Abstract
Improving plant germination is essential to guarantee better quality seedlings. Thus, this research aimed to evaluate whether the seed priming with light quality (LIQ) and the aqueous extract of Cyperus rotundus (AEC) tuber could modulate the germination and initial growth of Moringa oleifera L. seedlings. The experimental design was a completely randomized in the 4x4 factorial scheme, composed of four LIQ conditions (white, blue, red, and distant red light) and four AEC concentrations (0, 25, 50 and 100%). Seed priming with red light reduced the average emergence time, while blue, red, and extreme red lights associated with 50% of aqueous extract of C. rotundus increased shoot initial length and photosynthetic pigment accumulation. Seed priming with blue light resulted in seedlings with a shorter final shoot length. However, application of 100% of aqueous extract of C. rotundus reversed this. The white light in combination with concentrations of 50 and 100% of AEC promoted a higher relative shoot growth rate of seedlings. The research revealed that seed priming with light quality and aqueous extracts of C. rotundus tubers modulates the germination and initial growth of M. oleifera seedlings. More work needs to be done to determine the responsible compounds in AEC that is responsible for priming growth as phytohormones.
Keywords:
germination; light spectrum; plant extract; plant hormones
Resumo
A melhoria da germinação de plantas é fundamental para garantia de mudas de melhor qualidade. Assim, objetivou-se avaliar se o condicionamento de sementes com qualidade de luz (light quality - LIQ) e extrato aquoso de tubérculos de Cyperus rotundus (AEC) modula a germinação e o crescimento inicial de plântulas de Moringa oleifera. Utilizou-se delineamento inteiramente casualizado, em esquema fatorial 4x4, sendo quatro condições de LIQ (luz branca, azul, vermelha e vermelho distante) e quatro concentrações de AEC (0, 25, 50 e 100%). O condicionamento de sementes com luz vermelha reduziu o tempo médio de emergência, enquanto que as luzes azul, vermelha e vermelho extremo associadas a 50% de extrato aquoso de C. rotundus aumentaram o comprimento inicial da parte aérea e o acúmulo de pigmentos fotossintéticos. Condicionamento de sementes com luz azul induziu a formação de plântulas com menor comprimento final da parte aérea, no entanto, a aplicação de 100% de extrato aquoso de C. rotundus reverteu o menor crescimento. A luz branca associada às concentrações de 50 e 100% de fitormônios promoveu maior taxa de crescimento relativo da parte aérea de plântulas. Nossa pesquisa mostra que o condicionamento de sementes com radiação espectral de luz e extrato aquoso de tubérculos de Cyperus rotundus modula a germinação e o crescimento inicial de plântulas de Moringa oleifera. Mais trabalhos precisam ser feitos para determinar os compostos do AEC responsáveis que que atuam como fitormônios e são responsáveis pelo crescimento inicial.
Palavras-chave:
germinação; espectro de luz; extrato vegetal; hormônios vegetais
1. Introduction
Improving plant germination is essential to guarantee better quality seedlings, especially under abiotic stress conditions (Silva et al., 2024SILVA, J.E.S.B., TORRES, S.B., LEAL, C.C.P., LEITE, M.S., GUIRRA, K.S., DANTAS, B.F., MORAIS, M.B. and GUIRRA, B.S., 2024. Pre-germination treatments of melon seeds for the production of seedlings irrigated with biosaline water. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 84, no. 1, p. e257314. PMid:35043840.). However, physiological treatments can improve seed performance under these conditions (Sá et al., 2022SÁ, F.V.S., OLIVEIRA, F.S., TORRES, S.B., PAIVA, E.P., NOGUEIRA, N.W., SARMENTO, E.C.S. and MELO, A.S., 2022. Hydric and saline stress on Phaseolus lunatus L. seeds. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 82, no. 11, p. e233550. http://dx.doi.org/10.1590/1519-6984.233550. PMid:34133547.
http://dx.doi.org/10.1590/1519-6984.2335...
). Physiological conditioning has been the most recent and interesting treatment for this purpose. This treatment synchronizes germination as much as possible through the activation of seed metabolism, seeking to reach a uniform level and as close as possible to the stage of primary root protrusion, via controlled hydration (Costa et al., 2022COSTA, A.A., PAIVA, E.P., TORRES, S.B., SOUZA NETA, M.L., PEREIRA, K.T.O., LEITE, M.S., SÁ, F.V.S. and BENEDITO, C.P., 2022. Osmoprotection in Salvia hispanica L. seeds under water stress attenuators. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 82, no. 1, p. e233547. http://dx.doi.org/10.1590/1519-6984.233547. PMid:34105656.
http://dx.doi.org/10.1590/1519-6984.2335...
).
The abovementioned situation sets pressure on the agricultural sector to set aside and prepare more arable land and production factors as water, fertilizers and pesticides, whose use which has been intensely by the negative effects of human actions on agro-ecosystems and climate change (Martins et al., 2019MARTINS, J.J.F., SOARES, A.M.V.M., AZEITEIRO, U.M. and CORREIA, M.L.T., 2019. Anthropic action effects caused by soybean farmers in a watershed of Tocantins—Brazil and its connections with climate change. In: P. CASTRO, A. AZUL, W. LEAL FILHO and U. AZEITEIRO, eds. Climate change-resilient agriculture and agroforestry. Cham: Springer, pp. 257-281. http://dx.doi.org/10.1007/978-3-319-75004-0_15.
http://dx.doi.org/10.1007/978-3-319-7500...
). Thus, the use of plant extracts can be a viable alternative, as it has lower cost and greater availability, such as the extract of Cyperus rotundus (Cyperaceae) tubers (Cavalcante et al., 2018CAVALCANTE, J.A., LOPES, K.P., PEREIRA, N.A.E., SILVA, J.G., PINHEIRO, R.M. and MARQUES, R.L.L., 2018. Extrato aquoso de bulbos de tiririca sobre a germinação e crescimento inicial de plântulas de rabanete. Revista Verde de Agroecologia e Desenvolvimento Sustentável, vol. 13, no. 1, pp. 39-44. http://dx.doi.org/10.18378/rvads.v13i1.5701.
http://dx.doi.org/10.18378/rvads.v13i1.5...
; Santos et al., 2021SANTOS, M.G.M., SILVA, W.C., RIBEIRO, P.H.P., BARRETTO, V.C.M., ROCHA, E.C., OLIVEIRA, R.C., LUZ, J.M.Q. and ARRUDA, A.S., 2021. Clonal propagation of Eucalyptus urophyllaunder effect of Cyperus rotundus extract and indole-3-acetic acid. Scientia Plena, vol. 17, no. 10, pp. 1-8. http://dx.doi.org/10.14808/sci.plena.2021.100201.
http://dx.doi.org/10.14808/sci.plena.202...
). Changes in climate variables such as temperature and light can influence seed germination and plant development, mainly due to variations in light quality and its relationship with plant photoreceptors (Bornman et al., 2019BORNMAN, J.F., BARNES, P.W., ROBSON, T.M., ROBINSON, S.A., JANSEN, M.A.K., BALLARÉ, C.L. and FLINT, S.D., 2019. Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems. Photochemical & Photobiological Sciences, vol. 18, no. 3, pp. 681-716. http://dx.doi.org/10.1039/C8PP90061B. PMid:30810560.
http://dx.doi.org/10.1039/C8PP90061B...
; Weber et al., 2019WEBER, S., DAMEROW, L., KUNZ, A. and BLANKE, M., 2019. Anthocyanin synthesis and light utilisation can be enhanced by reflective mulch – visualisation of light penetration into a tree canopy. Journal of Plant Physiology, vol. 233, pp. 52-57. http://dx.doi.org/10.1016/j.jplph.2018.12.008. PMid:30597476.
http://dx.doi.org/10.1016/j.jplph.2018.1...
).
Growing plants with potential for multiple applications (functional foods) is an important strategy to ensure sustainability and food security. Those plants have genotypic and phenotypic resilience to adjust to much more diverse agroecosystems as under an integrated crop-livestock-forest system (Bussoni et al., 2019BUSSONI, A., ALVAREZ, J., CUBBAGE, F., FERREIRA, G. and PICASSO, V., 2019. Diverse strategies for integration of forestry and livestock production. Agroforestry Systems, vol. 93, no. 1, pp. 333-344. http://dx.doi.org/10.1007/s10457-017-0092-7.
http://dx.doi.org/10.1007/s10457-017-009...
; Cortner et al., 2019CORTNER, O., GARRETT, R.D., VALENTIM, J.F., FERREIRA, J., NILES, M.T., REIS, J. and GIL, J., 2019. Perceptions of integrated crop-livestock systems for sustainable intensification in the Brazilian Amazon. Land Use Policy, vol. 82, no. 3, pp. 841-853. http://dx.doi.org/10.1016/j.landusepol.2019.01.006.
http://dx.doi.org/10.1016/j.landusepol.2...
; Melo et al., 2022MELO, A.S., MELO, Y.L., LACERDA, C.F., VIEGAS, P.R.A., FERRAZ, R.L.S. and GHEYI, H.R., 2022. Water restriction in cowpea plants [Vigna unguiculata (L.) Walp.]: metabolic changes and tolerance induction. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 26, no. 3, pp. 190-197. http://dx.doi.org/10.1590/1807-1929/agriambi.v26n3p190-197.
http://dx.doi.org/10.1590/1807-1929/agri...
). In that respect, Moringa oleifera Lam. has attained global significance because all its parts can be used for food, medicines, and industrial purposes (Liu et al., 2019LIU, J., CAI, H.-H., LI, H.-Q., LIU, Z.-Y., ZHENG, C., SHI, C. and NIU, Y.-F., 2019. The chloroplast genome of Moringa oleifera (Moringaceae). Mitochondrial DNA Part B, vol. 4, no. 1, pp. 646-647. http://dx.doi.org/10.1080/23802359.2018.1545550.
http://dx.doi.org/10.1080/23802359.2018....
; Macário et al., 2020MACÁRIO, A.P.S., FERRAZ, R.L.S., COSTA, P.S., BRITO NETO, J.F., MELO, A.S. and DANTAS NETO, J., 2020. Allometric models for estimating Moringa oleifera leaflets area. Ciência e Agrotecnologia, vol. 44, p. e005220. http://dx.doi.org/10.1590/1413-7054202044005220.
http://dx.doi.org/10.1590/1413-705420204...
; Parveen et al., 2024PARVEEN, S., RASOOL, F., AKRAM, M.N., KHAN, N., ULLAH, M., MAHMOOD, S., RABBANI, G. and MANZOOR, K., 2024. Effect of Moringa olifera leaves on growth and gut microbiota of Nile tilapia (Oreochromis niloticus). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 84, no. 1, p. e250916. PMid:34705952.).
Moringa oleifera belongs to the Moringaceae family, native to India and Pakistan (Domenico et al., 2019DOMENICO, M., LINA, C. and FRANCESCA, B., 2019. Sustainable crops for food security: moringa (Moringa oleifera Lam.). Encyclopedia of Food Security and Sustainability, vol. 1, pp. 409-415. http://dx.doi.org/10.1016/B978-0-08-100596-5.22574-2.
http://dx.doi.org/10.1016/B978-0-08-1005...
