Abstract
Neutrophil extracellular traps (NETs) were first reported as a microbicidal strategy for activated neutrophils. Through an immunologic response against several stimuli, neutrophils release their DNA together with proteins from granules, nucleus, and cytoplasm (e.g., elastase and myeloperoxidase). To date, NETs have been implicated in tissue damage during intense inflammatory processes, mainly when their release is dependent on oxygen radical generation. Flavonoids are antioxidant and anti-inflammatory agents; of these, quercetin is commonly found in our daily diet. Therefore, quercetin could exert some protective activity against tissue damage induced by NETs. In our in vitro assays, quercetin reduced NETs, myeloperoxidase (MPO), and elastase release from neutrophils stimulated with phorbol 12-myristate 13-acetate (PMA). The activity of these enzymes also decreased in the presence of quercetin. Quercetin also reduced the cytotoxic effect of NETs on alveolar cells (A549 cell line). Further, in silico assays indicated favorable interactions between quercetin and NET proteins (MPO and elastase). Overall, our results demonstrate that quercetin decreases deleterious cellular effects of NETs by reducing their release from activated neutrophils, and diminishing the enzymatic activity of MPO and elastase, possibly through direct interaction.
Keywords:
inflammation; damaged tissue; flavonoids; quercetin; myeloperoxidase; elastase
Resumo
As armadilhas extracelulares de neutrófilos (NETs) foram relatadas pela primeira vez como uma estratégia microbicida para neutrófilos ativados. Por meio de uma resposta imunológica contra vários estímulos, os neutrófilos liberam seu DNA ligado a proteínas de grânulos, núcleo e citoplasma (por exemplo, elastase e mieloperoxidase). Até o momento, os NETs têm sido implicadas em danos aos tecidos durante processos inflamatórios intensos, principalmente quando sua liberação depende da geração de radicais de oxigênio. Os flavonóides são agentes antioxidantes e anti-inflamatórios, e destes, a quercetina é comumente encontrada em nossa dieta diária. Portanto, a quercetina pode exercer alguma atividade protetora contra o dano tecidual induzido por NETs. Em nossos ensaios in vitro, a quercetina reduziu a liberação das NETs, mieloperoxidase (MPO) e elastase a partir de neutrófilos estimulados com forbol 12-miristato 13-acetato (PMA). A atividade dessas enzimas também foi diminuída na presença de quercetina. A quercetina também reduziu o efeito citotóxico dos NETs sobre células alveolares (linha celular A549). Além disso, os ensaios in silico indicaram interações favoráveis entre a quercetina e as proteínas da NET (MPO e elastase). No geral, nossos resultados demonstram que a quercetina diminui os efeitos deletérios das NETs, reduzindo sua liberação à partir de neutrófilos ativados e diminuindo a atividade enzimática de MPO e elastase, possivelmente por meio de interação direta.
Palavras-chave:
inflamação; dano tecidual; flavonoides; quercetina; mieloperoxidase; elastase
1. Introduction
Neutrophils are the most abundant leukocytes in blood and are the first cells to arrive at infected sites, where they facilitate host defense through phagocytosis and the release of several inflammatory mediators. The persistence of inflammatory stimuli induces neutrophils to release their DNA into the extracellular environment in the form of traps. Neutrophil extracellular traps (NETs) consist of DNA associated with intracellular proteins such as elastase, histones, and myeloperoxidase. NETs have microbicidal activity, specifically related to the capture and/or death of several pathogens (Delgado-Rizo et al., 2017DELGADO-RIZO, V., MARTÍNEZ-GUZMÁN, M.A., IÑIGUEZ-GUTIERREZ, L., GARCÍA-OROZCO, A., ALVARADO-NAVARRO, A. and FAFUTIS-MORRIS, M., 2017. Neutrophil extracellular traps and its implications in inflammation: an overview. Frontiers in Immunology, vol. 8, p. 81. http://dx.doi.org/10.3389/fimmu.2017.00081. PMid:28220120.
http://dx.doi.org/10.3389/fimmu.2017.000...
). Further, their beneficial role has been investigated in several infectious and non-infectious human diseases including rheumatoid arthritis, systemic lupus erythematosus, diabetes, cancer, and thrombosis (Jorch and Kubes, 2017JORCH, S.K. and KUBES, P., 2017. An emerging role for neutrophil extracellular traps in noninfectious disease. Nature Medicine, vol. 23, no. 3, pp. 279-287. http://dx.doi.org/10.1038/nm.4294. PMid:28267716.
http://dx.doi.org/10.1038/nm.4294...
). In these cases, NETs modulate the inflammatory process and induce tissue damage, release of autoantigens, intensification of oxidative burst, alteration of the coagulation cascade, and occlusion of the vasculature or airways (Erpenbeck and Schön, 2017ERPENBECK, L. and SCHÖN, M.P., 2017. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene, vol. 36, no. 18, pp. 2483-2490. http://dx.doi.org/10.1038/onc.2016.406. PMid:27941879.
http://dx.doi.org/10.1038/onc.2016.406...
).
Several neutrophil activators act as inducers of NET release. In most cases, NET release depends on reactive oxygen species (ROS) generation via NADPH activation. Simultaneously, loss of granular membranes facilitates the migration of enzymes from the cytoplasm to the nucleus, where they induce chromatin decondensation. Thus, free DNA reaches the cytoplasm, where it mixes with cytoplasmic and granular proteins before being released to the extracellular environment. Increasing studies have been aiming to preserve the benefits of NETs and minimize their harmful effects (Porto and Stein, 2016PORTO, B.N. and STEIN, R.T., 2016. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Frontiers in Immunology, vol. 7, p. 311. http://dx.doi.org/10.3389/fimmu.2016.00311. PMid:27574522.
http://dx.doi.org/10.3389/fimmu.2016.003...
). Antioxidant molecules or enzyme inhibitors (e.g., elastase and myeloperoxidase) may be beneficial for treating pathologies exacerbated by NETs.
Flavonoids are natural anti-inflammatory and antioxidant agents with considerable scientific and therapeutic interest. Among them, quercetin (3,5,7,3’-4’-pentahidroxi flavone) is widely known and frequently present in a variety of foods including tomatoes, apples, shallots, Brassica vegetables, onions, capers, and grapes, as well as in the seeds, leaves, barks, nuts, and flowers of many plants. Quercetin is also found in medicinal plants such as Sambucus canadensis (elder), Ginkgo biloba, and Hypericum perforatum (St. John’s wort) (Kelly, 2011KELLY, G.S., 2011. Quercetin. Monograph. Alternative Medicine Review, vol. 16, no. 2, pp. 172-194. PMid:21649459.). Quercetin appears to have many beneficial effects on human health (allergy, asthma, cancer, cardiovascular, atopic and gastric diseases, diabetes, hypertension, immunity and infections, pain, arthritis, metabolic syndrome traits, obesity, and mood disorders). The mechanisms underlying quercetin activities involve decrease in ROS production, inhibition of granulocyte degranulation, repression of inflammatory mediator release, blockage of enzymes, improvement of endothelial function, and prevention of myocardial cell injury caused by Ca2+ overload (Kelly, 2011KELLY, G.S., 2011. Quercetin. Monograph. Alternative Medicine Review, vol. 16, no. 2, pp. 172-194. PMid:21649459.; Batiha et al., 2020BATIHA, G.E.-S., BESHBISHY, A.M., IKRAM, M., MULLA, Z.S., EL-HACK, M.E.A., TAHA, A.E., ALGAMMAL, A.M. and ELEWA, Y.H.A., 2020. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods, vol. 9, no. 3, p. 374. http://dx.doi.org/10.3390/foods9030374. PMid:32210182.
http://dx.doi.org/10.3390/foods9030374...
). Overall, those previous reports suggest that quercetin may inhibit NET release and their cytotoxic activity.
In the present study, we used in vitro and in silico approaches to evaluate the effect of quercetin on NET release and its protective effect on A549 alveolar cells incubated with NETs. Our data demonstrate that quercetin can reduce the release of NETs and some enzymes (elastase and myeloperoxidase), without modifying neutrophil viability. Quercetin also preserved A549 cell viability after incubation with NETs. Moreover, in silico analyses indicated that the formation of the quercetin/elastase and quercetin/myeloperoxidase complexes is thermodynamically favorable, and this event could help minimizing the enzymatic/cytotoxic activity of these enzymes. Finally, we suggest that quercetin can be used to treat tissue damage caused by NETs due to its ability to hamper the overproduction of NETs and its cytotoxicity effects.
