Abstract
Bioaccumulation of toxic heavy metals in the human body can give rise to adverse health effects, the severity of which depends upon their dosage and duration of exposure. In this study, yearlings of two different species of edible fish, i.e., Tor putitora (Mahseer) and Ctenopharyngodon Idella (grass carp), were exposed to different concentrations of lead nitrate in a controlled environment of aquarium for three different lengths of duration (14, 28, and 60 days). The bioaccumulation of lead in different organs, including gills, skin, muscles, liver, intestine, and swim bladder of the fish, was assessed using atomic absorption spectrometry. Generally, the highest lead concentration was observed in the gills and lowest in the muscles for both species at each experimental dosage and duration. In 14-days exposure, the relative pattern of bioaccumulation in different organs was observed as gill > liver > skin > intestine > swim bladder > muscle for both fish species. Similarly, the pattern of bioaccumulation observed in 28-days exposure was as: gill > liver > intestine > skin > swim bladder > muscle in both species. Whereas, pattern in 60-days exposure was observed as gill > liver > intestine > swim bladder > muscle > skin. The data shows that grass carp had stored higher concentrations of lead than Mahseer, which may be attributed to the fact that they are omnivorous. Furthermore, the lowest bioaccumulation was recorded in the muscles until the 56th day of the exposure, after which the concentration steadily increased in the muscles. The observed pattern highlights the importance of exposure’s duration to lead; chronic exposure could result in its bioaccumulation at toxic concentrations in the muscles, which is particularly of concern because the fish muscles are heavily consumed as food worldwide.
Keywords:
heavy metal accumulation in water; lead bioaccumulation of heavy metals in fish; Ctenopharyngodon idella; Tor putitora; lead toxicity
Resumo
A bioacumulação de metais pesados tóxicos no corpo humano pode causar efeitos adversos à saúde, cuja gravidade depende de sua dosagem e duração da exposição. Neste estudo, filhotes de duas espécies diferentes de peixes comestíveis, ou seja, Tor putitora (Mahseer) e Ctenopharyngodon idella (carpa-capim), foram expostos a diferentes concentrações de nitrato de chumbo em um ambiente controlado de aquário por três diferentes períodos de duração (14, 28 e 60 dias). A bioacumulação de chumbo em diferentes órgãos, incluindo brânquias, pele, músculos, fígado, intestino e bexiga natatória dos peixes, foi avaliada por espectrometria de absorção atômica. Geralmente, a maior concentração de chumbo foi observada nas brânquias e a menor nos músculos para ambas as espécies em cada dosagem e duração experimental. Na exposição de 14 dias, o padrão relativo de bioacumulação em diferentes órgãos foi observado como brânquia > fígado > pele > intestino > bexiga natatória > músculo para ambas as espécies de peixes. Da mesma forma, o padrão de bioacumulação observado em 28 dias de exposição foi: brânquia > fígado > intestino > pele > bexiga natatória > músculo em ambas as espécies. Já o padrão de exposição de 60 dias foi observado como brânquia > fígado > intestino > bexiga natatória > músculo > pele. Os dados mostram que a carpa-capim armazenou maiores concentrações de chumbo do que a Mahseer, o que pode ser atribuído ao fato de serem onívoras. Além disso, a menor bioacumulação foi registrada nos músculos até o 56º dia de exposição, após o que a concentração aumentou de forma constante nos músculos. O padrão observado destaca a importância da duração da exposição ao chumbo; a exposição crônica pode resultar em sua bioacumulação em concentrações tóxicas nos músculos, o que é particularmente preocupante porque os músculos dos peixes são muito consumidos como alimento em todo o mundo.
Palavras-chave:
acúmulo de metais pesados na água; bioacumulação de metais pesados por chumbo em peixes; Ctenopharyngodon idella; Tor putitora; toxicidade por chumbo
1. Introduction
The metals and metalloids having a density of at least 5 g cm−3 are known as heavy metals, and the heavy metals that are toxic even at ppb levels are broadly termed toxic heavy metals (Yadav et al., 2019YADAV, M., GUPTA, R. and SHARMA, R.K., 2019. Green and sustainable pathways for wastewater purification. In: S. AHUJA, ed. Advances in water purification techniques: meeting the needs of developed and developing countries. Amsterdam: Elsevier, pp. 355-383. http://dx.doi.org/10.1016/B978-0-12-814790-0.00014-4.
http://dx.doi.org/10.1016/B978-0-12-8147...
). Heavy metals are divided into two types: (i), essential: such as manganese, copper, iron, zinc, and nickel; (ii), non-essential: such as lead, cadmium, mercury, and arsenic. Heavy metals in a proper amount play a vital role in life processes, but their lower or higher than normal levels can create health problem/s (Chiba et al., 2011CHIBA, W.A.C., PASSERINI, M.D. and TUNDISI, J.G., 2011. Metal contamination in benthic macroinvertebrates in a sub-basin in the southeast of Brazil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 71, no. 2, pp. 391-399. http://dx.doi.org/10.1590/S1519-69842011000300008. PMid:21755156.
http://dx.doi.org/10.1590/S1519-69842011...
; Tabinda et al., 2013TABINDA, A.B., ZAFAR, S., ABDULLAH, Y. and MUNIR, S., 2013. Metals concentration in water, fodder, milk, meat, blood, kidney and liver of livestock and associated health impacts by intake of contaminated milk and meat. Pakistan Journal of Zoology, vol. 45, no. 4, pp. 1147-1150.). Because heavy metals have found numerous industrial, agricultural, domestic, pharmaceutical, and technological applications, humans are at an increased risk of exposure to these metals. Usually, heavy metals enter the water bodies through human activities like untreated domestic wastes, industrial effluents, and agriculture runoff (Seiyaboh and Izah, 2017SEIYABOH, E.I. and IZAH, S.C., 2017. Review of impact of anthropogenic activities in surface water resources in the Niger delta region of Nigeria: a case of Bayelsa state. International Journal of Ecotoxicology and Ecobiology, vol. 2, no. 2, pp. 61-73. http://dx.doi.org/10.11648/j.ijee.20170202.12.
http://dx.doi.org/10.11648/j.ijee.201702...
). Natural sources like landslides, cyclones, earthquakes, and rocks’ erosion are equally fundamental causes of heavy metal pollution in water bodies. Industrial effluents are one of the main contributors to heavy metals in water sources and their inclusion in the aqueous ecosystem where different aquatic organisms ingest them.
