brasil, 2019BRASIL. Ministério da Saúde. Secretaria de Vigilância em Saúde, 2019. Vigilância em saúde no Brasil 2003/2019: da criação da Secretaria de Vigilância em Saúde aos dias atuais. Brasília: Ministério da Saúde, Boletim Epidemiológico, vol. 50, no. esp.).
In Alagoas, during the study period, the mesoregion with the highest number of reported cases was sertão Alagoano, with 150 cases, corresponding to 42.6% of the reported cases, followed by the agreste Alagoano with 30.4% and East Alagoas with 27% of the cases. Considering the number of cases reported by municipalities, São José da Tapera and Palmeira dos Índios each presented 29 cases, both corresponding to 16.48%. Of the 66 municipalities with notifications of AVL cases, only 10 of them account for more than half of the state's case numbers, corresponding to 50.27%, these municipalities are: São José da Tapera (n=29; 8.24%), Palmeira dos Índios (n=29; 8.24%), Maceió (n=19; 5.40%), Estrela de Alagoas (n=16; 4.54%), Santana do Ipanema (n=16; 4.54%), Traipu (n=16; 4.54%), Girado do Pociano (n=15; 4.26%), Senador Rui Palmeira (n=13; 3.69%), Maragogi (n=12; 3.41%) and Pão de Açucar (n=12; 3.41%) (Table 1).
Number of reported cases of AVL in the microregions and municipalities of Alagoas, 2008-2017.
Regarding the microregions, it is observed that Santana do Ipanema presents the largest number of cases (n=92), with approximately 26.14% of the total, followed by the microregions of Palmeira dos Índios with 56 cases (15.91%), Maceió with 44 cases (12.5%), Arapiraca with 33 cases (9.37%) and Batalha with 27 cases (7.67%). The microregion of Serra dos Quilombos presented the lowest number of cases in the study period with 2 cases (0.57%) (Table 1).
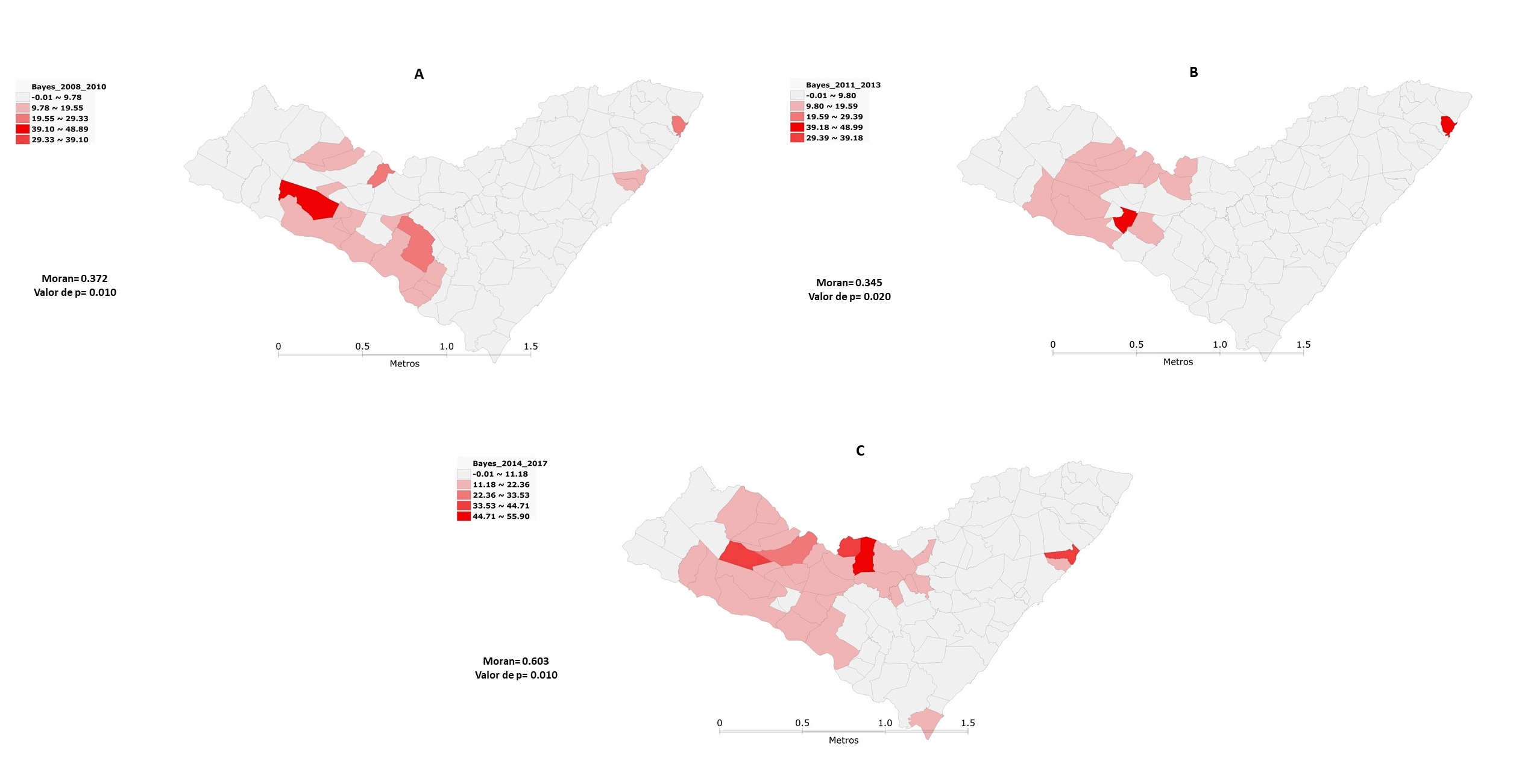
Regarding the temporal evolution of AVL cases in Alagoas, it can be seen that between 2008 and 2010, it was shown a higher incidence in the municipality of São José da Tapera, which was responsible not only for the highest number of cases (n=17), but also for the higher incidence, with 48.88 cases/100,000 inhabitants, followed by the municipalities of Japaratinga (27.38%) and Dois Riachos (24.58%), noting that the highest incidences are concentrated in the microregions of Santana do Ipanema and Batalha (Figure 2A). Between 2011 and 2013, there was a considerable expansion in the incidence of cases in the microregion of Santana do Ipanema in almost its entirety and we can see a slight reduction in the microregion of Batalha, despite having the municipality of Jacaré dos Homens with the second highest incidence of this period with 43.55 cases/100,000 inhabitants, second only to Japaratinga with 48.98 cases/100,000 inhabitants (Figure 2B).