2. Materials and Methods
2.1. Reagents
Quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one) (Sigma-Aldrich, St. Louis, MO, USA), dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich), was stored at -80°C and protected from light for one month. Based on previous work (Jablonska et al., 2020JABLONSKA, E., GARLEY, M., SURAZYNSKI, A., GRUBCZAK, K., IWANIUK, A., BORYS, J., MONIUSZKO, M. and RATAJCZAK-WRONA, W., 2020. Neutrophil extracellular traps (NETs) formation induced by TGF-β in oral lichen planus – possible implications for the development of oral cancer. Immunobiology, vol. 225, no. 2, p. 151901. http://dx.doi.org/10.1016/j.imbio.2019.151901. PMid:31882256.
http://dx.doi.org/10.1016/j.imbio.2019.1...
; Kanashiro et al., 2007KANASHIRO, A., SOUZA, J.G., KABEYA, L.M., AZZOLINI, A.E.C.S. and LUCISANO-VALIM, Y.M., 2007. Elastase release by stimulated neutrophils inhibited by flavonoids: importance of the catechol group. Zeitschrift fur Naturforschung. C, Journal of Biosciences, vol. 62, no. 5-6, pp. 357-361. http://dx.doi.org/10.1515/znc-2007-5-607. PMid:17708440.
http://dx.doi.org/10.1515/znc-2007-5-607...
; Lu et al., 2018LU, N., SUI, Y., TIAN, R. and PENG, Y.-Y., 2018. Inhibitive effects of quercetin on myeloperoxidase-dependent hypochlorous acid formation and vascular endothelial injury. Journal of Agricultural and Food Chemistry, vol. 66, no. 19, pp. 4933-4940. http://dx.doi.org/10.1021/acs.jafc.8b01537. PMid:29708335.
http://dx.doi.org/10.1021/acs.jafc.8b015...
; Santos et al., 2014SANTOS, E.O.L., KABEYA, L.M., FIGUEIREDO-RINHEL, A.S.G., MARCHI, L.F., ANDRADE, M.F., PIATESI, F., PAOLIELLO-PASCHOALATO, A.B., AZZOLINI, A.E.C.S. and LUCISANO-VALIM, Y.M., 2014. Flavonols modulate the effector functions of healthy individuals’ immune complex-stimulated neutrophils: a therapeutic perspective for rheumatoid arthritis. International Immunopharmacology, vol. 21, no. 1, pp. 102-111. http://dx.doi.org/10.1016/j.intimp.2014.04.014. PMid:24797916.
http://dx.doi.org/10.1016/j.intimp.2014....
), quercetin was tested at a maximum concentration of 50 μM in the assays described below.
2.2. Human neutrophil isolation
Human neutrophils were isolated and prepared according to a previously described method (Souza et al., 2018SOUZA, P.S.S., BARBOSA, L.V., DINIZ, L.F.A., SILVA, G.S., LOPES, B.R.P., SOUZA, P.M.R., ARAUJO, G.C., PESSOA, D., OLIVEIRA, J., SOUZA, F.P. and TOLEDO, K.A., 2018. Neutrophil extracellular traps possess anti-human respiratory syncytial virus activity: possible interaction with the viral F protein. Virus Research, vol. 251, pp. 68-77. http://dx.doi.org/10.1016/j.virusres.2018.04.001. PMid:29621602.
http://dx.doi.org/10.1016/j.virusres.201...
). We collected 10 mL of peripheral blood from healthy volunteers and placed it in BD Vacutainer® tubes (BD Biosciences, Franklin Lakes, NJ, USA) containing sodium citrate. The blood was processed in a double density gradient (Histopaque 1119 and 1077, Sigma-Aldrich) according to the manufacturer’s instructions. Trypan blue (Sigma) exclusion test determined 90–95% cell viability. All experimental procedures were evaluated and approved by the local Research Ethics Committee of the Faculty of Science and Letters of Assis (approval number: CEP–57233716.8.0000.5401). As suggested by the Committee, written informed consent was obtained from each volunteer before initiating any research procedures.
2.3. Cell culture
The adenocarcinomic human alveolar basal epithelial cell line (A549; Cell Bank of Rio de Janeiro, Rio de Janeiro, RJ, Brazil) was cultured in DMEM-F12 (Sigma-Aldrich) containing 10% fetal bovine serum (FBS; Cultilab, Campinas, SP, Brazil). The medium was supplemented with antibiotics and antimycotics (Invitrogen, Carlsbad, CA, USA). The incubator was maintained at 37°C with 5% CO2.
2.4. Neutrophil Extracellular Traps (NETs) release
Human neutrophils were incubated with or without different concentrations of quercetin for 30 min at 37°C and then stimulated with PMA (50 nM) for 4h at 37°C. DMEM-F12 FBS-free medium was used because of the presence of DNAse, which could degrade NETs (Von Köckritz-Blickwede et al., 2009VON KÖCKRITZ-BLICKWEDE, M., CHOW, O.A. and NIZET, V., 2009. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood, vol. 114, no. 25, pp. 5245-5246. http://dx.doi.org/10.1182/blood-2009-08-240713. PMid:20007813.
http://dx.doi.org/10.1182/blood-2009-08-...
). The cells were kept in a humidified incubator maintained at 37°C and 5% CO2 for 3h. Then, the supernatant was discarded from the culture and NETs were collected. They were submitted to additional incubation for 24 h at 40°C (Souza et al., 2018SOUZA, P.S.S., BARBOSA, L.V., DINIZ, L.F.A., SILVA, G.S., LOPES, B.R.P., SOUZA, P.M.R., ARAUJO, G.C., PESSOA, D., OLIVEIRA, J., SOUZA, F.P. and TOLEDO, K.A., 2018. Neutrophil extracellular traps possess anti-human respiratory syncytial virus activity: possible interaction with the viral F protein. Virus Research, vol. 251, pp. 68-77. http://dx.doi.org/10.1016/j.virusres.2018.04.001. PMid:29621602.
http://dx.doi.org/10.1016/j.virusres.201...
). DNA was then quantified using a Qubit 2.0 fluorimeter (Invitrogen Inc., Grand Island, NY, USA) and PicoGreen® dsDNA Assay Kit (Invitrogen). Samples were stored at −25°C.
2.5. Neutrophilic degranulation
Neutrophilic degranulation was monitored by assaying elastase and myeloperoxidase release from neutrophils stimulated with PMA in the presence or absence of quercetin. Cells (2 × 105) were suspended in Hank's solution and incubated with different quercetin concentrations for 30 min before activation with PMA (50 nM). After 3 h at 37°C, the neutrophils were centrifuged (437 × g, 5 min) and the supernatant was collected and incubated with 1 mM elastase substrate (N-Methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide) (Sigma) or 1 mM myeloperoxidase substrate (TMB; 3,3,5,5-tetramethylbenzidine) for 30 min. The reaction was stopped with 1N sulfuric acid, and the absorbance was measured at 405 nm using a Multiskan FC microplate reader (Thermo Scientific).
2.6. Cell viability (MTT assay)
The colorimetric MTT (3-(4,5-dimethylthiazol-2-yl) 2,5-Diphenyl tetrazolium bromide) (Sigma) method was used to measure cell viability. Living cells metabolize MTT salts via mitochondrial enzyme activity. In 96-well culture plates, human neutrophils (2 × 105 cells/well) and different concentrations of quercetin were co-incubated (1–4 h at 37°C). The medium was replaced with MTT (0.5 mg/mL) and incubated for 4h at 37 °C. After adding DMSO to dissolve the formazan crystals, optical density (OD) was measured using a spectrophotometer (560 nm). Neutrophils incubated only with RPMI-1640 (Sigma) and 50 μM H2O2 (Stocco et al., 2012STOCCO, B., TOLEDO, K., SALVADOR, M., PAULO, M., KOYAMA, N. and TOLOI, M.R.T., 2012. Dose-dependent effect of resveratrol on bladder cancer cells: chemoprevention and oxidative stress. Maturitas, vol. 72, no. 1, pp. 72-78. http://dx.doi.org/10.1016/j.maturitas.2012.02.004. PMid:22386766.
http://dx.doi.org/10.1016/j.maturitas.20...
) were used as negative and positive controls (100% viable) for cell death, respectively.
The viability of A549 cells, treated or not with NETs, was also evaluated using the MTT assay. For this purpose, the cells (5 × 104/well) were incubated with quercetin (24 h), NETs (24 h), or NETs (1 h) previous to quercetin (24 h) at 37°C.
