Abstract
Chronic stress (CS) can contribute to dysfunction in several organs including liver and kidney.
This study was performed to investigate the changes in serum biochemistry, histological structure, as well as in localization of tyrosine phosphorylated proteins (TyrPho) and Heat shock protein 70 (Hsp-70) in liver and kidney tissues of CS rats induced by two stressors (restrained and force swimming) for 60 consecutive days. Samples of blood, liver, and kidney were collected from adult male Sprague–Dawley rats in each group. Our results showed that serum biochemical parameters including corticosterone, blood sugar, urea nitrogen, creatinine, cholesterol, triglyceride, HDL-C, LDL-C, ALT, AST, alkaline phosphatase in CS group were significantly different from that in normal group in both liver and kidney tissues. Although histological structure was not changed. TyrPho expression was significantly increased in liver lysate but significantly decreased in kidney. Hsp-70 expression in liver increased whereas in kidney decreased. In conclusion, CS can induce changes in liver and kidney functions.
Keywords:
chronic stress; tyrosine phosphorylation; heat shock protein 70; liver; kidney
Resumo
O estresse crônico (SC) pode contribuir para a disfunção em vários órgãos, incluindo fígado e rim.
Este estudo foi realizado para investigar as alterações na bioquímica sérica, estrutura histológica, bem como na localização de proteínas tirosina fosforiladas (TyrPho) e proteína de choque térmico 70 (Hsp-70) em tecidos hepáticos e renais de ratos CS induzidas por dois estressores (restrito e natação forçada) por 60 dias consecutivos. Amostras de sangue, fígado e rim foram coletadas de ratos Sprague-Dawley machos adultos em cada grupo. Nossos resultados mostraram que os parâmetros bioquímicos séricos, incluindo corticosterona, glicemia, nitrogênio ureico, creatinina, colesterol, triglicerídeos, HDL-C, LDL-C, ALT, AST, fosfatase alcalina no grupo CS foram significativamente diferentes do grupo normal em ambos os fígados e tecidos renais. Embora a estrutura histológica não tenha sido alterada, a expressão de TyrPho aumentou significativamente no lisado hepático, mas diminuiu significativamente no rim. A expressão de Hsp-70 no fígado aumentou, enquanto que no rim diminuiu. Em conclusão, a CS pode induzir alterações nas funções hepáticas e renais.
Palavras-chave:
estresse crônico; fosforilação de tirosina; proteína de choque térmico 70; fígado; rim
1. Introduction
It is known that many chronic stresses (CS) conditions can cause physiological dysregulations including neuropsychiatric disorder, reproductive dysfunction, and gastrointestinal disease, especially hepatic disorder (Joung et al., 2019JOUNG, J.Y., CHO, J.H., KIM, Y.H., CHOI, S.H. and SON, C.G., 2019. A literature review for the mechanisms of stress-induced liver injury. Brain and Behavior, vol. 9, no. 3, pp. e01235. http://dx.doi.org/10.1002/brb3.1235. PMid:30761781.
http://dx.doi.org/10.1002/brb3.1235...
) and renal impairment (Marchon et al., 2018MARCHON, R.G., RIBEIRO, C.T., COSTA, W.S., SAMPAIO, F.J.B., PEREIRA-SAMPAIO, M.A. and SOUZA, D.B., 2018. Immediate and late effects of stress on kidneys of prepubertal and adult rats. Kidney & Blood Pressure Research, vol. 43, no. 6, pp. 1919-1926. http://dx.doi.org/10.1159/000496004. PMid:30566954.
http://dx.doi.org/10.1159/000496004...
). Previously, CS has been demonstrated to alter physiology and morphology of many organs such as brain, reproductive, liver, and kidney tissues. Indeed, changes of hepatic biochemistry parameters were revealed in stress patients with viral hepatitis (Nagano et al., 2004NAGANO, J., NAGASE, S., SUDO, N. and KUBO, C., 2004. Psychosocial stress, personality, and the severity of chronic hepatitis C. Psychosomatics, vol. 45, no. 2, pp. 100-106. http://dx.doi.org/10.1176/appi.psy.45.2.100. PMid:15016922.
http://dx.doi.org/10.1176/appi.psy.45.2....
) and autoimmune hepatitis (Srivastava and Boyer, 2010SRIVASTAVA, S. and BOYER, J.L., 2010. Psychological stress is associated with relapse in type 1 autoimmune hepatitis. Liver International, vol. 30, no. 10, pp. 1439-1447. http://dx.doi.org/10.1111/j.1478-3231.2010.02333.x. PMid:20849437.
http://dx.doi.org/10.1111/j.1478-3231.20...
). In addition, Tseilikman et al. (2012) TSEILIKMAN, V., KOZOCHKIN, D., SYNITSKY, A., SIBIRIAK, S., TSEILIKMAN, O., KATASHINSKY, E., and SIMBIRTSEV, A., 2012. Does stress‐induced release of interleukin‐1 cause liver injury?.Cellular and Molecular Neurobiology, vol. 32, no. 7, pp. 1069–1078. doi: 10.1007/s10571-012-9866-7.
https://doi.org/doi: 10.1007/s10571-012-...
have shown the liver injury in animal models of restraint stress and electric foot shock stress. The CS could also reduce the blood flow in hepatic tissues via secretion of corticotropin-releasing factor from the hypothalamus (Chida et al., 2005CHIDA, Y., SUDO, N. and KUBO, C., 2005. Psychological stress impairs he- patic blood flow via central CRF receptors in mice. Life Sciences, vol. 76, no. 15, pp. 1707-1712. http://dx.doi.org/10.1016/j.lfs.2004.08.032. PMid:15698849.
http://dx.doi.org/10.1016/j.lfs.2004.08....
). Moreover, stressed animals have reported to have necrosis of hepatocytes caused by mitochondrial hypoxia (Joung et al., 2019JOUNG, J.Y., CHO, J.H., KIM, Y.H., CHOI, S.H. and SON, C.G., 2019. A literature review for the mechanisms of stress-induced liver injury. Brain and Behavior, vol. 9, no. 3, pp. e01235. http://dx.doi.org/10.1002/brb3.1235. PMid:30761781.
http://dx.doi.org/10.1002/brb3.1235...
). Interestingly, previous study showed the immediate and late effects of stress on reduction of the kidney weight and volume in both prepuberty and puberty (Marchon et al., 2018MARCHON, R.G., RIBEIRO, C.T., COSTA, W.S., SAMPAIO, F.J.B., PEREIRA-SAMPAIO, M.A. and SOUZA, D.B., 2018. Immediate and late effects of stress on kidneys of prepubertal and adult rats. Kidney & Blood Pressure Research, vol. 43, no. 6, pp. 1919-1926. http://dx.doi.org/10.1159/000496004. PMid:30566954.
http://dx.doi.org/10.1159/000496004...