) and has been introduced as a cultivated crop in arid and semi-arid regions. It is an alternative for human and animal nutritional security due to its potential to provide essential amino acids, macronutrients, and micronutrients (Karthickeyan, 2019KARTHICKEYAN, V., 2019. Effect of cetane enhancer on Moringa oleifera biodiesel in a thermal coated direct injection diesel engine. Fuel, vol. 235, no. 1, pp. 538-550. http://dx.doi.org/10.1016/j.fuel.2018.08.030.
http://dx.doi.org/10.1016/j.fuel.2018.08...
; Páramo-Calderón et al., 2019PÁRAMO-CALDERÓN, D.E., APARICIO-SAGUILÁN, A., AGUIRRE-CRUZ, A., CARRILLO-AHUMADA, J., HERNÁNDEZ-URIBE, J.P., ACEVEDO-TELLO, S. and TORRUCO-UCO, J.G., 2019. Tortilla added with Moringa oleífera flour: physicochemical, texture properties and antioxidant capacity. LWT, vol. 100, pp. 409-415. http://dx.doi.org/10.1016/j.lwt.2018.10.078.
http://dx.doi.org/10.1016/j.lwt.2018.10....
). Furthermore, it can be used for water purification and has antifungal, analgesic, anti-inflammatory, antioxidant, antidiabetic, antitumor, and antibacterial activity (Garcia et al., 2019GARCIA, T.B., SOARES, A.A., COSTA, J.H., COSTA, H.P.S., NETO, J.X.S., ROCHA-BEZERRA, L.C.B., SILVA, F.D.A., ARANTES, M.R., SOUSA, D.O.B., VASCONCELOS, I.M. and OLIVEIRA, J.T.A., 2019. Gene expression and spatiotemporal localization of antifungal chitin-binding proteins during Moringa oleifera seed development and germination. Planta, vol. 249, no. 5, pp. 1503-1519. http://dx.doi.org/10.1007/s00425-019-03103-8. PMid:30706136.
http://dx.doi.org/10.1007/s00425-019-031...
).
Although M. oleifera is resilient and can with stand climate changes, however, events such as abiotic stresses that occur during its seed germination may influence seedlings' growth and development, showing a reduction in phytomass accumulation and fruit and seed yield (Hasan et al., 2019HASAN, M.M., ALHARBY, H.F., HAJAR, A.S., HAKEEM, K.R. and ALZAHRANI, Y., 2019. The effect of magnetized water on the growth and physiological conditions of Moringa species under drought stress. Polish Journal of Environmental Studies, vol. 28, no. 3, pp. 1145-1155. http://dx.doi.org/10.15244/pjoes/85879.
http://dx.doi.org/10.15244/pjoes/85879...
). Availability, quality, and time of exposure to light are critical for seed germination. Thus, M. oleifera reacts to light variations, chiefly because light influences factors such as soil temperature and humidity, air, and plant metabolism (Ahmed et al., 2014AHMED, L.T., WARRAG, E.I. and ABDELGADIR, A.Y., 2014. Effect of shade on seed germination on early seedling growth of Moringa oleifera Lam. Forest Products Industries, vol. 3, no. 1, pp. 20-26.; Silva et al., 2020SILVA, A.E., FERRAZ, R.L.S., SILVA, J.P., COSTA, P.S., VIÉGAS, P.R.A., BRITO NETO, J.F., MELO, A.S., MEIRA, K.S., SOARES, C.S., MAGALHÃES, I.D. and MEDEIROS, A.S., 2020. Microclimate changes, photomorphogenesis and water consumption of Moringa oleifera cuttings under different light spectrums and exogenous phytohormone concentrations. Australian Journal of Crop Science, vol. 14, no. 5, pp. 751-760. http://dx.doi.org/10.21475/ajcs.20.14.05.p2096.
http://dx.doi.org/10.21475/ajcs.20.14.05...
).
The response of photoreceptors to light plays a crucial role in plants' physiological processes. Light signals transduction involves important biochemical events for biosynthesis and action of phytohormones responsible for photomorphogenic changes and tolerance to abiotic stresses (Matsuo et al., 2019MATSUO, S., NANYA, K., IMANISHI, S., HONDA, I. and GOTO, E., 2019. Effects of blue and red lights on gibberellin metabolism in tomato seedlings. The Horticulture Journal, vol. 88, no. 1, pp. 76-82. http://dx.doi.org/10.2503/hortj.UTD-005.
http://dx.doi.org/10.2503/hortj.UTD-005...
; Polesi et al., 2019POLESI, L.G., FRAGA, H.P.F., VIEIRA, L.N., HERINGER, A.S., ORNELLAS, T.S., SANTOS, H.P., GUERRA, M.P. and PESCADOR, R., 2019. Chloroplast ultrastructure and hormone endogenous levels are differently affected under light and dark conditions during in vitro culture of Guadua chacoensis (Rojas) Londoño & P. M. Peterson. Acta Physiologiae Plantarum, vol. 41, no. 1, p. 10. http://dx.doi.org/10.1007/s11738-018-2804-7.
http://dx.doi.org/10.1007/s11738-018-280...
; Vaishak et al., 2019VAISHAK, K.P., YADUKRISHNAN, P., BAKSHI, S., KUSHWAHA, A.K., RAMACHANDRAN, H., JOB, N., BABU, D. and DATTA, S., 2019. The B-box bridge between light and hormones in plants. Journal of Photochemistry and Photobiology B: Biology, vol. 191, no. 2, pp. 164-174. http://dx.doi.org/10.1016/j.jphotobiol.2018.12.021. PMid:30640143.
http://dx.doi.org/10.1016/j.jphotobiol.2...
). Despite the importance of the interaction between light and phytohormones in plant development, little is known about the combined effect of those constituents on seed germination and initial seedling growth of M. oleifera.
Auxins, gibberellins, cytokinins, abscisic acid, ethylene, brassinosteroids, and strigolactones are phytohormones that regulate seed germination and plant growth and development (Pawela et al., 2019PAWELA, A., BANASIAK, J., BIAŁA, W., MARTINOIA, E. and JASIŃSKI, M., 2019. MtABCG20 is an ABA exporter influencing root morphology and seed germination of Medicago truncatula. The Plant Journal, vol. 98, no. 3, pp. 511-523. http://dx.doi.org/10.1111/tpj.14234. PMid:30661269.
http://dx.doi.org/10.1111/tpj.14234...
; Peres et al., 2019PERES, A.L.G.L., SOARES, J.S., TAVARES, R.G., RIGHETTO, G., ZULLO, M.A.T., MANDAVA, N.B. and MENOSSI, M., 2019. Brassinosteroids, the sixth class of phytohormones: a molecular view from the discovery to hormonal interactions in plant development and stress adaptation. International Journal of Molecular Sciences, vol. 20, no. 2, p. 331. http://dx.doi.org/10.3390/ijms20020331. PMid:30650539.
http://dx.doi.org/10.3390/ijms20020331...
). These hormones have been obtained from synthetic and natural sources such as 3-indol butyric acid (auxin), present in Cyperus rotundus tubers, which can be utilized as a technology to promote dormancy break and seed germination (Cavalcante et al., 2018CAVALCANTE, J.A., LOPES, K.P., PEREIRA, N.A.E., SILVA, J.G., PINHEIRO, R.M. and MARQUES, R.L.L., 2018. Extrato aquoso de bulbos de tiririca sobre a germinação e crescimento inicial de plântulas de rabanete. Revista Verde de Agroecologia e Desenvolvimento Sustentável, vol. 13, no. 1, pp. 39-44. http://dx.doi.org/10.18378/rvads.v13i1.5701.
http://dx.doi.org/10.18378/rvads.v13i1.5...
; Rifna et al., 2019RIFNA, E.J., RAMANAN, K.R. and MAHENDRAN, R., 2019. Emerging technology applications for improving seed germination. Trends in Food Science & Technology, vol. 86, no. 4, pp. 95-108. http://dx.doi.org/10.1016/j.tifs.2019.02.029.
http://dx.doi.org/10.1016/j.tifs.2019.02...
).
Seed germination is a complex physiological process that starts with water absorption and ends with radicle protrusion (Tuan et al., 2019TUAN, P.A., SUN, M., NGUYEN, T.N., PARK, S. and AYELE, B.T., 2019. Molecular mechanisms of seed germination. In: H. FENG, B. NEMZER and J.W. DEVRIES, eds. Sprouted grains: nutritional value, production and applications. Cambridge: AACC International Press, pp. 1-24. http://dx.doi.org/10.1016/B978-0-12-811525-1.00001-4.
http://dx.doi.org/10.1016/B978-0-12-8115...
). Hence, seed pre-treatment consists of controlling the water content of these germinative structures to partially activate the germination processes, however it is observed that the seed prime can prevent the total seed germination and those may return to quiescence phase (Sano and Seo, 2019SANO, N. and SEO, M., 2019. Cell cycle inhibitors improve seed storability after priming treatments. Journal of Plant Research, vol. 132, no. 2, pp. 263-271. http://dx.doi.org/10.1007/s10265-018-01084-5. PMid:30637553.
http://dx.doi.org/10.1007/s10265-018-010...
).
Based on the above considerations, it has been predicted that seed priming, varying the light spectrum radiation conditions and concentration of aqueous extract from C. rotundus tubers, can increase seed germination, seedling growth, and the balance of chloroplast pigments. Thus, the objective was to evaluate whether the seed priming with light spectral radiation and aqueous extract from C. rotundus tubers modulates seeds' physiological quality and growth and chloroplast pigments accumulation in M. oleifera seedlings.
2. Material and Method
2.1. Geographical localization
The research was carried out between October and December 2018, at the Phytopathology Laboratory, in a screen-protected environment at the Center for Agricultural and Environmental Sciences (CCAA) of Paraiba State University (UEPB), located in the municipality of Lagoa Seca - PB, in coordinates Latitude 7º 09 'S, Longitude 35º 52' W, and 634 m of altitude (Soares et al., 2017SOARES, C.S., SILVA, J.A. and SILVA, G.N., 2017. Produção de coentro em diferentes espaçamentos dos canais hidropônicos. Pesquisa Agropecuária Pernambucana, vol. 22, pp. 1-5. http://dx.doi.org/10.12661/pap.2017.001.
http://dx.doi.org/10.12661/pap.2017.001...
). The climate of the region according to the Köppen classification is As' (humid tropical), with an average annual temperature of 22 ºC, with minimum and maximum temperatures of 18 ºC and 33 ºC respectively, rainfall of 800 mm, and relative humidity 80% (Silva et al., 2020SILVA, A.E., FERRAZ, R.L.S., SILVA, J.P., COSTA, P.S., VIÉGAS, P.R.A., BRITO NETO, J.F., MELO, A.S., MEIRA, K.S., SOARES, C.S., MAGALHÃES, I.D. and MEDEIROS, A.S., 2020. Microclimate changes, photomorphogenesis and water consumption of Moringa oleifera cuttings under different light spectrums and exogenous phytohormone concentrations. Australian Journal of Crop Science, vol. 14, no. 5, pp. 751-760. http://dx.doi.org/10.21475/ajcs.20.14.05.p2096.
http://dx.doi.org/10.21475/ajcs.20.14.05...