2.7. Inhibition of enzymatic activity from elastase and myeloperoxidase
Elastase-and myeloperoxidase-enriched supernatants were obtained from neutrophils (2 × 105) incubated with PMA (50 nM) for 3 h at 37°C. After centrifugation, the supernatants were incubated with quercetin for 30 min at 37°C, and enzymatic activity was evaluated by adding 1 mM elastase substrate (N-Methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide) (Sigma) or 1 mM myeloperoxidase substrate (TMB; 3,3,5,5-tetramethylbenzidine) for 30 min. The reaction was stopped with 1N sulfuric acid and the absorbance was measured at 405 nm using a Multiskan FC microplate reader (Thermo Scientific).
2.8. Molecular docking
Protein-ligand docking was performed on a local workstation using Auto-Dock Vina software (version 1.1.2). Structures of elastase (PDB: 3Q76), and myeloperoxidase (PDB: 5FIW) receptors were obtained from the Protein Data Bank. The structure of the ligand, quercetin pentaacetate, was obtained from PubChem database (CID: 5280343), converted to a PDB file, and completed with hydrogen using the UCSF Chimera program. The UCSF Chimera program was also used to minimize the structures of the compounds. For elastase a docking box of 22.5 × 22.1 × 19.35 (X, Y, Z axes sizes) was centralized at the catalytic triad HIS 57, ASP 102, and SER 195. For myeloperoxidase the dimensions were 66.7 × 50.3 × 73.8, centralized in the heme cluster of chain A. The docking simulation was performed using the Broyden-Fletcher-Goldfarb-Shanno local gradient optimization method. The affinity of the docked complexes was calculated using the scoring function of AutoDock Vina (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3041641/pdf/nihms270965.pdf). Van Der Waals (VDW) surface overlaps with a cutoff ≥ -0.4 Å were considered as contacts.
2.9. Statistical analysis
Experimental data were processed using GraphPad Prism 6, who pointed out their Gaussian distribution. So, experimental data were evaluated using variance analysis (one-way ANOVA) followed by the Bonferroni test. The significance level was set at 5%. All assays were performed in triplicate, at least three times, independently.
3. Results and Discussion
NETs are structures released from activated neutrophils. They are composed of DNA and proteins from granules, the nucleus, and cytoplasm (i.e., elastase and myeloperoxidase) and play a microbicidal role by trapping and inactivating several pathogens. However, NETs have deleterious effects because they can increase inflammatory processes by intensifying tissue damage. Because flavonoids are known for their antioxidant and anti-inflammatory activities, we examined whether quercetin could exert protective activity against NETs. Our in vitro results showed that quercetin reduced NET release and cytotoxicity induced by it. Furthermore, quercetin inhibited the enzymatic activities of MPO and elastase.
NETs are often released under ROS-dependent stimuli such as PMA, lipopolysaccharide (LPS), and IL-8. Some stimuli can also induce ROS-independent NET release (Gabriel et al., 2010GABRIEL, C., MCMASTER, W.R., GIRARD, D. and DESCOTEAUX, A., 2010. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. Journal of Immunology, vol. 185, no. 7, pp. 4319-4327. http://dx.doi.org/10.4049/jimmunol.1000893. PMid:20826753.
http://dx.doi.org/10.4049/jimmunol.10008...
; Pilsczek et al., 2010PILSCZEK, F.H., SALINA, D., POON, K.K.H., FAHEY, C., YIPP, B.G., SIBLEY, C.D., ROBBINS, S.M., GREEN, F.H.Y., SURETTE, M.G., SUGAI, M., BOWDEN, M.G., HUSSAIN, M., ZHANG, K. and KUBES, P., 2010. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. Journal of Immunology, vol. 185, no. 12, pp. 7413-7425. http://dx.doi.org/10.4049/jimmunol.1000675. PMid:21098229.
http://dx.doi.org/10.4049/jimmunol.10006...
; Farley et al., 2012FARLEY, K., STOLLEY, J.M., ZHAO, P., COOLEY, J. and REMOLD-O’DONNELL, E., 2012. A SerpinB1 regulatory mechanism is essential for restricting neutrophil extracellular trap generation. Journal of Immunology, vol. 189, no. 9, pp. 4574-4581. http://dx.doi.org/10.4049/jimmunol.1201167. PMid:23002442.
http://dx.doi.org/10.4049/jimmunol.12011...
). These stimuli activate the NADPH-oxidase complex, ROS production, and activation of PAD14 (arginine protein deiminase 4). Consequently, the enzymes elastase and MPO migrate to the nucleus, where they assist in chromatin decondensation. The DNA is then released into the cytosol, where it mixes with the present granular and cytosolic proteins (Jorch and Kubes, 2017JORCH, S.K. and KUBES, P., 2017. An emerging role for neutrophil extracellular traps in noninfectious disease. Nature Medicine, vol. 23, no. 3, pp. 279-287. http://dx.doi.org/10.1038/nm.4294. PMid:28267716.
http://dx.doi.org/10.1038/nm.4294...
). We observed that prior incubation (30 min) of neutrophils with quercetin (50 μM) reduced NET release from PMA-stimulated neutrophils (Figure 1A). Other quercetin concentrations, in combination with PMA or alone, did not show any statistically significant effect in reducing NET release. Similar results were observed when MPO and elastase release was evaluated. Previous incubation with quercetin (50 μM) decreased elastase release by PMA-activated neutrophils (Figure 1C), whereas quercetin at both 25 μM and 50 μM diminished MPO release (Figure 1B). It is important to emphasize that all the effects of quercetin on neutrophils demonstrated in this study occurred in the absence of cell death (Figure 1D). In the first hour, quercetin appeared to improve cell metabolism, though the data were not statistically significant. Several roles have been attributed to quercetin, which may explain the reduction of NETs, MPO, and elastase release, as described above. Quercetin has antioxidant activity, which scavenges reactive oxygen species (Boots et al., 2008BOOTS, A.W., HAENEN, G.R.M.M. and BAST, A., 2008. Health effects of quercetin: from antioxidant to nutraceutical. European Journal of Pharmacology, vol. 585, no. 2-3, pp. 325-337. http://dx.doi.org/10.1016/j.ejphar.2008.03.008. PMid:18417116.
http://dx.doi.org/10.1016/j.ejphar.2008....
) and also reduces ROS overproduction (Xu et al., 2019XU, D., HU, M.-J., WANG, Y.-Q. and CUI, Y.-L., 2019. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules, vol. 24, no. 6, p. 1123. http://dx.doi.org/10.3390/molecules24061123. PMid:30901869.
http://dx.doi.org/10.3390/molecules24061...
). As mentioned previously, ROS production is an important step during NET formation, including the release of DNA and granule proteins (i.e., MPO and elastase). Inhibition of NET release has also been reported for other antioxidant flavonoids. For instance, epicatechin, catechin hydrate, and rutin trihydrate inhibit NET formation (Kirchner et al., 2013KIRCHNER, T., HERMANN, E., MÖLLER, S., KLINGER, M., SOLBACH, W., LASKAY, T. and BEHNEN, M., 2013. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediators of Inflammation, vol. 2013, p. 710239. http://dx.doi.org/10.1155/2013/710239. PMid:24381411.
http://dx.doi.org/10.1155/2013/710239...
). Recently, quercetin and luteolin have been shown to inhibit NET formation in patients with oral lichen planus (OLP), a potentially malignant oral disorder (Jablonska et al., 2020JABLONSKA, E., GARLEY, M., SURAZYNSKI, A., GRUBCZAK, K., IWANIUK, A., BORYS, J., MONIUSZKO, M. and RATAJCZAK-WRONA, W., 2020. Neutrophil extracellular traps (NETs) formation induced by TGF-β in oral lichen planus – possible implications for the development of oral cancer. Immunobiology, vol. 225, no. 2, p. 151901. http://dx.doi.org/10.1016/j.imbio.2019.151901. PMid:31882256.
http://dx.doi.org/10.1016/j.imbio.2019.1...
). Further, previous studies have implicated quercetin in the in vitro inhibition of elastase release from neutrophils activated by other stimuli such as fMLP plus cytochalasin B (Kanashiro et al., 2007KANASHIRO, A., SOUZA, J.G., KABEYA, L.M., AZZOLINI, A.E.C.S. and LUCISANO-VALIM, Y.M., 2007. Elastase release by stimulated neutrophils inhibited by flavonoids: importance of the catechol group. Zeitschrift fur Naturforschung. C, Journal of Biosciences, vol. 62, no. 5-6, pp. 357-361. http://dx.doi.org/10.1515/znc-2007-5-607. PMid:17708440.
http://dx.doi.org/10.1515/znc-2007-5-607...