Heavy metal contamination is considered a severe threat to aquatic fauna, flora, and human health due to industrialization globally and locally (Kutty and Al-Mahaqeri, 2016KUTTY, A.A. and AL-MAHAQERI, S.A., 2016. An investigation of the levels and distribution of selected heavy metals in sediments and plant species within the vicinity of ex-iron mine in Bukit Besi. Journal of Chemistry, vol. 2016, p. 2096147. http://dx.doi.org/10.1155/2016/2096147.
http://dx.doi.org/10.1155/2016/2096147...
). The term bioaccumulation means an increase in the concentration of a substance that is not biodegradable in living organisms in such a way that they have a comparatively high concentration of the substance than that in its environment (Adepoju-Bello and Alabi, 2005ADEPOJU-BELLO, A.A. and ALABI, O.M., 2005. Heavy metals: a review. The Nigerian Journal of Pharmaceutics, vol. 37, pp. 41-45.). A fish gets heavy metals such as lead, copper, cadmium, and nickel from water bodies and becomes a potential source of the heavy metal for its consumer (Kouba et al., 2010KOUBA, A., BUŘIČ, M. and KOZÁK, P., 2010. Bioaccumulation and effects of heavy metals in crayfish: a review. Water, Air, and Soil Pollution, vol. 211, no. 1, pp. 5-16. http://dx.doi.org/10.1007/s11270-009-0273-8.
http://dx.doi.org/10.1007/s11270-009-027...
). Since they are not biodegradable and digestible and living bodies do not have specialized systems to excrete them, they accumulate in different organs. As most fish species are edible, the final sink is the human body as humans consume them for food. Due to enormous industrial developments in the 1960s and onward, they gradually concentrated in the aquatic system as initially no importance was given to their potential toxicity. Upon the emergence of severe health complications in humans due to the bioaccumulation of heavy metals, the scientific community responded, but it was too late. Presently, governments of different countries have taken measures, but the threshold of these metals in an ecosystem has already been reached. Worldwide, strategies have to be developed to remediate aquatic ecosystems, and preventive measures are essential to keep humans safe from toxic effects (Adepoju-Bello and Alabi, 2005ADEPOJU-BELLO, A.A. and ALABI, O.M., 2005. Heavy metals: a review. The Nigerian Journal of Pharmaceutics, vol. 37, pp. 41-45.; Tabinda et al., 2013TABINDA, A.B., ZAFAR, S., ABDULLAH, Y. and MUNIR, S., 2013. Metals concentration in water, fodder, milk, meat, blood, kidney and liver of livestock and associated health impacts by intake of contaminated milk and meat. Pakistan Journal of Zoology, vol. 45, no. 4, pp. 1147-1150.). Fish are eaten in almost all parts of the world, and their use is considered highly healthy and safe because of their constituent fat-soluble vitamins, high-quality proteins, and omega-3 fatty acids (Isangedighi and David, 2019ISANGEDIGHI, I.A. and DAVID, G.S., 2019. Heavy metals contamination in fish: effects on human health. Journal of Aquatic Science and Marine Biology, vol. 2, no. 4, pp. 7-12.). Screening different species of wild edible fish for heavy metal bioaccumulation is the need of the hour, and reporting such data would help prevent heavy metals toxicities in humans.
In this regard, proper check of the water resources is crucial where toxic, and trace metals are present in a narrow gap between their essentiality and poisonousness that could ultimately lead to deposition in the human body in one way or the other (Yousafzai and Shakoori, 2008YOUSAFZAI, A.M. and SHAKOORI, A.R., 2008. Heavy metal accumulation in the gills of an endangered south Asian freshwater fish as an indicator of aquatic pollution. Pakistan Journal of Zoology, vol. 40, no. 6, pp. 423-430.). Fish muscles are the central organ of interest in this connection as it is edible and consumed by humans (Taweel et al., 2013TAWEEL, A., SHUHAIMI-OTHMAN, M. and AHMAD, A.K., 2013. Assessment of heavy metals in tilapia fish (Oreochromis niloticus) from the Langat river and Engineering lake in Bangi, Malaysia, and evaluation of the health risk from tilapia consumption. Ecotoxicology and Environmental Safety, vol. 93, pp. 45-51. http://dx.doi.org/10.1016/j.ecoenv.2013.03.031. PMid:23642778.
http://dx.doi.org/10.1016/j.ecoenv.2013....
). Fish have been considered detectors of heavy metals in water bodies (Siraj et al., 2016SIRAJ, M., KHISROON, M. and KHAN, A., 2016. Bioaccumulation of heavy metals in different organs of Wallago attu from river Kabul Khyber Pakhtunkhwa, Pakistan. Biological Trace Element Research, vol. 172, no. 1, pp. 242-250. http://dx.doi.org/10.1007/s12011-015-0572-4. PMid:26637495.
http://dx.doi.org/10.1007/s12011-015-057...
), and the quality of water could be checked by assessing various biochemical/hematological/ histological parameters of the fish living in the specific water body. For instance, the accumulation of mercury in water bodies could be assessed through catfish. Likewise, Cyprinus carpio is considered a bio-indicator for Pb and Fe levels in water (Siregar et al., 2012SIREGAR, Y.I., ZAMRI, A. and PUTRA, H., 2012. Penyerapan timbal (Pb) pada sistim organ Ikan Mas (Cyprinus carpio L). Jurnal Ilmu Lingkungan, vol. 6, no. 1, pp. 43-51.). Heavy metals may enter the fish body through gills, skin, and the alimentary canal. Gills and skin are the sites of interaction between dissolved heavy metals and the fish body (Ahmed and Bibi, 2010AHMED, M.S. and BIBI, S., 2010. Uptake and bioaccumulation of waterborne lead (Pb) in the fingerlings of a freshwater cyprinid, Catla catla L. Journal of Animal and Plant Sciences, vol. 20, no. 3, pp. 201-207.). The thinner epithelium of gills allows the metal ions to pass through its membrane, where they could easily bind to the negative charges upon the gills’ surface (Siraj et al., 2014SIRAJ, M., SHAHEEN, M., STHANADAR, A.A., KHAN, A., CHIVERS, D.P. and YOUSAFZAI, A.M., 2014. A comparative study of bioaccumulation of heavy metals in two fresh water species, Aorichthys seenghala and Ompok bimaculatous at river Kabul, Khyber Pakhtunkhwa, Pakistan. Journal of Biodiversity and Environmental Sciences, vol. 4, no. 3, pp. 40-54.). A high concentration of heavy metal in gills is often indicative of its presence in the surrounding water (Pandey et al., 2017PANDEY, M., PANDEY, A.K., MISHRA, A. and TRIPATHI, B.D., 2017. Assessment of metal bioaccumulation in Mastacembelus armatus (eel) and exposure evaluation in human. Environmental Nanotechnology, Monitoring & Management, vol. 7, pp. 103-109. http://dx.doi.org/10.1016/j.enmm.2017.02.002.
http://dx.doi.org/10.1016/j.enmm.2017.02...