). Particularly, it was found that animals induced by immobilization stress revealed the decrease of renal glomerular volume density (Souza et al., 2011SOUZA, D.B., SILVA, D., SILVA, C.M.C., SAMPAIO, F.J.B., COSTA, W.S. and CORTEZ, C.M., 2011. Effects of immobilization stress on kidneys of Wistar male rats: a morphometrical and stereological analysis. Kidney & Blood Pressure Research, vol. 34, no. 6, pp. 424-429. http://dx.doi.org/10.1159/000328331. PMid:21709423.
http://dx.doi.org/10.1159/000328331...
). Such glomerular volume loss in stress condition was shown to associate with hypertension (Hoy et al., 2008HOY, W.E., BERTRAM, J.F., DENTON, R.D., ZIMANYI, M., SAMUEL, T. and HUGHSON, M.D., 2008. Nephron number, glomerular volume, renal disease and hypertension. Current Opinion in Nephrology and Hypertension, vol. 17, no. 3, pp. 258-265. http://dx.doi.org/10.1097/MNH.0b013e3282f9b1a5. PMid:18408476.
http://dx.doi.org/10.1097/MNH.0b013e3282...
). Currently, Chaiyamoon et al. (2020)CHAIYAMOON, A., BUNSUEB, S. and IAMSAARD, S., 2020. Changes of tyrosine phosphorylation in liver and kidney of polycystic ovarian rats induced by letrozole. International Journal of Morphology, vol. 38, no. 4, pp. 919-923. http://dx.doi.org/10.4067/S0717-95022020000400919.
http://dx.doi.org/10.4067/S0717-95022020...
have reported not only the changes of functional parameters of liver and kidney, but also alterations of the tyrosine phosphorylation in polycystic ovary syndrome, a stress condition of women. However, such changes especially expressions of tyrosine phosphorylated proteins in liver and kidney of male have never been elucidated.
Tyrosine phosphorylated (TyrPho) proteins are documented to play important roles in many biological and physiological processes. Particularly, TyrPho proteins have been localized and investigated for individual function in the reproductive system (Tangsrisakda and Iamsaard, 2020TANGSRISAKDA, N. and IAMSAARD, S., 2020. Effect of ethanol on the changes in testicular protein expression in adult male rats. Andrologia, vol. 52, no. 10, pp. e13784. http://dx.doi.org/10.1111/and.13784. PMid:32721052.
http://dx.doi.org/10.1111/and.13784...
; Tongpan et al., 2019TONGPAN, S., SUKHORUM, W., ARUN, S., SAWATPHANICH, T. and IAMSAARD, S., 2019. Valproic acid changes the expression of tyrosine-phosphorylated proteins in rat seminal vesicle. Andrologia, vol. 51, no. 7, pp. e13303. http://dx.doi.org/10.1111/and.13303. PMid:31033021.
http://dx.doi.org/10.1111/and.13303...
; Sawatpanich et al., 2018SAWATPANICH, T., ARUN, S., TONGPAN, S., CHAICHUN, A., SAMPANNANG, A., SUKHORUM, W., MANEENIN, C., BURAWAT, J. and IAMSAARD, S., 2018. Localization and changes of tyrosine phosphorylated proteins and ß actin in epididymis of rats treated with valproic acid. International Journal of Morphology, vol. 36, no. 3, pp. 835-840. http://dx.doi.org/10.4067/S0717-95022018000300835.
http://dx.doi.org/10.4067/S0717-95022018...
; Iamsaard et al., 2018IAMSAARD, S., WELBAT, J.U., SUKHORUM, W., KRUTSRI, S., ARUN, S. and SAWATPANICH, T., 2018. Methotrexate changes the testicular tyrosine phosphorylated protein expression and seminal vesicle epithelia of adult rats. International Journal of Morphology, vol. 36, no. 2, pp. 737-742. http://dx.doi.org/10.4067/S0717-95022018000200737.
http://dx.doi.org/10.4067/S0717-95022018...
; Sampannang et al., 2019SAMPANNANG, A., ARUN, S., BURAWAT, J., SUKHORUM, W. and IAMSAARD, S., 2019. Expression of testicular phosphorylated proteins in types 1 and 2 dia- betes mellitus in mice: an experimental study. International Journal of Reproductive Biomedicine, vol. 17, no. 8, pp. 567-576. PMid:31583374.). Indeed, the changes of TyrPho protein expressions have been elucidated in testicular and epididymal tissues of adult male rats induced by acute and chronic stress conditions (Arun et al., 2018ARUN, S., BURAWAT, J., YANNASITHINON, S., SUKHORUM, W., LIMPONGSA, A. and IAMSAARD, S., 2018. Phyllanthus emblica leaf extract ameliorates testicular damage in rats with chronic stress. Journal of Zhejiang University. Science. B., vol. 19, no. 12, pp. 948-959. http://dx.doi.org/10.1631/jzus.B1800362. PMid:30507078.
http://dx.doi.org/10.1631/jzus.B1800362...
, 2021ARUN, S., CHAIYAMOON, A., LAPYUNEYONG, N., BUNSUEB, S., WU, A.T. and IAMSAARD, S., 2021. Chronic stress affects tyrosine phosphorylated protein expression and secretion of male rat epididymis. Andrologia, vol. 53, no. 3, pp. e13981. http://dx.doi.org/10.1111/and.13981. PMid:33469986.
http://dx.doi.org/10.1111/and.13981...
). Such changes were associated with increase of heat shock protein 70 (HSP-70) expression (Xun et al., 2015XUN, W., SHI, L., CAO, T., ZHAO, C., YU, P., WANG, D., HOU, G. and ZHOU, H., 2015. Dual functions in response to heat stress and spermato- genesis: characterization of expression profile of small heat shock proteins 9 and 10 in goat testis. BioMed Research International, vol. 2015, pp. 686239. http://dx.doi.org/10.1155/2015/686239. PMid:25685801.
http://dx.doi.org/10.1155/2015/686239...
; Wang et al., 2016WANG, W., BELOSAY, A., YANG, X., HARTMAN, J.A., SONG, H., IWANIEC, U.T., TURNER, R.T., CHURCHWELL, M.I., DOERGE, D.R. and HELFERICH, W.G., 2016. Effects of letrozole on breast cancer micro-metastatic tumor growth in bone and lung in mice inoculated with murine 4T1 cells. Clinical & Experimental Metastasis, vol. 33, no. 5, pp. 475-485. http://dx.doi.org/10.1007/s10585-016-9792-z. PMid:27209469.
http://dx.doi.org/10.1007/s10585-016-979...
). Consequently, the HSP-70 expression has been shown to be increased under heat stress or radiation (Wang et al., 2016WANG, W., BELOSAY, A., YANG, X., HARTMAN, J.A., SONG, H., IWANIEC, U.T., TURNER, R.T., CHURCHWELL, M.I., DOERGE, D.R. and HELFERICH, W.G., 2016. Effects of letrozole on breast cancer micro-metastatic tumor growth in bone and lung in mice inoculated with murine 4T1 cells. Clinical & Experimental Metastasis, vol. 33, no. 5, pp. 475-485. http://dx.doi.org/10.1007/s10585-016-9792-z. PMid:27209469.
http://dx.doi.org/10.1007/s10585-016-979...
