Abstract
Due to extensive application of antibiotics as growth promoters in animal feed, antimicrobial resistance has been increased. To overcome this challenge, rumen microbiologists search for new probiotics to improve the rate of livestock production. The present study was aimed to isolate and evaluate breed-specific lactic acid bacteria (LAB) as potential animal probiotics. The current study was conducted during 10 months from July 2020 to April 2021, in which a total of n=12 strains were isolated from different samples including milk, rumen, and feces of Nilli Ravi Buffaloes. These isolates were evaluated for their antimicrobial potential against common animal pathogens (Bacillus spp., E. coli, Staphylococcus aureus, Salmonella spp., Listeria spp.). All the isolates were identified using 16S rRNA gene sequencing and the phylogenetic analyses inferred that these strains showed close relations to the species of various genera; Enterococcus lactis, Pediococcus pentosaceus, Bacillus subtilis Weissella cibaria, Weissella soli, Bacillus tequilensis, Weissella bombi, Bacillus licheniformis, Lactococcus lactis, Bacillus megaterium, Lactobacillus ruminis, and Lactococcus lactis. NMCC-Ru2 has exhibited the enormous potential of antimicrobial activity, 28 mm, for Salmonella typhimurium;23 mm for Listeria monocytogenes 21 mm for E.coil. Highest resistance was seen in NMCC-Ru2 agasint test antbiotic, like 25.5 mm for Tetracycline. Overall results revesl that the probiotic profile of isolates was achieved using standard criteria, particularly with animal probiotic properties
Keywords:
antibiotic resistance; probiotics; 16S rRNA gene sequencing; lactic acid bacteria (LAB)
Resumo
Devido à extensa aplicação de antibióticos como promotores de crescimento na alimentação animal, a resistência aos antimicrobianos aumentou. Para superar esse desafio, os microbiologistas do rúmen buscam novos probióticos para melhorar a produtividade do gado. O presente estudo teve como objetivo isolar e avaliar bactérias lácticas específicas de raças (BAL) como potenciais probióticos animais. 12 cepas foram isoladas de diferentes amostras, incluindo leite, rúmen e fezes de búfalos Nilli Ravi. Esses isolados foram avaliados quanto ao seu potencial antimicrobiano contra patógenos animais comuns (Bacillus spp., E. coli, Staphylococcus aureus, Salmonella spp., Listeria spp.). Todos os isolados foram identificados por meio do sequenciamento do gene 16S rRNA e as análises filogenéticas inferiram que essas cepas apresentaram estreita relação com as espécies de vários gêneros; Enterococcus lactis, Pediococcus pentosaceus, Bacillus subtilis, Weissella cibaria, Weissella soli, Bacillus tequilensis, Weissella bombi, Bacillus licheniformis, Lactococcus lactis, Bacillus megaterium, Lactobacillus ruminis e Lactococcus lactis. O perfil probiótico dos isolados foi obtido usando critérios padrão, particularmente com propriedades probióticas animais.
Palavras-chave:
resistência a antibióticos; probióticos; sequenciamento do gene 16S rRNA; bactéria láctica (LAB)
1. Introduction
In Pakistan, 23.2% of the GDP (gross domestic product) is earned by agriculture, hence, considered as a principal sector of the economy. Livestock (a foremost sub-sector of agriculture) plays a vital role in improving the quality of food and enhancing the export earnings that make our economy strong. But still per animal production, income and social condition of animal breeder are unsatisfactory. Due to all these reasons, some public health issues such as malnutrition, etc arise; (Casewell et al., 2003CASEWELL, M., FRIIS, C., MARCO, E., MCMULLIN, P. and PHILLIPS, I., 2003. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. The Journal of Antimicrobial Chemotherapy, vol. 52, no. 2, pp. 159-161. http://dx.doi.org/10.1093/jac/dkg313. PMid:12837737.
http://dx.doi.org/10.1093/jac/dkg313...
). So, animal growth promoters are used to avoid these challenges as well as to enhance animal production. The extensive use of these antimicrobial agents is associated with the emerging resistance among bacterial population which ultimately develops health crises globally. To meet the demands of local markets, Livestock sector is looking for an alternate to these antibiotics that could be used safely. In lactating animals, milk production, weight gain, and nutrient digestibility depend on microbial population present in their gastrointestinal tract (GIT) (Jami and Mizrahi, 2012JAMI, E. and MIZRAHI, I., 2012. Composition and similarity of bovine rumen microbiota across individual animals. PLoS One, vol. 7, no. 3, pp. e33306. http://dx.doi.org/10.1371/journal.pone.0033306. PMid:22432013.
http://dx.doi.org/10.1371/journal.pone.0...
). It is recommended to improve microbial balance in GIT by the application of microbial growth promoters (probiotics) (Dowarah et al., 2017DOWARAH, R., VERMA, A. and AGARWAL, N., 2017. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: a review. Animal Nutrition, vol. 3, no. 1, pp. 1-6. http://dx.doi.org/10.1016/j.aninu.2016.11.002. PMid:29767055.
http://dx.doi.org/10.1016/j.aninu.2016.1...
).
Probiotic is an array of microorganisms showing health beneficial effects directly by reducing the prevalence of diseases and by increasing resistance towards intestinal pathogens. Probiotics secret various compounds such as hydrogen peroxide, bacteriocins, and organic acids to inhibit the growth of pathogenic strains in host (Garcia-Gutierrez et al., 2019GARCIA-GUTIERREZ, E., MAYER, M.J., COTTER, P.D. and NARBAD, A., 2019. Gut microbiota as a source of novel antimicrobials. Gut Microbes, vol. 10, no. 1, pp. 1-21. http://dx.doi.org/10.1080/19490976.2018.1455790. PMid:29584555.
http://dx.doi.org/10.1080/19490976.2018....
). Several microorganisms have been used as probiotics (Grochowska et al., 2019GROCHOWSKA, M., LASKUS, T. and RADKOWSKI, M., 2019. Gut microbiota in neurological disorders. Archivum Immunologiae et Therapiae Experimentalis, vol. 67, no. 6, pp. 375-383. http://dx.doi.org/10.1007/s00005-019-00561-6. PMid:31578596.
http://dx.doi.org/10.1007/s00005-019-005...
). The utmost common ruminant probiotic products available in market are bacterial probiotics (lactic acid bacteria (LAB), Bacillus, Bifidobacterium, Propionibacterium, and yeast probiotics (Saccharomyces cerevisiae) have been applied as feed supplements in adult ruminants (Ghazanfar, 2016GHAZANFAR, S., 2016. Study on the effect of dietary supplementation of saccharomyces cerevisiae on performance of dairy cattle and heifers. Pakistan: Quaid-i-Azam University Islamabad.; Uyeno et al., 2015UYENO, Y., SHIGEMORI, S. and SHIMOSATO, T., 2015. Effect of probiotics/prebiotics on cattle health and productivity. Microbes and Environments, vol. 30, no. 2, pp. 126-132. http://dx.doi.org/10.1264/jsme2.ME14176. PMid:26004794.
http://dx.doi.org/10.1264/jsme2.ME14176...
). Among LAB, Lactobacillus, L. acidophilus, L. plantarum, L. casei, are used (Emmanuel et al., 2007EMMANUEL, D., JAFARI, A., BEAUCHEMIN, K., LEEDLE, J. and AMETAJ, B., 2007. Feeding live cultures of Enterococcus faecium and Saccharomyces cerevisiae induces an inflammatory response in feedlot steers. Journal of Animal Science, vol. 85, no. 1, pp. 233-239. http://dx.doi.org/10.2527/jas.2006-216. PMid:17179561.
http://dx.doi.org/10.2527/jas.2006-216...
; Peterson et al., 2007PETERSON, C., RUCH, W., BEERMANN, U., PARK, N. and SELIGMAN, M.E., 2007. Strengths of character, orientations to happiness, and life satisfaction. The Journal of Positive Psychology, vol. 2, no. 3, pp. 149-156. http://dx.doi.org/10.1080/17439760701228938.
http://dx.doi.org/10.1080/17439760701228...