).
2.2. Experimental design
Seed priming application was performed in a completely randomized experimental design, in a 4x4 factorial scheme, with four replications with 24 seeds (Pereira et al., 2015PEREIRA, K.T.O., SANTOS, B.R.V., BENEDITO, C.P., LOPES, E.G. and AQUINO, G.S.M., 2015. Germinação e vigor de sementes de Moringa oleifera Lam. em diferentes substratos e temperaturas. Revista Caatinga, vol. 28, no. 2, pp. 92-99.). The factors consisted of four conditions light quality (LIQ) measured with a digital lux meter model LD-400 (WL = white light with emission of 202 lumens m-2, BL = blue light with emission of 108 lumens m-2 and wavelengths from 400 to 485 nm, RL = red light with emission of 184 lumens m-2 and wavelength 600 to 680 nm, and FR = distant red light with emission of 32 lumens m-2 and wavelength 680 to 720 nm) plus four concentrations of aqueous extract of C. rotundus (AEC0 = control 0%, AEC25 = 25%, AEC50 = 50% and AEC100 = 100%).
2.3. Light quality treatments
Biochemical Oxygen Demand (B.O.D.) germinating chamber equipped with LED strips with white light (emission of 205 lumens m-2) was used as a light source. Transparent gearboxes (11x11x3.5 cm in length, width and height) were used to obtain the light quality. For obtain of white, blue and red lights, the boxes were covered with four layers of clear, blue and red cellophane, respectively. For obtain of distant red light, the boxes were covered with two layers of blue and two red cellophane, for a total of four layers (Yamashita et al., 2011YAMASHITA, O.M., GUIMARÃES, S.C. and CAVENAGHI, A.L., 2011. Germinação das sementes de Conyza canadensis e Conyza bonariensis em função da qualidade de luz. Planta Daninha, vol. 29, no. 4, pp. 737-743. http://dx.doi.org/10.1590/S0100-83582011000400003.
http://dx.doi.org/10.1590/S0100-83582011...
).
2.4. Preparation of aqueous extract from Cyperus rotundus tuber
Cyperus rotundus tubers were obtained at CCAA/UEPB Experimental Field (Latitude 7 ° 09 'S, Longitude 35 ° 52' W, and altitude 634 m) (Silva Filho et al., 2016). Fresh tubers without shot and roots were washed with running water and neutral detergent and dried to a constant weight under shed. The dried plant material was ground into powder. The powder, 10.0 g was extracted with 200 mL of distilled water on a sonication bath for 1hr followed by filtration through a Whatman No. 1 filter paper to obtain a stock solution with 100% of extract concentration (AEC100) (Simões et al., 2003SIMÕES, C.M.O., SCHENKEL, E.P., GOSMANN, G., MELLO, J.C.P., MENTZ, L.A. and PETROVICK, P.R., 2003. Farmacognosia: da planta ao medicamento. 5th ed. Florianópolis: Editora da UFSC, 1102 p.).
Concentrations corresponding to each treatment were obtained by diluting the stock solution (AEC100) with distilled water as follows: control treatment (AEC0 = 0%) just distilled water, (AEC25 = 25%) dilution with 75% distilled water + 25% stock solution, (AEC50 = 50%) dilution with 50% distilled water + 50% stock solution, (AEC100 = 100%) stock solution without dilution with distilled water (Rezende et al., 2013REZENDE, F.P.F., ZUFFELLATO-RIBAS, K.C. and KOEHLER, H.S., 2013. Aplicação de extratos de folhas e tubérbulos de Cyperus rotundus L. e de auxinas sintéticas na estaquia caulinar de Duranta repens L. Revista Brasileira de Plantas Medicinais, vol. 15, no. 4, suppl. 1, pp. 639-645. http://dx.doi.org/10.1590/S1516-05722013000500003.
http://dx.doi.org/10.1590/S1516-05722013...
; Scariot et al., 2017SCARIOT, E., BONOME, L.T.S., BITTENCOURT, H.V.H. and LIMA, C.S.M., 2017. Extrato aquoso de Cyperus rotundus no enraizamento de estacas lenhosas de Prunus persica cv. ‘Chimarrita’. Revista de Ciências Agroveterinárias, vol. 16, no. 2, pp. 195-200. http://dx.doi.org/10.5965/223811711622017195.
http://dx.doi.org/10.5965/22381171162201...
).
2.5. Seed priming application
Seeds of Moringa oleifera Lam., obtained from a domesticated variety (2018 harvest), lot 00016, were used in the experiment. Initially, 4 samples (100 seeds weighing 30 g) were characterized by length 10.59 ± 1.58 mm, width 9.61 ± 0.90 mm, weighing 0.29 ± 0.06 g, with electrical conductivity of 172 16 ± 41.82 μS cm-1 g-1, moisture content of 11.57 ± 0.30%, 96% pureness, 4% inert matter, and 90% germination. Afterward, seeds went through a cleaning process with sodium hypochlorite (1%) for 3 minutes (Carvalho and Carvalho, 2009CARVALHO, D.B. and CARVALHO, R.I.N., 2009. Qualidade fisiológica de sementes de guanxuma em influência do envelhecimento acelerado e da luz. Acta Scientiarum. Agronomy, vol. 31, no. 3, pp. 489-494. http://dx.doi.org/10.4025/actasciagron.v31i3.585.
http://dx.doi.org/10.4025/actasciagron.v...
). The procedure was carried out under a green light, recognized as a safety light because it does not influence phytochromes (Parreira et al., 2011PARREIRA, M.C., CARDOZO, N.P., GIANCOTTI, P.R.F. and ALVES, P.L.A.S., 2011. Germinação de sementes de melão-de-são-caetano sob variação de água, luz e temperatura. Bioscience Journal, vol. 27, no. 3, pp. 363-370.).
Each box received a substrate composed of two layers of 'germitest' paper sheet, sprayed with solutions matching each AEC concentration with added volume corresponding to approximately 2.5 times germitest paper dry mass (Ferreira et al., 2017FERREIRA, D.T.R.G., SILVA, V.M., SILVA, I.C., ARAÚJO NETO, J.C., SOUZA, R.C. and FERREIRA, V.M., 2017. Germinação de três Euphorbiaceae influenciada pela luz e níveis de palhada. Revista Agro@mbiente On-line, vol. 11, no. 3, pp. 215-222. http://dx.doi.org/10.18227/1982-8470ragro.v11i3.3852.
http://dx.doi.org/10.18227/1982-8470ragr...
). The boxes and seeds were stored in a Biochemical Oxygen Demand (B.O.D.) germinating chamber, at a temperature of 30 ± 5 °C and photoperiod of 8 hours (Pereira et al., 2015PEREIRA, K.T.O., SANTOS, B.R.V., BENEDITO, C.P., LOPES, E.G. and AQUINO, G.S.M., 2015. Germinação e vigor de sementes de Moringa oleifera Lam. em diferentes substratos e temperaturas. Revista Caatinga, vol. 28, no. 2, pp. 92-99.).
Seed priming was applied for 24 h, the time necessary for soaking the seeds (phase II) without concluding the germination process (Guimarães et al., 2008GUIMARÃES, M.A., DIAS, D.C.F.S. and LOUREIRO, M.E., 2008. Hidratação de sementes. Revista Trópica – Ciências Agrárias e Biológicas, vol. 2, no. 1, pp. 31-39.). Next, seeds were dried on polyethylene trays (30 cm x 20 cm x 5 cm length, width, and height, respectively) for 24 h. The trays were covered with two layers of absorbent paper and covered with cellophane in the colors matching the same lighting conditions applied in the gearboxes.
2.6. Seed sowing
After these priming treatments, 48 hours after drying, seeds were sown at a depth of 0.02 m in polyethylene trays, filled with 3.0 dm3 of autoclaved sandy substrate, and its humidity maintained between 90 and 100% of field capacity (CC). Trays were kept in shaded conditions with a 15% reduction of natural light. Irrigation management was carried out every day by the weighing method (Silva et al., 2020SILVA, A.E., FERRAZ, R.L.S., SILVA, J.P., COSTA, P.S., VIÉGAS, P.R.A., BRITO NETO, J.F., MELO, A.S., MEIRA, K.S., SOARES, C.S., MAGALHÃES, I.D. and MEDEIROS, A.S., 2020. Microclimate changes, photomorphogenesis and water consumption of Moringa oleifera cuttings under different light spectrums and exogenous phytohormone concentrations. Australian Journal of Crop Science, vol. 14, no. 5, pp. 751-760. http://dx.doi.org/10.21475/ajcs.20.14.05.p2096.
http://dx.doi.org/10.21475/ajcs.20.14.05...
).
2.7. Variables evaluated
Assessments were done to determine the percentage of emerged seedlings (PES, %), emergence speed index (ESI, dimensionless), average emergence time (AET, days), initial shoot average length (ISL, cm), final shoot average length (FSL, cm), relative shoot growth rate (RSGR, cm cm-1 day-1), initial root average length (IRL, cm), final root average length (FRL, cm), relative root growth rate (RRGR, cm cm-1 day-1), initial shoot phytomass (ISP, mg), final shoot phytomass (FSP, mg), shoot phytomass relative gain (SPRG, mg mg-1 day-1), initial root phytomass (IRP, mg), final root phytomass (FRP, mg), root phytomass relative gain (RPRG, mg mg-1 day-1) (Ferraz et al., 2017FERRAZ, R.L.S., BARBOSA, M.A., MAGALHÃES, I.D., MELO, A.S., ROCHA, M.S. and COSTA, P.S., 2017. Atributos qualitativos de sementes de algodoeiro hidrocondicionadas em soluções de silício. Científica, vol. 45, no. 1, pp. 85-94. http://dx.doi.org/10.15361/1984-5529.2017v45n1p85-94.
http://dx.doi.org/10.15361/1984-5529.201...
).
2.8. Seedling emergence
Seedling emergence was evaluated at 24-hour intervals as those that appeared on the substrate surface (epicotyl ≥ 2.0 mm). Percentage of emerged plants was calculated per evaluations done within 24 days (Equation 1):
where: PES = percentage of emergence of seedlings, N1= total number of seeds sawn, and N2= number of seedlings emerged.
Emergence speed index (ESI) estimates the average number of seedlings that emerge per day and, the higher the value obtained from the ESI, the greater the emergence speed and, consequently, greater the seedlings' vigor (Maguire, 1962MAGUIRE, J.D., 1962. Speed of germination aid in selec-tion and evoluation for seedling and vigour. Crop Science, vol. 2, no. 2, pp. 176-177. http://dx.doi.org/10.2135/cropsci1962.0011183X000200020033x.
http://dx.doi.org/10.2135/cropsci1962.00...
). ESI was calculated by Equation 2
where: N = number of seeds sowed, n = number of seedlings emerged on day j (j = number of days after sowing) (A-As-Saqui and Corleto, 1978A-AS-SAQUI, M. and CORLETO, A., 1978. Effect of seed presowing hardening on seedling emergence of four forage species. Seed Science and Technology, vol. 6, no. 3, pp. 701-709.).