In the last study period, between 2014 and 2017, there was a territorial advance for the entire microregion of Santana do Ipanema and for the microregions of Batalha, Traipu and Palmeira dos Índios, which presented the municipality with the highest incidence, represented by Estrela de Alagoas with about 55.89 cases/100,000 inhabitants (Figure 2C). It is noticed that with the temporal evolution, the incidence of cases of AVL was concentrated in most municipalities in the microregion of Santana do Ipanema and in some municipalities of the microregion of Batalha, that is, in the Mesoregion of Sertão Alagoano (Figure 2). In the spatial statistical analysis observed in Figure 2 by moran's method, he presented indices between 2008 and 2010 (I=0.372; p=0.01); 2011 to 2013 (I=0.345; p=0.02) and from 2014 to 2017 (I=0.603; p<0.01), showing the presence of clusters with autocorrelation between several microregions of the state of Alagoas.
These indicators showed more significant clusters located in the microregions of Santana do Ipanema, Batalha and Traipu, covering part of the Serrana do Sertão Alagoano and Sertão do São Francisco, during the period from 2008 to 2010 (Figure 3A).
Between 2011 and 2013, there was a permanence of the most significant clusters in the regions mentioned in the previous period, except in Traipu (Figure 3B). In the last period, between 2014 and 2017, there was an expansion to the microregion of Palmeira dos Índios (Figure 3C). We can also observe a concentration of clusters with clusters of low incidence in the three study periods, mainly concentrated in the Microregions of São Miguel dos Campos, Maceió, Serrana dos Quilombos and Mata Alagoana (Figure 3).
A published study indicates that 1.69 billion people live in adequate areas for VL transmission and this points to the importance of maps that estimate the risk of the disease and provide a basis for evaluating future incidence in the identified high-risk regions, in order to guide other public health actions (Pigott et al., 2014PIGOTT, D.M., BHATT, S., GOLDING, N., DUDA, K.A., BATTLE, E., BRADY, O.J., MESSINA, J.P., BALARD, Y., BASTIEN, P., PRATLONG, F., BROWNSTEIN, J.S., FREIFELD, C.C., MEKARU, S.R., GETHING, P.W., GEORGE, D.B., MYERS, M.F., REITHINGER, R. and HAY, S.I., 2014. Global distribution maps of the leishmaniases. eLife, vol. 3, pp. 1-21. http://dx.doi.org/10.7554/eLife.02851. PMid:24972829.
http://dx.doi.org/10.7554/eLife.02851...
).