; Qadis et al., 2014QADIS, A.Q., GOYA, S., IKUTA, K., YATSU, M., KIMURA, A., NAKANISHI, S. and SATO, S., 2014. Effects of a bacteria-based probiotic on ruminal pH, volatile fatty acids, and bacterial flora of holstein calves. The Journal of Veterinary Medical Science, vol. 76, no. 6, pp. 877-885. http://dx.doi.org/10.1292/jvms.14-0028. PMid:24614603.
http://dx.doi.org/10.1292/jvms.14-0028...
; Stanford and Bottini, 2014STANFORD, S.M. and BOTTINI, N., 2014. PTPN22: the archetypal non-HLA autoimmunity gene. Nature Reviews. Rheumatology, vol. 10, no. 10, pp. 602-611. http://dx.doi.org/10.1038/nrrheum.2014.109. PMid:25003765.
http://dx.doi.org/10.1038/nrrheum.2014.1...
). Research showed that, the animal health and production can be improveby using the probiotic as feed supplements. In ruminants’ probiotics promote health by reducing acidosis, improving food digestion, eliminating pathogenic bacteria, and enhancing weight gain (Elghandour et al., 2020ELGHANDOUR, M.M.Y., TAN, Z.L., ABU HAFSA, S.H., ADEGBEYE, M.J., GREINER, R., UGBOGU, E.A., CEDILLO MONROY, J. and SALEM, A.Z.M., 2020. Saccharomyces cerevisiae as a probiotic feed additive to non and pseudo‐ruminant feeding: a review. Journal of Applied Microbiology, vol. 128, no. 3, pp. 658-674. http://dx.doi.org/10.1111/jam.14416. PMid:31429174.
http://dx.doi.org/10.1111/jam.14416...
). For maximum colonization probiotics must be isolated and fed to same host. The GIT of buffalo contains diverse beneficial bacteria that should be isolated and fed to gain maximum efficiency. Therefore, the current research was objectively designed to isolate and evaluate probiotic strains from buffalo gut for their possible application in wellbeing of animal health.
2. Materials and Methods
2.1. Specimens collection and identification
The current study was conducted during 10 months from July 2020 to April 2021 in which a total of n=30 specimens, including feces, milk and rumen, were aseptically collected in sterile tubes from different buffalo’s houses of Islamabad. All the collected specimens were taken to the laboratory via insulated boxes and stored at 4°C to process within 24 hours. 1ml of each specimen was serially diluted in 9ml of phosphate buffer saline solution (BPS) and allowed to homogenise for 1 minute. The dilution from 10-1 to 10-5 was inoculated on De Man Rogosa and Sharpe agar (MRS, Oxoid) and was allowed to incubate for 24-48 hours at 37°C (De Man et al., 1960DE MAN, J., ROGOSA, D. and SHARPE, M.E., 1960. A medium for the cultivation of lactobacilli. The Journal of Applied Bacteriology, vol. 23, no. 1, pp. 130-135. http://dx.doi.org/10.1111/j.1365-2672.1960.tb00188.x.
http://dx.doi.org/10.1111/j.1365-2672.19...
). After morphological identification, the colonies were then purified via sub-culturing and were further identified via biochemical tests including catalase, oxidase, etc. as prescribed by (Wali et al., 2021WALI, N., QAYYUM, H.T., WAHEED, F., ALI, G. and SHEHBAZ, M., 2021. Isolation and molecular characterization of exo-polysaccharide producing weissella confusa from buffalo ruminal gut. International Journal of Modern Agriculture, vol. 10, no. 3, pp. 35-42.).
2.2. Molecular identification of selected isolates
Bacterial DNA was extracted from pure isolates as followed and supported by the previous study (Naeem et al., 2018NAEEM, M., AHMED, I., AHMED, S., AHMED, Z., RIAZ, M. and GHAZANFAR, S., 2018. Screening of cattle gut associated Bacillus strains for their potential use as animal probiotic. Indian Journal of Animal Research, no. of. http://dx.doi.org/10.18805/ijar.B-948.
http://dx.doi.org/10.18805/ijar.B-948...
). The colony comprising of a single bacterial strain was suspended and mixed in micro-PCR strips containing 20µl tris EDTA buffer and was run in thermocycler machine at 95°C for 10 minutes. The resulting mixture was then centrifuged at 6000 rpm for 3 minutes and the obtained supernatant was used as DNA template whereas the pellet was discarded. The 16S rRNA gene present on DNA template was amplified in Polymerase Chain Reaction (PCR). The PCR conditions were set as, primary denaturation at 94°C for 2 minutes proceeded by 30 cycles of denaturation at 94°C for 1 min, the annealing at 50°C for 1 min, the extension at 72°C for 1.5 mins and the last extension at 72°C for 5 mins. The final PCR product was run along with 1kb ladder on agarose gel (0.8%) and was examined via gel documentation system. The desired bands were then sent for sequencing through commercial sequencing service Macrogen Inc. (Seoul Korea).
2.3. Growth determination at different temperature
The growth of isolated strains was observed at different temperatures as adapted by the previous study (Kavitha and Devasena, 2013KAVITHA, J. and DEVASENA, T., 2013. Isolation, characterization, determination of probiotic properties of lactic acid bacteria from human milk. OSR J Pharm Biol Sci, vol. 7, no. 3, pp. 1-7. http://dx.doi.org/10.9790/3008-0730107.
http://dx.doi.org/10.9790/3008-0730107...
). Briefly, 2ml of fresh culture was inoculated to each test tube containing 110ml MRS broth medium and were allowed to incubate at different temperatures including 6°C, 22°C, 30°C, 37°C, and 44°C. The ideal growth temperature was examined after 24-48 hours.
2.4. Phylogenetic analysis
Following gene sequencing of 16S rRNA, the complete sequenced data of bacterial strains were aligned using Clusta lX software. BioEdit software was applied for assembling the sequences of the 16S rRNA gene. The classification of bacterial isolates at species level was done using nBLAST search using GenBank internet service. For permitting public access to these probiotic strains. The 16S rRNA gene sequences were submitted to GenBank database (www.ncbi.nlm.nih.gov/projects/genome/clone/). To construct the phylogenetic tree, the Ez-BioCloud service was applied to retrieve the sequences of closely related bacterial strains. For molecular and phylogenetic evolutionary analysis of the bacterial strains, MEGA7 Molecular evolutionary Genetic Analysis software was utilized.
2.5. Probiotic characteristics of LAB isolates
The presumptive probiotic bacterial isolates were screened for tolerance to bile salt and acidic environment using MRS broth. The broth was concentrated in different tubes with different concentrations of bile salt such as 0.8, 1.3, 1.8, and 2.0% by adjusting pH 2, 3, and 7. Each tube containing MRS broth was inoculated with fresh culture of Lab isolates and was allowed to incubate at 37°C for 72 hours. After incubation, the resultant growth was inoculated on MRS agar and was allowed to incubate for 24 hours at 37°C. The next day, the grown colonies were counted (Lee et al., 2012LEE, K.W., PARK, J.Y., JEONG, H.R., HEO, H.J., HAN, N.S. and KIM, J.H., 2012. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe, vol. 18, no. 1, pp. 96-102. http://dx.doi.org/10.1016/j.anaerobe.2011.12.015. PMid:22200451.
http://dx.doi.org/10.1016/j.anaerobe.201...
).
The antimicrobial activity of the isolated strains was assessed by agar well diffusion method. Five pathogens E. coli, salmonella, listeria, Staphylococcus aureus, and Bacillus cereus were used in this method. The presumptive probiotic strains were inoculated in MRS broth (Oxide, UK) and incubated at 37ºC for 24 hours. After incubation, (cell density 108 cfu/mL the MRS broth culture was shifted into Eppendorf tubes. It was then centrifuged at 8000 rpm for 20 minutes and supernatant was collected, to obtain cell-free supernatant (CFS) it was then passed through a 0.22 mm syringe filter and was placed at 4ºC. Pathogens culture after dilution in PBS was properly spread on surface of MHA media and punctured well into media using 1000µl sterile pipette tip. 10µl soft agar was poured into the bottom of wells, then each well was inoculated with 20-35µl probiotic strains cell-free supernatant that was passed through a sterile filter syringe. The plates were incubated at 37°C for 24-48hrs in upright direction. The antimicrobial activity of isolated strains was interpreted by measuring its zone diameter (mm) using scale.