). The vertebrate’s brain, gills, and liver control essential life processes such as excretion, detoxification, respiration, and metabolism. The pesticides and chemical pollutants such as heavy metals severely affect these organs’ morphology and normal function and physiology of all vertebrate animals (Ghaffar et al., 2016GHAFFAR, A., HUSSAIN, R., ASLAM, M., ABBAS, G. and KHAN, A., 2016. Arsenic and urea in combination alters the hematology, biochemistry and protoplasm in exposed rahu fish (Labeo rohita) (Hamilton, 1822). Turkish Journal of Fisheries and Aquatic Sciences, vol. 16, no. 2, pp. 289-296. http://dx.doi.org/10.4194/1303-2712-v16_2_09.
http://dx.doi.org/10.4194/1303-2712-v16_...
). It is also reported that heavy metals like nickel, chromium, zinc, mercury, copper, and lead adversely affect the kidney, liver, muscles, and other organs, disrupt metabolism, and retard fish growth (Birge et al., 2000BIRGE, W.J., PRICE, D.J., SHAW, J.R., SPROMBERG, J.A., WIGGINTON, A.J. and HOGSTRAND, C., 2000. Metal body burden and biological sensors as ecological indicators. Environmental Toxicology and Chemistry: An International Journal, vol. 19, no. 4, pp. 1199-1212. http://dx.doi.org/10.1002/etc.5620190454.
http://dx.doi.org/10.1002/etc.5620190454...
).
Heavy metal causes toxic effects in humans such as hypertension, abdominal pain, kidney and liver problems, intellectual disabilities, irritability, carcinogenesis, anemia, nerve damage, and headache (Ambreen et al., 2015AMBREEN, F., JAVED, M. and BATOOL, U., 2015. Tissue specific heavy metals uptake in economically important fish, Cyprinus carpio at acute exposure of metals mixtures. Pakistan Journal of Zoology, vol. 47, no. 2, pp. 399-407.). Humans either use the contaminated water through which heavy metal/s enter their bodies directly or consume the affected fish through which heavy metal accumulates. Thus, it is important to know about heavy metal levels in freshwater concerning food chain transfer of these elements and the associated risk to human health (Scalon et al., 2010SCALON, M.C.S., RECHENMACHER, C., SIEBEL, A.M., KAYSER, M.L., RODRIGUES, M.T., MALUF, S.W., RODRIGUES, M.A.S. and SILVA, L.B.D., 2010. Evaluation of Sinos river water genotoxicity using the comet assay in fish. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 70, no. 4, (suppl.), pp. 1217-1222. http://dx.doi.org/10.1590/S1519-69842010000600011. PMid:21225163.
http://dx.doi.org/10.1590/S1519-69842010...
; Tunçsoy et al., 2016TUNÇSOY, M., DURAN, S., AY, Ö., CICIK, B. and ERDEM, C., 2016. Accumulation of copper in gill, liver, spleen, kidney and muscle tissues of Clarias gariepinus exposed to the metal singly and in mixture with chitosan. Bulletin of Environmental Contamination and Toxicology, vol. 97, no. 4, pp. 486-489. http://dx.doi.org/10.1007/s00128-016-1900-x. PMid:27502412.
http://dx.doi.org/10.1007/s00128-016-190...
).
Lead is the toxic metal that fundamentally targets the nervous system in adults and children. The lead is primarily present in inorganic form and has several oxidation states in the environment (Kasthuri and Chandran, 1997KASTHURI, J. and CHANDRAN, M.R., 1997. Sublethal effect of lead on feeding energetics, growth performance, biochemical composition and accumulation of the estuarine catfish, Mystus gulio(Hamilton). Journal of Environmental Biology, vol. 18, no. 1, pp. 95-101.). The sub-lethal concentration of lead results in de- creased growth rate, feeding energy, and deranged biochemical parameters of fish (Yousafzai and Shakoori, 2008YOUSAFZAI, A.M. and SHAKOORI, A.R., 2008. Heavy metal accumulation in the gills of an endangered south Asian freshwater fish as an indicator of aquatic pollution. Pakistan Journal of Zoology, vol. 40, no. 6, pp. 423-430.). A high lead level also affects neurochemicals development, such as ions channels and neurotransmitters (Amdur et al., 1993AMDUR, M.O., DOULL, J.M.D., and KLAASSEN, C.D., 1993. Casarett and Doull's Toxicology, Journal of Occupational Medicine, vol. 35, no. 1, pp. 76.). For better understanding, the current study was designed to investigate the lead concentration in Ctenopharyngodon Idella (grass carp), and Tor putitora (Mahseer) exposed to a specific concentration of lead nitrate in a controlled environment. The study’s primary objectives were to investigate the effect of lead accumulation in different organs.
2. Materials and Methods
2.1. Fish collection
The study was designed to quantify the heavy metal accumulation in the two fish species. The experimental work included 30 uniform yearlings of grass carp bought from the Mardan carp hatchery and 30 uniform yearlings of Mahseer brought from Mahseer hatchery at Thana Malakand. Fish were transported to the University of Malakand in oxygenated bags with care. The fish were placed in two separate large aquariums already set up for them. The sizes were 60-70 g in weight, and 6-7.5 cm in length measured accurately. The yearlings were acclimatized for about 14 days sustaining all the required conditions in their optimal range. All the aquariums were placed in the plan steel holding cages, one was labelled as the control group, and the other was treated. In each experiment, three fish were utilized. The amount of water in each aquarium was 50 litres. The fish of both the groups were fed with artificial fish feed twice a day at 3% of their body weight during the experimental period. Approval of the study was taken from Ethical Committee vide notification Biochem/EC-ChMu/22-11/01 as per approved “Animal Bye-Laws 2008, Scientific Procedures Issue-I of the University of Malakand, Pakistan.