The mean emergence time (MET) corresponds to the weighted average of the time required for the emergence of seedlings, that is, the shorter that time, the greater the emergence speed (Edmond and Drapala, 1958EDMOND, J.B. and DRAPALA, W.J., 1958. The effects of temperature, sand and soil, and acetone on germination of okra seeds. Proceedings of the American Society for Horticultural Science, vol. 71, pp. 428-434.). It has used the following relationship to estimate the average emergency (Equation 3):
where: n = number of emerged seedlings and t = number of days after sowing (Labouriau and Valadares, 1976LABOURIAU, L.G. and VALADARES, M.B., 1976. On the germination of seeds of Calotropis procera (Ait.) Ait.f. Anais da Academia Brasileira de Ciências, vol. 48, pp. 174-186.).
2.9. Seedling growth
Two standardized seedlings were selected per plot after thirteen days from the beginning of the experiment. These seedlings were measured to get the initial shoot (ISL) and root average length (IRL) with a graduated ruler (mm). Afterward, the two seedlings were separated into shoot and root parts. These materials were packed in paper bags and stored in a forced air circulation oven at 70 ºC until stable weight and weighed on an analytical scale to determine the initials shoot (ISP) and root phytomass (IRP) (Ferraz et al., 2017FERRAZ, R.L.S., BARBOSA, M.A., MAGALHÃES, I.D., MELO, A.S., ROCHA, M.S. and COSTA, P.S., 2017. Atributos qualitativos de sementes de algodoeiro hidrocondicionadas em soluções de silício. Científica, vol. 45, no. 1, pp. 85-94. http://dx.doi.org/10.15361/1984-5529.2017v45n1p85-94.
http://dx.doi.org/10.15361/1984-5529.201...
).
Twenty-one days after the beginning of the experiment, two seedlings were selected per plot among the remaining ones and measured to obtain the finals shoot (FSL) and root average lengths (FRL). Afterward, plant material was sectioned, packed in a paper bag, and dry to determine the final shoot (FSP) and root phytomass (FRP) (Ferraz et al., 2017FERRAZ, R.L.S., BARBOSA, M.A., MAGALHÃES, I.D., MELO, A.S., ROCHA, M.S. and COSTA, P.S., 2017. Atributos qualitativos de sementes de algodoeiro hidrocondicionadas em soluções de silício. Científica, vol. 45, no. 1, pp. 85-94. http://dx.doi.org/10.15361/1984-5529.2017v45n1p85-94.
http://dx.doi.org/10.15361/1984-5529.201...
).
Relative shoot (RSGR) and root growth rate (RRGR), relative shoot (SPRG) and root phytomass gain (RPRG) were obtained by the relationship (Equation 4):
where: R= growth rate or relative gain; ln = Neperian logarithm; W1 = initial length or phytomass; W2 = final length or phytomass; t1 = initial time, and t2 = final time (Echer et al., 2010ECHER, F.R., CUSTÓDIO, C.C., HOSSOMI, S.T., DOMINATO, J.C. and MACHADO NETO, N.B., 2010. Estresse hídrico induzido por manitol em cultivares de algodão. Ciência Agronômica, vol. 41, no. 4, pp. 638-645. http://dx.doi.org/10.1590/S1806-66902010000400018.
http://dx.doi.org/10.1590/S1806-66902010...
; Ferraz et al., 2017FERRAZ, R.L.S., BARBOSA, M.A., MAGALHÃES, I.D., MELO, A.S., ROCHA, M.S. and COSTA, P.S., 2017. Atributos qualitativos de sementes de algodoeiro hidrocondicionadas em soluções de silício. Científica, vol. 45, no. 1, pp. 85-94. http://dx.doi.org/10.15361/1984-5529.2017v45n1p85-94.
http://dx.doi.org/10.15361/1984-5529.201...
).
2.10. Photosynthetic pigments
Twenty-four days after setting up the experiment, among remaining seedlings, were collected leaflets from two standardized seedlings per plot. The leaflets were wrapped in aluminum film and stored in a refrigerated environment at -20 °C. Pigments extraction was assessed with 0.5 g of leaflet tissue, digested in 6.0 mL of acetone (80%) for 24 hours. Subsequently, the samples have diluted on a 2:1 basis with 2 ml of acetone (80%) for each mL of sample. The extract absorbances were analyzed in a spectrophotometer at the wavelengths of 663, 646, and 470 nm, for chlorophyll a (Chl a), chlorophyll b (Chl b), total carotenoids (Cart), and total chlorophylls (Chl t), respectively. The pigment contents have obtained by Lichtenthaler and Buschmann's equation (Lichtenthaler and Buschmann, 2001LICHTENTHALER, H.K. and BUSCHMANN, C., 2001. Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Current Protocols in Food Analytical Chemistry, vol. 1, no. 1, pp. F4.3.1-F4.3.8. http://dx.doi.org/10.1002/0471142913.faf0403s01.
http://dx.doi.org/10.1002/0471142913.faf...
) namely (Equations 5-8):
The values obtained were multiplied by 6.0 mL (volume of the digestion tube) and divided by the sample mass (0.5 g) for conversion of µg mL-1 to µg g-1. With these data were calculated Chl a/Chl b and Chl a/Car t relationships.
2.11. Statistical analysis
Original variables data were submitted to the Shapiro-Wilk normality test (Shapiro and Wilk, 1965SHAPIRO, S.S. and WILK, M.B., 1965. An analysis of variance test for normality (complete samples). Biometrika Trust, vol. 52, no. 3-4, pp. 591-609. http://dx.doi.org/10.2307/2333709.
http://dx.doi.org/10.2307/2333709...
). When postulates of normality were achieved, each variable data were standardized to obtain the variable Z with null mean ( = 0.0) and unit variance (σ2 = 1.0). Equation 9:
Where: X is each observation of the variable's data set, is the mean, and σ2 is the variance of the data set.
Converted data were subjected to the Principal Component Analysis (PCA) exploratory procedure. The choice of Principal Components (PCs) was based on eigenvalues greater than one (λ> 1.0), which explained a percentage greater than 10% of total variance (Govaerts et al., 2007GOVAERTS, B., SAYRE, K.D., LICHTER, K., DENDOOVEN, L. and DECKERS, J., 2007. Influence of permanent raised bed planting and residue management on physical and chemical soil quality in rain fed maize/wheat systems. Plant and Soil, vol. 291, no. 1-2, pp. 39-54. http://dx.doi.org/10.1007/s11104-006-9172-6.
http://dx.doi.org/10.1007/s11104-006-917...
; Hair Junior et al., 2009). Original data from each PC were submitted to multivariate analysis of variance (MANOVA) by Roy’s test. Afterward, seed primings were grouped based on variables from each PC by Ward's minimum variance hierarchical method.
Variables, not associated with any of the PCs, were removed from PCA and subjected to univariate analysis of variance (ANOVA) by the F test with 95% confidence (Barbosa and Maldonado Júnior, 2015). Statistica v. 7.0 (Statsoft Inc., 2004STATSOFT INC., 2004. Statistica: data analysis software system. Version 7 [software]. Available from: www.statsoft.com.) was used to perform the analyzes.
3. Results
3.1. Principal components and multivariate variance
Four PCs with λ > 1 and σ2 > 10% were formed from the linear combination of the 18 original variables because of the combination of LIQ and AEC with 78.74% of total σ2. PC1 represents 27.91% of σ2, composed by the combination of ISL, RSGR, IRL, RRGR, ISP, SPRG, and Cart; PC2 represents 21.44% of σ2, formed by FRL, FSP, Chl a, and Chl t; PC3 represents 16.61% of σ2 composed by RPRG, Chl b, Chl a/Chl b, and Chl a/Car t; and PC4 represents 12.79% of σ2, made by the combination of PES, ESI, and FRP. MET, FSL, and IRP Variables did not match in any PCs and, therefore, were excluded from PCA to be assessed by a univariate analysis. There was a significant interaction between LIQ and AEC combinations in the four PCs (MANOVA results) (Table 1).
Correlation among original variables and principal components, eigenvalues, explained and accumulated variance, and probability significance of hypothesis test in the first four main interaction components (PCs 1, 2, 3 and 4) among light quality levels and aqueous extract concentrations of Cyperus rotundus on Moringa oleifera seeds.
In two-dimensional projection of the first two PCs (Figures 1A-1B), it was observed that in PC1 the seed priming with LIQ – light quality in the BL - blue light, RL - red light, and FR - distant red light region, combined with AEC - aqueous extract of C. rotundus, triggered different processes in M. oleifera seedlings in contrast to WL - white light. Seeds irradiated with distant red light without the addition of AEC (FR-0) plus the addition of 50% AEC (FR-50) induced seedlings with higher ISL, IRL, and ISP with consequent reduction in RSGR, RRGR, and SPRG that increased in seedlings irradiated with white light and the addition of 50% (WL-50) and 100% (WL-100) of AEC; while RL-25 and RL-100 increased Cart contents.
Two-dimensional projection of factorials scores (A and C) and variables (B and D) in the first four main interaction components (CPs 1, 2, 3, and 4) among light quality levels and aqueous extract concentrations of Cyperus rotundus (0, 25, 50 and 100%) on Moringa oleifera seeds. WL - white light, BL - blue light, RL - red light, FR - distant red light, PES - percentage emerged seedlings, ESI - emergency speed index, ISL - initial shoot length, RSGR - relative shoot growth rate, IRL - initial root length, FRL - final root length, RRGR - relative root growth rate, ISP - initial shoot phytomass, FSP - final shoot phytomass, SPRG - shoot phytomass relative gain, FRP - final root phytomass, RPRG - root phytomass relative gain, Chla - chlorophyll a content, Chlb - chlorophyll b content, Cart - total carotenoid content, Chlt - total chlorophyll content, Chla/Chlb - relationship between chlorophylls a and b, Chla/Cart - relationship between Chla and Cart.
It was observed that in PC2 that seeds under irradiation with blue light and the addition of 25% (BL-25) and 50% (BL-50) of AEC combined with RL-50 originated seedlings with the higher levels of Chla and Chlt, whereas, irradiation with BL, without addition of AEC, significantly decreases the level of these pigments. It is possible to note that the seedlings from seeds irradiated with FR-100 and BL-0 had higher FRL and FSP in PC2.
In the two-dimensional projection of the third and fourth PC (Figures 1C-1D), in PC3, it was observed that irradiation with RL had a synergistic effect on 50% of AEC concentration, which triggered a process of rising Chla/Chlb and Chla/Cart due to raised Chla contents and the decrease of Chlb and Cart contents. It was observed that in PC4 the emergence of seedlings occurred in higher percentages (PES) and speed (ESI) under irradiation of seeds, WL potentiated by using 100% of AEC. This could be attributed to the reduction of final root phytomass accumulation (FRP), which showed the partition of a greater amount of energy supply to hypocotyl growth and lesser to root extension.