The susceptibility pattern of Lab isolates was examined via Kirby-Bauer disc diffusion method using Mueller Hinton Agar (MHA) (Bauer et al., 1966BAUER, A.W., KIRBY, W.M., SHERRIS, J.C. and TURCK, M., 1966. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology, vol. 45, no. 4, pp. 493-496. http://dx.doi.org/10.1093/ajcp/45.4_ts.493. PMid:5325707.
http://dx.doi.org/10.1093/ajcp/45.4_ts.4...
). Before inoculation, the isolated strains were adjusted up to 0.5 MacFarland index in phosphate buffer solution (BPS). The inoculum was then inoculated on MHA medium using sterilized cotton swabs and was allowed to dry. Commercially available antibiotics (Oxoid) were exposed and were allowed to overnight incubation for 37°C. After incubation, the zone of inhibition of each antibiotic against each bacterial strain was examined, measured and checked with disc diffusion chart for Lab, to obtain results as sensitive, intermediate and resistant (CLSI, 2016CLINICAL AND LABORATORY STANDARDS INSTITUTE – CLSI, 2016. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement. 26th ed. Wayne, Pa, USA: CLSI. CLSI Document M100S. vol 36.).
The proteolytic activity of each isolate was determined using skim milk agar. Fresh culture of Lab isolates was inoculated on skim milk agar and was allowed to incubate 37°C for 48 hours. After incubation, the plates were examined for translucent halos around the colonies (Pailin et al., 2001PAILIN, T., KANG, D., SCHMIDT, K. and FUNG, D., 2001. Detection of extracellular bound proteinase in EPS‐producing lactic acid bacteria cultures on skim milk agar. Letters in Applied Microbiology, vol. 33, no. 1, pp. 45-49. http://dx.doi.org/10.1046/j.1472-765X.2001.00954.x. PMid:11442814.
http://dx.doi.org/10.1046/j.1472-765X.20...
). For lipase activity, the presumptive probiotic strains were inoculated on amalgam of tween 80 medium, TSA medium and phenol red as elucidated by a previous study (Sierra, 1957SIERRA, G., 1957. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie van Leeuwenhoek, vol. 23, no. 1, pp. 15-22. http://dx.doi.org/10.1007/BF02545855. PMid:13425509.
http://dx.doi.org/10.1007/BF02545855...
). The petri dishes were incubated at 37°C for 48 hours. The culture plates were examined carefully for changing color from red to yellow-orange. The amylolytic activity of Lab isolate was screened using starch agar medium. The isolated strains were inoculated on starch agar medium in a straight line and were allowed for incubation at 37°C for overnight incubation. After incubation, the culture plates were flushed with 1%-gram iodine to observe the bright zones near the cultured lines (Bernfeld, 1955BERNFELD, P., 1955. Amylase assays. Methods in Enzymology, vol. 1, pp. 149-158. http://dx.doi.org/10.1016/0076-6879(55)01021-5.
http://dx.doi.org/10.1016/0076-6879(55)0...
).
Probiotic with haemolytic capability is assumed as disadvantage. LAB cultures were grown overnight, inoculated on petri dishes having nutrient agar (NA) media and 4% based agar (Hi media). The plates were incubated at 37°C for 48 hours. After incubation, the presence or absence of hydrolysis zone near the cultured colonies were observed (Naeem et al., 2018NAEEM, M., AHMED, I., AHMED, S., AHMED, Z., RIAZ, M. and GHAZANFAR, S., 2018. Screening of cattle gut associated Bacillus strains for their potential use as animal probiotic. Indian Journal of Animal Research, no. of. http://dx.doi.org/10.18805/ijar.B-948.
http://dx.doi.org/10.18805/ijar.B-948...
). The results for haemolytic activity were reported as α-haemolysis (slight hydrolysis involving appearance of green zones around the cultured isolates), β-haemolysis (formation of clean zones of hydrolysis around the colonies) and γ-haemolysis (without any change in the media).
3. Results and Discussion
3.1. Isolation and identification of potential probiotics
While being proposed as a probiotic, the comprehensive resemblance concerning the safety profile and functional property of bacterial strains is a critical stage particularly when the source of isolation is an animal (Naeem et al., 2018NAEEM, M., AHMED, I., AHMED, S., AHMED, Z., RIAZ, M. and GHAZANFAR, S., 2018. Screening of cattle gut associated Bacillus strains for their potential use as animal probiotic. Indian Journal of Animal Research, no. of. http://dx.doi.org/10.18805/ijar.B-948.
http://dx.doi.org/10.18805/ijar.B-948...
). Animal gut, and its milk are a rich source for the isolation of the unique probiotic strains.Nilli Ravi Buffalo is the black gold of Pakistan. The ruminal gut/milk of the aimal may contain many useful probiotic strains.
In the present study, 30 samples from different sources of water buffalo i.e., milk, rumen, and fecal were taken, serially diluted in PBS buffer, and then cultured aseptically on De Man, Rogosa, and Sharpe agar for selective growth of Lactobacilli. The subsequent sub-culturing of bacterial colonies produced pure cultures which were used for further research. Initially, the bacterial strains were identified using phenotypic methods. The gram reaction of the isolated bacterial strains was determined by observing it under a phase contrast microscope (Phase Contrast 2, Nikon, Japan) after performing gram staining, following the procedure recommended by the manufacturers. Lactic acid bacteria were observed as gram-positive, catalase-negative. 12 bacterial isolates were identified based on their morphology and biochemical properties namely, NMCC-Ru1, NMCC-M1, NMCC-Ru2, NMCC-f5, NMCC-Ru3, NMCC-f12, NMCC-f13, NMCC-f14, NMCC-f15, NMCC-f16, NMCC-f17, and NMCC-M16, all exhibited either coccus or rod shape and were further viewed under Scanning Electron Microscope (Mira 3, Tescan SEM). The ideal growth temperature for NMCC-Ru1, NMCC-M1, NMCC-Ru2, NMCC-f5, NMCC-Ru3 and NMCC-M16 was observed to be 37ºC, whereas for NMCC-f12, NMCC-f13, NMCC-f14, NMCC-f15, NMCC-f16 and NMCC-f17 was observed to be 30ºC (Table 1 and Figure 1).
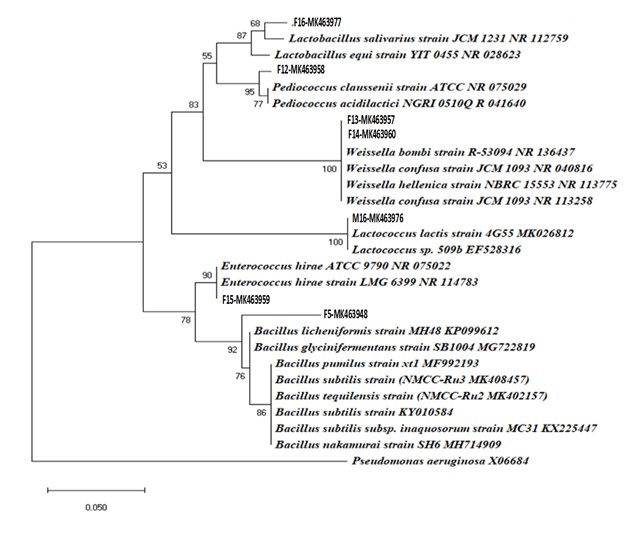
Probiotic starins identification is a basic step for the prepration of the unique probiotic. We used the 16 s rRNA gene sequences for the indentification of the microbial starins. Based on 16S rRNA, six major genera were molecularly identified including, species of Weissella, Lactococcus, Enterococcus, Bacillus, Lactobacillus, and Pediococcus. The bacterial species isolated from faecal sample were presumed to be; Bacillus megaterium (NMCC-f5), Pediococcus pentosaceus (NMCC-f12), Weissella bombi (NMCC-f13), Weissella cibaria (NMCC-f14), Enterococcus lactis (NMCC-f15), Lactobacillus ruminis (NMCC-f16), Weissella soli (NMCC-f17) and Weissella confuse (NMCC-M1). Three bacterial species were isolated from rumen including Bacillus licheniformis (NMCC-Ru1), Bacillus tequilensis (NMCC-Ru2) and Bacillus subtilis (NMCC-Ru3), whereas, Lactococcus lactis (NMCC-M16) was isolated from milk sample (Table 2 and Figure 2). Althoughlarge variety of bacterial strains that have been introduced as a probiotic in the market; however, the heavily claimed beneficial potential of such bacterial strains has not been evaluated in most cases. The autochthonous bacterial strains may prove more compatible with the animal gut microflora; therefore, investigation of the probiotic potential of new indigenous strains is indispensable (Naeem et al., 2018NAEEM, M., AHMED, I., AHMED, S., AHMED, Z., RIAZ, M. and GHAZANFAR, S., 2018. Screening of cattle gut associated Bacillus strains for their potential use as animal probiotic. Indian Journal of Animal Research, no. of. http://dx.doi.org/10.18805/ijar.B-948.
http://dx.doi.org/10.18805/ijar.B-948...