2.2. Determination of lead toxicity in selected fish species in the aquarium
According to the present experimental plan, the yearlings of both fish species were exposed to lead nitrate in separate aquariums for different durations. Based on the duration of exposure to lead nitrate, yearlings of the two species were divided into three groups, each containing three fish. Of each species, the first group was exposed to 08 mg/50 lit lead nitrate for 14 days; the second group was exposed to the same amount of lead nitrate for 28 days; whereas the third group of fish was exposed to the same amount of lead nitrate for 56 days.
2.3. Organ collection
At the end of each experimental duration/cycle, the fish were sacrificed, and 0.5 g of each of the different organs, including skin, gills, muscles, liver, intestine, and swim bladder, were separated and washed with distilled water. After drying in an oven at 80-90°C, the tissues were subjected to acid digestion. Muscles were freed from their skin by cutting through knives and scissors behind the dorsal fin from the dorsal side (Rosseland et al., 2017ROSSELAND, B.O., TEIEN, H.C., BASNET, S., BORGSTRØM, R. and SHARMA, C.M., 2017. Trace elements and organochlorine pollutants in selected fish species from lake Phewa, Nepal. Toxicological and Environmental Chemistry, vol. 99, no. 3, pp. 390-401. http://dx.doi.org/10.1080/02772248.2016.1189915.
http://dx.doi.org/10.1080/02772248.2016....
). The skin was separated from the lateral side behind the anal fin and made free from muscles by hooks carefully.
2.4. Acid digestion
The acid digestion method of Javed and Usmani was followed with slight modification (Shahid et al., 2019SHAHID, A.J., JAVED, A.U., MUSHTAQUE, A.J., FARKHANDA, Z.D., and HAFIZULLAH, M., 2019. Investigation of different biochemical parameters in three economical important fish species captured from ponds of district Kashmore, Sindh, Pakistan. Int. J. Biosci, vol. 14, no. 4, pp. 387-392.). To each of the collected organs (0.5 g), 7.5mL of nitric acid was added. The beakers were labeled properly, made airtight, and kept still overnight. On the next day, 2.5 mL of HClO4 (70%) was added to each beaker and heated 80-1000C in an electric oven for 60 minutes. The mixture was heated until all the acid was converted into brown fumes of NO2 and the remaining solution became clear. After the solution in each beaker was cooled down at room temperature, it was filtered through Whatman filter paper. The filtrates were collected in labelled plastic bottles and diluted up to 50 ml using distilled water. The sample was analyzed for lead detection through atomic absorption spectrophotometer (Perkin-Elmer model no: 2380).
3. Results
3.1. Comparison of bioaccumulation of lead in different organs of Grass carp and Mahseer
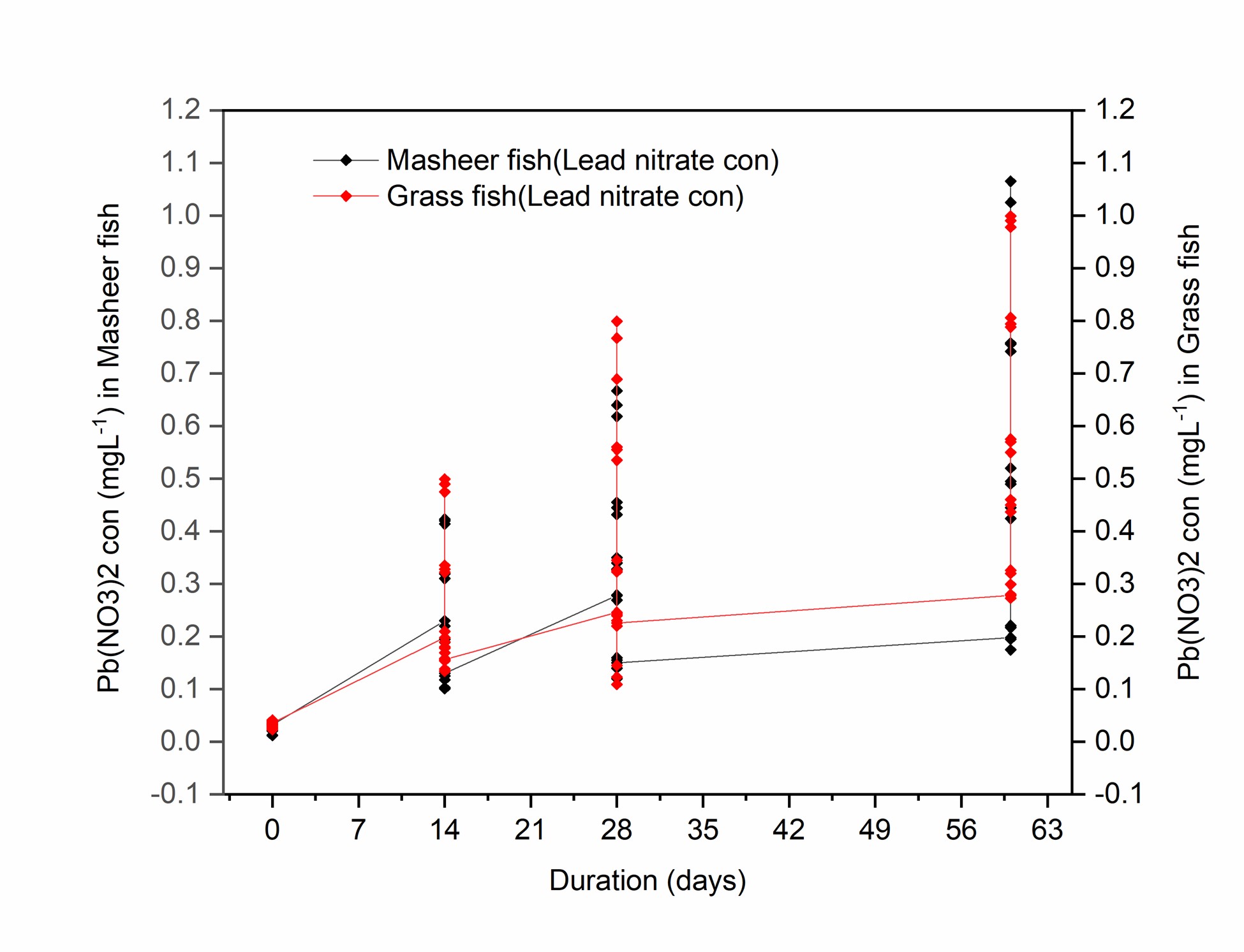
Lead accumulation in the different organs of both species was calculated in terms of lead nitrate concentration in the organs and is presented in Figure 1. The obtained results have been summarized in Table 1 whereas the week wise has been presented in Table 2 for Mahseer and in Table 3 for Grass carp.