Studies similar to ours were conducted in Petrolina (Maia et al., 2014MAIA, C.S., PIMENTEL, D.S., SANTANA, M.A., OLIVEIRA, G.M., PEDROSA, N.A., NASCIMENTO, L.A., FAUSTINO, M.A.G. and ALVES, L.C., 2014. Análise espacial da leishmaniose visceral americana no município de Petrolina, Pernambuco, Brasil. Hygeia, vol. 10, no. 18, pp. 167-176.) and Ceará (Maia et al., 2014MAIA, C.S., PIMENTEL, D.S., SANTANA, M.A., OLIVEIRA, G.M., PEDROSA, N.A., NASCIMENTO, L.A., FAUSTINO, M.A.G. and ALVES, L.C., 2014. Análise espacial da leishmaniose visceral americana no município de Petrolina, Pernambuco, Brasil. Hygeia, vol. 10, no. 18, pp. 167-176.) where during the spatiotemporal analysis of VL in these cities, clusters were detected. In another study conducted in Sergipe, during spatial analysis between 2010 and 2015, demonstrating that clusters were formed with positive spatial self-relations between the municipalities, revealing similar epidemiological findings (Araújo, 2017ARAÚJO, D.M., 2017. Análise espacial dos casos humanos de leishmaniose visceral. Arq. Ciência & Saúde, vol. 24, no. 2, pp. 71-75.). The studies cited showed that there was a marked geographical expansion of VL, with the emergence of new foci in addition to the persistence of the old areas of occurrence of the disease, as is the case of the spatial-temporal analysis of VL in the city of Natal (Barbosa, 2016BARBOSA, I.R., 2016. Leishmaniose Visceral Humana no município de Natal-RN: análise clínico epidemiológica e espacial. Rev Cienc Plural, vol. 2, no. 1, pp. 89-101. http://dx.doi.org/10.21680/2446-7286.2016v2n1ID8559.
http://dx.doi.org/10.21680/2446-7286.201...
).
Visceral leihsmaniasis is a disease of focal occurrence and, thus, local epidemiology can differ widely depending on the level of observation, being crucial to understand the structure of each focus for the adoption of efficient control measures directed to the reservoirs of infection and vectors (Dantas-Torres and Brandão-Filho, 2006DANTAS-TORRES, F. and BRANDÃO-FILHO, S.P., 2006. Visceral leishmaniasis in Brazil: revisiting paradigms of epidemiology and control. Revista do Instituto de Medicina Tropical de São Paulo, vol. 48, no. 3, pp. 151-156. http://dx.doi.org/10.1590/S0036-46652006000300007. PMid:16847505.
http://dx.doi.org/10.1590/S0036-46652006...
). Thus, the finding of cases of VL in the urban and rural area of Maceió corroborates the process of expansion of the disease that has been occurring in several Brazilian regions, with cases of the disease being recorded in fully urbanized areas or in the urbanization phase. During the spatiotemporal analysis, it was also verified the presence of clusters both in the rural area and in the urban area.
References
- AGUIAR, P.F. and RODRIGUES, R.K., 2017. Leishmaniose visceral no Brasil: artigo de revisão. Rev Unimontes Cient, vol. 19, no. 1, pp. 191-204.
- ARAÚJO, D.M., 2017. Análise espacial dos casos humanos de leishmaniose visceral. Arq. Ciência & Saúde, vol. 24, no. 2, pp. 71-75.
- BARBOSA, I.R., 2016. Leishmaniose Visceral Humana no município de Natal-RN: análise clínico epidemiológica e espacial. Rev Cienc Plural, vol. 2, no. 1, pp. 89-101. http://dx.doi.org/10.21680/2446-7286.2016v2n1ID8559
» http://dx.doi.org/10.21680/2446-7286.2016v2n1ID8559 - BRASIL. Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis, 2015. Manual de recomendações para diagnóstico, tratamento e acompanhamento de pacientes com a coinfecção leishmania-HIV Brasília: Ministério da Saúde.
- BRASIL. Ministério da Saúde. Secretaria de Vigilância em Saúde, 2019. Vigilância em saúde no Brasil 2003/2019: da criação da Secretaria de Vigilância em Saúde aos dias atuais Brasília: Ministério da Saúde, Boletim Epidemiológico, vol. 50, no. esp.
- BRASIL. Ministério da Saúde. Secretaria de Vigilância em Saúde. Coordenação-Geral de Desenvolvimento da Epidemiologia em Serviços, 2017. Guia de vigilância em saúde Brasília: Ministério da Saúde, vol. 3.
- DANTAS-TORRES, F. and BRANDÃO-FILHO, S.P., 2006. Visceral leishmaniasis in Brazil: revisiting paradigms of epidemiology and control. Revista do Instituto de Medicina Tropical de São Paulo, vol. 48, no. 3, pp. 151-156. http://dx.doi.org/10.1590/S0036-46652006000300007 PMid:16847505.