), i.e. Lactobacillus ruminis (MK463977), Pediococcus pentosaceus (MK463958), Weissella soli (MK463978). Weissella bombi (MK463957).
Phylogenetic tree of the bacterial isolates exhibiting the inter-relationship of most closely related type species inferred from 16S rRNA analysis
3.2. Acid tolerance
It is important to understand that once in transit through the gastrointestinal tract, resistance and survivability of probiotic bacteria extremely depend on bacterial strain. Furthermore, it is well established that each strain has its unique acid tolerance property. This exclusive property varies immensely between strains and species of lactic acid bacteria (Gu et al., 2008GU, R.X., YANG, Z.Q., LI, Z.H., CHEN, S.L. and LUO, Z.L., 2008. Probiotic properties of lactic acid bacteria isolated from stool samples of longevous people in regions of Hotan, Xinjiang and Bama, Guangxi, China. Anaerobe, vol. 14, no. 6, pp. 313-317. http://dx.doi.org/10.1016/j.anaerobe.2008.06.001. PMid:18634896.
http://dx.doi.org/10.1016/j.anaerobe.200...
). While selection of a good probiotic is an important criterion that it must be resistant to low pH. As it is difficult for microorganisms to sustain in unfavorable conditions (pH 2–3) of stomach during transport, where it takes 2-4 hours for the processing of food (Montoro et al., 2016MONTORO, B.P., BENOMAR, N., LAVILLA LERMA, L., CASTILLO GUTIÉRREZ, S., GÁLVEZ, A. and ABRIOUEL, H., 2016. Fermented Aloreña table olives as a source of potential probiotic Lactobacillus pentosus strains. Frontiers in Microbiology, vol. 7, pp. 1583. http://dx.doi.org/10.3389/fmicb.2016.01583. PMid:27774088.
http://dx.doi.org/10.3389/fmicb.2016.015...
). All the tested bacterial strains in this study exhibited stability at pH 2 for 2-3 hours deprived of showing any substantial decline in viability. Our results supported by the previous two studies as they had observed survivability of probiotic bacteria at pH 3 for 2.5 hours (Lee et al., 2012LEE, K.W., PARK, J.Y., JEONG, H.R., HEO, H.J., HAN, N.S. and KIM, J.H., 2012. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe, vol. 18, no. 1, pp. 96-102. http://dx.doi.org/10.1016/j.anaerobe.2011.12.015. PMid:22200451.
http://dx.doi.org/10.1016/j.anaerobe.201...
; Patel et al., 2014PATEL, A., PRAJAPATI, J., HOLST, O. and LJUNGH, A., 2014. Determining probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from vegetables and traditional Indian fermented food products. Food Bioscience, vol. 5, pp. 27-33. http://dx.doi.org/10.1016/j.fbio.2013.10.002.
http://dx.doi.org/10.1016/j.fbio.2013.10...
). This huge acid tolerance capacity of bacterial isolates might be relying upon the source and H+-ATPase activity (Matsumoto et al., 2004MATSUMOTO, M., OHISHI, H. and BENNO, Y., 2004. H+-ATPase activity in Bifidobacterium with special reference to acid tolerance. International Journal of Food Microbiology, vol. 93, no. 1, pp. 109-113. http://dx.doi.org/10.1016/j.ijfoodmicro.2003.10.009. PMid:15135587.
http://dx.doi.org/10.1016/j.ijfoodmicro....
).
3.3. Antimicrobial susceptibility assay
The bacterial strains have displayed various spectrum of antagonistic properties towards foodborne bacteria, NMCC-Ru2 has shown the strongest antibacterial action towards Salmonella typhimurium followed by NMCC-f12, NMCC-M1 and NMCC-Ru1 (Table 3 and Figure 3). The high antimicrobial mechanism against Listeria monocytogenes was displayed by NMCC-f12, NMCC-Ru2, NMCC-f16, NMCC-f13 and temperate activity by NMCC-M16. NMCC-Ru7 has elicited the strongest competitive exclusion mechanism against Staphylococcus aureus, while NMCC-M1 displayed moderate activity. In case of Escherichia coli, Ru7 exhibited potent antibacterial activity followed by NMCC-f5, NMCC-f13, NMCC-f14 and NMCC-f15. Overall, NMCC-Ru2 displayed maximum competitive inhibition against the tested pathogenic strains. This conclusion corresponded with the previous research; Bacillus tequilensis has been revealed to exhibit high antilisterial activity and purification of the antilisterial peptide has revealed it to be subtilisin A (Parveen Rani et al., 2016PARVEEN RANI, R., ANANDHARAJ, M., HEMA, S., DEEPIKA, R. and DAVID RAVINDRAN, A., 2016. Purification of antilisterial peptide (Subtilosin A) from novel Bacillus tequilensis FR9 and demonstrate their pathogen invasion protection ability using human carcinoma cell line. Frontiers in Microbiology, vol. 7, pp. 1910. http://dx.doi.org/10.3389/fmicb.2016.01910. PMid:27990138.
http://dx.doi.org/10.3389/fmicb.2016.019...
). One possible mechanism for showing excellent antibacterial activity from presumptive probiotic bacteria might be the secretion of some useful antimicrobial compounds such as niacin, bacteriocin, and lactic acid (Fisher and Phillips, 2009FISHER, K. and PHILLIPS, C., 2009. The ecology, epidemiology and virulence of Enterococcus. Microbiology, vol. 155, no. Pt 6, pp. 1749-1757. http://dx.doi.org/10.1099/mic.0.026385-0. PMid:19383684.
http://dx.doi.org/10.1099/mic.0.026385-0...
). The usage of bacteriocins in food items acts against pathogens and prolongs their shelf life, therefore the production of bacteriocins is considered an invaluable probiotic feature (Yang et al., 2014YANG, S.C., LIN, C.H., SUNG, C.T. and FANG, J.Y., 2014. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Frontiers in Microbiology, vol. 5, pp. 241. PMid:24904554.). In addition, bacteriocins have a key role as auspicious alternatives to fight against emergent resistance shown by microorganisms (Cotter et al., 2013COTTER, P.D., ROSS, R.P. and HILL, C., 2013. Bacteriocins: a viable alternative to antibiotics? Nature Reviews. Microbiology, vol. 11, no. 2, pp. 95-105. http://dx.doi.org/10.1038/nrmicro2937. PMid:23268227.
http://dx.doi.org/10.1038/nrmicro2937...
; Hammami et al., 2013HAMMAMI, R., FERNANDEZ, B., LACROIX, C. and FLISS, I., 2013. Anti-infective properties of bacteriocins: an update. Cellular and Molecular Life Sciences, vol. 70, no. 16, pp. 2947-2967. http://dx.doi.org/10.1007/s00018-012-1202-3. PMid:23109101.
http://dx.doi.org/10.1007/s00018-012-120...
). Overall, NMCC-Ru2 has exhibited the enormous potential of antimicrobial activity against the tested pathogens.
The antimicrobial susceptibility spectrum of bacterial isolates against pathogens and their inhibitory zones diameter (mm).