). Taken together, CS can induce some structural damages and change functional parameters of liver and kidney but the complete mechanism on this issue especially alteration of tyrosine phosphorylation has never been reported. To gain our knowledges, this study aimed to investigate the effects of chronic stress on associations among histological features, serum biochemical parameters, and expressions of TyrPho and HSP-70 proteins in liver and kidney of adult male rats.
2. Materials and Methods
2.1. Animals and experimental design
Twenty adult male Sprague–Dawley rats were obtained from the Animal unit, Faculty of Medicine Khon Kaen University, Thailand. All animals were housed in plastic cages under the experimental room with controlled condition (12 hr. light/dark cycle, temperature 23 ± 2°C, humidity 30-60% RH, sound < 85 decibels, and light intensity 350-400 lux) at Laboratory Animal Unit, Faculty of Medicine, Khon Kaen University, KhonKaen, Thailand. The rats received the commercial food pellets and water ad libitum. This study has been approved by Animal Ethics Committee Research of Faculty of Medicine Khon Kaen University, based on the Ethics of Animal Experimentation of National Research Council of Thailand (Record No. IACUC-KKU-15/64)
2.2. Stress induction
After a week of acclimatization, animals were randomly divided into control and chronic stress (CS) groups. Control rats were maintained without any stress disturbance, whereas the rats in CS group were exposed with two stressors (restrained and force swimming), based on the procedure described by Nirupama et al. (2013) NIRUPAMA, M., DEVAKI, M., NIRUPAMA, R. and YAJURVED, H.N., 2013. Chronic intermittent stress-induced alterations in the spermatogenesis and antioxidant status of the testis are irreversible in albino rat. Journal of Physiology and Biochemistry, vol. 69, no. 1, pp. 59-68. http://dx.doi.org/10.1007/s13105-012-0187-6. PMid:22820994.
http://dx.doi.org/10.1007/s13105-012-018...
and Arun et al. (2016)ARUN, S., BURAWAT, J., SUKHORUM, W., SAMPANNANG, A., MANEENIN, C. and IAMSAARD, S., 2016. Chronic restraint stress induces sperm acrosome reaction and changes in testicular tyrosine phosphorylated proteins in rats. International Journal of Reproductive Biomedicine, vol. 14, no. 7, pp. 443-52. PMid: 27525328.. In brief, the rats were restrained within the restraint cage for consecutive4 hour before forced swimming for 15 minutes) in the cold water (approximately 15 °C). This CS induction was performed for consecutive 60 days. At the end of experiment, all animals of both groups were anesthetized by thiobarbital sodium (60 mg/KgBW via intraperitoneal injection) and euthanatized by cervical dislocation before collections of blood, liver and kidney for further analyses.
2.3. Biochemical analyses of blood serum for determination of liver and kidney functions
After euthanization, the blood from each rat was collected by cardiac puncture from the left ventricle and centrifuged at 12000 rpm at 4 °C for 15 min by Microfuge 22R (Biocompare Inc., USA) to separate the serum from the blood cells. Subsequently, blood serum was sent to the Radiology Unit, Srina- garind Hospital, Faculty of Medicine, Khon Kaen University, Thailand for determination of serum corticosterone levels and biochemical analyses of liver and renal function parameters by electrochemiluminescence immunoassay (ECLIA).
2.4. Histological evaluation by Masson’s trichrome staining
After weighing liver and kidney organs, each tissue was fixed into 10% formalin fixative and routinely processed for making of paraffin tissue blocks. Then, all liver and kidney tissues were sectioned approximately 5-7 um thickness. Subsequently, the tissue sections were further stained with Masson's trichrome (Catalogue no. HT15, Sigma-Aldrich, Inc., USA) to observe collagen accumulation in liver or kidney tissues because previous study has demonstrated that chronic stress caused the fibrosis in liver and penile corpus cavernosum of stress rats (Li et al., 2016LI, M., SUN, Q., LI, S., ZHAI, Y., WANG, J., CHEN, B. and LU, J., 2016. Chronic restraint stress reduces carbon tetrachloride-induced liver fibrosis. Experimental and Therapeutic Medicine, vol. 11, no. 6, pp. 2147-2152. http://dx.doi.org/10.3892/etm.2016.3205. PMid:27284296.
http://dx.doi.org/10.3892/etm.2016.3205...
; Souza et al., 2012SOUZA, D.B., SILVA, D., CORTEZ, C.M., COSTA, W.S. and SAMPAIO, F.J., 2012. Effects of chronic stress on penile corpus cavernosum of rats. Journal of Andrology, vol. 33, no. 4, pp. 735-739. http://dx.doi.org/10.2164/jandrol.111.014225. PMid:21940985.
http://dx.doi.org/10.2164/jandrol.111.01...
). Briefly, the tissue sections were deparaffinized with xylene and rehydrated with the serial descending alcohols. Then sections were fixed in Bouin's solution at 56 °C for 15 minutes. Then, sections were washed with tap water for 20 minutes and stained with Weigert's Iron Hematoxylin for 5 minutes. After rinsing with tap water for 5 minutes the sections were stained with biebrich scarlet-acid fuchsin solution for 5 minutes. After washing, sections were stained in phosphomolybdic-phosphotungstic acid solution for 5 minutes and incubated with aniline blue solution (HT1S-04) for 10 minutes before dropping with 1% acetic acid.
2.5. Western blot analysis
The total proteins of liver and kidney from both groups were homogenized and extracted with ice-cold RIPA buffer (catalogue no. GR3333912-1, Cell Signalling Technology, Inc., USA). Then the total proteins (100 µg) were loaded and separated on SDS-PAGE (10% polyacrylamide resolving gel). The separated proteins were transferred onto the nitrocellulose membrane before incubated with 5% BSA in 0.1% TBST (0.1% Tween-20, TBS, pH 7.4) for 45 minutes to block the non-specific binding proteins. All membranes of liver and kidney protein lysates were incubated with primary antibody (anti-tyrosine phosphorylated; 1:2000 dilution, catalogue no.05-321, Merck Millipore Co., USA) at 4°C overnight followed by washing and incubating with secondary antibody (anti-mouse,1:5000 dilution, TM catalogue number G-21234, Invitrogen, USA), in 0.1% TBST, for 1 h, room temperature. The tyrosine phosphorylated proteins on each membrane were detected using ECL and visualized under exposure to the gel documentation 4 (ImageQuant 400, GH Healthcare, USA). For quantitative analysis, the relative intensity of protein expression was measured and analyzed by using the ImageJ program (version 1.49).
2.6. Statistical analysis
All data were expressed as means ± standard deviation (SD). To compare the difference between two groups, the independent t-test was used for normally- distributed data or the Mann- Whitney U test was used for not normally- distributed data by using the program IBM SPSS statistics 19.0 software data analysis. The P-value < 0.05 was considered as a statistically significant difference between control and CS groups.