Concentrations of Pb (NO3)2 in different organs of Mahseer and grass carp measured after different durations of exposure.
Bioaccumulation of lead nitrate in different organs of Mahseer after 14, 28, and 60 days of exposure to 08 mg/50 litres of lead nitrate.
Bioaccumulation of lead nitrate in different organs of grass carp after 14, 28, and 60-day exposure to 08 mg/50 lit of lead nitrate.
3.1.1. Skin
The mean lead concentration calculated was 0.199±0.010 in Mahseer and 0.215±.018 in grass carp after14 days of exposure. After 28 days, the accumulation was 0.275±.005 in Mahseer and 0.24±0.008 in grass carp; whereas, in 60-days exposure, the concentration was observed as 0.289±0.012 and 0.277±0.003, respectively. The maximum lead concentration was reported in the 60-days exposure period due to maximum exposure time.
3.1.2. Gill
The mean lead concentration in the gill of Mahseer was observed to be 0.419±0.004, and that of grass carp was 0.488±0.012 after 14-days exposure. After 28 days of the exposure, the concentration was 0.641±0.02 and 0.126±0.018 whereas, after 60-days exposure, it was recorded as 0.978±0.03 and 0.989±0.010 in the gills of Mahseer and grass carp, respectively. The maximum concentration of lead was reported in a 60-days exposure duration.
3.1.3. Muscles
The mean lead concentration in the muscles of Mahseer was 0.101±0.001 and 0.136±0.002 in grass carp after 14 days exposure; after 28 days, the concentration was 0.126±0.0183 in the muscles of Mahseer and in that of grass carp was 0.136±0.002. After 60 days of exposure, it was recorded as 0.319±0.002 and 0.315±0.014 in Mahseer and grass carp muscles, respectively. As clear from the results, the maximum lead concentration was reported in the 60-days exposure duration.
3.1.4. Liver
The mean lead concentration in the liver of Mahseer was quantified as 0.321±0.01 and 0.328±0.006 in the liver of Mahseer and grass carp, respectively, in 14-day exposure duration. The concentration in 28-day exposure was recorded to be 0.544±0.011in Mahseer’s liver and 0.55±0.013 in grass carp’s liver; while after 60 days, it was 0.785±0.008 and 0.796±0.009 in grass carp and Mahseer, respectively. The maximum concentration of lead was reported in a 60-day duration.
3.1.5. Intestine
In the intestine, the mean lead concentration of Mahseer was 0.185±0.006, and in grass fish, it was 0.176±0.005 after 14 days of exposure. In 28-day exposure, the accumulation was 0.339±0.11 and 0.332±0.011 in Mahseer and grass carp; while after 60 days, it was 0.565±0.013 in Mahseer and 0.565±0.013 in grass carp, respectively. Again, the maximum concentration of lead was observed in 60-day exposure.
3.1.6. Swim bladder
The mean lead concentration in the swim bladder of Mahseer was 0.122±0.006 that of grass fish was 0.156±0.002 after 14 days of exposure. After 28 days, the accumulation was 0.21±0.005 in Mahseer and 0.229±0.010 in the swim bladder of grass carp. After 60 days, the concentration was 0.44±0.013 in the swim bladder of Mahseer and 0.449±0.011 in that of grass carp. The maximum concentration of lead was observed after 60 days of exposure.
The order of lead nitrate bioaccumulation in the organs of both Mahseer and grass carp was gill > liver > skin > intestine > swim bladder > muscle in 14-days exposure to 8 mg/50 litres lead nitrate. After 28-days exposure, the order was gill > liver > intestine > skin > swim bladder > muscle in both fish species. Similarly, the pattern of bioaccumulation of lead nitrate in the organs of both species was gill > liver > intestine > swim bladder > muscle > skin in 60 days exposure to 8 mg/litres of lead nitrate.
The overall pattern of metal burden in Mahseer and grass carp in all exposure durations were observed as gill > liver > intestine.
4. Discussion
The threat of heavy metals toxic amounts in an environment, especially in water sources, has increased and is still increasing rapidly. Because of the non-biodegradable nature of these metals, they have a high potential of getting accumulated in the fish and other biota living in heavy-metal contaminated water bodies. The humans consuming those contaminated fish as food are equally at the risk of heavy metal accumulation that could lead to severe hazardous effects in the human body, including damage to the central nervous system and vital organs of the body. In the present study, the bioaccumulation profile of lead in the different organs of freshwater yearlings of grass carp and Mahseer was analyzed in different durations of exposure to lead nitrate (14, 28, and 60 days). The concentration of lead in the organs was analyzed using atomic absorption spectrophotometer. The general pattern of lead bioaccumulation observed uniformly is gill > liver > intestine observed in all durations of exposure; the lowest accumulation was observed in the muscles of both fish species in all exposure durations.
Results of the present studies are consistent with that of several reported studies, some of which are given as follows. Zhang et al. reported the same results (highest bioaccumulation of lead in gills and lowest in muscles) from their study to evaluate bioaccumulation of lead and its toxic effect in different organs of grass carp (Zhang et al., 2018ZHANG, J., ZHU, L., LI, F., LIU, C., QIU, Z., XIAO, M. and CAI, Y., 2018. Comparison of toxic metal distribution characteristics and health risk between cultured and wild fish captured from Honghu city, China. International Journal of Environmental Research and Public Health, vol. 15, no. 2, p. 334. http://dx.doi.org/10.3390/ijerph15020334. PMid:29443869.
http://dx.doi.org/10.3390/ijerph15020334...