3.4. Antibiotic resistance profile
Bacterial strains were tested using the procedure provided by Clinical and Laboratory Standards Institute (CLSI, 2016CLINICAL AND LABORATORY STANDARDS INSTITUTE – CLSI, 2016. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement. 26th ed. Wayne, Pa, USA: CLSI. CLSI Document M100S. vol 36.) for their resistance towards commonly used antibiotics. The antibiotics used in this research included Tetracycline, Ampicillin, Kanamycin, Chloramphenicol, Streptomycin and Gentamycin. The sensitivity of bacterial isolates against antibiotics can be seen in Table 4. Various patterns of resistance had shown by the isolated bacterial strains against the tested antibiotics. The indiscriminate use of antibiotics as growth promoters in human and veterinary medicine leads to the production of drug-resistant strains among microorganisms (Robredo et al., 2000ROBREDO, B., SINGH, K.V., BAQUERO, F., MURRAY, B.E. and TORRES, C., 2000. Vancomycin-resistant enterococci isolated from animals and food. International Journal of Food Microbiology, vol. 54, no. 3, pp. 197-204. http://dx.doi.org/10.1016/S0168-1605(99)00195-6. PMid:10777070.
http://dx.doi.org/10.1016/S0168-1605(99)...
). Strong susceptibility had shown by NMCC-M16 towards all the selected antibiotics, while for ampicillin it showed intermediate resistance previous finding is correspondent with the susceptibility spectrum of Lactococcus lactis (Khemariya et al., 2013KHEMARIYA, P., SINGH, S., NATH, G. and GULATI, A.K., 2013. Isolation, identification and antibiotic susceptibility of nis+ Lactococcus lactis from dairy and non-dairy sources. Czech Journal of Food Sciences, vol. 31, no. 4, pp. 323-331. http://dx.doi.org/10.17221/316/2012-CJFS.
http://dx.doi.org/10.17221/316/2012-CJFS...
; Liasi et al., 2009LIASI, S., AZMI, T., HASSAN, M., SHUHAIMI, M., ROSFARIZAN, M. and ARIFF, A., 2009. Antimicrobial activity and antibiotic sensitivity of three isolates of lactic acid bacteria from fermented fish product, Budu. Malaysian Journal of Microbiology, vol. 5, no. 1, pp. 33-37.; Ozdogan et al., 2012OZDOGAN, D.K., AKCELIK, N., ASLIM, B., SULUDERE, Z. and AKCELIK, M., 2012. Probiotic and antioxidative properties of L. lactis LL27 isolated from milk. Biotechnology, Biotechnological Equipment, vol. 26, no. 1, pp. 2750-2758. http://dx.doi.org/10.5504/BBEQ.2011.0091.
http://dx.doi.org/10.5504/BBEQ.2011.0091...
). In contrast NMCC-f13, NMCC-f17 and NMCC-M1 are sensitive to Chloramphenicol, Gentamycin, Kanamycin and Tetracycline, but mainly resistant to ampicillin and streptomycin. These figures were in accordance with previously cited literature (Lee et al., 2012LEE, K.W., PARK, J.Y., JEONG, H.R., HEO, H.J., HAN, N.S. and KIM, J.H., 2012. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe, vol. 18, no. 1, pp. 96-102. http://dx.doi.org/10.1016/j.anaerobe.2011.12.015. PMid:22200451.
http://dx.doi.org/10.1016/j.anaerobe.201...
) excluding the statistic that they described Weissella strains are sensitive towards ampicillin; whereas NMCC-f14 displayed resistance against ampicillin, gentamycin, kanamycin and streptomycin and sensitivity to tetracycline and chloramphenicol. Various pattern of resistance towards ampicillin and sensitivity against tetracycline, chloramphenicol and gentamycin was shown by NMCC-Ru1, NMCC-Ru2, NMCC-Ru3 and NMCC-f5. Yet NMCC-Ru1 and NMCC-f5 showed resistance towards streptomycin and kanamycin; NMCC-Ru2 and NMCC-Ru3 have displayed sensitivity for these two drugs. Likewise, NMCC-f16 was sensitive against all the antibiotics used in this study but it has shown intermediate resistance for ampicillin. The antibiotic resistance spectrum exhibited by Lactobacillus and Bacillus species was also reported by different researchers (Anandharaj et al., 2015ANANDHARAJ, M., SIVASANKARI, B., SANTHANAKARUPPU, R., MANIMARAN, M., RANI, R.P. and SIVAKUMAR, S., 2015. Determining the probiotic potential of cholesterol-reducing Lactobacillus and Weissella strains isolated from gherkins (fermented cucumber) and south Indian fermented koozh. Research in Microbiology, vol. 166, no. 5, pp. 428-439. http://dx.doi.org/10.1016/j.resmic.2015.03.002. PMid:25839996.
http://dx.doi.org/10.1016/j.resmic.2015....
; Nguyen et al., 2015NGUYEN, A.T.V., NGUYEN, D.V., TRAN, M.T., NGUYEN, L.T., NGUYEN, A.H. and PHAN, T.N., 2015. Isolation and characterization of B acillus subtilis CH 16 strain from chicken gastrointestinal tracts for use as a feed supplement to promote weight gain in broilers. Letters in Applied Microbiology, vol. 60, no. 6, pp. 580-588. http://dx.doi.org/10.1111/lam.12411. PMid:25754534.
http://dx.doi.org/10.1111/lam.12411...
; Parveen Rani et al., 2016PARVEEN RANI, R., ANANDHARAJ, M., HEMA, S., DEEPIKA, R. and DAVID RAVINDRAN, A., 2016. Purification of antilisterial peptide (Subtilosin A) from novel Bacillus tequilensis FR9 and demonstrate their pathogen invasion protection ability using human carcinoma cell line. Frontiers in Microbiology, vol. 7, pp. 1910. http://dx.doi.org/10.3389/fmicb.2016.01910. PMid:27990138.
http://dx.doi.org/10.3389/fmicb.2016.019...
). NMCC-f15 has shown to be more sensitive against applied antibiotics excluding ampicillin (Braïek et al., 2018BRAÏEK, O.B., MORANDI, S., CREMONESI, P., SMAOUI, S., HANI, K. and GHRAIRI, T., 2018. Biotechnological potential, probiotic and safety properties of newly isolated enterocin-producing Enterococcus lactis strains. LWT, vol. 92, pp. 361-370. http://dx.doi.org/10.1016/j.lwt.2018.02.045.
http://dx.doi.org/10.1016/j.lwt.2018.02....
). NMCC-f12 demonstrated sensitivity to chloramphenicol, gentamycin, tetracycline and resistance to ampicillin, kanamycin, and streptomycin (Cao et al., 2016CAO, Z., PAN, H., TONG, H., GU, D., LI, S., XU, Y., GE, C. and LIN, Q., 2016. In vitro evaluation of probiotic potential of Pediococcus pentosaceus L1 isolated from paocai: a Chinese fermented vegetable. Annals of Microbiology, vol. 66, no. 3, pp. 963-971. http://dx.doi.org/10.1007/s13213-015-1182-2.
http://dx.doi.org/10.1007/s13213-015-118...
; Cavicchioli et al., 2019CAVICCHIOLI, V.Q., CAMARGO, A.C., TODOROV, S.D. and NERO, L.A., 2019. Potential control of Listeria monocytogenes by bacteriocinogenic Enterococcus hirae ST57ACC and pediococcus pentosaceus ST65ACC strains isolated from artisanal cheese. Probiotics and Antimicrobial Proteins, vol. 11, no. 2, pp. 696-704. http://dx.doi.org/10.1007/s12602-018-9449-0. PMid:30069686.
http://dx.doi.org/10.1007/s12602-018-944...
; Fernandes et al., 2018FERNANDES, M.L., PERIN, L.M., TODOROV, S.D., NERO, L.A., DE ALENCAR, E.R. and FERREIRA, M.D.A., 2018. In vitro evaluation of the safety and probiotic and technological potential of Pediococcus pentosaceus isolated from sheep milk. Semina: Ciências Agrárias, vol. 39, no. 1, pp. 113-132. http://dx.doi.org/10.5433/1679-0359.2018v39n1p113.
http://dx.doi.org/10.5433/1679-0359.2018...
), whether antibiotic resistance among putative probiotic strains is a desirable attribute or not is an extremely controversial subject. Some researchers have the opinion that probiotic bacteria having antibiotic resistance is mainly dangerous as they can pass these resistance determinants to the host gut microflora (Angmo et al., 2016ANGMO, K., KUMARI, A., SAVITRI. and BHALLA, T.C., 2016. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. Lebensmittel-Wissenschaft + Technologie, vol. 66, pp. 428-435. http://dx.doi.org/10.1016/j.lwt.2015.10.057.
http://dx.doi.org/10.1016/j.lwt.2015.10....