3. Results
3.1. Effect of chronic stress (CS) on weights of body, liver, and kidney
The results in Table 1 showed that CS significantly decreased the weights of body, liver, and kidney in both absolute and relative weights as compared to those of control (P < 0.05).
3.2. Effect of chronic stress on serum biochemical parameters of liver and kidney functions
As shown in the Table 2, it was found that the CS significantly decreased the serum levels of creatinine, cholesterol, triglyceride, HLD-C, and LDL-C, respectively. In contrast, the levels of corticosterone, blood sugar, urea nitrogen, and ALT in CS group were significantly increased as compared to the control (P<0.05, Table 2).
Serum biochemical parameters determining liver and kidney functions compared between control and chronic stress rats.
3.3. Histology of liver and kidney induced by chronic stress
Under investigating the histopathology of liver and kidney stained with Masson’s trichrome, no cellular damage in hepatic portal triad or fibrotic tissue in renal glomerulus was obviously found in CS groups as compared to control (Figure 1A and Figure 1B).
Representative histology of liver and kidney tissues stained by Masson’s trichrome of control (A, C) and CS groups (B, D).
3.4. Changes of tyrosine phosphorylated (TyrPho) protein expressions in liver and kidney
The SDS-PAGE results demonstrating total proteins profiles of both liver (Figure 2A) and kidney (Figure 3A) lysates showed no difference as compared between control and CS groups. In the liver lysates, it was obviously demonstrated that the expressions and intensities of 80, 43, and 39 TyrPho proteins in CS group were significantly increased as compared to control (Figure 2B and Figure 2C). In contrast, the expressions of 165 and 95 TyrPho proteins were significantly decreased in kidney of CS group as compared to those of control (Figure 3B and Figure 3C).
Showing total protein profiles of liver lysates revealed by SDS‐PAGE (A), expression (B), and intensities (C) of tyrosine phosphorylated (TyrPho) proteins demonstrated by western blotting compared between control and CS groups. *p < 0.05, statistically significant difference as compared between the control and CS groups. Con; control, CS; chronic stress; EGF; epidermal growth factor used as a positive control, kDa; kilodalton, MW; molecular weight.
Showing total protein profiles of kidney lysates revealed by SDS‐PAGE (A), expression (B), and intensities (C) of tyrosine phosphorylated (TyrPho) proteins demonstrated by western blotting compared between control and CS groups. *p < 0.05, statistically significant difference as compared between the control and CS groups. Con; control, CS; chronic stress; EGF; epidermal growth factor used as a positive control, kDa; kilodalton, MW; molecular weight.
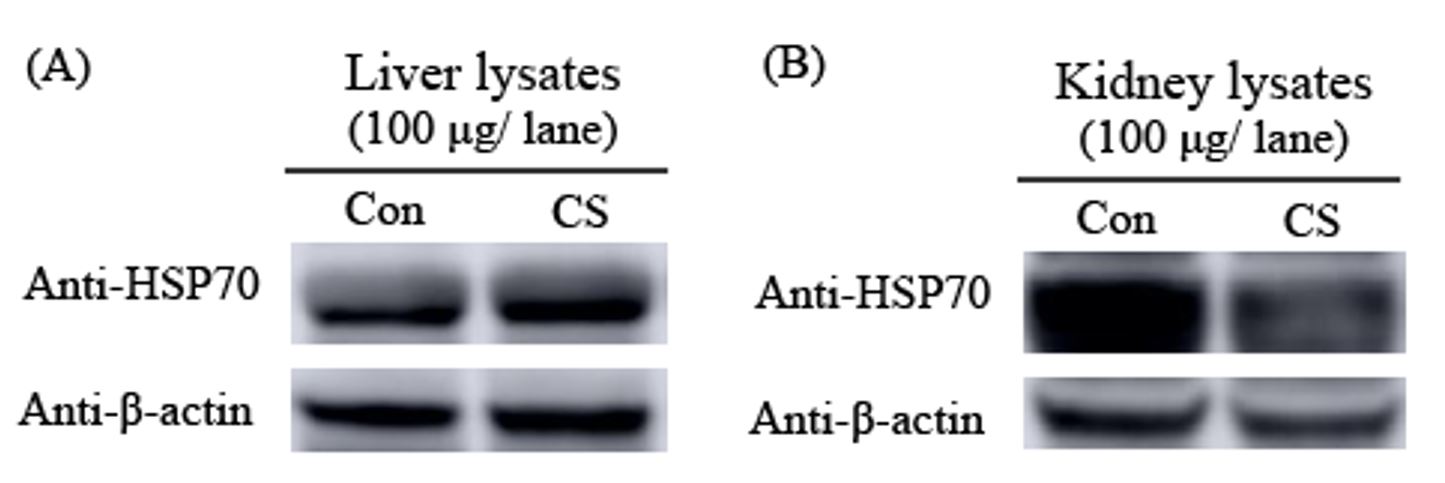
3.5. Expression of Heat Shock Protein 70 (Hsp70) in liver and kidney
Figure 4A showed the increased expressions of Hsp70 expression in the liver induced by chronic stress. Compared to the control, we found that CS obviously decrerased the expression of Hsp70 in kidney as demonstrated in Figure 4B.
Expression of heat shock protein 70 (Hsp70) in liver (A) and kidney (B) lysates compared between the control and CS groups. β- actin used as an internal control. Con; control, CS; chronic stress.
4. Discussion
The chronic stress (CS) of animals used in this study was induced by restraining and forced swimming for consecutive 60 days. The development of CS was confirmed by increased level of serum corticosterone in comparison to control animals. Our results were similar to a previous study demonstrating that the weights of body, liver, and kidney of CS rats were significantly decreased as compared to healthy animals (Roberts et al., 2007ROBERTS, C., TROOP, N., CONNAN, F., TREASURE, J. and CAMPBELL, I.C., 2007. The effects of stress on body weight: biological and psychological predictors of change in BMI. Obesity, vol. 15, no. 12, pp. 3045-3055. http://dx.doi.org/10.1038/oby.2007.363. PMid:18198314.
http://dx.doi.org/10.1038/oby.2007.363...
). Although the food consumption of stress rats was not recorded in our study, it is assumed to be reduced as reported by Roberts et al. (2007)ROBERTS, C., TROOP, N., CONNAN, F., TREASURE, J. and CAMPBELL, I.C., 2007. The effects of stress on body weight: biological and psychological predictors of change in BMI. Obesity, vol. 15, no. 12, pp. 3045-3055. http://dx.doi.org/10.1038/oby.2007.363. PMid:18198314.
http://dx.doi.org/10.1038/oby.2007.363...
. Particularly, the decrease of kidney weight in this investigation are corresponded to previous studies (Souza et al., 2011SOUZA, D.B., SILVA, D., SILVA, C.M.C., SAMPAIO, F.J.B., COSTA, W.S. and CORTEZ, C.M., 2011. Effects of immobilization stress on kidneys of Wistar male rats: a morphometrical and stereological analysis. Kidney & Blood Pressure Research, vol. 34, no. 6, pp. 424-429. http://dx.doi.org/10.1159/000328331. PMid:21709423.
http://dx.doi.org/10.1159/000328331...