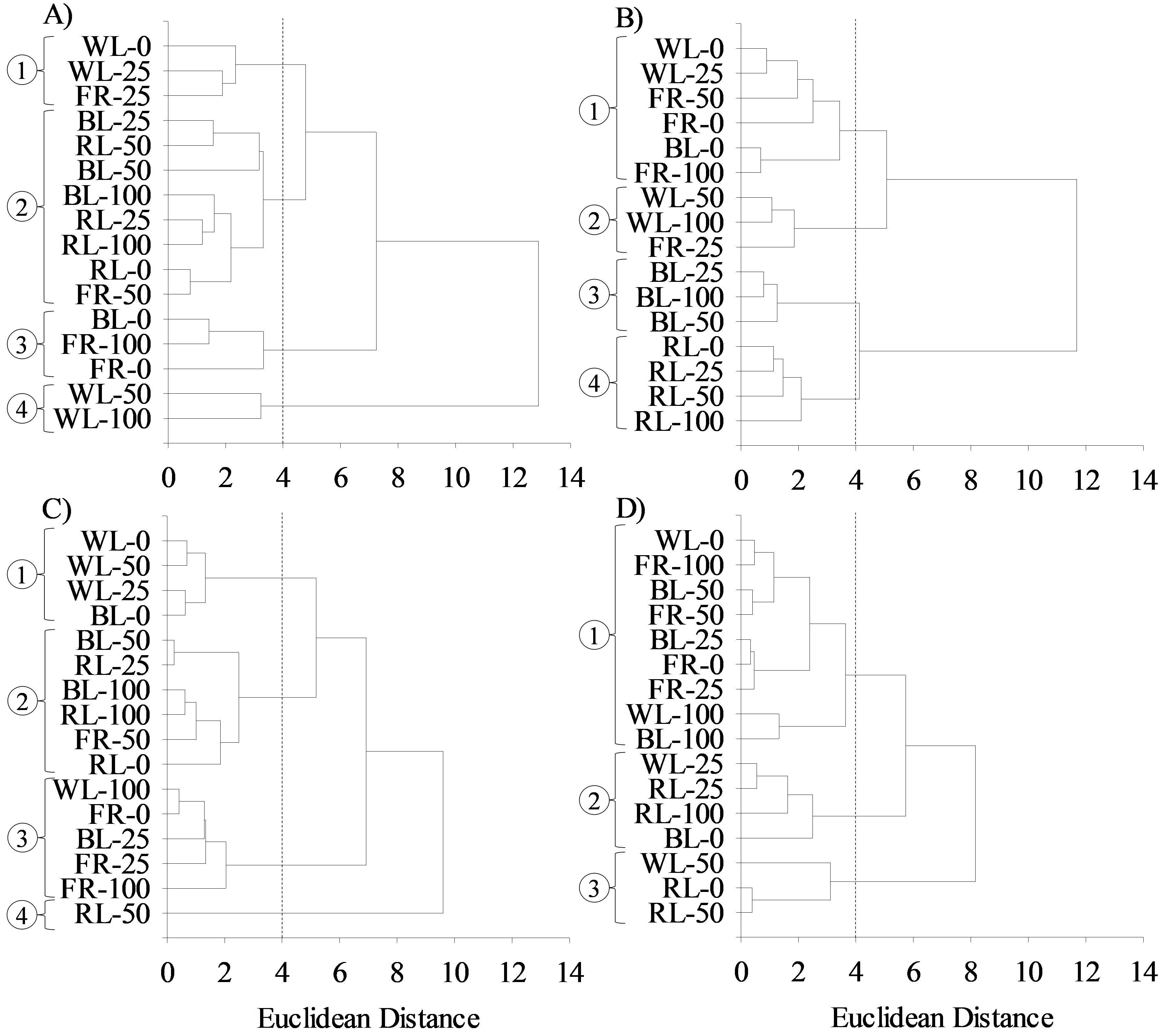
Applying Euclidean Distance (ED) as a measure of dissimilarity and subjective visual inspection criteria to establish the cutoff point with ED = 4, the seed priming from LIQ and AEC combination was grouped into four groups (1, 2, 3 and 4) of PC1 (Figure 2A), PC2 (Figure 2B) and PC3 (Figure 2C) and in three groups of PC4 (Figure 2D). Those groups are characterized by having greater homogeneity (similarity) between seed priming of each group and higher heterogeneity (dissimilarity) among groups regarding variables considered because Ward's method (Minimum Variance Method) minimizes the sum of squares within the group.
Grouping dendrograms of seed priming of Moringa oleifera assembled with PC1 (A), PC2 (B), PC3 (C), and PC4 (D) variables according to the interaction among light quality levels and aqueous extract concentrations of Cyperus rotundus (0, 25, 50 and 100%). WL - white light, BL - blue light, RL - red light, FR - distant red light.
Moringa oleifera seedlings had a shorter average emergence time (0.086 days) when seeds were submitted to seed priming in red light (Figure 3A). Moringa oleifera seeds irradiated with WL and bio induced with 100% of AEC gave rise to seedlings with higher FSL (10.55 cm), followed by those generated from the combination of FR with 50% AEC that had FSL of 10.31 cm (Figure 3B). Seeds not treated with AEC generated seedlings with a greater initial root phytomass (IRP) accumulation when irradiated with RL (50.38 mg) and BL (45.75 mg), respectively. Seedlings originated from seeds treated with 100% AEC showed higher IRP when irradiated by RL and FR with 49.13 mg and 45.38 mg, respectively (Figure 3C).
Average emergence time (A), average shoot final length (B), and initial root phytomass (C) of Moringa oleifera seedlings as a function of the interaction among light quality levels and aqueous extract concentrations Cyperus rotundus.
Original averages data of all individual variables assessed in this study and the F (Fc) test are in Table 2.
Variables averages evaluated in function of the interaction among light quality levels and aqueous extract concentrations of Cyperus rotundus.
4. Discussion
Plant plasticity is closely related to environmental conditions and endogenous phytohormones balances (Melo et al., 2022MELO, A.S., MELO, Y.L., LACERDA, C.F., VIEGAS, P.R.A., FERRAZ, R.L.S. and GHEYI, H.R., 2022. Water restriction in cowpea plants [Vigna unguiculata (L.) Walp.]: metabolic changes and tolerance induction. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 26, no. 3, pp. 190-197. http://dx.doi.org/10.1590/1807-1929/agriambi.v26n3p190-197.
http://dx.doi.org/10.1590/1807-1929/agri...
). Interactions between LIQ and AEC, in germination, growth, and accumulation of photosynthetic pigment variables, occur because light is the main environmental factor that influences cell elongation, division, differentiation and induces plant photomorphogenesis. This is mainly because of photoreceptors excitation that trigger the synthesis of phytohormones (Bîlc and Luchian, 2020BÎLC, B.-A. and LUCHIAN, M.-R., 2020. Aspects related to the relationship between phytochrome and phytohormones. Biostudent, vol. 3, no. 1, pp. 81-102.).
The intrinsic mechanisms by which light interacts with phytohormones in M. oleifera are not yet clear, principally when applied seed priming. However, shifts in LIQ levels promote microclimate changes which influence photomorphogenesis and water consumption by M. oleifera mini-cuttings when treated with AEC in a vegetative propagation system. This response occurs because of the reduction in air temperature under BL and the increase in air temperature and relative air humidity, and soil temperature under RL and FR (Silva et al., 2020SILVA, A.E., FERRAZ, R.L.S., SILVA, J.P., COSTA, P.S., VIÉGAS, P.R.A., BRITO NETO, J.F., MELO, A.S., MEIRA, K.S., SOARES, C.S., MAGALHÃES, I.D. and MEDEIROS, A.S., 2020. Microclimate changes, photomorphogenesis and water consumption of Moringa oleifera cuttings under different light spectrums and exogenous phytohormone concentrations. Australian Journal of Crop Science, vol. 14, no. 5, pp. 751-760. http://dx.doi.org/10.21475/ajcs.20.14.05.p2096.
http://dx.doi.org/10.21475/ajcs.20.14.05...
).
Photomorphogenesis is regulated by a set of light signal photoreceptors (Kami et al., 2010KAMI, C., LORRAIN, S., HORNITSCHEK, P. and FANKHAUSER, C., 2010. Light-regulated plant growth and development. In: M.C.P. TIMMERMANS, ed. Plant development. Amsterdam: Elsevier, Current Topics in Developmental Biology, vol. 91, pp. 29-66. http://dx.doi.org/10.1016/S0070-2153(10)91002-8.
http://dx.doi.org/10.1016/S0070-2153(10)...
; Neff, 2012NEFF, M.M., 2012. Light-mediated seed germination: connecting phytochrome B to gibberellic acid. Developmental Cell, vol. 22, no. 4, pp. 687-688. http://dx.doi.org/10.1016/j.devcel.2012.04.003. PMid:22516192.
http://dx.doi.org/10.1016/j.devcel.2012....
), thus can be inferred that seed priming with RL and FR influenced the phytohormones activity, for example, phyA, phyB, phyC, phyD, and phyE since these photoreceptors are responsible for light reception and modulation and gene expression by way of signal transduction systems (Oka and Yamamoto, 2019OKA, Y. and YAMAMOTO, K., 2019. Photoreceptor-mediated plant development. In: M. ANPO, H. FUKUDA and T. WADA, eds. Plant factory using artificial light: adapting to environmental disruption and clues to agricultural innovation. Amsterdam: Elsevier, pp. 111-117. http://dx.doi.org/10.1016/B978-0-12-813973-8.00011-7.
http://dx.doi.org/10.1016/B978-0-12-8139...
). This explains PES and ESI reduction and ISL, IRL, and ISP increase, which may be related to the enhanced production of phenolic and flavonoid compounds and the resulting photoprotection mentioned in M. oleifera.
Possibly, seed priming with BL, RL, and FR plus doses of AEC increased the action of phytochrome interaction factors (PIFs) from rapid phosphorylation, ubiquitination, and proteasome-mediated degradation, which accelerated the transition state from skotomorphogenesis to photomorphogenic development (Liang et al., 2020LIANG, S., GAO, X., WANG, Y., ZHANG, H., YIN, K., CHEN, S., ZHANG, M. and ZHAO, R., 2020. Phytochrome-interacting factors regulate seedling growth through ABA signaling. Biochemical and Biophysical Research Communications, vol. 526, no. 4, pp. 1100-1105. http://dx.doi.org/10.1016/j.bbrc.2020.04.011. PMid:32307082.
http://dx.doi.org/10.1016/j.bbrc.2020.04...
). The interaction between LIQ and AEC supported the activity of phyA under FR and stimulated the action of phyB under RL, which promoted greater germination and growth (Oh et al., 2020OH, J., PARK, E., SONG, K., BAE, G. and CHOI, G., 2020. Phytochrome interacting factor8 inhibits phytochrome A-mediated far-red light responses in Arabidopsis. The Plant Cell, vol. 32, no. 1, pp. 186-205. http://dx.doi.org/10.1105/tpc.19.00515. PMid:31732705.
http://dx.doi.org/10.1105/tpc.19.00515...
), mainly under seed priming with WL-100, because WL is composed of all colors balance.