). Likewise, Shovon studied bioaccumulation of lead and other heavy metals in the different organs of three species of fish viz., Labeo rohita, Gibelion Catla, and Pangasius hypophthalmus in Bangladesh. Their results also confirmed the highest amount of lead accumulation in the gills of Pangasius hypophthalmus and lowest in the muscles of all the three species (Shovon et al., 2017SHOVON, M.N.H., MAJUMDAR, B.C. and RAHMAN, Z., 2017. Heavy metals (lead, cadmium and nickel) concentration in different organs of three commonly consumed fishes in Bangladesh. Fisheries and Aquaculture Journal, vol. 8, no. 3, pp. 1-6.). Jakhrani et al. studied bioaccumulation of heavy metals and traced elements (iron, zinc, copper, manganese, nickel, cadmium, and chromium) in three different fish species viz., Catla catla, Cirrhinus mirigala and Labeo rohita caught from ponds in district Kashmore, Pakistan. The results showed that the bioaccumulation of heavy metals and trace elements was higher in gills than liver and muscles (Jakhrani et al., 2019JAKHRANI, S.A., UJJAN, J.A., JAKHRANI, M.A., DAYO, F.Z. and MAZARI, H., 2019. Investigation of different biochemical parameters in three economical important fish species captured from ponds of district Kashmore, Sindh, Pakistan. International Network for Natural Sciences, vol. 14, no. 4, pp. 387-392.). Iqbal also reported a similar bioaccumulation trend as in the present study, i.e., highest in the gills and lowest in the muscles when he carried out the studies in two fish species Labeo rohita and Wallago attu caught from Taunsa barrage of the Indus river in Pakistan (Iqbal et al., 2017IQBAL, A., TABINDA, A.B., YASAR, A. and MAHFOOZ, Y., 2017. Heavy metal uptake and toxicity in tissues of commercially important freshwater fish (Labeo rohita and Wallago attu) from the Indus river, Pakistan. Polish Journal of Environmental Studies, vol. 26, no. 2, pp. 627-633. http://dx.doi.org/10.15244/pjoes/66850.
http://dx.doi.org/10.15244/pjoes/66850...
).
The highest bioaccumulation of heavy metals in gills as reported in several studies could be attributed to two main reasons. First, because of its thinnest epithelium among all the organs of the body through which metals can easily pass (Mastan, 2014MASTAN, S.A., 2014. Heavy metals concentration in various tissues of two freshwater fishes, Labeo rohita and Channa striatus. African Journal of Environmental Science and Technology, vol. 8, no. 2, pp. 166-170. http://dx.doi.org/10.5897/AJEST2013.1540.
http://dx.doi.org/10.5897/AJEST2013.1540...
) and second, the surfaces of the gills are negatively charged whereas, the metal ions are positive charges due to which there is high affinity to the bonding between them (Siraj et al., 2016SIRAJ, M., KHISROON, M. and KHAN, A., 2016. Bioaccumulation of heavy metals in different organs of Wallago attu from river Kabul Khyber Pakhtunkhwa, Pakistan. Biological Trace Element Research, vol. 172, no. 1, pp. 242-250. http://dx.doi.org/10.1007/s12011-015-0572-4. PMid:26637495.
http://dx.doi.org/10.1007/s12011-015-057...
.
The bioaccumulation of heavy metals in the freshwater fish muscles is especially of concern because these are rich protein sources and are vastly consumed as human food (Tunçsoy et al., 2016TUNÇSOY, M., DURAN, S., AY, Ö., CICIK, B. and ERDEM, C., 2016. Accumulation of copper in gill, liver, spleen, kidney and muscle tissues of Clarias gariepinus exposed to the metal singly and in mixture with chitosan. Bulletin of Environmental Contamination and Toxicology, vol. 97, no. 4, pp. 486-489. http://dx.doi.org/10.1007/s00128-016-1900-x. PMid:27502412.
http://dx.doi.org/10.1007/s00128-016-190...
). As indicated by the results, the present study infers the accumulation of the lowest amount of lead in the muscles compared to the other organs, and several studies report the same.
Yousafzai studied the bioaccumulation of different heavy metals in Cyprinus carpio and Labeo rohita from river Kabul and reported the lowest bioaccumulation of different heavy metals in the muscles compared to other organs of the two species (Yousafzai and Shakoori, 2008YOUSAFZAI, A.M. and SHAKOORI, A.R., 2008. Heavy metal accumulation in the gills of an endangered south Asian freshwater fish as an indicator of aquatic pollution. Pakistan Journal of Zoology, vol. 40, no. 6, pp. 423-430.). Mulk studied the different heavy metals bioaccumulation in the Mahseer inhabiting in water bodies at Buner district, Pakistan, where the effluents of marble industry enter frequently. He, too, reported the lowest concentration of heavy metals in the muscles of Mahseer (Mulk et al., 2016MULK, S., KORAI, A.L., AZIZULLAH, A. and KHATTAK, M.N.K., 2016. Decreased fish diversity found near marble industry effluents in river Barandu, Pakistan. Ecotoxicology, vol. 25, no. 1, pp. 132-140. http://dx.doi.org/10.1007/s10646-015-1575-9. PMid:26497021.
http://dx.doi.org/10.1007/s10646-015-157...
).
Likewise, in Suyatna’s studies for comparative bioaccumulation of different heavy metals, the results followed the pattern as gill > liver > muscles (Suyatna, 2017).
Javeed and Afridi, in their studies carried out in different fish species for analysis of comparative bioaccumulation of heavy metals in different fish organs, also reported the lowest bioaccumulation of heavy metals in the muscles (Ambreen et al., 2015AMBREEN, F., JAVED, M. and BATOOL, U., 2015. Tissue specific heavy metals uptake in economically important fish, Cyprinus carpio at acute exposure of metals mixtures. Pakistan Journal of Zoology, vol. 47, no. 2, pp. 399-407.; Afridi et al., 2017AFRIDI, A.J., ZUBERI, A., REHMAN, H.U., KHAN, A., SAEED, K., ACHAKZAI, W.M., SADDOZAI, S., USMAN, K., ATEEQ, M. and AKBAR, N.U., 2017. Effect of the aquatic environment of different water bodies on metal contents of common carp (Cypriniuscarpio) collected from two different water bodies. Journal of Entomology and Zoology Studies, vol. 5, no. 1, pp. 388-399.).
5. Conclusion
The increasing industrial, domestic, and agricultural use of metal and metal-containing compounds has led to their higher-than-normal levels in an environment, especially in water bodies. Given their potential bioaccumulation of fish and the potential hazards in the human body after consuming these contaminated fish, screening fish found in different water bodies is the need of the hour. In this connection, the current study was designed to assess the bioaccumulation of lead in the different organs of Mahseer and grass carp after a different length of exposure to lead nitrate. The study showed that grass carp were more sensitive than Mahseer to lead toxicity. Compared to the other organs, the bioaccumulation of lead in gills was more pronounced than other organs in both the fish species in all exposure durations. The lowest heavy metal bioaccumulation was reported in the muscles, except after 56 days of exposure, the accumulation in the muscles was observed significantly high than observed previously. The observed results could be because the lead can be channelized through several ways and accumulate in muscles. The pattern of bioaccumulation in different organs of both the fish species in 14-day exposure to lead nitrate was gill > liver > skin > intestine > swim bladder > muscle. In 28-days exposure the pattern was gill > liver > intestine > skin > swim bladder > muscle; whereas, in 56 days, the pattern was gill > liver > intestine > swim bladder > muscle > skin. The probability of bioaccumulation in different organs increases with the duration of exposure to lead. The effect of length of duration was more noticeable in muscles, which is a matter of concern because it is the part that humans consume. Governments should take measures to ensure the safety measures before dumping the industrial effluents and sewage into water bodies to minimize the entry of heavy metals into water bodies so the hazards associated with heavy metal toxicity to aquatic biota and eventually to humans may be prevented or at least decreased.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP-2021/96), King Saud University, Riyadh, Saudi Arabia.