; Marchon et al., 2018MARCHON, R.G., RIBEIRO, C.T., COSTA, W.S., SAMPAIO, F.J.B., PEREIRA-SAMPAIO, M.A. and SOUZA, D.B., 2018. Immediate and late effects of stress on kidneys of prepubertal and adult rats. Kidney & Blood Pressure Research, vol. 43, no. 6, pp. 1919-1926. http://dx.doi.org/10.1159/000496004. PMid:30566954.
http://dx.doi.org/10.1159/000496004...
). We assumed that the excess corticosterone level may disrupt some functions of liver and kidney on metabolic pathway in anabolism of cells, resulted in inhibition of protein and glycogen syntheses especially in hepatocytes of liver. It is possible that increased corticosterone could early damage such function before later destroying cellular structures. Consequently, no histopathology was observed in both kidney and liver tissues. However, the actual mechanism on such issue is still not known. Possibly, such absolute organ weights may corroborate with their relative weights since CS animals have less intake. In contrast to the weight results, the histological structures of both renal and hepatic tissues revealed no difference as compared between control and CS group. It indicates that chronic stress did not obviously affect the microstructures of liver and kidney which similar to that stress caused by drug effect called “letrozol” as demonstrated previously (Chaiyamoon et al., 2020CHAIYAMOON, A., BUNSUEB, S. and IAMSAARD, S., 2020. Changes of tyrosine phosphorylation in liver and kidney of polycystic ovarian rats induced by letrozole. International Journal of Morphology, vol. 38, no. 4, pp. 919-923. http://dx.doi.org/10.4067/S0717-95022020000400919.
http://dx.doi.org/10.4067/S0717-95022020...
). In contrast, a previous study has revealed that the glomerular volume of kidney in CS animals was significantly decreased (Marchon et al., 2018MARCHON, R.G., RIBEIRO, C.T., COSTA, W.S., SAMPAIO, F.J.B., PEREIRA-SAMPAIO, M.A. and SOUZA, D.B., 2018. Immediate and late effects of stress on kidneys of prepubertal and adult rats. Kidney & Blood Pressure Research, vol. 43, no. 6, pp. 1919-1926. http://dx.doi.org/10.1159/000496004. PMid:30566954.
http://dx.doi.org/10.1159/000496004...
). Although our results did not show any histopathology in vital tissues in CS rats, their biochemical parameters in the blood serum were indeed changed from the control health animals. It is suggested that the CS extremely affect the functions but not structures of liver and kidney, possibly resulting in anorexia, dyspepsis, and anuria.
Since the tyrosine phosphorylation especially TyrPho proteins has been shown to be involved in many physiological systems of mammalian animals, including liver and kidney. It is well known that theTyrPho protein have been reported to express and play the important roles in the reproductive organs of many models, for examples, stress model (Arun et al., 2021ARUN, S., CHAIYAMOON, A., LAPYUNEYONG, N., BUNSUEB, S., WU, A.T. and IAMSAARD, S., 2021. Chronic stress affects tyrosine phosphorylated protein expression and secretion of male rat epididymis. Andrologia, vol. 53, no. 3, pp. e13981. http://dx.doi.org/10.1111/and.13981. PMid:33469986.
http://dx.doi.org/10.1111/and.13981...
; Iamsaard et al., 2021IAMSAARD, S., TONGPAN, S., YANNASITHINON, S., ARUN, S., WU, A.T.H. and SUKHORUM, W., 2021. Effect of chronic stress on expression and secretion of seminal vesicle proteins in adult rats. Andrologia, vol. 53, no. 1, pp. e13800. http://dx.doi.org/10.1111/and.13800. PMid:32816406.
http://dx.doi.org/10.1111/and.13800...
), diabetic mellitus model (Sampannang et al., 2019SAMPANNANG, A., ARUN, S., BURAWAT, J., SUKHORUM, W. and IAMSAARD, S., 2019. Expression of testicular phosphorylated proteins in types 1 and 2 dia- betes mellitus in mice: an experimental study. International Journal of Reproductive Biomedicine, vol. 17, no. 8, pp. 567-576. PMid:31583374.), and cancer drug models (Iamsaard et al., 2017IAMSAARD, S., SUKHORUM, W., ARUN, S., PHUNCHAGO, N., UABUNDIT, N., BOONRUANGSRI, P. and NAMKING, M., 2017. Valproic acid induces histologic changes and decreases androgen receptor levels of testis and epididymis in rats. International Journal of Reproductive Biomedicine, vol. 15, no. 4, pp. 217-224. http://dx.doi.org/10.29252/ijrm.15.4.217. PMid:28835938.
http://dx.doi.org/10.29252/ijrm.15.4.217...
). In our result, that the changes of TyrPho protein expressions were obviously investigated in CS liver. Previous study has demonstrated that TyrPho protein has important role in response to regeneration in liver injury (Pagano et al., 2012PAGANO, M.A., TIBALDI, E., GRINGERI, E. and BRUNATI, A.M., 2012. Tyrosine phosphorylation and liver regeneration: A glance at intracellular transducers. IUBMB Life, vol. 64, no. 1, pp. 27-35. http://dx.doi.org/10.1002/iub.576. PMid:22184095.
http://dx.doi.org/10.1002/iub.576...
). Our results support the idea that the changes of liver TyrPho proteins may be involved in abnormal function of the CS liver with alteration of serum biochemical parameters. In kidney, the expression of TyrPho proteins have been demonstrated to be decreased in the stress induced by valpoic acid (Maneenin et al., 2019MANEENIN, C., LAPYUNEYONG, N., TONGPAN, S., YANNASITHINON, S., BURAWAT, J., MANEENIN, N., SUKHORUM, W., ARUN, S. and IAMSAARD, S., 2019. The alterations of microvasculature, tyrosine phosphorylation, and lipid peroxidation in kidney of rats treated with valproic acid. International Journal of Morphology, vol. 37, no. 1, pp. 65-70. http://dx.doi.org/10.4067/S0717-95022019000100065.
http://dx.doi.org/10.4067/S0717-95022019...
). Such decrease of such Typho proteins can cause the minimal s nephrosis (Uchida et al., 2008UCHIDA, K., SUZUKI, K., IWAMOTO, M., KAWACHI, H., OHNO, M., HORITA, S. and NITTA, K., 2008. Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney International, vol. 73, no. 8, pp. 926-932. http://dx.doi.org/10.1038/ki.2008.19. PMid:18256598.
http://dx.doi.org/10.1038/ki.2008.19...
