Abstract
To mitigate the deleterious effects of salt stress, substances capable of acting as mitigators and/or inducers of tolerance to stress have been used, enabling the use of saline waters and contributing to the development of irrigated agriculture. In this context, the aim of the present study was to evaluate the effect of foliar spraying with hydrogen peroxide as an attenuator of salt stress effects on soursop morphophysiology. The experiment was conducted under greenhouse conditions in Campina Grande - PB, Brazil, using a randomized block design, in a 4 × 4 factorial arrangement, whose treatments resulted from the combination of four levels of electrical conductivity of irrigation water - ECw (0.8 – control, 1.6, 2.4, and 3.2 dS m-1) and four concentrations of hydrogen peroxide - H2O2 (0, 10, 20, and 30 μM), with three replicates. Foliar application of hydrogen peroxide at a concentration of 10 µM increased growth, chlorophyll synthesis, and relative water content in the leaves and consequently reduced the foliar water saturation deficit of soursop irrigated with ECw up to 1.6 dS m-1. The concentration of hydrogen peroxide of 30 µM intensified the salt stress on the electrolyte leakage in the leaf blade and the photosynthetic pigments of soursop, 270 days after transplanting.
Keywords:
Annona muricata L.; abiotic stress; mitigation; H2O2
Resumo
Para mitigar os efeitos deletérios do estresse salino, tem se utilizado substâncias capazes de atuar como atenuantes e/ou indutoras de tolerância ao estresse, viabilizando o uso de águas salinas e contribuindo para desenvolvimento da agricultura irrigada. Neste contexto, objetivou-se com presente estudo, avaliar o efeito da pulverização foliar com peróxido de hidrogênio como atenuante do estresse salino sobre a morfofisiologia da gravioleira. O estudo foi conduzido sob condições de casa de vegetação, em Campina Grande - PB, utilizando-se o delineamento de blocos casualizados, no arranjo fatorial 4 × 4, cujos tratamentos resultaram da combinação de quatro níveis de condutividade elétrica da água de irrigação - CEa (0,8 – controle; 1,6; 2,4 e 3,2 dS m-1) e quatro concentrações de peróxido de hidrogênio - H2O2 (0; 10; 20 e 30 µM), com três repetições. A aplicação foliar de peróxido de hidrogênio na concentração de 10 µM aumentou o crescimento, a síntese de clorofila e o teor relativo de água nas folhas e consequentemente reduziu o déficit de saturação hídrica foliar da gravioleira irrigada com CEa de até 1,6 dS m-1. A concentração de peroxido de hidrogênio de 30 µM intensificou o estresse salino sobre o extravasamento de eletrólitos no limbo foliar e os pigmentos fotossintéticos da gravioleira, aos 270 dias após o transplantio.
Palavras-chave:
Annona muricata L.; estresse abiótico; mitigação; H2O2
1. Introduction
Brazil has stood out as one of the largest fruit producers in the world, occupying third place in production, only behind China and India (FAO, 2021FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS – FAO, 2021 [viewed 5 December 2021]. FAOSTAT [online]. Available from: https://www.fao.org/faostat/en/#data/QCL/visualize.
https://www.fao.org/faostat/en/#data/QCL...
). The Northeast region has ideal edaphic conditions, temperature, and luminosity for the cultivation of fruit crops of tropical climate, among which soursop (Annona muricata L.) has gained space, especially in the State of Bahia, standing out as an alternative for income generation (Lemos, 2014LEMOS, E.E.P., 2014. A produção de anonáceas no Brasil. Revista Brasileira de Fruticultura, vol. 36, Spe 1, pp. 77-85. http://dx.doi.org/10.1590/S0100-29452014000500009.
http://dx.doi.org/10.1590/S0100-29452014...
; Caliman et al., 2021CALIMAN, M.E., SILVA, D.C., PEREIRA, J. and BEZERRA, J.L., 2021. Biologia e patogenicidade de Prillieuxina winteriana em pomares de gravioleira na Bahia. Summa Phytopathologica, vol. 47, no. 1, pp. 27-33. http://dx.doi.org/10.1590/0100-5405/182617.
http://dx.doi.org/10.1590/0100-5405/1826...
).