References
- ADEPOJU-BELLO, A.A. and ALABI, O.M., 2005. Heavy metals: a review. The Nigerian Journal of Pharmaceutics, vol. 37, pp. 41-45.
- AFRIDI, A.J., ZUBERI, A., REHMAN, H.U., KHAN, A., SAEED, K., ACHAKZAI, W.M., SADDOZAI, S., USMAN, K., ATEEQ, M. and AKBAR, N.U., 2017. Effect of the aquatic environment of different water bodies on metal contents of common carp (Cypriniuscarpio) collected from two different water bodies. Journal of Entomology and Zoology Studies, vol. 5, no. 1, pp. 388-399.
- AHMED, M.S. and BIBI, S., 2010. Uptake and bioaccumulation of waterborne lead (Pb) in the fingerlings of a freshwater cyprinid, Catla catla L. Journal of Animal and Plant Sciences, vol. 20, no. 3, pp. 201-207.
- AMBREEN, F., JAVED, M. and BATOOL, U., 2015. Tissue specific heavy metals uptake in economically important fish, Cyprinus carpio at acute exposure of metals mixtures. Pakistan Journal of Zoology, vol. 47, no. 2, pp. 399-407.
- AMDUR, M.O., DOULL, J.M.D., and KLAASSEN, C.D., 1993. Casarett and Doull's Toxicology, Journal of Occupational Medicine, vol. 35, no. 1, pp. 76.
- BIRGE, W.J., PRICE, D.J., SHAW, J.R., SPROMBERG, J.A., WIGGINTON, A.J. and HOGSTRAND, C., 2000. Metal body burden and biological sensors as ecological indicators. Environmental Toxicology and Chemistry: An International Journal, vol. 19, no. 4, pp. 1199-1212. http://dx.doi.org/10.1002/etc.5620190454
» http://dx.doi.org/10.1002/etc.5620190454 - CHIBA, W.A.C., PASSERINI, M.D. and TUNDISI, J.G., 2011. Metal contamination in benthic macroinvertebrates in a sub-basin in the southeast of Brazil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 71, no. 2, pp. 391-399. http://dx.doi.org/10.1590/S1519-69842011000300008 PMid:21755156.
» http://dx.doi.org/10.1590/S1519-69842011000300008 - GHAFFAR, A., HUSSAIN, R., ASLAM, M., ABBAS, G. and KHAN, A., 2016. Arsenic and urea in combination alters the hematology, biochemistry and protoplasm in exposed rahu fish (Labeo rohita) (Hamilton, 1822). Turkish Journal of Fisheries and Aquatic Sciences, vol. 16, no. 2, pp. 289-296. http://dx.doi.org/10.4194/1303-2712-v16_2_09
» http://dx.doi.org/10.4194/1303-2712-v16_2_09 - IQBAL, A., TABINDA, A.B., YASAR, A. and MAHFOOZ, Y., 2017. Heavy metal uptake and toxicity in tissues of commercially important freshwater fish (Labeo rohita and Wallago attu) from the Indus river, Pakistan. Polish Journal of Environmental Studies, vol. 26, no. 2, pp. 627-633. http://dx.doi.org/10.15244/pjoes/66850
» http://dx.doi.org/10.15244/pjoes/66850 - ISANGEDIGHI, I.A. and DAVID, G.S., 2019. Heavy metals contamination in fish: effects on human health. Journal of Aquatic Science and Marine Biology, vol. 2, no. 4, pp. 7-12.
- JAKHRANI, S.A., UJJAN, J.A., JAKHRANI, M.A., DAYO, F.Z. and MAZARI, H., 2019. Investigation of different biochemical parameters in three economical important fish species captured from ponds of district Kashmore, Sindh, Pakistan. International Network for Natural Sciences, vol. 14, no. 4, pp. 387-392.
- KASTHURI, J. and CHANDRAN, M.R., 1997. Sublethal effect of lead on feeding energetics, growth performance, biochemical composition and accumulation of the estuarine catfish, Mystus gulio(Hamilton). Journal of Environmental Biology, vol. 18, no. 1, pp. 95-101.
- KOUBA, A., BUŘIČ, M. and KOZÁK, P., 2010. Bioaccumulation and effects of heavy metals in crayfish: a review. Water, Air, and Soil Pollution, vol. 211, no. 1, pp. 5-16. http://dx.doi.org/10.1007/s11270-009-0273-8
» http://dx.doi.org/10.1007/s11270-009-0273-8 - KUTTY, A.A. and AL-MAHAQERI, S.A., 2016. An investigation of the levels and distribution of selected heavy metals in sediments and plant species within the vicinity of ex-iron mine in Bukit Besi. Journal of Chemistry, vol. 2016, p. 2096147. http://dx.doi.org/10.1155/2016/2096147
» http://dx.doi.org/10.1155/2016/2096147 - MASTAN, S.A., 2014. Heavy metals concentration in various tissues of two freshwater fishes, Labeo rohita and Channa striatus. African Journal of Environmental Science and Technology, vol. 8, no. 2, pp. 166-170. http://dx.doi.org/10.5897/AJEST2013.1540
» http://dx.doi.org/10.5897/AJEST2013.1540 - MULK, S., KORAI, A.L., AZIZULLAH, A. and KHATTAK, M.N.K., 2016. Decreased fish diversity found near marble industry effluents in river Barandu, Pakistan. Ecotoxicology, vol. 25, no. 1, pp. 132-140. http://dx.doi.org/10.1007/s10646-015-1575-9 PMid:26497021.