). In normal condition, TyrPho also has been observed in the rat kidney (Zhang et al., 2008ZHANG, J., BROWN, R.P., SHAW, M., VAIDYA, V.S., ZHOU, Y., ESPANDIARI, P., SADRIEH, N., STRATMEYER, M., KEENAN, J., KILTY, C.G., BONVENTRE, J.V. and GOERING, P.L., 2008. Immunolocalization of Kim-1, RPA-1, and RPA-2 in kidney of gentamicin-, mercury-, or chromium-treated rats: relationship to renal distributions of iNOS and nitrotyrosine. Toxicologic Pathology, vol. 36, no. 3, pp. 397-409. http://dx.doi.org/10.1177/0192623308315832. PMid:18441258.
http://dx.doi.org/10.1177/01926233083158...
). Moreover, it was documented that the expression of TyrPho proteins in the liver and kidney was totally changed with other functional parameters in stress rats with polycystic ovarian (Chaiyamoon et al., 2020CHAIYAMOON, A., BUNSUEB, S. and IAMSAARD, S., 2020. Changes of tyrosine phosphorylation in liver and kidney of polycystic ovarian rats induced by letrozole. International Journal of Morphology, vol. 38, no. 4, pp. 919-923. http://dx.doi.org/10.4067/S0717-95022020000400919.
http://dx.doi.org/10.4067/S0717-95022020...
). For chronic stress model in this study, we suggested that TyrPho proteins also play the essential roles for liver and kidney functions although the individual protein need to be further identified and characterized to elucidate the actual mechanism. It is possible that some TyrPho proteins may regulate the signalling pathways in liver and kidney tissues. In cellular level, it is known that tyrosine phosphorylation has dual functions (turn in and turn on off various proteins) based on individually physiological pathway. As shown in chronic stress models, many TyrPho proteins were changed in reproductive tissues (Arun etal., 2021ARUN, S., CHAIYAMOON, A., LAPYUNEYONG, N., BUNSUEB, S., WU, A.T. and IAMSAARD, S., 2021. Chronic stress affects tyrosine phosphorylated protein expression and secretion of male rat epididymis. Andrologia, vol. 53, no. 3, pp. e13981. http://dx.doi.org/10.1111/and.13981. PMid:33469986.
http://dx.doi.org/10.1111/and.13981...
; Bunsueb et al., 2021; Choowong-In et al., 2021CHOOWONG-IN, P., SATTAYASAI, J., POODENDAEN, C. and IAMSAARD, S., 2021. Decreased expression of AKAP4 and TyrPho proteins in testis, epididymis, and spermatozoa with low sexual performance of mice induced by modified CUMS. Andrologia, vol. 53, no. 3, pp. e13977. http://dx.doi.org/10.1111/and.13977. PMid:33486757.
http://dx.doi.org/10.1111/and.13977...
; Iamsaard et al., 2021IAMSAARD, S., TONGPAN, S., YANNASITHINON, S., ARUN, S., WU, A.T.H. and SUKHORUM, W., 2021. Effect of chronic stress on expression and secretion of seminal vesicle proteins in adult rats. Andrologia, vol. 53, no. 1, pp. e13800. http://dx.doi.org/10.1111/and.13800. PMid:32816406.
http://dx.doi.org/10.1111/and.13800...
)
It is well known that stress condition causes the increase of the heat shock protein 70 (Hsp70) expression which is a chaperone for protecting cellular stress. The Hsp 70 has been documented in response to liver regeneration (Wolf et al., 2014WOLF, J.H., BHATTI, T.R., FOURASCHEN, S., CHAKRAVORTY, S., WANG, L., KURIAN, S., SALOMON, D., OLTHOFF, K.M., HANCOCK, W.W. and LEVINE, M.H., 2014. Heat shock protein 70 is required for optimal liver regeneration after partial hepatectomy in mice. Liver Transplantation, vol. 20, no. 3, pp. 376-385. http://dx.doi.org/10.1002/lt.23813. PMid:24357103.
http://dx.doi.org/10.1002/lt.23813...
). Particularly, the levels of Hsp-70 expression in liver have been shown to be increased in the heat shock condition (Oka et al., 2013OKA, Y., AKAGI, Y., KINUGASA, T., ISHIBASHI, N., IWAKUMA, N., SHIRATSUCHI, I. and SHIROUZU, K., 2013. Pre-treatment reduces liver injury and aids liver recovery after partial hepatectomy in mice. Anticancer Research, vol. 33, no. 7, pp. 2887-2894. PMid:23780975.). Similar to the previous literature, our results have demonstrated that the Hsp-70 expression in liver lysate of CS group was obviously increased as compared to that of control. However, this protein was shown to be decreased in CS kidney for the first time, suggesting that renal HSP-70 was not involved in CS condition but it expression may response to chronic glomerulonephritis and lupus nephritis as previously reported (Chebotareva et al., 2017CHEBOTAREVA, N., BOBKOVA, I. and SHILOV, E., 2017. Heat shock proteins and kidney disease: perspectives of HSP therapy. Cell Stress & Chaperones, vol. 22, no. 3, pp. 319-343. http://dx.doi.org/10.1007/s12192-017-0790-0. PMid:28409327.
http://dx.doi.org/10.1007/s12192-017-079...
).
Conclusion
In conclusion, CS induced by restraint and forced swimming in rat model caused not only weights of body and vital organs like liver and kidney but also decreased the functionally biochemical parameters of such vital organs. To gain our knowledge, CS also changed the functional proteins in liver and kidney especially TyrPho and Hsp 70 proteins.
Acknowledgements
This research project was financial supported by young researcher development project of Khon Kaen University, Thailand to Dr. Arada Chaiyamoon and Dr. Sitthichai Iamsaard.
References
- ARUN, S., BURAWAT, J., SUKHORUM, W., SAMPANNANG, A., MANEENIN, C. and IAMSAARD, S., 2016. Chronic restraint stress induces sperm acrosome reaction and changes in testicular tyrosine phosphorylated proteins in rats. International Journal of Reproductive Biomedicine, vol. 14, no. 7, pp. 443-52. PMid: 27525328.
- ARUN, S., BURAWAT, J., YANNASITHINON, S., SUKHORUM, W., LIMPONGSA, A. and IAMSAARD, S., 2018. Phyllanthus emblica leaf extract ameliorates testicular damage in rats with chronic stress. Journal of Zhejiang University. Science. B., vol. 19, no. 12, pp. 948-959. http://dx.doi.org/10.1631/jzus.B1800362 PMid:30507078.
» http://dx.doi.org/10.1631/jzus.B1800362 - ARUN, S., CHAIYAMOON, A., LAPYUNEYONG, N., BUNSUEB, S., WU, A.T. and IAMSAARD, S., 2021. Chronic stress affects tyrosine phosphorylated protein expression and secretion of male rat epididymis. Andrologia, vol. 53, no. 3, pp. e13981. http://dx.doi.org/10.1111/and.13981 PMid:33469986.