Although the semi-arid region of northeastern Brazil has favorable conditions for soursop cultivation, water restrictions in terms of quality and quantity make its cultivation dependent on irrigation, which is often carried out with waters containing high salt levels (Silva et al., 2019SILVA, A.A.R., LIMA, G.S., AZEVEDO, C.A.V., GHEYI, H.R., SOUZA, L.P. and VELOSO, L.L.S.A., 2019. Gas exchanges and growth of passion fruit seedlings under salt stress and hydrogen peroxide. Pesquisa Agropecuária Tropical, vol. 49, no. 1, p. e55671. http://dx.doi.org/10.1590/1983-40632019v4955671.
http://dx.doi.org/10.1590/1983-40632019v...
).
The excess of salts in the irrigation water promotes the reduction of plant growth, because, while absorbing water, plants simultaneously absorb Na+ and Cl- ions, which cause damage to membranes, inactivation of biochemical pathways, consumption of reserves, and alterations in osmotic and ionic homeostase (Sá et al., 2022SÁ, F.V.S., OLIVEIRA, F.S., TORRES, S.B., PAIVA, E.P., NOGUEIRA, N.W., SARMENTO, E.C.S. and MELO, A.S., 2022. Hydric and saline stress on Phaseolus lunatus L. seeds. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 82, no. 1, p. e233550. http://dx.doi.org/10.1590/1519-6984.233550. PMid:34133547.
http://dx.doi.org/10.1590/1519-6984.2335...
; Sousa et al., 2024SOUSA, V.F.O., SANTOS, A.S., SALES, W.S., SILVA, A.J., GOMES, F.A.L., DIAS, T.J., GONÇALVES-NETO, A.C., FARAZ, A., SANTOS, J.P.O., SANTOS, G.L., CRUZ, J.M.F.L., SILVA, L.D.R. and ARAÚJO, J.R.E.S., 2024. Exogenous application of salicylic acid induces salinity tolerance in eggplant seedlings. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 84, no. 1, p. e257739. PMid:35081218.). Salt stress also causes structural changes in photosynthetic pigments, compromising the excitation energy efficiency of the light-harvesting antenna and causing damage to photosystem II reaction centers (Tatagiba et al., 2014TATAGIBA, S.D., MORAES, G.A.B.K., NASCIMENTO, K.J.T. and PELOSO, A.F., 2014. Limitações fotossintéticas em folhas de plantas de tomateiro submetidas a crescentes concentrações salinas. Engenharia na Agricultura, vol. 22, no. 2, pp. 138-149. http://dx.doi.org/10.13083/reveng.v22i2.488.
http://dx.doi.org/10.13083/reveng.v22i2....
), affecting leaf turgor potential and inducing membrane damage.
Despite the negative effects of salts on plants, the use of saline water in irrigation can be made possible through the use of hydrogen peroxide (H2O2) (Andrade et al., 2019ANDRADE, E.M.G., LIMA, G.S., LIMA, V.L.A., SILVA, S.S., GHEYI, H.R. and SILVA, A.A.R., 2019. Gas exchanges and growth of passion fruit under saline water irrigation and H2O2 application. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 23, no. 12, pp. 945-951. http://dx.doi.org/10.1590/1807-1929/agriambi.v23n12p945-951.
http://dx.doi.org/10.1590/1807-1929/agri...
). H2O2 can act in acclimatization to salt stress, through metabolic changes in plants that will be responsible for increasing their tolerance to a new exposure to stress (Gohari et al., 2020GOHARI, G., ALAVI, Z., ESFANDIARI, E., PANAHIRAD, S., HAJIHOSEINLOU, S. and FOTOPOULOS, V., 2020. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiologia Plantarum, vol. 168, no. 2, pp. 361-373. PMid:31433490.), antioxidant metabolism, and reduce lipid peroxidation in leaves and roots (Hossain et al., 2015HOSSAIN, M.A., BHATTACHARJEE, S., ARMIN, S.M., QIAN, P., XIN, W., LI, H.Y., BURRITT, D.J., FUJITA, M. and TRAN, L.S.P., 2015. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Frontiers in Plant Science, vol. 6, no. 1, p. 420. http://dx.doi.org/10.3389/fpls.2015.00420. PMid:26136756.
http://dx.doi.org/10.3389/fpls.2015.0042...
). In this context, the aim of this study was to evaluate the effect of foliar spraying of hydrogen peroxide as an attenuator of salt stress effects on soursop morphophysiology.