), fMLP, solid phase IgG, or zymosan-treated serum (Blackburn Junior et al., 1987), and cytochalasin B plus calcium ionophore, A23187 (Carini et al., 2001CARINI, M., STEFANI, R., ALDINI, G., OZIOLI, M. and FACINO, R.M., 2001. Procyanidins from Vitis vinifera seeds inhibit the respiratory burst of activated human neutrophils and lysosomal enzyme release. Planta Medica, vol. 67, no. 8, pp. 714-717. http://dx.doi.org/10.1055/s-2001-18353. PMid:11731911.
http://dx.doi.org/10.1055/s-2001-18353...
). Quercetin also inhibited MPO release from neutrophils stimulated by LPS (Lu et al., 2018LU, N., SUI, Y., TIAN, R. and PENG, Y.-Y., 2018. Inhibitive effects of quercetin on myeloperoxidase-dependent hypochlorous acid formation and vascular endothelial injury. Journal of Agricultural and Food Chemistry, vol. 66, no. 19, pp. 4933-4940. http://dx.doi.org/10.1021/acs.jafc.8b01537. PMid:29708335.
http://dx.doi.org/10.1021/acs.jafc.8b015...
), cytochalasin B plus PMA (Nosáľ et al., 2014NOSÁĽ, R., DRÁBIKOVÁ, K., JANČINOVÁ, V., PEREČKO, T., AMBROŽOVÁ, G., ČÍŽ, M., LOJEK, A., PEKAROVÁ, M., ŠMIDRKAL, J. and HARMATHA, J., 2014. On the molecular pharmacology of resveratrol on oxidative burst inhibition in professional phagocytes. Oxidative Medicine and Cellular Longevity, vol. 2014, p. 706269. http://dx.doi.org/10.1155/2014/706269. PMid:24672638.
http://dx.doi.org/10.1155/2014/706269...
) and immune complexes (Santos et al., 2014SANTOS, E.O.L., KABEYA, L.M., FIGUEIREDO-RINHEL, A.S.G., MARCHI, L.F., ANDRADE, M.F., PIATESI, F., PAOLIELLO-PASCHOALATO, A.B., AZZOLINI, A.E.C.S. and LUCISANO-VALIM, Y.M., 2014. Flavonols modulate the effector functions of healthy individuals’ immune complex-stimulated neutrophils: a therapeutic perspective for rheumatoid arthritis. International Immunopharmacology, vol. 21, no. 1, pp. 102-111. http://dx.doi.org/10.1016/j.intimp.2014.04.014. PMid:24797916.
http://dx.doi.org/10.1016/j.intimp.2014....
). In all the studies cited above, quercetin did not show cytotoxic effects on neutrophils. Interestingly, quercetin inhibits acetylation (Xiao et al., 2011XIAO, X., SHI, D., LIU, L., WANG, J., XIE, X., KANG, T. and DENG, W., 2011. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS One, vol. 6, no. 8, p. e22934. http://dx.doi.org/10.1371/journal.pone.0022934. PMid:21857970.
http://dx.doi.org/10.1371/journal.pone.0...
) and reduces the demethylation of histones (Abdulla et al., 2013ABDULLA, A., ZHAO, X. and YANG, F., 2013. Natural polyphenols inhibit lysine-specific demethylase-1 in vitro. Journal of Biochemical and Pharmacological Research, vol. 1, no. 1, pp. 56-63. PMid:23662249.). These events, combined with the inhibition of MPO and elastase enzymatic action by quercetin, can hamper NET release because they favor chromatin compaction. Future studies might clarify these issues.
Quercetin reduces NETs, MPO, and elastase in the absence of cell death. (A-C) Neutrophils were incubated for 4 h, at 37°C, with medium, Quercetin alone (QCT 50 μM), PMA (50 nM) alone, or in the presence of quercetin (3.1–50 μM). The supernatant was collected to measure the release of (A) NETs, (B) elastase and (C) MPO. The results represent the mean values from three independent assays performed in triplicate ± standard deviation (#p < 0.05 compared to the positive control, PMA-activated neutrophils). (D) Neutrophils were incubated with medium, H2O2 (50 μM), or Quercetin (3.1–50 μM) for 1 h or 4 h. Cell viability was measured using the MTT assay. The results represent the mean values from three independent assays performed in triplicate ± standard deviation.
Although NETs were initially described as microbicidal structures, a growing number of studies have shown that NETs are also responsible for deleterious effects on various tissues. NETs exacerbate inflammatory processes during rheumatoid arthritis, systemic lupus erythematosus, diabetes, cancer, and thrombosis (Jorch and Kubes, 2017JORCH, S.K. and KUBES, P., 2017. An emerging role for neutrophil extracellular traps in noninfectious disease. Nature Medicine, vol. 23, no. 3, pp. 279-287. http://dx.doi.org/10.1038/nm.4294. PMid:28267716.
http://dx.doi.org/10.1038/nm.4294...
). MPO and elastase have been partially implicated in the deleterious effects of NETs on tissues (Saffarzadeh et al., 2012SAFFARZADEH, M., JUENEMANN, C., QUEISSER, M.A., LOCHNIT, G., BARRETO, G., GALUSKA, S.P., LOHMEYER, J. and PREISSNER, K.T., 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One, vol. 7, no. 2, p. e32366. http://dx.doi.org/10.1371/journal.pone.0032366. PMid:22389696.
http://dx.doi.org/10.1371/journal.pone.0...
). Therefore, it is necessary to search for alternatives in the therapeutic treatment of diseases exacerbated by the presence of NETs. Our results, and data from others, suggest that quercetin has low cytotoxic potential and high potential to inhibit the release of NETs and their microbicidal enzymes.
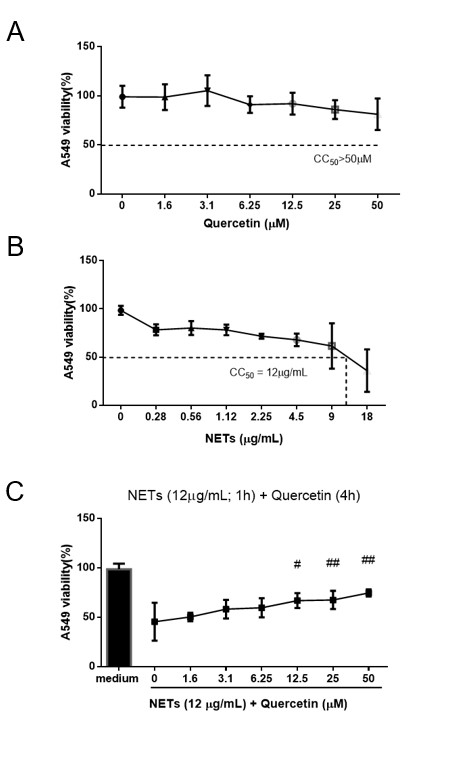
As quercetin inhibited NET production, we performed assays to evaluate its role in the cytotoxicity induced by NETs (Figure 2). For this, we selected the A549 cell line because NETs have been demonstrated to induce cell death in this cellular lineage (Saffarzadeh et al., 2012SAFFARZADEH, M., JUENEMANN, C., QUEISSER, M.A., LOCHNIT, G., BARRETO, G., GALUSKA, S.P., LOHMEYER, J. and PREISSNER, K.T., 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One, vol. 7, no. 2, p. e32366. http://dx.doi.org/10.1371/journal.pone.0032366. PMid:22389696.
http://dx.doi.org/10.1371/journal.pone.0...