This data reported here, on the LIQ and AEC effect, is important for making decisions on seedling management of M. oleifera because there may be competition for light in dense crops which can stimulate stem elongation, decrease photoassimilates allocation to leaves, and reduce seedlings growth, a phenomenon that occurs more frequently at a greater FR rate (Kong et al., 2018KONG, Y., STASIAK, M., DIXON, M.A. and ZHENG, Y., 2018. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: a comparison with red light in four bedding plant species. Environmental and Experimental Botany, vol. 155, pp. 345-359. http://dx.doi.org/10.1016/j.envexpbot.2018.07.021.
http://dx.doi.org/10.1016/j.envexpbot.20...
; Shibuya et al., 2020SHIBUYA, T., ENDO, R., KITAYA, Y. and TSUCHIDA, M., 2020. Far-red light interacts with plant density to change photosynthate allocation of cucumber seedlings and their subsequent early growth after transplanting. Horticultural Science, vol. 55, no. 9, pp. 1433-1437.). FR increases the quantum efficiency of photosystems (PSI and PSII) due to the enhanced electron flow and H+ in the thylakoid lumen and the synthesis of adenosine triphosphate (ATP) in chloroplasts stroma of plants under light availability fluctuations (Kono et al., 2020KONO, M., KAWAGUCHI, H., MIZUSAWA, N., YAMORI, W., SUZUKI, Y. and TERASHIMA, I., 2020. Far-red light accelerates photosynthesis in the low-light phases of fluctuating light. Plant & Cell Physiology, vol. 61, no. 1, pp. 192-202. http://dx.doi.org/10.1093/pcp/pcz191. PMid:31617558.
http://dx.doi.org/10.1093/pcp/pcz191...
).
The knowledge acquired in this research is important to induce abiotic stresses tolerance in M. oleifera and allow its cultivation at restrictive environments because the luminous stress, caused by seed priming, can stimulate the synthesis and action of phytohormones as auxin, gibberellic acid, cytokinins, ethylene, and abscisic acid by regulating plant defense mechanisms (Banerjee and Roychoudhury, 2016BANERJEE, A. and ROYCHOUDHURY, A., 2016. Plant responses to light stress: oxidative damages, photoprotection, and role of phytohormones. In: G. AHAMMED and J.Q. YU, eds. Plant hormones under challenging environmental factors. Berlin: Springer/Dordrecht, pp. 181-213. http://dx.doi.org/10.1007/978-94-017-7758-2_8.
http://dx.doi.org/10.1007/978-94-017-775...
). Our results propose that the interaction between LIQ and AEC induced further chloroplasts development and describe the rise of chlorophyll biosynthesis as observed on Arabidopsis thaliana and Camellia sinensis (Liu et al., 2020LIU, L., LIN, N., LIU, X., YANG, S., WANG, W. and WAN, X., 2020. From chloroplast biogenesis to chlorophyll accumulation: the interplay of light and hormones on gene expression in Camellia sinensis cv. Shuchazao leaves. Frontiers in Plant Science, vol. 11, p. 256. http://dx.doi.org/10.3389/fpls.2020.00256. PMid:32218794.
http://dx.doi.org/10.3389/fpls.2020.0025...
).
Light induces endogenous phytohormones synthesis. However, their activity can be initiated by natural phytohormones obtained from species as C. rotundus. If applied in supra-optimal amounts, these auxin-producing species can provide a herbicide-like effect that reduces plant growth (LV et al., 2019LV, B., YAN, Z., TIAN, H., ZHANG, X. and DING, Z., 2019. Local auxin biosynthesis mediates plant growth and development. Trends in Plant Science, vol. 24, no. 1, pp. 6-9. http://dx.doi.org/10.1016/j.tplants.2018.10.014. PMid:30448230.
http://dx.doi.org/10.1016/j.tplants.2018...
; Bieleszová et al., 2019BIELESZOVÁ, K., PAŘÍZKOVÁ, B., KUBEŠ, M., HUSIČKOVÁ, A., KUBALA, M., MA, Q., SEDLÁŘOVÁ, M., ROBERT, S., DOLEŽAL, K., STRNAD, M., NOVÁK, O. and ŽUKAUSKAITĖ, A., 2019. New fluorescently labeled auxins exhibit promising anti-auxin activity. New Biotechnology, vol. 48, no. 1, pp. 44-52. http://dx.doi.org/10.1016/j.nbt.2018.06.003. PMid:29953966.
http://dx.doi.org/10.1016/j.nbt.2018.06....
). This response may explain the lower performance of M. oleifera under WL combined with 50 and 100% of AEC, respectively.
Seed priming with WL activated the photo perception and triggered adaptive responses of M. oleifera seedlings resulting in a higher relative growth rate. Although wavelengths in the BL (400-500 nm) and RL (600-700 nm) regions from the visible spectrum are more efficient in capturing CO2 and releasing O2. Additions seen in RSGR under WL and AEC may be related to the fact that up to 50% of white light spectral composition is between 500 and 600 nm, comprising blue, green, yellow, red, and extreme red lights (Mickens et al., 2018MICKENS, M.A., SKOOG, E.J., REESE, L.E., BARNWELL, P.L., SPENCER, L.E., MASSA, G.D. and WHEELER, R.M., 2018. A strategic approach for investigating light recipes for ‘Outredgeous’ red romaine lettuce using white and monochromatic LEDs. Life Sciences in Space Research, vol. 19, no. 11, pp. 53-62. http://dx.doi.org/10.1016/j.lssr.2018.09.003. PMid:30482283.
http://dx.doi.org/10.1016/j.lssr.2018.09...
).
5. Conclusion
Seed priming with red light reduced the average emergence time while blue, red, and extreme red lights, associated with 50% of aqueous extract of C. rotundus, resulted in an increase in initial shoots length and photosynthetic pigments accumulation. Seed priming with blue light exhibited a shorter final shoot length seedlings, although using 100% of the aqueous extract of C. rotundus reversed that lower growth. The association among white light to 50 and 100% of extract promoted a higher rate of seedlings relative shoot growth. Our research shows that seed priming modulates the ecophysiology of M. oleifera seedlings when applied in combination with light spectral radiation and the aqueous extract of C. rotundus tubers. More work to determine the mechanisms of these actions are needed.
Acknowledgements
Authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq for funding this work under Research project No. 160862/2019-1. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
References
- A-AS-SAQUI, M. and CORLETO, A., 1978. Effect of seed presowing hardening on seedling emergence of four forage species. Seed Science and Technology, vol. 6, no. 3, pp. 701-709.
- AHMED, L.T., WARRAG, E.I. and ABDELGADIR, A.Y., 2014. Effect of shade on seed germination on early seedling growth of Moringa oleifera Lam. Forest Products Industries, vol. 3, no. 1, pp. 20-26.
- BANERJEE, A. and ROYCHOUDHURY, A., 2016. Plant responses to light stress: oxidative damages, photoprotection, and role of phytohormones. In: G. AHAMMED and J.Q. YU, eds. Plant hormones under challenging environmental factors Berlin: Springer/Dordrecht, pp. 181-213. http://dx.doi.org/10.1007/978-94-017-7758-2_8
» http://dx.doi.org/10.1007/978-94-017-7758-2_8 - BARBOSA, J.C. and MALDONADO JÚNIOR, W., 2015. Experimentação agronômica & AgroEstat – sistema para análises estatísticas de ensaios agronômicos Jaboticabal: Funep, 396 p.
- BIELESZOVÁ, K., PAŘÍZKOVÁ, B., KUBEŠ, M., HUSIČKOVÁ, A., KUBALA, M., MA, Q., SEDLÁŘOVÁ, M., ROBERT, S., DOLEŽAL, K., STRNAD, M., NOVÁK, O. and ŽUKAUSKAITĖ, A., 2019. New fluorescently labeled auxins exhibit promising anti-auxin activity. New Biotechnology, vol. 48, no. 1, pp. 44-52. http://dx.doi.org/10.1016/j.nbt.2018.06.003 PMid:29953966.
» http://dx.doi.org/10.1016/j.nbt.2018.06.003 - BÎLC, B.-A. and LUCHIAN, M.-R., 2020. Aspects related to the relationship between phytochrome and phytohormones. Biostudent, vol. 3, no. 1, pp. 81-102.
- BORNMAN, J.F., BARNES, P.W., ROBSON, T.M., ROBINSON, S.A., JANSEN, M.A.K., BALLARÉ, C.L. and FLINT, S.D., 2019. Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems. Photochemical & Photobiological Sciences, vol. 18, no. 3, pp. 681-716. http://dx.doi.org/10.1039/C8PP90061B PMid:30810560.
» http://dx.doi.org/10.1039/C8PP90061B - BUSSONI, A., ALVAREZ, J., CUBBAGE, F., FERREIRA, G. and PICASSO, V., 2019. Diverse strategies for integration of forestry and livestock production. Agroforestry Systems, vol. 93, no. 1, pp. 333-344. http://dx.doi.org/10.1007/s10457-017-0092-7
» http://dx.doi.org/10.1007/s10457-017-0092-7 - CARVALHO, D.B. and CARVALHO, R.I.N., 2009. Qualidade fisiológica de sementes de guanxuma em influência do envelhecimento acelerado e da luz. Acta Scientiarum. Agronomy, vol. 31, no. 3, pp. 489-494. http://dx.doi.org/10.4025/actasciagron.v31i3.585
» http://dx.doi.org/10.4025/actasciagron.v31i3.585 - CAVALCANTE, J.A., LOPES, K.P., PEREIRA, N.A.E., SILVA, J.G., PINHEIRO, R.M. and MARQUES, R.L.L., 2018. Extrato aquoso de bulbos de tiririca sobre a germinação e crescimento inicial de plântulas de rabanete. Revista Verde de Agroecologia e Desenvolvimento Sustentável, vol. 13, no. 1, pp. 39-44. http://dx.doi.org/10.18378/rvads.v13i1.5701
» http://dx.doi.org/10.18378/rvads.v13i1.5701 - CORTNER, O., GARRETT, R.D., VALENTIM, J.F., FERREIRA, J., NILES, M.T., REIS, J. and GIL, J., 2019. Perceptions of integrated crop-livestock systems for sustainable intensification in the Brazilian Amazon. Land Use Policy, vol. 82, no. 3, pp. 841-853. http://dx.doi.org/10.1016/j.landusepol.2019.01.006
» http://dx.doi.org/10.1016/j.landusepol.2019.01.006 - COSTA, A.A., PAIVA, E.P., TORRES, S.B., SOUZA NETA, M.L., PEREIRA, K.T.O., LEITE, M.S., SÁ, F.V.S. and BENEDITO, C.P., 2022. Osmoprotection in Salvia hispanica L. seeds under water stress attenuators. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 82, no. 1, p. e233547. http://dx.doi.org/10.1590/1519-6984.233547 PMid:34105656.