2. Material and Methods
2.1. Experimental location
The experiment was carried out during April 2020 and September 2021 in a greenhouse, belonging to the Academic Unit of Agricultural Engineering - UAEA of the Federal University of Campina Grande - UFCG, in Campina Grande, Paraíba, Brazil, at the geographical coordinates 7°15’18” South latitude, 35°52’28” West longitude and an average altitude of 550 m. The data of air temperature (maximum and minimum) and relative air humidity during the experimental period are shown in Figure 1.
Mean air temperature (maximum and minimum) and mean Relative humidity of the air in the internal area of the greenhouse during the experimental period.
2.2. Treatment and experimental design
The treatments consisted of four levels of electrical conductivity of irrigation water - ECw (0.8, 1.6, 2.4, and 3.2 dS m-1) and four concentrations of hydrogen peroxide - H2O2 (0, 10, 20, and 30 μM), in a 4 × 4 factorial arrangement, distributed in a randomized block design, with three replicates. H2O2 concentrations were established according to a study conducted by Veloso et al. (2019)VELOSO, L.L.S.A., AZEVEDO, C.A.V., SILVA, A.A.R., LIMA, G.S., GHEYI, H.R., NÓBREGA, R.A., PINHEIRO, F.W.A. and LUCENA, R.C.M., 2019. Effects of saline water and exogenous application of hydrogen peroxide (H2O2) on soursop (Annona muricata L.) at vegetative stage. Australian Journal of Crop Science, vol. 13, no. 3, pp. 472-479. http://dx.doi.org/10.21475/ajcs.19.13.03.p1583.
http://dx.doi.org/10.21475/ajcs.19.13.03...
and water salinity levels were defined according to Silva et al. (2020)SILVA, A.A.R., LIMA, G.S., AZEVEDO, C.A.V., VELOSO, L.L.S.A. and GHEYI, H.R., 2020. Salicylic acid as an attenuator of salt stress in soursop. Revista Caatinga, vol. 33, no. 4, pp. 1092-1101. http://dx.doi.org/10.1590/1983-21252020v33n424rc.
http://dx.doi.org/10.1590/1983-21252020v...
.
The adoption of the randomized block design was due to the heterogeneity of luminosity inside the greenhouse, due to the shading effect of another greenhouse located on the left-hand side, taking into account the three basic principles of experimentation: repetition, randomization, and local control.
2.3. Establishment and management of the experiment
The seedlings were propagated sexually and the formation period was of 180 days. After this period, the seedlings were transplanted into plastic pots. The experiment was conducted using plastic pots adapted as drainage lysimeters, with a capacity of 200 L, filled with a 1.0-kg layer of crushed stone followed by 230 kg of soil classified as Entisol, collected at a depth of 0-30 cm, from the municipality of Riachão do Bacamarte - PB, whose physical-chemical characteristics (Table 1) were determined according to Teixeira et al. (2017)TEIXEIRA, P.C., DONAGEMMA, G.K., FONTANA, A. and TEIXEIRA, W.G., 2017. Manual de métodos de análise de solo. 3rd ed. Brasília: Embrapa Solos, 212 p..
Chemical and physical attributes of the soil (0-0.30 m layer), used in the experiment, before the application of the treatments. Campina Grande, PB.
The irrigation waters with different electrical conductivity were prepared by dissolving NaCl, CaCl2.2H2O, and MgCl2.6H2O salts, in the equivalent proportion of 7:2:1, respectively, in water from the municipal supply (ECw = 0.38 dS m-1). Irrigation waters were prepared considering the relationship between ECw and the sum of cations (Richards, 1954RICHARDS, L.A., 1954. Diagnosis and improvement of saline and alkali soils. Washington: U.S. Department of Agriculture, 160 p.), according to Equation 1:
Where:
Q - sum of cations (mmolc L-1)
ECw - electrical conductivity of water (dS m-1)
Irrigation events with saline waters started 45 days after transplanting, this time was necessary for the acclimatization of the plants to the drainage lysimeters and the climatic conditions of the greenhouse. A three-day irrigation interval was adopted, taking into account the soil's water retention capacity, climatic conditions, and the water requirements of plants. The applied volume was estimated by the soil water balance, determined by Equation 2:
Where:
VI - volume of water to be used in the irrigation event (mL);
Va - volume applied in the previous irrigation event (mL);
Vd - volume drained in the previous irrigation event (mL);
LF - leaching fraction of 0.15, applied every 30 days to avoid excessive accumulation of salts.