» http://dx.doi.org/10.1111/and.13981 - BUNSUEB, S., LAPYUNEYONG, N., TONGPAN, S., ARUN, S. and IAMSAARD, S., 2021. Chronic stress increases the tyrosine phosphorylation in female reproductive organs: an experimental study. International Journal of Reproductive Biomedicine, vol. 19, no. 1, pp. 87-96. http://dx.doi.org/10.18502/ijrm.v19i1.8183 PMid:33554006.
» http://dx.doi.org/10.18502/ijrm.v19i1.8183 - CHAIYAMOON, A., BUNSUEB, S. and IAMSAARD, S., 2020. Changes of tyrosine phosphorylation in liver and kidney of polycystic ovarian rats induced by letrozole. International Journal of Morphology, vol. 38, no. 4, pp. 919-923. http://dx.doi.org/10.4067/S0717-95022020000400919
» http://dx.doi.org/10.4067/S0717-95022020000400919 - CHEBOTAREVA, N., BOBKOVA, I. and SHILOV, E., 2017. Heat shock proteins and kidney disease: perspectives of HSP therapy. Cell Stress & Chaperones, vol. 22, no. 3, pp. 319-343. http://dx.doi.org/10.1007/s12192-017-0790-0 PMid:28409327.
» http://dx.doi.org/10.1007/s12192-017-0790-0 - CHIDA, Y., SUDO, N. and KUBO, C., 2005. Psychological stress impairs he- patic blood flow via central CRF receptors in mice. Life Sciences, vol. 76, no. 15, pp. 1707-1712. http://dx.doi.org/10.1016/j.lfs.2004.08.032 PMid:15698849.
» http://dx.doi.org/10.1016/j.lfs.2004.08.032 - CHOOWONG-IN, P., SATTAYASAI, J., POODENDAEN, C. and IAMSAARD, S., 2021. Decreased expression of AKAP4 and TyrPho proteins in testis, epididymis, and spermatozoa with low sexual performance of mice induced by modified CUMS. Andrologia, vol. 53, no. 3, pp. e13977. http://dx.doi.org/10.1111/and.13977 PMid:33486757.
» http://dx.doi.org/10.1111/and.13977 - HOY, W.E., BERTRAM, J.F., DENTON, R.D., ZIMANYI, M., SAMUEL, T. and HUGHSON, M.D., 2008. Nephron number, glomerular volume, renal disease and hypertension. Current Opinion in Nephrology and Hypertension, vol. 17, no. 3, pp. 258-265. http://dx.doi.org/10.1097/MNH.0b013e3282f9b1a5 PMid:18408476.
» http://dx.doi.org/10.1097/MNH.0b013e3282f9b1a5 - IAMSAARD, S., SUKHORUM, W., ARUN, S., PHUNCHAGO, N., UABUNDIT, N., BOONRUANGSRI, P. and NAMKING, M., 2017. Valproic acid induces histologic changes and decreases androgen receptor levels of testis and epididymis in rats. International Journal of Reproductive Biomedicine, vol. 15, no. 4, pp. 217-224. http://dx.doi.org/10.29252/ijrm.15.4.217 PMid:28835938.
» http://dx.doi.org/10.29252/ijrm.15.4.217 - IAMSAARD, S., TONGPAN, S., YANNASITHINON, S., ARUN, S., WU, A.T.H. and SUKHORUM, W., 2021. Effect of chronic stress on expression and secretion of seminal vesicle proteins in adult rats. Andrologia, vol. 53, no. 1, pp. e13800. http://dx.doi.org/10.1111/and.13800 PMid:32816406.
» http://dx.doi.org/10.1111/and.13800 - IAMSAARD, S., WELBAT, J.U., SUKHORUM, W., KRUTSRI, S., ARUN, S. and SAWATPANICH, T., 2018. Methotrexate changes the testicular tyrosine phosphorylated protein expression and seminal vesicle epithelia of adult rats. International Journal of Morphology, vol. 36, no. 2, pp. 737-742. http://dx.doi.org/10.4067/S0717-95022018000200737
» http://dx.doi.org/10.4067/S0717-95022018000200737 - JOUNG, J.Y., CHO, J.H., KIM, Y.H., CHOI, S.H. and SON, C.G., 2019. A literature review for the mechanisms of stress-induced liver injury. Brain and Behavior, vol. 9, no. 3, pp. e01235. http://dx.doi.org/10.1002/brb3.1235 PMid:30761781.
» http://dx.doi.org/10.1002/brb3.1235 - LI, M., SUN, Q., LI, S., ZHAI, Y., WANG, J., CHEN, B. and LU, J., 2016. Chronic restraint stress reduces carbon tetrachloride-induced liver fibrosis. Experimental and Therapeutic Medicine, vol. 11, no. 6, pp. 2147-2152. http://dx.doi.org/10.3892/etm.2016.3205 PMid:27284296.
» http://dx.doi.org/10.3892/etm.2016.3205 - MANEENIN, C., LAPYUNEYONG, N., TONGPAN, S., YANNASITHINON, S., BURAWAT, J., MANEENIN, N., SUKHORUM, W., ARUN, S. and IAMSAARD, S., 2019. The alterations of microvasculature, tyrosine phosphorylation, and lipid peroxidation in kidney of rats treated with valproic acid. International Journal of Morphology, vol. 37, no. 1, pp. 65-70. http://dx.doi.org/10.4067/S0717-95022019000100065
» http://dx.doi.org/10.4067/S0717-95022019000100065 - MARCHON, R.G., RIBEIRO, C.T., COSTA, W.S., SAMPAIO, F.J.B., PEREIRA-SAMPAIO, M.A. and SOUZA, D.B., 2018. Immediate and late effects of stress on kidneys of prepubertal and adult rats. Kidney & Blood Pressure Research, vol. 43, no. 6, pp. 1919-1926. http://dx.doi.org/10.1159/000496004 PMid:30566954.
» http://dx.doi.org/10.1159/000496004 - NAGANO, J., NAGASE, S., SUDO, N. and KUBO, C., 2004. Psychosocial stress, personality, and the severity of chronic hepatitis C. Psychosomatics, vol. 45, no. 2, pp. 100-106. http://dx.doi.org/10.1176/appi.psy.45.2.100 PMid:15016922.
» http://dx.doi.org/10.1176/appi.psy.45.2.100 - NIRUPAMA, M., DEVAKI, M., NIRUPAMA, R. and YAJURVED, H.N., 2013. Chronic intermittent stress-induced alterations in the spermatogenesis and antioxidant status of the testis are irreversible in albino rat. Journal of Physiology and Biochemistry, vol. 69, no. 1, pp. 59-68. http://dx.doi.org/10.1007/s13105-012-0187-6 PMid:22820994.
» http://dx.doi.org/10.1007/s13105-012-0187-6 - OKA, Y., AKAGI, Y., KINUGASA, T., ISHIBASHI, N., IWAKUMA, N., SHIRATSUCHI, I. and SHIROUZU, K., 2013. Pre-treatment reduces liver injury and aids liver recovery after partial hepatectomy in mice. Anticancer Research, vol. 33, no. 7, pp. 2887-2894. PMid:23780975.