). Thus, A549 cells were incubated for 24 h with quercetin (1.6–50 μM), NETs (0.1–18 μg/mL), or NETs plus quercetin. Different concentrations of quercetin did not affect A549 cell viability (Figure 2A). In contrast, NETs were cytotoxic to A549 cells in a concentration-dependent manner (Figure 2B). The calculated data from linear regression showed 12 μg/mL of NETs as the CC50 in these cells. This was an important result for designing the next assay. To evaluate the effects of quercetin in the treatment of NET-damaged tissue, we incubated A549 cells with NETs (12 μg/mL) for 1 h, and then added quercetin (1.6–50 μM; 24 h) (Figure 2C). Cells incubated with medium only were considered to have 100% viability. The presence of NETs (12 μg/mL) induced 50% cell death. Quercetin at 12.5–50 μM, was able to reduce the cytotoxic effects of NETs, whereas lower concentrations (1.6–6.25 μM) showed no effects. Previous studies examining the effects of quercetin on A549 cells also demonstrated that quercetin (< 200 μM) maintains 72.3% of cell viability after 24 h of incubation (Robaszkiewicz et al., 2007ROBASZKIEWICZ, A., BALCERCZYK, A. and BARTOSZ, G., 2007. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biology International, vol. 31, no. 10, pp. 1245-1250. http://dx.doi.org/10.1016/j.cellbi.2007.04.009. PMid:17583542.
http://dx.doi.org/10.1016/j.cellbi.2007....
). These results agree with our findings. Similarly, incubation of A549 cells with NETs (10.1 μg/mL) for 16 h revealed that ~50% of the cells were positive for Annexin-V (Saffarzadeh et al., 2012SAFFARZADEH, M., JUENEMANN, C., QUEISSER, M.A., LOCHNIT, G., BARRETO, G., GALUSKA, S.P., LOHMEYER, J. and PREISSNER, K.T., 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One, vol. 7, no. 2, p. e32366. http://dx.doi.org/10.1371/journal.pone.0032366. PMid:22389696.
http://dx.doi.org/10.1371/journal.pone.0...
). Quercetin acted positively by reducing the cytotoxic effects of NETs on A549 cells in a concentration-dependent manner. As the cytotoxicity of NETs on A549 cells was attributed to major protein components in the composition of NETs (Saffarzadeh et al., 2012SAFFARZADEH, M., JUENEMANN, C., QUEISSER, M.A., LOCHNIT, G., BARRETO, G., GALUSKA, S.P., LOHMEYER, J. and PREISSNER, K.T., 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One, vol. 7, no. 2, p. e32366. http://dx.doi.org/10.1371/journal.pone.0032366. PMid:22389696.
http://dx.doi.org/10.1371/journal.pone.0...
), we hypothesized that the protective effect of quercetin would result from its interaction with some of these molecules.
Quercetin reduces cell damage induced by NETs. A549 cells were seeded into a 96-well plate and incubated for 4 h with (A) quercetin (1.6–50 μM) or (B) NETs (0.28–18 μg/mL). In (C), cells were previously incubated with NETs (12 μg/mL) for 1 h, and then with quercetin (1.6–50 μM) for 4 h. Cell viability was measured using MTT assay. The dashed line represents CC50 for quercetin (> 50 μM) and NETs (12 μg/mL) in A and B. The results represent the mean values from three independent assays, performed in triplicate ± standard deviation (#p < 0.05 and ##p < 0.01 compared to the control, NETs 12 μg/mL plus Quercetin 0 μM).
In particular, during chronic lung inflammation, there is activation and mass migration of neutrophils into the alveolar space, which is controlled through chemokines produced by epithelial cells, macrophages, and neutrophils (Kasama et al., 2005KASAMA, T., MIWA, Y., ISOZAKI, T., ODAI, T., ADACHI, M. and KUNKEL, S.L., 2005. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Current Drug Targets - Inflammation & Allergy, vol. 4, no. 3, pp. 273-279. http://dx.doi.org/10.2174/1568010054022114. PMid:16101533.
http://dx.doi.org/10.2174/15680100540221...
). The cell activation process is negatively modulated by pulmonary surfactant proteins. In this context, there is a deficiency in the cleaning of nucleic acids present in the tissue, which results in the permanence of NETs and damage to local cells (Douda et al., 2011DOUDA, D.N., JACKSON, R., GRASEMANN, H. and PALANIYAR, N., 2011. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. Journal of Immunology, vol. 187, no. 4, pp. 1856-1865. http://dx.doi.org/10.4049/jimmunol.1004201. PMid:21724991.
http://dx.doi.org/10.4049/jimmunol.10042...
; Nayak et al., 2012NAYAK, A., DODAGATTA-MARRI, E., TSOLAKI, A.G. and KISHORE, U., 2012. An insight into the diverse roles of surfactant proteins, SP-A and SP-D in innate and adaptive immunity. Frontiers in Immunology, vol. 3, p. 131. http://dx.doi.org/10.3389/fimmu.2012.00131. PMid:22701116.
http://dx.doi.org/10.3389/fimmu.2012.001...
). Further, the protein components of NETs are potential risk factors for the lung; elastase increases the permeability of the alveolus-capillary barrier by cleaving actin in the endothelial cytoskeleton, which induces epithelial cell apoptosis and the pro-inflammatory cytokine release, thus aggravating inflammation (Lee and Downey, 2001LEE, W.L. and DOWNEY, G.P., 2001. Leukocyte elastase: physiological functions and role in acute lung injury. American Journal of Respiratory and Critical Care Medicine, vol. 164, no. 5, pp. 896-904. http://dx.doi.org/10.1164/ajrccm.164.5.2103040. PMid:11549552.
http://dx.doi.org/10.1164/ajrccm.164.5.2...
; Kawabata et al., 2002KAWABATA, K., HAGIO, T. and MATSUOKA, S., 2002. The role of neutrophil elastase in acute lung injury. European Journal of Pharmacology, vol. 451, no. 1, pp. 1-10. http://dx.doi.org/10.1016/S0014-2999(02)02182-9. PMid:12223222.
http://dx.doi.org/10.1016/S0014-2999(02)...
). ROS production by MPO causes damage to pulmonary epithelial cells, leading to apoptosis and necrosis (Grommes and Soehnlein, 2011GROMMES, J. and SOEHNLEIN, O., 2011. Contribution of neutrophils to acute lung injury. Molecular Medicine, vol. 17, no. 3-4, pp. 293-307. http://dx.doi.org/10.2119/molmed.2010.00138. PMid:21046059.
http://dx.doi.org/10.2119/molmed.2010.00...
; Nishinaka et al., 2011NISHINAKA, Y., ARAI, T., ADACHI, S., TAKAORI-KONDO, A. and YAMASHITA, K., 2011. Singlet oxygen is essential for neutrophil extracellular trap formation. Biochemical and Biophysical Research Communications, vol. 413, no. 1, pp. 75-79. http://dx.doi.org/10.1016/j.bbrc.2011.08.052. PMid:21871447.
http://dx.doi.org/10.1016/j.bbrc.2011.08...
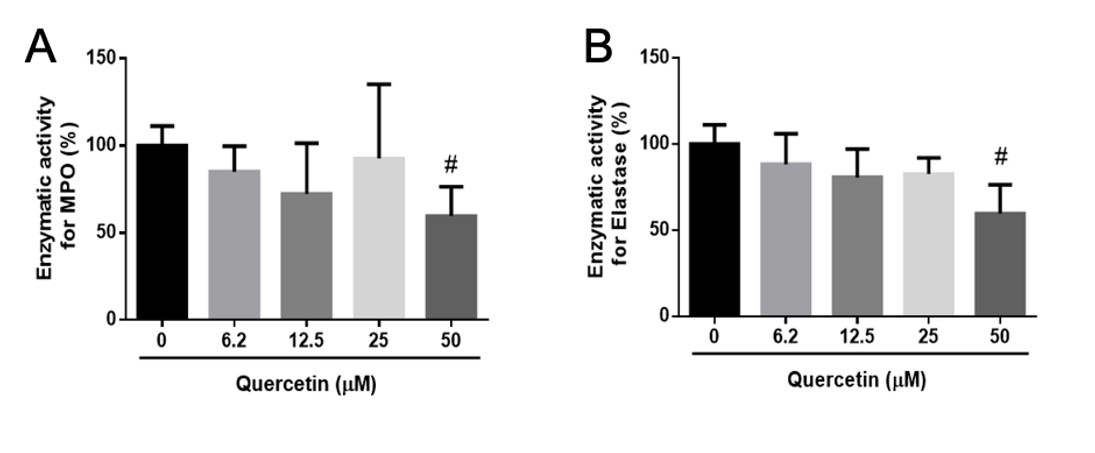
). We observed that quercetin reduced the release of NETs, MPO, and elastase. We further sought to evaluate whether quercetin could inhibit the enzymatic activity of these enzymes (Figure 3).