). Whereas, others claim that it is useful as the antibiotics along with probiotics may enhance the healthy microflora that was worn during antibiotic treatments.
Antibiotic resistance profiles of isolated strains against commonly used antibacterial compounds.
3.5. Lipolytic activity screening
Lipases hold much attention since they involve in a wide range of reactions; moreover, lipases play a significant role in many industrial processes including food industry in enhancing flavour, texture and aroma of products. Hence, lactic acid bacterial strains having lipolytic potential are of vital importance (Lopes et al., 2002LOPES, M.F.S., LEITÃO, A.L., REGALLA, M., FIGUEIREDO MARQUES, J.J., CARRONDO, M.J.T. and CRESPO, M.T.B., 2002. Characterization of a highly thermostable extracellular lipase from Lactobacillus plantarum. International Journal of Food Microbiology, vol. 76, no. 1-2, pp. 107-115. http://dx.doi.org/10.1016/S0168-1605(02)00013-2. PMid:12038566.
http://dx.doi.org/10.1016/S0168-1605(02)...
). The selected bacterial strains had shown variable responses to in vitro screening of lipase production. NMCC-M1, NMCC-f15, NMCC-f16 and NMCC-M16 had shown strong in vitro lipolytic activity which has been documented before. Whereas NMCC-f17, NMCC-f14, NMCC-f13, and NMCC-f12 also bear lipase activity as seen in other studies. While all of the putative probiotic strains had shown considerable lipolytic potential NMCC-RU1, NMCC-RU2, NMCC-RU3, and NMCC-f5 are an exception and this also concurs with previous studies (Table 5 and Figure 4).
Enzymatic Potential of P. pentosaceus SPARC2: (A) Amylolytic (B) Lipolytic (C) Proteolytic.
3.6. Screening for proteolytic potential
Proteolytic arsenal by LAB is not only crucial for their growth in milk but it also has a key role in the development of taste and aroma in fermented foods (Siezen, 1999SIEZEN, R.J., 1999. Multi-domain, cell-envelope proteinases of lactic acid bacteria. Lactic Acid Bacteria: Genetics, Metabolism and Applications. Springer.). Since the milk products do not contain a sufficient amount of low molecular peptides and amino acids which are necessary for LAB growth .To hydrolyse milk proteins into free amino acids s an active system of proteases is required (de Souza and Dias, 2017DE SOUZA, J.V. and DIAS, F.S., 2017. Protective, technological, and functional properties of select autochthonous lactic acid bacteria from goat dairy products. Current Opinion in Food Science, vol. 13, pp. 1-9. http://dx.doi.org/10.1016/j.cofs.2017.01.003.
http://dx.doi.org/10.1016/j.cofs.2017.01...
; Leboš Pavunc et al., 2012LEBOŠ PAVUNC, A., BEGANOVIĆ, J., KOS, B., UROIĆ, K., BLAŽIĆ, M. and ŠUŠKOVIĆ, J., 2012. Characterization and application of autochthonous starter cultures for fresh cheese production. Food Technology and Biotechnology, vol. 50, no. 2, pp. 141-151.). Impressively, all the LAB strains tested in this study have exhibited strong proteolytic properties with NMCC-RU1, NMCC-RU2, NMCC-R3, NMCC-f5, NMCC-f12, NMCC-f13, NMCC-f14, NMCC-M16, and NMCC-M1 displaying the highest potential subsequently followed by NMCC-f17, NMCC-f15, and NMCC-f16 (Table 5 and Figure 4).
3.7. Screening for amylolytic potential
Amylolytic Lactic acid bacteria (ALAB) have exceptional significance because they produce alpha amylases and lactic acid by modifying the structural properties of starch and are therefore used for a broad spectrum of industrial applications (Sundarram and Murthy, 2014SUNDARRAM, A. and MURTHY, T.P.K., 2014. α-amylase production and applications: a review. Journal of Applied & Environmental Microbiology, vol. 2, no. 2, pp. 166-175.). With the prevention of chemical oxidants like potassium bromate (used as a bread improver), screening and selection of suitable amylase-producing strains is required (Amapu et al., 2016AMAPU, T., AMEH, J., ADO, S., ABDULLAHI, I. and DAPIYA, H., 2016. Amylolytic potential of lactic acid bacteria isolated from wet milled cereals, cassava flour and fruits. Microbiology Research Journal International, vol. 5, pp. 1-8.). All the selected LAB strains had shown maximum amylolytic activity with NMCC-M1, NMCC-f5, NMCC-f12, NMCC-f13, NMCC-f14, NMCC-f16, NMCC-f17, NMCC-M16, NMCC-RU1, NMCC-RU2 and NMCC-RU3 whereas NMCC-f15 had also been able for hydrolysis of starch substrate with considerable potential. The amylolytic potential spectrum has been given in Table 5 and Figure 4.
3.8. Screening of haemolysin production
The most crucial characteristic of a probiotic strain is the absence of virulence traits. Production of hemolysin has to be screened progressively for the selection of a probiotic strain. The tested LAB strains exhibited negative results for the production of both α-and β-hemolysis.
4. Conclusion
The frequent use of antibiotics as animal growth promoter is causing huge anxiety, to overcome this problem, microbiologists are trying to introduce new alternatives agents having least adverse reactions on host health and production. According to present research, live microbial feed supplements (probiotics) could be used as an best perfromaing alternative of antibiotics if they are isolated and fed to the same host. This is the first report on isolation and evaluation of probiotics from Nili Ravi buffalo in Pakistan. Our work exposed that LAB isolated from buffalo gut were tolerated to acid and bile condition, showed excellent probiotic potentials and antagonistic activity towards tested animal pathogens. This research revealed that the isolated probiotics may have a role in balancing the stability between pathogenic bacteria and normal microflora of animals. This study has suggested that probiotics in animal feed can reduce certain enteric infections like diarrhoea, food indigestion, gastroenteritis, and rumen acidosis.
Acknowledgements
Authors appreciate Researchers Support Project number (RSP-2021/293) King Saud University, Riyadh, Saudi Arabia for support.
References
- AMAPU, T., AMEH, J., ADO, S., ABDULLAHI, I. and DAPIYA, H., 2016. Amylolytic potential of lactic acid bacteria isolated from wet milled cereals, cassava flour and fruits. Microbiology Research Journal International, vol. 5, pp. 1-8.
- ANANDHARAJ, M., SIVASANKARI, B., SANTHANAKARUPPU, R., MANIMARAN, M., RANI, R.P. and SIVAKUMAR, S., 2015. Determining the probiotic potential of cholesterol-reducing Lactobacillus and Weissella strains isolated from gherkins (fermented cucumber) and south Indian fermented koozh. Research in Microbiology, vol. 166, no. 5, pp. 428-439. http://dx.doi.org/10.1016/j.resmic.2015.03.002 PMid:25839996.
» http://dx.doi.org/10.1016/j.resmic.2015.03.002 - ANGMO, K., KUMARI, A., SAVITRI. and BHALLA, T.C., 2016. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. Lebensmittel-Wissenschaft + Technologie, vol. 66, pp. 428-435. http://dx.doi.org/10.1016/j.lwt.2015.10.057
» http://dx.doi.org/10.1016/j.lwt.2015.10.057 - BAUER, A.W., KIRBY, W.M., SHERRIS, J.C. and TURCK, M., 1966. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology, vol. 45, no. 4, pp. 493-496. http://dx.doi.org/10.1093/ajcp/45.4_ts.493 PMid:5325707.