H2O2 applications began 30 days after transplantation (DAT), on the abaxial and adaxial sides of the leaves, and the subsequent ones were performed at 30-day intervals using a backpack sprayer, between 17:00 and 17:45 h. Nitrogen, phosphorus, and potassium fertilization were based on the recommendation of Cavalcanti (2008)CAVALCANTI, F.J.A., 2008. Recomendação de adubação para o estado de Pernambuco: 2a. aproximação. 3rd ed. Recife: Instituto Agronômico de Pernambuco, 212 p. for the soursop crop, applying 33,3 kg of nitrogen, 20 kg of phosphorus, and 13,3 kg of potassium ha-1 for the first year of cultivation, split into 24 portions and applied at 15-day intervals. Ammonium sulfate (21% N), monoammonium phosphate (61% P2O5, 12% N), and potassium chloride (60% K2O) were used as sources of nitrogen, phosphorus and potassium, respectively.
Every two weeks, a backpack sprayer was used to apply a Dripsol® micro solution on the adaxial and abaxial sides of the leaves, to meet the micronutrient requirement at the concentration of 1.0 g L-1, with the following composition: Mg (1.1%), Zn (4.2%), B (0.85%), Fe (3.4%), Mn (3.2%), Cu (0.5%), and Mo (0.05%).
2.4. Variables analyzed
At 270 days after transplanting (DAT), when the plants were in full vegetative development, growth was evaluated based on stem diameter (SD), crown height (CRH), crown volume (CRV), crown diameter (CRD), and vegetative vigor index (VVI). At the same time, electrolyte leakage (% EL) in the leaf blade, relative water content (RWC), and water saturation deficit (WSD) in the leaf blade, and photosynthetic pigments (chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids) were determined.
CRH was measured by taking as reference the distance from the plant collar to the insertion of the apical meristem. Stem diameter was determined at 3 cm height from the plant collar with a digital caliper. CRD was obtained from the average crown diameter observed in the directions of the planting row (DR) and inter-row (DIR). Crown volume (CRV) was calculated from crown height (CRH), DR, and DIR, using Equation 3, and VVI was calculated using Equation 4, according to Portella et al. (2016)PORTELLA, C.R., MARINHO, C.S., AMARAL, B.D., CARVALHO, W.S.G., CAMPOS, G.S., SILVA, M.P.S.D. and SOUSA, M.C.D., 2016. Desempenho de cultivares de citros enxertadas sobre o trifoliateiro ‘flying Dragon’e limoeiro ‘cravo’ em fase de formação do pomar. Bragantia, vol. 75, no. 1, pp. 70-75. http://dx.doi.org/10.1590/1678-4499.267.
http://dx.doi.org/10.1590/1678-4499.267...
.
Where:
CRV - crown volume (m3);
CRD - crown diameter (m);
VVI - vegetative vigor index;
CRH - crown height (m);
DR - crown diameter in the row direction (m);
DIR - crown diameter in the inter-row direction (m); and
SD - stem diameter (mm).
The vegetative vigor index (VVI), considering plant height, crown, and stem diameter, despite the evident correlation of its components, is possibly the parameter that best reflects the vegetative vigor of plants (Bordignon et al., 2003BORDIGNON, R., MEDINA FILHO, H.P., SIQUEIRA, W.J. and PIO, R.M., 2003. Características da laranjeira Valência sobre clones e híbridos de porta-enxertos tolerantes à tristeza. Bragantia, vol. 62, no. 3, pp. 381-395. http://dx.doi.org/10.1590/S0006-87052003000300005.
http://dx.doi.org/10.1590/S0006-87052003...
).
In order to determine the percentage of intercellular electrolyte leakage (% IEL) in the leaf blade, a copper hole punch was used to obtain five-leaf discs with an area of 1.54 cm2 each, per experimental unit, which were washed and placed in Erlenmeyer® flasks containing 50 mL of distilled water. After being closed with aluminum foil, the Erlenmeyer® flasks were kept at a temperature of 25 °C for 90 minutes, and then the initial conductivity of the medium (Xi) was measured using a benchtop conductivity meter (MB11, MS Techonopon®). Then, the Erlenmeyer® flasks were subjected to a temperature of 90 °C for 90 minutes in a drying oven (SL100/336, SOLAB®) and, after cooling their contents, the final conductivity (Xf) was measured. The percentage of intercellular electrolyte leakage was expressed as the percentage of initial electrical conductivity relative to the electrical conductivity after treatment for 90 minutes at 90 ºC, according to Equation 5 (Scotti-Campos et al., 2013SCOTTI-CAMPOS, P., PHAM-THI, A.-T., SEMEDO, J.N., PAIS, I.P., RAMALHO, J.C. and MATOS, M.C., 2013. Physiological responses and membrane integrity in three Vigna genotypes with contrasting drought tolerance. Emirates Journal of Food and Agriculture, vol. 25, no. 12, pp. 1002-1013. http://dx.doi.org/10.9755/ejfa.v25i12.16733.