- PAGANO, M.A., TIBALDI, E., GRINGERI, E. and BRUNATI, A.M., 2012. Tyrosine phosphorylation and liver regeneration: A glance at intracellular transducers. IUBMB Life, vol. 64, no. 1, pp. 27-35. http://dx.doi.org/10.1002/iub.576 PMid:22184095.
» http://dx.doi.org/10.1002/iub.576 - ROBERTS, C., TROOP, N., CONNAN, F., TREASURE, J. and CAMPBELL, I.C., 2007. The effects of stress on body weight: biological and psychological predictors of change in BMI. Obesity, vol. 15, no. 12, pp. 3045-3055. http://dx.doi.org/10.1038/oby.2007.363 PMid:18198314.
» http://dx.doi.org/10.1038/oby.2007.363 - SAMPANNANG, A., ARUN, S., BURAWAT, J., SUKHORUM, W. and IAMSAARD, S., 2019. Expression of testicular phosphorylated proteins in types 1 and 2 dia- betes mellitus in mice: an experimental study. International Journal of Reproductive Biomedicine, vol. 17, no. 8, pp. 567-576. PMid:31583374.
- SAWATPANICH, T., ARUN, S., TONGPAN, S., CHAICHUN, A., SAMPANNANG, A., SUKHORUM, W., MANEENIN, C., BURAWAT, J. and IAMSAARD, S., 2018. Localization and changes of tyrosine phosphorylated proteins and ß actin in epididymis of rats treated with valproic acid. International Journal of Morphology, vol. 36, no. 3, pp. 835-840. http://dx.doi.org/10.4067/S0717-95022018000300835
» http://dx.doi.org/10.4067/S0717-95022018000300835 - SOUZA, D.B., SILVA, D., CORTEZ, C.M., COSTA, W.S. and SAMPAIO, F.J., 2012. Effects of chronic stress on penile corpus cavernosum of rats. Journal of Andrology, vol. 33, no. 4, pp. 735-739. http://dx.doi.org/10.2164/jandrol.111.014225 PMid:21940985.
» http://dx.doi.org/10.2164/jandrol.111.014225 - SOUZA, D.B., SILVA, D., SILVA, C.M.C., SAMPAIO, F.J.B., COSTA, W.S. and CORTEZ, C.M., 2011. Effects of immobilization stress on kidneys of Wistar male rats: a morphometrical and stereological analysis. Kidney & Blood Pressure Research, vol. 34, no. 6, pp. 424-429. http://dx.doi.org/10.1159/000328331 PMid:21709423.
» http://dx.doi.org/10.1159/000328331 - SRIVASTAVA, S. and BOYER, J.L., 2010. Psychological stress is associated with relapse in type 1 autoimmune hepatitis. Liver International, vol. 30, no. 10, pp. 1439-1447. http://dx.doi.org/10.1111/j.1478-3231.2010.02333.x PMid:20849437.
» http://dx.doi.org/10.1111/j.1478-3231.2010.02333.x - TANGSRISAKDA, N. and IAMSAARD, S., 2020. Effect of ethanol on the changes in testicular protein expression in adult male rats. Andrologia, vol. 52, no. 10, pp. e13784. http://dx.doi.org/10.1111/and.13784 PMid:32721052.
» http://dx.doi.org/10.1111/and.13784 - TONGPAN, S., SUKHORUM, W., ARUN, S., SAWATPHANICH, T. and IAMSAARD, S., 2019. Valproic acid changes the expression of tyrosine-phosphorylated proteins in rat seminal vesicle. Andrologia, vol. 51, no. 7, pp. e13303. http://dx.doi.org/10.1111/and.13303 PMid:31033021.
» http://dx.doi.org/10.1111/and.13303 - TSEILIKMAN, V., KOZOCHKIN, D., SYNITSKY, A., SIBIRIAK, S., TSEILIKMAN, O., KATASHINSKY, E., and SIMBIRTSEV, A., 2012. Does stress‐induced release of interleukin‐1 cause liver injury?.Cellular and Molecular Neurobiology, vol. 32, no. 7, pp. 1069–1078. doi: 10.1007/s10571-012-9866-7.
» https://doi.org/doi: 10.1007/s10571-012-9866-7 - UCHIDA, K., SUZUKI, K., IWAMOTO, M., KAWACHI, H., OHNO, M., HORITA, S. and NITTA, K., 2008. Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney International, vol. 73, no. 8, pp. 926-932. http://dx.doi.org/10.1038/ki.2008.19 PMid:18256598.
» http://dx.doi.org/10.1038/ki.2008.19 - WANG, W., BELOSAY, A., YANG, X., HARTMAN, J.A., SONG, H., IWANIEC, U.T., TURNER, R.T., CHURCHWELL, M.I., DOERGE, D.R. and HELFERICH, W.G., 2016. Effects of letrozole on breast cancer micro-metastatic tumor growth in bone and lung in mice inoculated with murine 4T1 cells. Clinical & Experimental Metastasis, vol. 33, no. 5, pp. 475-485. http://dx.doi.org/10.1007/s10585-016-9792-z PMid:27209469.
» http://dx.doi.org/10.1007/s10585-016-9792-z - WOLF, J.H., BHATTI, T.R., FOURASCHEN, S., CHAKRAVORTY, S., WANG, L., KURIAN, S., SALOMON, D., OLTHOFF, K.M., HANCOCK, W.W. and LEVINE, M.H., 2014. Heat shock protein 70 is required for optimal liver regeneration after partial hepatectomy in mice. Liver Transplantation, vol. 20, no. 3, pp. 376-385. http://dx.doi.org/10.1002/lt.23813 PMid:24357103.
» http://dx.doi.org/10.1002/lt.23813 - XUN, W., SHI, L., CAO, T., ZHAO, C., YU, P., WANG, D., HOU, G. and ZHOU, H., 2015. Dual functions in response to heat stress and spermato- genesis: characterization of expression profile of small heat shock proteins 9 and 10 in goat testis. BioMed Research International, vol. 2015, pp. 686239. http://dx.doi.org/10.1155/2015/686239 PMid:25685801.
» http://dx.doi.org/10.1155/2015/686239 - ZHANG, J., BROWN, R.P., SHAW, M., VAIDYA, V.S., ZHOU, Y., ESPANDIARI, P., SADRIEH, N., STRATMEYER, M., KEENAN, J., KILTY, C.G., BONVENTRE, J.V. and GOERING, P.L., 2008. Immunolocalization of Kim-1, RPA-1, and RPA-2 in kidney of gentamicin-, mercury-, or chromium-treated rats: relationship to renal distributions of iNOS and nitrotyrosine. Toxicologic Pathology, vol. 36, no. 3, pp. 397-409. http://dx.doi.org/10.1177/0192623308315832 PMid:18441258.
» http://dx.doi.org/10.1177/0192623308315832
Publication Dates
-
Publication in this collection
18 Feb 2022 -
Date of issue
2024
History
-
Received
27 July 2021 -
Accepted
30 Dec 2021