» http://dx.doi.org/10.1093/ajcp/45.4_ts.493 - BERNFELD, P., 1955. Amylase assays. Methods in Enzymology, vol. 1, pp. 149-158. http://dx.doi.org/10.1016/0076-6879(55)01021-5
» http://dx.doi.org/10.1016/0076-6879(55)01021-5 - BRAÏEK, O.B., MORANDI, S., CREMONESI, P., SMAOUI, S., HANI, K. and GHRAIRI, T., 2018. Biotechnological potential, probiotic and safety properties of newly isolated enterocin-producing Enterococcus lactis strains. LWT, vol. 92, pp. 361-370. http://dx.doi.org/10.1016/j.lwt.2018.02.045
» http://dx.doi.org/10.1016/j.lwt.2018.02.045 - CAO, Z., PAN, H., TONG, H., GU, D., LI, S., XU, Y., GE, C. and LIN, Q., 2016. In vitro evaluation of probiotic potential of Pediococcus pentosaceus L1 isolated from paocai: a Chinese fermented vegetable. Annals of Microbiology, vol. 66, no. 3, pp. 963-971. http://dx.doi.org/10.1007/s13213-015-1182-2
» http://dx.doi.org/10.1007/s13213-015-1182-2 - CASEWELL, M., FRIIS, C., MARCO, E., MCMULLIN, P. and PHILLIPS, I., 2003. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. The Journal of Antimicrobial Chemotherapy, vol. 52, no. 2, pp. 159-161. http://dx.doi.org/10.1093/jac/dkg313 PMid:12837737.
» http://dx.doi.org/10.1093/jac/dkg313 - CAVICCHIOLI, V.Q., CAMARGO, A.C., TODOROV, S.D. and NERO, L.A., 2019. Potential control of Listeria monocytogenes by bacteriocinogenic Enterococcus hirae ST57ACC and pediococcus pentosaceus ST65ACC strains isolated from artisanal cheese. Probiotics and Antimicrobial Proteins, vol. 11, no. 2, pp. 696-704. http://dx.doi.org/10.1007/s12602-018-9449-0 PMid:30069686.
» http://dx.doi.org/10.1007/s12602-018-9449-0 - CLINICAL AND LABORATORY STANDARDS INSTITUTE – CLSI, 2016. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement 26th ed. Wayne, Pa, USA: CLSI. CLSI Document M100S. vol 36.
- COTTER, P.D., ROSS, R.P. and HILL, C., 2013. Bacteriocins: a viable alternative to antibiotics? Nature Reviews. Microbiology, vol. 11, no. 2, pp. 95-105. http://dx.doi.org/10.1038/nrmicro2937 PMid:23268227.
» http://dx.doi.org/10.1038/nrmicro2937 - DE MAN, J., ROGOSA, D. and SHARPE, M.E., 1960. A medium for the cultivation of lactobacilli. The Journal of Applied Bacteriology, vol. 23, no. 1, pp. 130-135. http://dx.doi.org/10.1111/j.1365-2672.1960.tb00188.x
» http://dx.doi.org/10.1111/j.1365-2672.1960.tb00188.x - DE SOUZA, J.V. and DIAS, F.S., 2017. Protective, technological, and functional properties of select autochthonous lactic acid bacteria from goat dairy products. Current Opinion in Food Science, vol. 13, pp. 1-9. http://dx.doi.org/10.1016/j.cofs.2017.01.003
» http://dx.doi.org/10.1016/j.cofs.2017.01.003 - DOWARAH, R., VERMA, A. and AGARWAL, N., 2017. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: a review. Animal Nutrition, vol. 3, no. 1, pp. 1-6. http://dx.doi.org/10.1016/j.aninu.2016.11.002 PMid:29767055.
» http://dx.doi.org/10.1016/j.aninu.2016.11.002 - ELGHANDOUR, M.M.Y., TAN, Z.L., ABU HAFSA, S.H., ADEGBEYE, M.J., GREINER, R., UGBOGU, E.A., CEDILLO MONROY, J. and SALEM, A.Z.M., 2020. Saccharomyces cerevisiae as a probiotic feed additive to non and pseudo‐ruminant feeding: a review. Journal of Applied Microbiology, vol. 128, no. 3, pp. 658-674. http://dx.doi.org/10.1111/jam.14416 PMid:31429174.
» http://dx.doi.org/10.1111/jam.14416 - EMMANUEL, D., JAFARI, A., BEAUCHEMIN, K., LEEDLE, J. and AMETAJ, B., 2007. Feeding live cultures of Enterococcus faecium and Saccharomyces cerevisiae induces an inflammatory response in feedlot steers. Journal of Animal Science, vol. 85, no. 1, pp. 233-239. http://dx.doi.org/10.2527/jas.2006-216 PMid:17179561.
» http://dx.doi.org/10.2527/jas.2006-216 - FERNANDES, M.L., PERIN, L.M., TODOROV, S.D., NERO, L.A., DE ALENCAR, E.R. and FERREIRA, M.D.A., 2018. In vitro evaluation of the safety and probiotic and technological potential of Pediococcus pentosaceus isolated from sheep milk. Semina: Ciências Agrárias, vol. 39, no. 1, pp. 113-132. http://dx.doi.org/10.5433/1679-0359.2018v39n1p113
» http://dx.doi.org/10.5433/1679-0359.2018v39n1p113 - FISHER, K. and PHILLIPS, C., 2009. The ecology, epidemiology and virulence of Enterococcus. Microbiology, vol. 155, no. Pt 6, pp. 1749-1757. http://dx.doi.org/10.1099/mic.0.026385-0 PMid:19383684.
» http://dx.doi.org/10.1099/mic.0.026385-0 - GARCIA-GUTIERREZ, E., MAYER, M.J., COTTER, P.D. and NARBAD, A., 2019. Gut microbiota as a source of novel antimicrobials. Gut Microbes, vol. 10, no. 1, pp. 1-21. http://dx.doi.org/10.1080/19490976.2018.1455790 PMid:29584555.
» http://dx.doi.org/10.1080/19490976.2018.1455790 - GHAZANFAR, S., 2016. Study on the effect of dietary supplementation of saccharomyces cerevisiae on performance of dairy cattle and heifers Pakistan: Quaid-i-Azam University Islamabad.
- GROCHOWSKA, M., LASKUS, T. and RADKOWSKI, M., 2019. Gut microbiota in neurological disorders. Archivum Immunologiae et Therapiae Experimentalis, vol. 67, no. 6, pp. 375-383. http://dx.doi.org/10.1007/s00005-019-00561-6 PMid:31578596.
» http://dx.doi.org/10.1007/s00005-019-00561-6 - GU, R.X., YANG, Z.Q., LI, Z.H., CHEN, S.L. and LUO, Z.L., 2008. Probiotic properties of lactic acid bacteria isolated from stool samples of longevous people in regions of Hotan, Xinjiang and Bama, Guangxi, China. Anaerobe, vol. 14, no. 6, pp. 313-317. http://dx.doi.org/10.1016/j.anaerobe.2008.06.001 PMid:18634896.
» http://dx.doi.org/10.1016/j.anaerobe.2008.06.001 - HAMMAMI, R., FERNANDEZ, B., LACROIX, C. and FLISS, I., 2013. Anti-infective properties of bacteriocins: an update. Cellular and Molecular Life Sciences, vol. 70, no. 16, pp. 2947-2967. http://dx.doi.org/10.1007/s00018-012-1202-3 PMid:23109101.
» http://dx.doi.org/10.1007/s00018-012-1202-3 - JAMI, E. and MIZRAHI, I., 2012. Composition and similarity of bovine rumen microbiota across individual animals. PLoS One, vol. 7, no. 3, pp. e33306. http://dx.doi.org/10.1371/journal.pone.0033306 PMid:22432013.
» http://dx.doi.org/10.1371/journal.pone.0033306 - KAVITHA, J. and DEVASENA, T., 2013. Isolation, characterization, determination of probiotic properties of lactic acid bacteria from human milk. OSR J Pharm Biol Sci, vol. 7, no. 3, pp. 1-7. http://dx.doi.org/10.9790/3008-0730107
» http://dx.doi.org/10.9790/3008-0730107 - KHEMARIYA, P., SINGH, S., NATH, G. and GULATI, A.K., 2013. Isolation, identification and antibiotic susceptibility of nis+ Lactococcus lactis from dairy and non-dairy sources. Czech Journal of Food Sciences, vol. 31, no. 4, pp. 323-331. http://dx.doi.org/10.17221/316/2012-CJFS
» http://dx.doi.org/10.17221/316/2012-CJFS - LEBOŠ PAVUNC, A., BEGANOVIĆ, J., KOS, B., UROIĆ, K., BLAŽIĆ, M. and ŠUŠKOVIĆ, J., 2012. Characterization and application of autochthonous starter cultures for fresh cheese production. Food Technology and Biotechnology, vol. 50, no. 2, pp. 141-151.