http://dx.doi.org/10.9755/ejfa.v25i12.16...
).
Where:
% IEL - percentage of intercellular electrolyte leakage;
Xi - initial electrical conductivity;
Xf - final electrical conductivity.
To determine the relative water content (RWC) and water saturation deficit (WSD), two leaves were collected from the middle third of the main branch to obtain five discs of 12-mm diameter from each leaf. Immediately after collection, the leaves were washed with distilled water and the discs were weighed, avoiding moisture loss, to obtain fresh mass (FM); then, these samples were placed in a beaker, immersed in 50 mL of distilled water, and stored for 90 minutes. After this period, excess water was removed from the discs with paper towels to obtain the turgid mass (TM) of the samples, which were dried in an oven at ≈ 65 ± 3 ºC until reaching constant weight to obtain the dry mass (DM). RWC and WSD were determined according to Lima et al. (2015)LIMA, G.S., GHEYI, H.R., NOBRE, R.G., SOARES, L.A.A., XAVIER, D.A. and SANTOS JUNIOR, J.A., 2015. Water relations and gas exchange in castor bean irrigated with saline water of distinct cationic nature. African Journal of Agricultural Research, vol. 10, no. 13, pp. 1581-1594. http://dx.doi.org/10.5897/AJAR2015.9606.
http://dx.doi.org/10.5897/AJAR2015.9606...
, by Equations 6-7:
Where:
RWC - relative water content (%);
WSD - water saturation deficit (%);
FM - fresh mass of discs (g);
TM - turgid mass of discs (g); and
DM - dry mass of discs (g).
The contents of photosynthetic pigments (chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids) were quantified according to Arnon (1949)ARNON, D.I., 1949. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiology, vol. 24, no. 1, pp. 1-15. http://dx.doi.org/10.1104/pp.24.1.1. PMid:16654194.
http://dx.doi.org/10.1104/pp.24.1.1...
, with plant extracts from samples of discs from the blade of the third mature leaf from the apex. In each sample, 6.0 mL of 80% acetone (A.R.) was used. Through these extracts, chlorophyll and carotenoid concentrations in the solutions were determined using a spectrophotometer at absorbance wavelength (ABS) (470, 647, and 663 nm) according to Equations 8-11.
Where:
Chl a - Chlorophyll a;
Chl b - Chlorophyll b;
Car - Carotenoids; and
Chl t - Total chlorophyll.
The values obtained for chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid contents in the leaves were expressed in mg g-1 of fresh matter (FM).
2.5. Statistical analysis
The multivariate structure of the results was evaluated employing principal component analysis (PCA), synthesizing the amount of relevant information contained in the original data set in a smaller number of dimensions, resulting from linear combinations of the original variables generated from the eigenvalues (λ ≥ 1.0) in the correlation matrix, explaining a percentage greater than 10% of the total variance (Govaerts et al., 2007GOVAERTS, B., SAYRE, K.D., LICHTER, K., DENDOOVEN, L. and DECKERS, J., 2007. Influence of permanent raised bed planting and residue management on physical and chemical soil quality in rain fed maize/wheat systems. Plant and Soil, vol. 291, no. 2, pp. 39-54. http://dx.doi.org/10.1007/s11104-006-9172-6.
http://dx.doi.org/10.1007/s11104-006-917...
).