» http://dx.doi.org/10.1590/1519-6984.233547 - DOMENICO, M., LINA, C. and FRANCESCA, B., 2019. Sustainable crops for food security: moringa (Moringa oleifera Lam.). Encyclopedia of Food Security and Sustainability, vol. 1, pp. 409-415. http://dx.doi.org/10.1016/B978-0-08-100596-5.22574-2
» http://dx.doi.org/10.1016/B978-0-08-100596-5.22574-2 - ECHER, F.R., CUSTÓDIO, C.C., HOSSOMI, S.T., DOMINATO, J.C. and MACHADO NETO, N.B., 2010. Estresse hídrico induzido por manitol em cultivares de algodão. Ciência Agronômica, vol. 41, no. 4, pp. 638-645. http://dx.doi.org/10.1590/S1806-66902010000400018
» http://dx.doi.org/10.1590/S1806-66902010000400018 - EDMOND, J.B. and DRAPALA, W.J., 1958. The effects of temperature, sand and soil, and acetone on germination of okra seeds. Proceedings of the American Society for Horticultural Science, vol. 71, pp. 428-434.
- FERRAZ, R.L.S., BARBOSA, M.A., MAGALHÃES, I.D., MELO, A.S., ROCHA, M.S. and COSTA, P.S., 2017. Atributos qualitativos de sementes de algodoeiro hidrocondicionadas em soluções de silício. Científica, vol. 45, no. 1, pp. 85-94. http://dx.doi.org/10.15361/1984-5529.2017v45n1p85-94
» http://dx.doi.org/10.15361/1984-5529.2017v45n1p85-94 - FERREIRA, D.T.R.G., SILVA, V.M., SILVA, I.C., ARAÚJO NETO, J.C., SOUZA, R.C. and FERREIRA, V.M., 2017. Germinação de três Euphorbiaceae influenciada pela luz e níveis de palhada. Revista Agro@mbiente On-line, vol. 11, no. 3, pp. 215-222. http://dx.doi.org/10.18227/1982-8470ragro.v11i3.3852
» http://dx.doi.org/10.18227/1982-8470ragro.v11i3.3852 - GARCIA, T.B., SOARES, A.A., COSTA, J.H., COSTA, H.P.S., NETO, J.X.S., ROCHA-BEZERRA, L.C.B., SILVA, F.D.A., ARANTES, M.R., SOUSA, D.O.B., VASCONCELOS, I.M. and OLIVEIRA, J.T.A., 2019. Gene expression and spatiotemporal localization of antifungal chitin-binding proteins during Moringa oleifera seed development and germination. Planta, vol. 249, no. 5, pp. 1503-1519. http://dx.doi.org/10.1007/s00425-019-03103-8 PMid:30706136.
» http://dx.doi.org/10.1007/s00425-019-03103-8 - GOVAERTS, B., SAYRE, K.D., LICHTER, K., DENDOOVEN, L. and DECKERS, J., 2007. Influence of permanent raised bed planting and residue management on physical and chemical soil quality in rain fed maize/wheat systems. Plant and Soil, vol. 291, no. 1-2, pp. 39-54. http://dx.doi.org/10.1007/s11104-006-9172-6
» http://dx.doi.org/10.1007/s11104-006-9172-6 - GUIMARÃES, M.A., DIAS, D.C.F.S. and LOUREIRO, M.E., 2008. Hidratação de sementes. Revista Trópica – Ciências Agrárias e Biológicas, vol. 2, no. 1, pp. 31-39.
- HAIR JUNIOR, J.F., BLACK, W.C., BABIN, B.J., ANDERSON, R.E. and TATHAM, R.L., 2009. Análise multivariada de dados 6th ed. Porto Alegre: Bookman, 688 p.
- HASAN, M.M., ALHARBY, H.F., HAJAR, A.S., HAKEEM, K.R. and ALZAHRANI, Y., 2019. The effect of magnetized water on the growth and physiological conditions of Moringa species under drought stress. Polish Journal of Environmental Studies, vol. 28, no. 3, pp. 1145-1155. http://dx.doi.org/10.15244/pjoes/85879
» http://dx.doi.org/10.15244/pjoes/85879 - KAMI, C., LORRAIN, S., HORNITSCHEK, P. and FANKHAUSER, C., 2010. Light-regulated plant growth and development. In: M.C.P. TIMMERMANS, ed. Plant development Amsterdam: Elsevier, Current Topics in Developmental Biology, vol. 91, pp. 29-66. http://dx.doi.org/10.1016/S0070-2153(10)91002-8
» http://dx.doi.org/10.1016/S0070-2153(10)91002-8 - KARTHICKEYAN, V., 2019. Effect of cetane enhancer on Moringa oleifera biodiesel in a thermal coated direct injection diesel engine. Fuel, vol. 235, no. 1, pp. 538-550. http://dx.doi.org/10.1016/j.fuel.2018.08.030
» http://dx.doi.org/10.1016/j.fuel.2018.08.030 - KONG, Y., STASIAK, M., DIXON, M.A. and ZHENG, Y., 2018. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: a comparison with red light in four bedding plant species. Environmental and Experimental Botany, vol. 155, pp. 345-359. http://dx.doi.org/10.1016/j.envexpbot.2018.07.021
» http://dx.doi.org/10.1016/j.envexpbot.2018.07.021 - KONO, M., KAWAGUCHI, H., MIZUSAWA, N., YAMORI, W., SUZUKI, Y. and TERASHIMA, I., 2020. Far-red light accelerates photosynthesis in the low-light phases of fluctuating light. Plant & Cell Physiology, vol. 61, no. 1, pp. 192-202. http://dx.doi.org/10.1093/pcp/pcz191 PMid:31617558.
» http://dx.doi.org/10.1093/pcp/pcz191 - LABOURIAU, L.G. and VALADARES, M.B., 1976. On the germination of seeds of Calotropis procera (Ait.) Ait.f. Anais da Academia Brasileira de Ciências, vol. 48, pp. 174-186.
- LIANG, S., GAO, X., WANG, Y., ZHANG, H., YIN, K., CHEN, S., ZHANG, M. and ZHAO, R., 2020. Phytochrome-interacting factors regulate seedling growth through ABA signaling. Biochemical and Biophysical Research Communications, vol. 526, no. 4, pp. 1100-1105. http://dx.doi.org/10.1016/j.bbrc.2020.04.011 PMid:32307082.
» http://dx.doi.org/10.1016/j.bbrc.2020.04.011 - LICHTENTHALER, H.K. and BUSCHMANN, C., 2001. Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Current Protocols in Food Analytical Chemistry, vol. 1, no. 1, pp. F4.3.1-F4.3.8. http://dx.doi.org/10.1002/0471142913.faf0403s01
» http://dx.doi.org/10.1002/0471142913.faf0403s01 - LIU, J., CAI, H.-H., LI, H.-Q., LIU, Z.-Y., ZHENG, C., SHI, C. and NIU, Y.-F., 2019. The chloroplast genome of Moringa oleifera (Moringaceae). Mitochondrial DNA Part B, vol. 4, no. 1, pp. 646-647. http://dx.doi.org/10.1080/23802359.2018.1545550
» http://dx.doi.org/10.1080/23802359.2018.1545550 - LIU, L., LIN, N., LIU, X., YANG, S., WANG, W. and WAN, X., 2020. From chloroplast biogenesis to chlorophyll accumulation: the interplay of light and hormones on gene expression in Camellia sinensis cv. Shuchazao leaves. Frontiers in Plant Science, vol. 11, p. 256. http://dx.doi.org/10.3389/fpls.2020.00256 PMid:32218794.
» http://dx.doi.org/10.3389/fpls.2020.00256 - LV, B., YAN, Z., TIAN, H., ZHANG, X. and DING, Z., 2019. Local auxin biosynthesis mediates plant growth and development. Trends in Plant Science, vol. 24, no. 1, pp. 6-9. http://dx.doi.org/10.1016/j.tplants.2018.10.014 PMid:30448230.
» http://dx.doi.org/10.1016/j.tplants.2018.10.014 - MACÁRIO, A.P.S., FERRAZ, R.L.S., COSTA, P.S., BRITO NETO, J.F., MELO, A.S. and DANTAS NETO, J., 2020. Allometric models for estimating Moringa oleifera leaflets area. Ciência e Agrotecnologia, vol. 44, p. e005220. http://dx.doi.org/10.1590/1413-7054202044005220
» http://dx.doi.org/10.1590/1413-7054202044005220 - MAGUIRE, J.D., 1962. Speed of germination aid in selec-tion and evoluation for seedling and vigour. Crop Science, vol. 2, no. 2, pp. 176-177. http://dx.doi.org/10.2135/cropsci1962.0011183X000200020033x
» http://dx.doi.org/10.2135/cropsci1962.0011183X000200020033x - MARTINS, J.J.F., SOARES, A.M.V.M., AZEITEIRO, U.M. and CORREIA, M.L.T., 2019. Anthropic action effects caused by soybean farmers in a watershed of Tocantins—Brazil and its connections with climate change. In: P. CASTRO, A. AZUL, W. LEAL FILHO and U. AZEITEIRO, eds. Climate change-resilient agriculture and agroforestry Cham: Springer, pp. 257-281. http://dx.doi.org/10.1007/978-3-319-75004-0_15
» http://dx.doi.org/10.1007/978-3-319-75004-0_15 - MATSUO, S., NANYA, K., IMANISHI, S., HONDA, I. and GOTO, E., 2019. Effects of blue and red lights on gibberellin metabolism in tomato seedlings. The Horticulture Journal, vol. 88, no. 1, pp. 76-82. http://dx.doi.org/10.2503/hortj.UTD-005
» http://dx.doi.org/10.2503/hortj.UTD-005 - MELO, A.S., MELO, Y.L., LACERDA, C.F., VIEGAS, P.R.A., FERRAZ, R.L.S. and GHEYI, H.R., 2022. Water restriction in cowpea plants [Vigna unguiculata (L.) Walp.]: metabolic changes and tolerance induction. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 26, no. 3, pp. 190-197. http://dx.doi.org/10.1590/1807-1929/agriambi.v26n3p190-197
» http://dx.doi.org/10.1590/1807-1929/agriambi.v26n3p190-197 - MICKENS, M.A., SKOOG, E.J., REESE, L.E., BARNWELL, P.L., SPENCER, L.E., MASSA, G.D. and WHEELER, R.M., 2018. A strategic approach for investigating light recipes for ‘Outredgeous’ red romaine lettuce using white and monochromatic LEDs. Life Sciences in Space Research, vol. 19, no. 11, pp. 53-62. http://dx.doi.org/10.1016/j.lssr.2018.09.003 PMid:30482283.
» http://dx.doi.org/10.1016/j.lssr.2018.09.003 - NEFF, M.M., 2012. Light-mediated seed germination: connecting phytochrome B to gibberellic acid. Developmental Cell, vol. 22, no. 4, pp. 687-688. http://dx.doi.org/10.1016/j.devcel.2012.04.003 PMid:22516192.
» http://dx.doi.org/10.1016/j.devcel.2012.04.003 - OH, J., PARK, E., SONG, K., BAE, G. and CHOI, G., 2020. Phytochrome interacting factor8 inhibits phytochrome A-mediated far-red light responses in Arabidopsis. The Plant Cell, vol. 32, no. 1, pp. 186-205. http://dx.doi.org/10.1105/tpc.19.00515 PMid:31732705.