- LEE, K.W., PARK, J.Y., JEONG, H.R., HEO, H.J., HAN, N.S. and KIM, J.H., 2012. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe, vol. 18, no. 1, pp. 96-102. http://dx.doi.org/10.1016/j.anaerobe.2011.12.015 PMid:22200451.
» http://dx.doi.org/10.1016/j.anaerobe.2011.12.015 - LIASI, S., AZMI, T., HASSAN, M., SHUHAIMI, M., ROSFARIZAN, M. and ARIFF, A., 2009. Antimicrobial activity and antibiotic sensitivity of three isolates of lactic acid bacteria from fermented fish product, Budu. Malaysian Journal of Microbiology, vol. 5, no. 1, pp. 33-37.
- LOPES, M.F.S., LEITÃO, A.L., REGALLA, M., FIGUEIREDO MARQUES, J.J., CARRONDO, M.J.T. and CRESPO, M.T.B., 2002. Characterization of a highly thermostable extracellular lipase from Lactobacillus plantarum. International Journal of Food Microbiology, vol. 76, no. 1-2, pp. 107-115. http://dx.doi.org/10.1016/S0168-1605(02)00013-2 PMid:12038566.
» http://dx.doi.org/10.1016/S0168-1605(02)00013-2 - MATSUMOTO, M., OHISHI, H. and BENNO, Y., 2004. H+-ATPase activity in Bifidobacterium with special reference to acid tolerance. International Journal of Food Microbiology, vol. 93, no. 1, pp. 109-113. http://dx.doi.org/10.1016/j.ijfoodmicro.2003.10.009 PMid:15135587.
» http://dx.doi.org/10.1016/j.ijfoodmicro.2003.10.009 - MONTORO, B.P., BENOMAR, N., LAVILLA LERMA, L., CASTILLO GUTIÉRREZ, S., GÁLVEZ, A. and ABRIOUEL, H., 2016. Fermented Aloreña table olives as a source of potential probiotic Lactobacillus pentosus strains. Frontiers in Microbiology, vol. 7, pp. 1583. http://dx.doi.org/10.3389/fmicb.2016.01583 PMid:27774088.
» http://dx.doi.org/10.3389/fmicb.2016.01583 - NAEEM, M., AHMED, I., AHMED, S., AHMED, Z., RIAZ, M. and GHAZANFAR, S., 2018. Screening of cattle gut associated Bacillus strains for their potential use as animal probiotic. Indian Journal of Animal Research, no. of. http://dx.doi.org/10.18805/ijar.B-948
» http://dx.doi.org/10.18805/ijar.B-948 - NGUYEN, A.T.V., NGUYEN, D.V., TRAN, M.T., NGUYEN, L.T., NGUYEN, A.H. and PHAN, T.N., 2015. Isolation and characterization of B acillus subtilis CH 16 strain from chicken gastrointestinal tracts for use as a feed supplement to promote weight gain in broilers. Letters in Applied Microbiology, vol. 60, no. 6, pp. 580-588. http://dx.doi.org/10.1111/lam.12411 PMid:25754534.
» http://dx.doi.org/10.1111/lam.12411 - OZDOGAN, D.K., AKCELIK, N., ASLIM, B., SULUDERE, Z. and AKCELIK, M., 2012. Probiotic and antioxidative properties of L. lactis LL27 isolated from milk. Biotechnology, Biotechnological Equipment, vol. 26, no. 1, pp. 2750-2758. http://dx.doi.org/10.5504/BBEQ.2011.0091
» http://dx.doi.org/10.5504/BBEQ.2011.0091 - PAILIN, T., KANG, D., SCHMIDT, K. and FUNG, D., 2001. Detection of extracellular bound proteinase in EPS‐producing lactic acid bacteria cultures on skim milk agar. Letters in Applied Microbiology, vol. 33, no. 1, pp. 45-49. http://dx.doi.org/10.1046/j.1472-765X.2001.00954.x PMid:11442814.
» http://dx.doi.org/10.1046/j.1472-765X.2001.00954.x - PARVEEN RANI, R., ANANDHARAJ, M., HEMA, S., DEEPIKA, R. and DAVID RAVINDRAN, A., 2016. Purification of antilisterial peptide (Subtilosin A) from novel Bacillus tequilensis FR9 and demonstrate their pathogen invasion protection ability using human carcinoma cell line. Frontiers in Microbiology, vol. 7, pp. 1910. http://dx.doi.org/10.3389/fmicb.2016.01910 PMid:27990138.
» http://dx.doi.org/10.3389/fmicb.2016.01910 - PATEL, A., PRAJAPATI, J., HOLST, O. and LJUNGH, A., 2014. Determining probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from vegetables and traditional Indian fermented food products. Food Bioscience, vol. 5, pp. 27-33. http://dx.doi.org/10.1016/j.fbio.2013.10.002
» http://dx.doi.org/10.1016/j.fbio.2013.10.002 - PETERSON, C., RUCH, W., BEERMANN, U., PARK, N. and SELIGMAN, M.E., 2007. Strengths of character, orientations to happiness, and life satisfaction. The Journal of Positive Psychology, vol. 2, no. 3, pp. 149-156. http://dx.doi.org/10.1080/17439760701228938
» http://dx.doi.org/10.1080/17439760701228938 - QADIS, A.Q., GOYA, S., IKUTA, K., YATSU, M., KIMURA, A., NAKANISHI, S. and SATO, S., 2014. Effects of a bacteria-based probiotic on ruminal pH, volatile fatty acids, and bacterial flora of holstein calves. The Journal of Veterinary Medical Science, vol. 76, no. 6, pp. 877-885. http://dx.doi.org/10.1292/jvms.14-0028 PMid:24614603.
» http://dx.doi.org/10.1292/jvms.14-0028 - ROBREDO, B., SINGH, K.V., BAQUERO, F., MURRAY, B.E. and TORRES, C., 2000. Vancomycin-resistant enterococci isolated from animals and food. International Journal of Food Microbiology, vol. 54, no. 3, pp. 197-204. http://dx.doi.org/10.1016/S0168-1605(99)00195-6 PMid:10777070.
» http://dx.doi.org/10.1016/S0168-1605(99)00195-6 - SIERRA, G., 1957. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie van Leeuwenhoek, vol. 23, no. 1, pp. 15-22. http://dx.doi.org/10.1007/BF02545855 PMid:13425509.
» http://dx.doi.org/10.1007/BF02545855 - SIEZEN, R.J., 1999. Multi-domain, cell-envelope proteinases of lactic acid bacteria. Lactic Acid Bacteria: Genetics, Metabolism and Applications. Springer.
- STANFORD, S.M. and BOTTINI, N., 2014. PTPN22: the archetypal non-HLA autoimmunity gene. Nature Reviews. Rheumatology, vol. 10, no. 10, pp. 602-611. http://dx.doi.org/10.1038/nrrheum.2014.109 PMid:25003765.
» http://dx.doi.org/10.1038/nrrheum.2014.109 - SUNDARRAM, A. and MURTHY, T.P.K., 2014. α-amylase production and applications: a review. Journal of Applied & Environmental Microbiology, vol. 2, no. 2, pp. 166-175.
- UYENO, Y., SHIGEMORI, S. and SHIMOSATO, T., 2015. Effect of probiotics/prebiotics on cattle health and productivity. Microbes and Environments, vol. 30, no. 2, pp. 126-132. http://dx.doi.org/10.1264/jsme2.ME14176 PMid:26004794.
» http://dx.doi.org/10.1264/jsme2.ME14176 - WALI, N., QAYYUM, H.T., WAHEED, F., ALI, G. and SHEHBAZ, M., 2021. Isolation and molecular characterization of exo-polysaccharide producing weissella confusa from buffalo ruminal gut. International Journal of Modern Agriculture, vol. 10, no. 3, pp. 35-42.
- YANG, S.C., LIN, C.H., SUNG, C.T. and FANG, J.Y., 2014. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Frontiers in Microbiology, vol. 5, pp. 241. PMid:24904554.
Publication Dates
-
Publication in this collection
11 Mar 2022 -
Date of issue
2024
History
-
Received
11 Dec 2021 -
Accepted
01 Feb 2022