Quercetin reduces enzymatic activity of MPO and elastase. (A–B) Neutrophils were incubated for 4 h at 37°C, with PMA (50 nM). The enriched supernatant with MPO and elastase was incubated (30 min) with quercetin (6.2–50 μM) to measure enzymatic activity. The results represent the mean values from three independent assays performed in triplicate ± standard deviation (#p < 0.05 compared to the positive control, Quercetin 0 μM).
Supernatants from PMA-activated neutrophils, enriched with MPO and elastase, were incubated with quercetin for 30 min. Enzymatic activity was then tested using the specific substrates of MPO and elastase. Quercetin (50 μM) was found to diminish the enzymatic activity of MPO (Figure 3A) and elastase (Figure 3B). Lower quercetin concentrations had no effect. Although some authors have demonstrated similar results, the detailed mechanisms involved in hampering enzymatic activity remain vague. Previous studies have suggested that quercetin could be a co-substrate for MPO (Shiba et al., 2008SHIBA, Y., KINOSHITA, T., CHUMAN, H., TAKETANI, Y., TAKEDA, E., KATO, Y., NAITO, M., KAWABATA, K., ISHISAKA, A., TERAO, J. and KAWAI, Y., 2008. Flavonoids as substrates and inhibitors of myeloperoxidase: molecular actions of aglycone and metabolites. Chemical Research in Toxicology, vol. 21, no. 8, pp. 1600-1609. http://dx.doi.org/10.1021/tx8000835. PMid:18620432.
http://dx.doi.org/10.1021/tx8000835...
; Santos et al., 2014SANTOS, E.O.L., KABEYA, L.M., FIGUEIREDO-RINHEL, A.S.G., MARCHI, L.F., ANDRADE, M.F., PIATESI, F., PAOLIELLO-PASCHOALATO, A.B., AZZOLINI, A.E.C.S. and LUCISANO-VALIM, Y.M., 2014. Flavonols modulate the effector functions of healthy individuals’ immune complex-stimulated neutrophils: a therapeutic perspective for rheumatoid arthritis. International Immunopharmacology, vol. 21, no. 1, pp. 102-111. http://dx.doi.org/10.1016/j.intimp.2014.04.014. PMid:24797916.
http://dx.doi.org/10.1016/j.intimp.2014....
). Thus, the interaction between quercetin and MPO oxidizes the flavonoid and induces irreversible enzyme inhibition. Further, quercetin is reported to interact with and inhibit elastase depending on the presence of the catechol group at the flavonoid B-ring (Kanashiro et al., 2007KANASHIRO, A., SOUZA, J.G., KABEYA, L.M., AZZOLINI, A.E.C.S. and LUCISANO-VALIM, Y.M., 2007. Elastase release by stimulated neutrophils inhibited by flavonoids: importance of the catechol group. Zeitschrift fur Naturforschung. C, Journal of Biosciences, vol. 62, no. 5-6, pp. 357-361. http://dx.doi.org/10.1515/znc-2007-5-607. PMid:17708440.
http://dx.doi.org/10.1515/znc-2007-5-607...
).
We performed molecular docking analysis to evaluate the possible interaction between quercetin and MPO (Figure 4A) or elastase (Figure 4B). The simulations demonstrated that the Quercetin/MPO (Vina Score -8.1) and Quercetin/Elastase (Vina Score -6.4) complexes are thermodynamically favorable and stable. The aromatic rings of quercetin can establish 71 VDW contacts with amino acids near the heme radical and the catalytic site region (Davey and Fenna, 1996DAVEY, C.A. and FENNA, R.E., 1996. 2.3 Å resolution X-ray crystal structure of the bisubstrate analogue inhibitor salicylhydroxamic acid bound to human myeloperoxidase: a model for a prereaction complex with hydrogen peroxide. Biochemistry, vol. 35, no. 33, pp. 10967-10973. http://dx.doi.org/10.1021/bi960577m. PMid:8718890.
http://dx.doi.org/10.1021/bi960577m...
; Cockroft et al., 2005COCKROFT, S.L., HUNTER, C.A., LAWSON, K.R., PERKINS, J. and URCH, C.J., 2005. Electrostatic control of aromatic stacking interactions. Journal of the American Chemical Society, vol. 127, no. 24, pp. 8594-8595. http://dx.doi.org/10.1021/ja050880n. PMid:15954755.
http://dx.doi.org/10.1021/ja050880n...
). Quercetin was found at an average distance of 2.66 Å (1.45 3.88 Å) from nine amino acids (Arg239, Phe99, Thr100, Glu102, Phe147, Glu116, Met441, Pro145, and Leu415). Quercetin also approached the elastase catalytic site (Allen et al., 1996ALLEN, K.N., BELLAMACINA, C.R., DING, X., JEFFERY, C.J., MATTOS, C., PETSKO, G.A. and RINGE, D., 1996. An experimental approach to mapping the binding surfaces of crystalline proteins. Journal of Physical Chemistry, vol. 100, no. 7, pp. 2605-2611. http://dx.doi.org/10.1021/jp952516o.
http://dx.doi.org/10.1021/jp952516o...
; Mattos et al., 2006MATTOS, C., BELLAMACINA, C.R., PEISACH, E., PEREIRA, A., VITKUP, D., PETSKO, G.A. and RINGE, D., 2006. Multiple solvent crystal structures: probing binding sites, plasticity and hydration. Journal of Molecular Biology, vol. 357, no. 5, pp. 1471-1482. http://dx.doi.org/10.1016/j.jmb.2006.01.039. PMid:16488429.
http://dx.doi.org/10.1016/j.jmb.2006.01....
) where it was able to establish 57 VDW contacts with eight amino acids (Phe215, Val216, His57, Phe192, Ser195, Gly193, Cys42, and Phe41), maintaining an average distance of 2.8 Å (1.865 – 3.735 Å). The average distance shown by Quercetin/MPO (2.66 Å) and Quercetin/Elastase (2.8 Å) complexes are in a distance range (2.0–3.2 Å) considered favorable for hydrogen bond formation (Mancini et al., 2004MANCINI, A.L., HIGA, R.H., OLIVEIRA, A., DOMINIQUINI, F., KUSER, P.R., YAMAGISHI, M.E.B., TOGAWA, R.C. and NESHICH, G., 2004. STING contacts: a web-based application for identification and analysis of amino acid contacts within protein structure and across protein interfaces. Bioinformatics, vol. 20, no. 13, pp. 2145-2147. http://dx.doi.org/10.1093/bioinformatics/bth203. PMid:15073001.
http://dx.doi.org/10.1093/bioinformatics...
). In fact, because of the atom types, geometric criteria, and degree of interaction between quercetin and the receptors, hydrogen bonds were also predicted in the quercetin/MPO (2 H-bonds) and quercetin/elastase (3 H-bonds) complexes (Mancini et al., 2004MANCINI, A.L., HIGA, R.H., OLIVEIRA, A., DOMINIQUINI, F., KUSER, P.R., YAMAGISHI, M.E.B., TOGAWA, R.C. and NESHICH, G., 2004. STING contacts: a web-based application for identification and analysis of amino acid contacts within protein structure and across protein interfaces. Bioinformatics, vol. 20, no. 13, pp. 2145-2147. http://dx.doi.org/10.1093/bioinformatics/bth203. PMid:15073001.
http://dx.doi.org/10.1093/bioinformatics...
). Together, these data may provide a possible theoretical explanation for the inhibition of MPO and elastase activities by quercetin.
Molecular docking analyses from the best complexes of quercetin/MPO and quercetin/Elastase. The structure and catalytic sites of MPO (A) and elastase (B) enzymes are highlighted on the left, the lowest scored energy interactions of Quercetin and the receptors are detailed on the right. Vina Score = AutoDock Vina Score function and VDW Contacts = Van Der Waals surface overlap.
4. Conclusions
NETs show beneficial microbicidal effects but also show deleterious cytotoxic effects. The discovery of drugs that can reduce such effects can be used in the treatment of inflammatory diseases. We have demonstrated for the first time that quercetin reduces the cytotoxic effects of NETs (Figure 5). In addition, quercetin reduces NET release, as well as the liberation and enzymatic activity of MPO and elastase. Because in vitro assays are great for carrying out controlled studies, but they show limitations when compared to in vivo conditions, we believe that future studies may clarify whether quercetin could be an alternative or complementary therapeutic treatment strategy for NET-mediated tissue diseases.
Main conclusions and perspectives. Performing in vitro assays, we demonstrated that quercetin reduces release and cytotoxic effects of NETs, as well as the liberation and enzymatic activity of MPO and elastase. Future studies could clarify whether quercetin could be an alternative or complementary therapeutic treatment strategy for NET-mediated lung diseases.