» http://dx.doi.org/10.1105/tpc.19.00515 - OKA, Y. and YAMAMOTO, K., 2019. Photoreceptor-mediated plant development. In: M. ANPO, H. FUKUDA and T. WADA, eds. Plant factory using artificial light: adapting to environmental disruption and clues to agricultural innovation Amsterdam: Elsevier, pp. 111-117. http://dx.doi.org/10.1016/B978-0-12-813973-8.00011-7
» http://dx.doi.org/10.1016/B978-0-12-813973-8.00011-7 - PÁRAMO-CALDERÓN, D.E., APARICIO-SAGUILÁN, A., AGUIRRE-CRUZ, A., CARRILLO-AHUMADA, J., HERNÁNDEZ-URIBE, J.P., ACEVEDO-TELLO, S. and TORRUCO-UCO, J.G., 2019. Tortilla added with Moringa oleífera flour: physicochemical, texture properties and antioxidant capacity. LWT, vol. 100, pp. 409-415. http://dx.doi.org/10.1016/j.lwt.2018.10.078
» http://dx.doi.org/10.1016/j.lwt.2018.10.078 - PARVEEN, S., RASOOL, F., AKRAM, M.N., KHAN, N., ULLAH, M., MAHMOOD, S., RABBANI, G. and MANZOOR, K., 2024. Effect of Moringa olifera leaves on growth and gut microbiota of Nile tilapia (Oreochromis niloticus). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 84, no. 1, p. e250916. PMid:34705952.
- PAWELA, A., BANASIAK, J., BIAŁA, W., MARTINOIA, E. and JASIŃSKI, M., 2019. MtABCG20 is an ABA exporter influencing root morphology and seed germination of Medicago truncatula. The Plant Journal, vol. 98, no. 3, pp. 511-523. http://dx.doi.org/10.1111/tpj.14234 PMid:30661269.
» http://dx.doi.org/10.1111/tpj.14234 - PEREIRA, K.T.O., SANTOS, B.R.V., BENEDITO, C.P., LOPES, E.G. and AQUINO, G.S.M., 2015. Germinação e vigor de sementes de Moringa oleifera Lam. em diferentes substratos e temperaturas. Revista Caatinga, vol. 28, no. 2, pp. 92-99.
- PARREIRA, M.C., CARDOZO, N.P., GIANCOTTI, P.R.F. and ALVES, P.L.A.S., 2011. Germinação de sementes de melão-de-são-caetano sob variação de água, luz e temperatura. Bioscience Journal, vol. 27, no. 3, pp. 363-370.
- PERES, A.L.G.L., SOARES, J.S., TAVARES, R.G., RIGHETTO, G., ZULLO, M.A.T., MANDAVA, N.B. and MENOSSI, M., 2019. Brassinosteroids, the sixth class of phytohormones: a molecular view from the discovery to hormonal interactions in plant development and stress adaptation. International Journal of Molecular Sciences, vol. 20, no. 2, p. 331. http://dx.doi.org/10.3390/ijms20020331 PMid:30650539.
» http://dx.doi.org/10.3390/ijms20020331 - POLESI, L.G., FRAGA, H.P.F., VIEIRA, L.N., HERINGER, A.S., ORNELLAS, T.S., SANTOS, H.P., GUERRA, M.P. and PESCADOR, R., 2019. Chloroplast ultrastructure and hormone endogenous levels are differently affected under light and dark conditions during in vitro culture of Guadua chacoensis (Rojas) Londoño & P. M. Peterson. Acta Physiologiae Plantarum, vol. 41, no. 1, p. 10. http://dx.doi.org/10.1007/s11738-018-2804-7
» http://dx.doi.org/10.1007/s11738-018-2804-7 - REZENDE, F.P.F., ZUFFELLATO-RIBAS, K.C. and KOEHLER, H.S., 2013. Aplicação de extratos de folhas e tubérbulos de Cyperus rotundus L. e de auxinas sintéticas na estaquia caulinar de Duranta repens L. Revista Brasileira de Plantas Medicinais, vol. 15, no. 4, suppl. 1, pp. 639-645. http://dx.doi.org/10.1590/S1516-05722013000500003
» http://dx.doi.org/10.1590/S1516-05722013000500003 - RIFNA, E.J., RAMANAN, K.R. and MAHENDRAN, R., 2019. Emerging technology applications for improving seed germination. Trends in Food Science & Technology, vol. 86, no. 4, pp. 95-108. http://dx.doi.org/10.1016/j.tifs.2019.02.029
» http://dx.doi.org/10.1016/j.tifs.2019.02.029 - SÁ, F.V.S., OLIVEIRA, F.S., TORRES, S.B., PAIVA, E.P., NOGUEIRA, N.W., SARMENTO, E.C.S. and MELO, A.S., 2022. Hydric and saline stress on Phaseolus lunatus L. seeds. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 82, no. 11, p. e233550. http://dx.doi.org/10.1590/1519-6984.233550 PMid:34133547.
» http://dx.doi.org/10.1590/1519-6984.233550 - SANO, N. and SEO, M., 2019. Cell cycle inhibitors improve seed storability after priming treatments. Journal of Plant Research, vol. 132, no. 2, pp. 263-271. http://dx.doi.org/10.1007/s10265-018-01084-5 PMid:30637553.
» http://dx.doi.org/10.1007/s10265-018-01084-5 - SANTOS, M.G.M., SILVA, W.C., RIBEIRO, P.H.P., BARRETTO, V.C.M., ROCHA, E.C., OLIVEIRA, R.C., LUZ, J.M.Q. and ARRUDA, A.S., 2021. Clonal propagation of Eucalyptus urophyllaunder effect of Cyperus rotundus extract and indole-3-acetic acid. Scientia Plena, vol. 17, no. 10, pp. 1-8. http://dx.doi.org/10.14808/sci.plena.2021.100201
» http://dx.doi.org/10.14808/sci.plena.2021.100201 - SCARIOT, E., BONOME, L.T.S., BITTENCOURT, H.V.H. and LIMA, C.S.M., 2017. Extrato aquoso de Cyperus rotundus no enraizamento de estacas lenhosas de Prunus persica cv. ‘Chimarrita’. Revista de Ciências Agroveterinárias, vol. 16, no. 2, pp. 195-200. http://dx.doi.org/10.5965/223811711622017195
» http://dx.doi.org/10.5965/223811711622017195 - SHAPIRO, S.S. and WILK, M.B., 1965. An analysis of variance test for normality (complete samples). Biometrika Trust, vol. 52, no. 3-4, pp. 591-609. http://dx.doi.org/10.2307/2333709
» http://dx.doi.org/10.2307/2333709 - SHIBUYA, T., ENDO, R., KITAYA, Y. and TSUCHIDA, M., 2020. Far-red light interacts with plant density to change photosynthate allocation of cucumber seedlings and their subsequent early growth after transplanting. Horticultural Science, vol. 55, no. 9, pp. 1433-1437.
- SILVA FILHO, A.M., FERRAZ, R.L.S., SILVA, E.S., SILVA, G.N., ANDRADE, L.O. and BARBOSA, M.A., 2016. Florística e potencial medicinal de plantas espontâneas em agroecossistema de Jatropha molíssima (Pohl) Baill sob condição salina. Revista Brasileira de Agroecologia, vol. 11, no. 1, pp. 45-53.
- SILVA, A.E., FERRAZ, R.L.S., SILVA, J.P., COSTA, P.S., VIÉGAS, P.R.A., BRITO NETO, J.F., MELO, A.S., MEIRA, K.S., SOARES, C.S., MAGALHÃES, I.D. and MEDEIROS, A.S., 2020. Microclimate changes, photomorphogenesis and water consumption of Moringa oleifera cuttings under different light spectrums and exogenous phytohormone concentrations. Australian Journal of Crop Science, vol. 14, no. 5, pp. 751-760. http://dx.doi.org/10.21475/ajcs.20.14.05.p2096
» http://dx.doi.org/10.21475/ajcs.20.14.05.p2096 - SILVA, J.E.S.B., TORRES, S.B., LEAL, C.C.P., LEITE, M.S., GUIRRA, K.S., DANTAS, B.F., MORAIS, M.B. and GUIRRA, B.S., 2024. Pre-germination treatments of melon seeds for the production of seedlings irrigated with biosaline water. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 84, no. 1, p. e257314. PMid:35043840.
- SIMÕES, C.M.O., SCHENKEL, E.P., GOSMANN, G., MELLO, J.C.P., MENTZ, L.A. and PETROVICK, P.R., 2003. Farmacognosia: da planta ao medicamento 5th ed. Florianópolis: Editora da UFSC, 1102 p.
- SOARES, C.S., SILVA, J.A. and SILVA, G.N., 2017. Produção de coentro em diferentes espaçamentos dos canais hidropônicos. Pesquisa Agropecuária Pernambucana, vol. 22, pp. 1-5. http://dx.doi.org/10.12661/pap.2017.001
» http://dx.doi.org/10.12661/pap.2017.001 - STATSOFT INC., 2004. Statistica: data analysis software system. Version 7 [software]. Available from: www.statsoft.com.
- TUAN, P.A., SUN, M., NGUYEN, T.N., PARK, S. and AYELE, B.T., 2019. Molecular mechanisms of seed germination. In: H. FENG, B. NEMZER and J.W. DEVRIES, eds. Sprouted grains: nutritional value, production and applications Cambridge: AACC International Press, pp. 1-24. http://dx.doi.org/10.1016/B978-0-12-811525-1.00001-4
» http://dx.doi.org/10.1016/B978-0-12-811525-1.00001-4 - VAISHAK, K.P., YADUKRISHNAN, P., BAKSHI, S., KUSHWAHA, A.K., RAMACHANDRAN, H., JOB, N., BABU, D. and DATTA, S., 2019. The B-box bridge between light and hormones in plants. Journal of Photochemistry and Photobiology B: Biology, vol. 191, no. 2, pp. 164-174. http://dx.doi.org/10.1016/j.jphotobiol.2018.12.021 PMid:30640143.
» http://dx.doi.org/10.1016/j.jphotobiol.2018.12.021 - WEBER, S., DAMEROW, L., KUNZ, A. and BLANKE, M., 2019. Anthocyanin synthesis and light utilisation can be enhanced by reflective mulch – visualisation of light penetration into a tree canopy. Journal of Plant Physiology, vol. 233, pp. 52-57. http://dx.doi.org/10.1016/j.jplph.2018.12.008 PMid:30597476.
» http://dx.doi.org/10.1016/j.jplph.2018.12.008 - YAMASHITA, O.M., GUIMARÃES, S.C. and CAVENAGHI, A.L., 2011. Germinação das sementes de Conyza canadensis e Conyza bonariensis em função da qualidade de luz. Planta Daninha, vol. 29, no. 4, pp. 737-743. http://dx.doi.org/10.1590/S0100-83582011000400003
» http://dx.doi.org/10.1590/S0100-83582011000400003
Publication Dates
-
Publication in this collection
29 Apr 2022 -
Date of issue
2024
History
-
Received
29 Aug 2021 -
Accepted
16 Mar 2022