» http://dx.doi.org/10.1007/s10646-015-1575-9 - PANDEY, M., PANDEY, A.K., MISHRA, A. and TRIPATHI, B.D., 2017. Assessment of metal bioaccumulation in Mastacembelus armatus (eel) and exposure evaluation in human. Environmental Nanotechnology, Monitoring & Management, vol. 7, pp. 103-109. http://dx.doi.org/10.1016/j.enmm.2017.02.002
» http://dx.doi.org/10.1016/j.enmm.2017.02.002 - ROSSELAND, B.O., TEIEN, H.C., BASNET, S., BORGSTRØM, R. and SHARMA, C.M., 2017. Trace elements and organochlorine pollutants in selected fish species from lake Phewa, Nepal. Toxicological and Environmental Chemistry, vol. 99, no. 3, pp. 390-401. http://dx.doi.org/10.1080/02772248.2016.1189915
» http://dx.doi.org/10.1080/02772248.2016.1189915 - SCALON, M.C.S., RECHENMACHER, C., SIEBEL, A.M., KAYSER, M.L., RODRIGUES, M.T., MALUF, S.W., RODRIGUES, M.A.S. and SILVA, L.B.D., 2010. Evaluation of Sinos river water genotoxicity using the comet assay in fish. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 70, no. 4, (suppl.), pp. 1217-1222. http://dx.doi.org/10.1590/S1519-69842010000600011 PMid:21225163.
» http://dx.doi.org/10.1590/S1519-69842010000600011 - SEIYABOH, E.I. and IZAH, S.C., 2017. Review of impact of anthropogenic activities in surface water resources in the Niger delta region of Nigeria: a case of Bayelsa state. International Journal of Ecotoxicology and Ecobiology, vol. 2, no. 2, pp. 61-73. http://dx.doi.org/10.11648/j.ijee.20170202.12
» http://dx.doi.org/10.11648/j.ijee.20170202.12 - SHAHID, A.J., JAVED, A.U., MUSHTAQUE, A.J., FARKHANDA, Z.D., and HAFIZULLAH, M., 2019. Investigation of different biochemical parameters in three economical important fish species captured from ponds of district Kashmore, Sindh, Pakistan. Int. J. Biosci, vol. 14, no. 4, pp. 387-392.
- SHOVON, M.N.H., MAJUMDAR, B.C. and RAHMAN, Z., 2017. Heavy metals (lead, cadmium and nickel) concentration in different organs of three commonly consumed fishes in Bangladesh. Fisheries and Aquaculture Journal, vol. 8, no. 3, pp. 1-6.
- SIRAJ, M., KHISROON, M. and KHAN, A., 2016. Bioaccumulation of heavy metals in different organs of Wallago attu from river Kabul Khyber Pakhtunkhwa, Pakistan. Biological Trace Element Research, vol. 172, no. 1, pp. 242-250. http://dx.doi.org/10.1007/s12011-015-0572-4 PMid:26637495.
» http://dx.doi.org/10.1007/s12011-015-0572-4 - SIRAJ, M., SHAHEEN, M., STHANADAR, A.A., KHAN, A., CHIVERS, D.P. and YOUSAFZAI, A.M., 2014. A comparative study of bioaccumulation of heavy metals in two fresh water species, Aorichthys seenghala and Ompok bimaculatous at river Kabul, Khyber Pakhtunkhwa, Pakistan. Journal of Biodiversity and Environmental Sciences, vol. 4, no. 3, pp. 40-54.
- SIREGAR, Y.I., ZAMRI, A. and PUTRA, H., 2012. Penyerapan timbal (Pb) pada sistim organ Ikan Mas (Cyprinus carpio L). Jurnal Ilmu Lingkungan, vol. 6, no. 1, pp. 43-51.
- SUYATNA, I., SULISTYAWATI, ADNAN, A., SYAHRIR, M., GHITARINA, G., ABDUNNUR, A., and SALEH, S., 2017. Heavy metal levels in water and fish samples from coastal waters of Mahakam Delta, Kutai Kartanegara District, East Kalimantan, Indonesia. AACL Bioflux, vol. 10, no. 5, pp. 1319-1329.
- TABINDA, A.B., ZAFAR, S., ABDULLAH, Y. and MUNIR, S., 2013. Metals concentration in water, fodder, milk, meat, blood, kidney and liver of livestock and associated health impacts by intake of contaminated milk and meat. Pakistan Journal of Zoology, vol. 45, no. 4, pp. 1147-1150.
- TAWEEL, A., SHUHAIMI-OTHMAN, M. and AHMAD, A.K., 2013. Assessment of heavy metals in tilapia fish (Oreochromis niloticus) from the Langat river and Engineering lake in Bangi, Malaysia, and evaluation of the health risk from tilapia consumption. Ecotoxicology and Environmental Safety, vol. 93, pp. 45-51. http://dx.doi.org/10.1016/j.ecoenv.2013.03.031 PMid:23642778.
» http://dx.doi.org/10.1016/j.ecoenv.2013.03.031 - TUNÇSOY, M., DURAN, S., AY, Ö., CICIK, B. and ERDEM, C., 2016. Accumulation of copper in gill, liver, spleen, kidney and muscle tissues of Clarias gariepinus exposed to the metal singly and in mixture with chitosan. Bulletin of Environmental Contamination and Toxicology, vol. 97, no. 4, pp. 486-489. http://dx.doi.org/10.1007/s00128-016-1900-x PMid:27502412.
» http://dx.doi.org/10.1007/s00128-016-1900-x - YADAV, M., GUPTA, R. and SHARMA, R.K., 2019. Green and sustainable pathways for wastewater purification. In: S. AHUJA, ed. Advances in water purification techniques: meeting the needs of developed and developing countries Amsterdam: Elsevier, pp. 355-383. http://dx.doi.org/10.1016/B978-0-12-814790-0.00014-4
» http://dx.doi.org/10.1016/B978-0-12-814790-0.00014-4 - YOUSAFZAI, A.M. and SHAKOORI, A.R., 2008. Heavy metal accumulation in the gills of an endangered south Asian freshwater fish as an indicator of aquatic pollution. Pakistan Journal of Zoology, vol. 40, no. 6, pp. 423-430.
- ZHANG, J., ZHU, L., LI, F., LIU, C., QIU, Z., XIAO, M. and CAI, Y., 2018. Comparison of toxic metal distribution characteristics and health risk between cultured and wild fish captured from Honghu city, China. International Journal of Environmental Research and Public Health, vol. 15, no. 2, p. 334. http://dx.doi.org/10.3390/ijerph15020334 PMid:29443869.
» http://dx.doi.org/10.3390/ijerph15020334
Publication Dates
-
Publication in this collection
15 Apr 2022 -
Date of issue
2024
History
-
Received
25 Jan 2022 -
Accepted
10 Mar 2022