Acknowledgements
We thank CAPES and FAPESP for providing financial assistance and UNESP for their structural support.
References
- ABDULLA, A., ZHAO, X. and YANG, F., 2013. Natural polyphenols inhibit lysine-specific demethylase-1 in vitro. Journal of Biochemical and Pharmacological Research, vol. 1, no. 1, pp. 56-63. PMid:23662249.
- ALLEN, K.N., BELLAMACINA, C.R., DING, X., JEFFERY, C.J., MATTOS, C., PETSKO, G.A. and RINGE, D., 1996. An experimental approach to mapping the binding surfaces of crystalline proteins. Journal of Physical Chemistry, vol. 100, no. 7, pp. 2605-2611. http://dx.doi.org/10.1021/jp952516o
» http://dx.doi.org/10.1021/jp952516o - BATIHA, G.E.-S., BESHBISHY, A.M., IKRAM, M., MULLA, Z.S., EL-HACK, M.E.A., TAHA, A.E., ALGAMMAL, A.M. and ELEWA, Y.H.A., 2020. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods, vol. 9, no. 3, p. 374. http://dx.doi.org/10.3390/foods9030374 PMid:32210182.
» http://dx.doi.org/10.3390/foods9030374 - BLACKBURN JUNIOR, W.D., HECK, L.W. and WALLACE, R.W., 1987. The bioflavonoid quercetin inhibits neutrophil degranulation, superoxide production, and the phosphorylation of specific neutrophil proteins. Biochemical and Biophysical Research Communications, vol. 144, no. 3, pp. 1229-1236. http://dx.doi.org/10.1016/0006-291X(87)91442-2 PMid:3034275.
» http://dx.doi.org/10.1016/0006-291X(87)91442-2 - BOOTS, A.W., HAENEN, G.R.M.M. and BAST, A., 2008. Health effects of quercetin: from antioxidant to nutraceutical. European Journal of Pharmacology, vol. 585, no. 2-3, pp. 325-337. http://dx.doi.org/10.1016/j.ejphar.2008.03.008 PMid:18417116.
» http://dx.doi.org/10.1016/j.ejphar.2008.03.008 - CARINI, M., STEFANI, R., ALDINI, G., OZIOLI, M. and FACINO, R.M., 2001. Procyanidins from Vitis vinifera seeds inhibit the respiratory burst of activated human neutrophils and lysosomal enzyme release. Planta Medica, vol. 67, no. 8, pp. 714-717. http://dx.doi.org/10.1055/s-2001-18353 PMid:11731911.
» http://dx.doi.org/10.1055/s-2001-18353 - COCKROFT, S.L., HUNTER, C.A., LAWSON, K.R., PERKINS, J. and URCH, C.J., 2005. Electrostatic control of aromatic stacking interactions. Journal of the American Chemical Society, vol. 127, no. 24, pp. 8594-8595. http://dx.doi.org/10.1021/ja050880n PMid:15954755.
» http://dx.doi.org/10.1021/ja050880n - DAVEY, C.A. and FENNA, R.E., 1996. 2.3 Å resolution X-ray crystal structure of the bisubstrate analogue inhibitor salicylhydroxamic acid bound to human myeloperoxidase: a model for a prereaction complex with hydrogen peroxide. Biochemistry, vol. 35, no. 33, pp. 10967-10973. http://dx.doi.org/10.1021/bi960577m PMid:8718890.
» http://dx.doi.org/10.1021/bi960577m - DELGADO-RIZO, V., MARTÍNEZ-GUZMÁN, M.A., IÑIGUEZ-GUTIERREZ, L., GARCÍA-OROZCO, A., ALVARADO-NAVARRO, A. and FAFUTIS-MORRIS, M., 2017. Neutrophil extracellular traps and its implications in inflammation: an overview. Frontiers in Immunology, vol. 8, p. 81. http://dx.doi.org/10.3389/fimmu.2017.00081 PMid:28220120.
» http://dx.doi.org/10.3389/fimmu.2017.00081 - DOUDA, D.N., JACKSON, R., GRASEMANN, H. and PALANIYAR, N., 2011. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. Journal of Immunology, vol. 187, no. 4, pp. 1856-1865. http://dx.doi.org/10.4049/jimmunol.1004201 PMid:21724991.
» http://dx.doi.org/10.4049/jimmunol.1004201 - ERPENBECK, L. and SCHÖN, M.P., 2017. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene, vol. 36, no. 18, pp. 2483-2490. http://dx.doi.org/10.1038/onc.2016.406 PMid:27941879.
» http://dx.doi.org/10.1038/onc.2016.406 - FARLEY, K., STOLLEY, J.M., ZHAO, P., COOLEY, J. and REMOLD-O’DONNELL, E., 2012. A SerpinB1 regulatory mechanism is essential for restricting neutrophil extracellular trap generation. Journal of Immunology, vol. 189, no. 9, pp. 4574-4581. http://dx.doi.org/10.4049/jimmunol.1201167 PMid:23002442.
» http://dx.doi.org/10.4049/jimmunol.1201167 - GABRIEL, C., MCMASTER, W.R., GIRARD, D. and DESCOTEAUX, A., 2010. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. Journal of Immunology, vol. 185, no. 7, pp. 4319-4327. http://dx.doi.org/10.4049/jimmunol.1000893 PMid:20826753.
» http://dx.doi.org/10.4049/jimmunol.1000893 - GROMMES, J. and SOEHNLEIN, O., 2011. Contribution of neutrophils to acute lung injury. Molecular Medicine, vol. 17, no. 3-4, pp. 293-307. http://dx.doi.org/10.2119/molmed.2010.00138 PMid:21046059.
» http://dx.doi.org/10.2119/molmed.2010.00138 - JABLONSKA, E., GARLEY, M., SURAZYNSKI, A., GRUBCZAK, K., IWANIUK, A., BORYS, J., MONIUSZKO, M. and RATAJCZAK-WRONA, W., 2020. Neutrophil extracellular traps (NETs) formation induced by TGF-β in oral lichen planus – possible implications for the development of oral cancer. Immunobiology, vol. 225, no. 2, p. 151901. http://dx.doi.org/10.1016/j.imbio.2019.151901 PMid:31882256.
» http://dx.doi.org/10.1016/j.imbio.2019.151901 - JORCH, S.K. and KUBES, P., 2017. An emerging role for neutrophil extracellular traps in noninfectious disease. Nature Medicine, vol. 23, no. 3, pp. 279-287. http://dx.doi.org/10.1038/nm.4294 PMid:28267716.
» http://dx.doi.org/10.1038/nm.4294 - KANASHIRO, A., SOUZA, J.G., KABEYA, L.M., AZZOLINI, A.E.C.S. and LUCISANO-VALIM, Y.M., 2007. Elastase release by stimulated neutrophils inhibited by flavonoids: importance of the catechol group. Zeitschrift fur Naturforschung. C, Journal of Biosciences, vol. 62, no. 5-6, pp. 357-361. http://dx.doi.org/10.1515/znc-2007-5-607 PMid:17708440.
» http://dx.doi.org/10.1515/znc-2007-5-607 - KASAMA, T., MIWA, Y., ISOZAKI, T., ODAI, T., ADACHI, M. and KUNKEL, S.L., 2005. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Current Drug Targets - Inflammation & Allergy, vol. 4, no. 3, pp. 273-279. http://dx.doi.org/10.2174/1568010054022114 PMid:16101533.
» http://dx.doi.org/10.2174/1568010054022114 - KAWABATA, K., HAGIO, T. and MATSUOKA, S., 2002. The role of neutrophil elastase in acute lung injury. European Journal of Pharmacology, vol. 451, no. 1, pp. 1-10. http://dx.doi.org/10.1016/S0014-2999(02)02182-9 PMid:12223222.
» http://dx.doi.org/10.1016/S0014-2999(02)02182-9 - KELLY, G.S., 2011. Quercetin. Monograph. Alternative Medicine Review, vol. 16, no. 2, pp. 172-194. PMid:21649459.
- KIRCHNER, T., HERMANN, E., MÖLLER, S., KLINGER, M., SOLBACH, W., LASKAY, T. and BEHNEN, M., 2013. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediators of Inflammation, vol. 2013, p. 710239. http://dx.doi.org/10.1155/2013/710239 PMid:24381411.