» http://dx.doi.org/10.1590/S0036-46652006000300007 - DINIZ, M.M.C.S.L., OVALLOS, F.G., GOMES, C.M.C., LAVITSCHKA, C.O. and GALATI, E.A.B., 2014. Hostbiting rate and susceptibility of some suspected vectors to Leishmania braziliensis. Parasites & Vectors, vol. 7, no. 1, pp. 139. http://dx.doi.org/10.1186/1756-3305-7-139 PMid:24684943.
» http://dx.doi.org/10.1186/1756-3305-7-139 - DIVISÃO DE PROCESSAMENTO DE IMAGENS – DPI. Instituto Nacional de Pesquisas Espaciais - INPE, 2020 [viewed 20 February 2022]. Divisão de Processamento de Imagens/Instituto Nacional de Pesquisas Espaciais. Portal da Vigilância em Saúde [online]. Available from: http://vigilancia.saude.mg.gov.br/index.php/download/instalador-terraview-windows-v-4-2-2/
» http://vigilancia.saude.mg.gov.br/index.php/download/instalador-terraview-windows-v-4-2-2/ - KARAGIANNIS-VOULES, D., SCHOLTE, R.G.C., GUIMARÃES, L.H., UTZINGER, J. and VOUNATSOU, P., 2013. Bayesian geostatistical modeling of leishmaniasis incidence in Brazil. PLoS Neglected Tropical Diseases, vol. 7, no. 5, pp. e2213. http://dx.doi.org/10.1371/journal.pntd.0002213 PMid:23675545.
» http://dx.doi.org/10.1371/journal.pntd.0002213 - LIMA, A.F., SANTOS, A.F.S., CALHEIROS, T.R.S.P., XAVIER JUNIOR, A.F.S. and OLIVEIRA, S.G., 2019. Leishmaniose visceral: perfil epidemiológico dos casos no município de Maceió, Alagoas no período de 2011 a 2016. Interfaces Científicas, vol. 7, no. 3, pp. 133-142. http://dx.doi.org/10.17564/2316-3798.2019v7n3p133-142
» http://dx.doi.org/10.17564/2316-3798.2019v7n3p133-142 - MAIA, C.S., PIMENTEL, D.S., SANTANA, M.A., OLIVEIRA, G.M., PEDROSA, N.A., NASCIMENTO, L.A., FAUSTINO, M.A.G. and ALVES, L.C., 2014. Análise espacial da leishmaniose visceral americana no município de Petrolina, Pernambuco, Brasil. Hygeia, vol. 10, no. 18, pp. 167-176.
- MARTINS-MELO, F.R., CARNEIRO, M., RAMOS JUNIOR, A.N., HEUKELBACH, J., RIBEIRO, A.L.P. and WERNECK, G.L., 2018. The burden of neglected tropical diseases in Brazil, 1990-2016: a subnational analysis from the Global Burden of Disease Study 2016. PLoS Neglected Tropical Diseases, vol. 12, no. 6, pp. e0006559. http://dx.doi.org/10.1371/journal.pntd.0006559 PMid:29864133.
» http://dx.doi.org/10.1371/journal.pntd.0006559 - OKWOR, I. and UZONNA, J., 2016. Social and economic burden of human leishmaniasis. The American Journal of Tropical Medicine and Hygiene, vol. 94, no. 3, pp. 489-493. http://dx.doi.org/10.4269/ajtmh.15-0408 PMid:26787156.
» http://dx.doi.org/10.4269/ajtmh.15-0408 - Organización Panamericana de la Salud – OPS, 2019. Communicable diseases and environmental determinants of health Washington, DC: OPS.
- PIGOTT, D.M., BHATT, S., GOLDING, N., DUDA, K.A., BATTLE, E., BRADY, O.J., MESSINA, J.P., BALARD, Y., BASTIEN, P., PRATLONG, F., BROWNSTEIN, J.S., FREIFELD, C.C., MEKARU, S.R., GETHING, P.W., GEORGE, D.B., MYERS, M.F., REITHINGER, R. and HAY, S.I., 2014. Global distribution maps of the leishmaniases. eLife, vol. 3, pp. 1-21. http://dx.doi.org/10.7554/eLife.02851 PMid:24972829.
» http://dx.doi.org/10.7554/eLife.02851 - QUEIROZ, M.J., ALVES, J.G. and CORREIA, J.B., 2004. Leishmaniose visceral: características clínico-epidemiológicas em crianças de área endêmica. Jornal de Pediatria, vol. 80, no. 2, pp. 141-146. http://dx.doi.org/10.2223/1154 PMid:15079185.
» http://dx.doi.org/10.2223/1154
Publication Dates
-
Publication in this collection
18 Feb 2022 -
Date of issue
2024
History
-
Received
08 June 2021 -
Accepted
02 Oct 2021