» http://dx.doi.org/10.1155/2013/710239 - LEE, W.L. and DOWNEY, G.P., 2001. Leukocyte elastase: physiological functions and role in acute lung injury. American Journal of Respiratory and Critical Care Medicine, vol. 164, no. 5, pp. 896-904. http://dx.doi.org/10.1164/ajrccm.164.5.2103040 PMid:11549552.
» http://dx.doi.org/10.1164/ajrccm.164.5.2103040 - LU, N., SUI, Y., TIAN, R. and PENG, Y.-Y., 2018. Inhibitive effects of quercetin on myeloperoxidase-dependent hypochlorous acid formation and vascular endothelial injury. Journal of Agricultural and Food Chemistry, vol. 66, no. 19, pp. 4933-4940. http://dx.doi.org/10.1021/acs.jafc.8b01537 PMid:29708335.
» http://dx.doi.org/10.1021/acs.jafc.8b01537 - MANCINI, A.L., HIGA, R.H., OLIVEIRA, A., DOMINIQUINI, F., KUSER, P.R., YAMAGISHI, M.E.B., TOGAWA, R.C. and NESHICH, G., 2004. STING contacts: a web-based application for identification and analysis of amino acid contacts within protein structure and across protein interfaces. Bioinformatics, vol. 20, no. 13, pp. 2145-2147. http://dx.doi.org/10.1093/bioinformatics/bth203 PMid:15073001.
» http://dx.doi.org/10.1093/bioinformatics/bth203 - MATTOS, C., BELLAMACINA, C.R., PEISACH, E., PEREIRA, A., VITKUP, D., PETSKO, G.A. and RINGE, D., 2006. Multiple solvent crystal structures: probing binding sites, plasticity and hydration. Journal of Molecular Biology, vol. 357, no. 5, pp. 1471-1482. http://dx.doi.org/10.1016/j.jmb.2006.01.039 PMid:16488429.
» http://dx.doi.org/10.1016/j.jmb.2006.01.039 - NAYAK, A., DODAGATTA-MARRI, E., TSOLAKI, A.G. and KISHORE, U., 2012. An insight into the diverse roles of surfactant proteins, SP-A and SP-D in innate and adaptive immunity. Frontiers in Immunology, vol. 3, p. 131. http://dx.doi.org/10.3389/fimmu.2012.00131 PMid:22701116.
» http://dx.doi.org/10.3389/fimmu.2012.00131 - NISHINAKA, Y., ARAI, T., ADACHI, S., TAKAORI-KONDO, A. and YAMASHITA, K., 2011. Singlet oxygen is essential for neutrophil extracellular trap formation. Biochemical and Biophysical Research Communications, vol. 413, no. 1, pp. 75-79. http://dx.doi.org/10.1016/j.bbrc.2011.08.052 PMid:21871447.
» http://dx.doi.org/10.1016/j.bbrc.2011.08.052 - NOSÁĽ, R., DRÁBIKOVÁ, K., JANČINOVÁ, V., PEREČKO, T., AMBROŽOVÁ, G., ČÍŽ, M., LOJEK, A., PEKAROVÁ, M., ŠMIDRKAL, J. and HARMATHA, J., 2014. On the molecular pharmacology of resveratrol on oxidative burst inhibition in professional phagocytes. Oxidative Medicine and Cellular Longevity, vol. 2014, p. 706269. http://dx.doi.org/10.1155/2014/706269 PMid:24672638.
» http://dx.doi.org/10.1155/2014/706269 - PILSCZEK, F.H., SALINA, D., POON, K.K.H., FAHEY, C., YIPP, B.G., SIBLEY, C.D., ROBBINS, S.M., GREEN, F.H.Y., SURETTE, M.G., SUGAI, M., BOWDEN, M.G., HUSSAIN, M., ZHANG, K. and KUBES, P., 2010. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. Journal of Immunology, vol. 185, no. 12, pp. 7413-7425. http://dx.doi.org/10.4049/jimmunol.1000675 PMid:21098229.
» http://dx.doi.org/10.4049/jimmunol.1000675 - PORTO, B.N. and STEIN, R.T., 2016. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Frontiers in Immunology, vol. 7, p. 311. http://dx.doi.org/10.3389/fimmu.2016.00311 PMid:27574522.
» http://dx.doi.org/10.3389/fimmu.2016.00311 - ROBASZKIEWICZ, A., BALCERCZYK, A. and BARTOSZ, G., 2007. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biology International, vol. 31, no. 10, pp. 1245-1250. http://dx.doi.org/10.1016/j.cellbi.2007.04.009 PMid:17583542.
» http://dx.doi.org/10.1016/j.cellbi.2007.04.009 - SAFFARZADEH, M., JUENEMANN, C., QUEISSER, M.A., LOCHNIT, G., BARRETO, G., GALUSKA, S.P., LOHMEYER, J. and PREISSNER, K.T., 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One, vol. 7, no. 2, p. e32366. http://dx.doi.org/10.1371/journal.pone.0032366 PMid:22389696.
» http://dx.doi.org/10.1371/journal.pone.0032366 - SANTOS, E.O.L., KABEYA, L.M., FIGUEIREDO-RINHEL, A.S.G., MARCHI, L.F., ANDRADE, M.F., PIATESI, F., PAOLIELLO-PASCHOALATO, A.B., AZZOLINI, A.E.C.S. and LUCISANO-VALIM, Y.M., 2014. Flavonols modulate the effector functions of healthy individuals’ immune complex-stimulated neutrophils: a therapeutic perspective for rheumatoid arthritis. International Immunopharmacology, vol. 21, no. 1, pp. 102-111. http://dx.doi.org/10.1016/j.intimp.2014.04.014 PMid:24797916.
» http://dx.doi.org/10.1016/j.intimp.2014.04.014 - SHIBA, Y., KINOSHITA, T., CHUMAN, H., TAKETANI, Y., TAKEDA, E., KATO, Y., NAITO, M., KAWABATA, K., ISHISAKA, A., TERAO, J. and KAWAI, Y., 2008. Flavonoids as substrates and inhibitors of myeloperoxidase: molecular actions of aglycone and metabolites. Chemical Research in Toxicology, vol. 21, no. 8, pp. 1600-1609. http://dx.doi.org/10.1021/tx8000835 PMid:18620432.
» http://dx.doi.org/10.1021/tx8000835 - SOUZA, P.S.S., BARBOSA, L.V., DINIZ, L.F.A., SILVA, G.S., LOPES, B.R.P., SOUZA, P.M.R., ARAUJO, G.C., PESSOA, D., OLIVEIRA, J., SOUZA, F.P. and TOLEDO, K.A., 2018. Neutrophil extracellular traps possess anti-human respiratory syncytial virus activity: possible interaction with the viral F protein. Virus Research, vol. 251, pp. 68-77. http://dx.doi.org/10.1016/j.virusres.2018.04.001 PMid:29621602.
» http://dx.doi.org/10.1016/j.virusres.2018.04.001 - STOCCO, B., TOLEDO, K., SALVADOR, M., PAULO, M., KOYAMA, N. and TOLOI, M.R.T., 2012. Dose-dependent effect of resveratrol on bladder cancer cells: chemoprevention and oxidative stress. Maturitas, vol. 72, no. 1, pp. 72-78. http://dx.doi.org/10.1016/j.maturitas.2012.02.004 PMid:22386766.
» http://dx.doi.org/10.1016/j.maturitas.2012.02.004 - VON KÖCKRITZ-BLICKWEDE, M., CHOW, O.A. and NIZET, V., 2009. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood, vol. 114, no. 25, pp. 5245-5246. http://dx.doi.org/10.1182/blood-2009-08-240713 PMid:20007813.
» http://dx.doi.org/10.1182/blood-2009-08-240713 - XIAO, X., SHI, D., LIU, L., WANG, J., XIE, X., KANG, T. and DENG, W., 2011. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS One, vol. 6, no. 8, p. e22934. http://dx.doi.org/10.1371/journal.pone.0022934 PMid:21857970.
» http://dx.doi.org/10.1371/journal.pone.0022934 - XU, D., HU, M.-J., WANG, Y.-Q. and CUI, Y.-L., 2019. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules, vol. 24, no. 6, p. 1123. http://dx.doi.org/10.3390/molecules24061123 PMid:30901869.
» http://dx.doi.org/10.3390/molecules24061123
Publication Dates
-
Publication in this collection
20 Apr 2022 -
Date of issue
2024
History
-
Received
03 June 2021 -
Accepted
21 Feb 2022