From the reduction of dimensions, the original data of the variables of each component were subjected to multivariate analysis of variance (MANOVA) by the Hotelling test (Hotelling et al., 1947HOTELLING, H., EISENHART, C., HASTAY, M.W. and WALLIS, W.A., 1947. Multivariate quality control: techniques of statistical analysis. New York: John Wiley & Sons, 73 p.) at a 0.05 probability level for salinity levels and hydrogen peroxide concentrations, as well as for the interaction between them. Only variables with correlation coefficient greater than or equal to 0.6 were maintained in each principal component (PC) (Hair et al., 2009HAIR, F.J., BLACK, W.C., BABIN, B.J., ANDERSON, R.E. and TATHAM, R.L., 2009. Análise multivariada de dados. 6th ed. Porto Alegre: The Bookman, 688 p.). The statistical analyses were performed using Statistica software v. 7.0 (Statsoft, 2004).
3. Results and Discussion
The multidimensional space of the original variables was reduced to two principal components (PC1 and PC2) with eigenvalues greater than λ ≥ 1.0, according to Kaiser (1960)KAISER, H.F., 1960. The application of electronic computers to factor analysis. Educational and Psychological Measurement, vol. 20, no. 1, pp. 141-151. http://dx.doi.org/10.1177/001316446002000116.
http://dx.doi.org/10.1177/00131644600200...
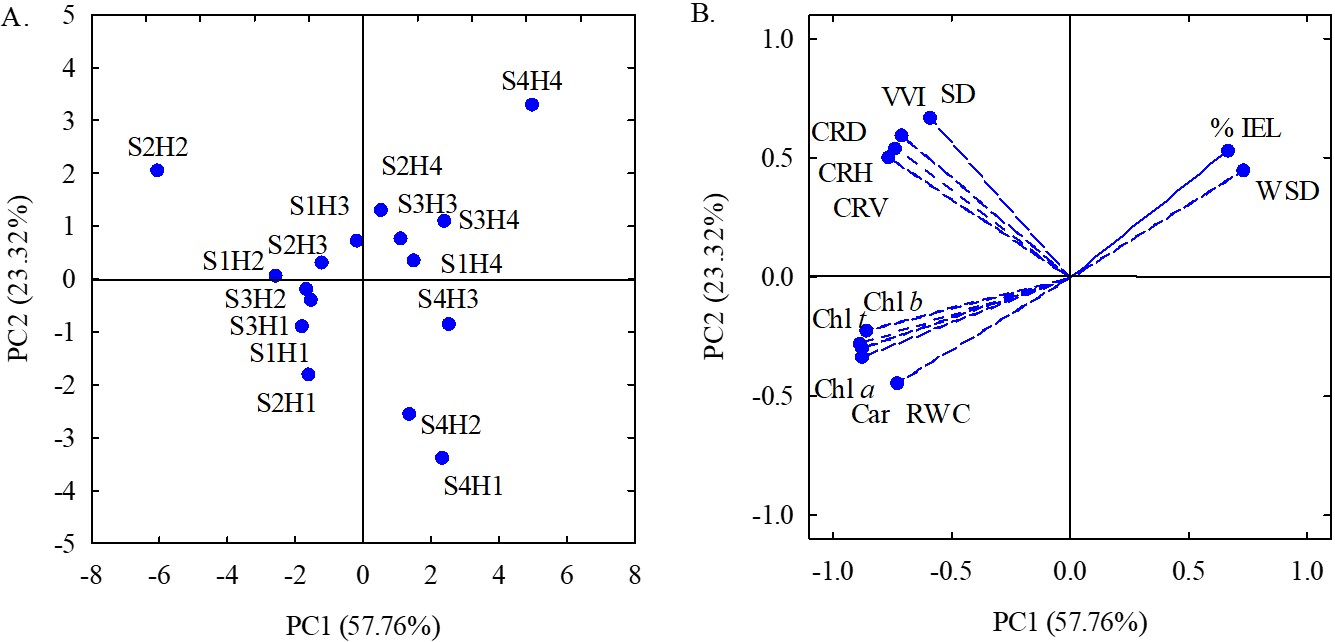
. The eigenvalues and percentage of variation explained for each component (Table 2) represented together 81.08% of the total variation. PC1 explained 57.76% of the total variance, formed by most of the variables analyzed, except for stem diameter (SD) and vegetative vigor index (VVI). PC2 represented 23.32% of the remaining variance, formed by SD and VVI.
Eigenvalues, percentage of total variance explained, in the multivariate analysis of variance (MANOVA) and correlations (r) between original variables and the principal components.
According to the multivariate analysis of variance (Table 2), there was a significant effect of the interaction between the electrical conductivity of irrigation water (ECw) and hydrogen peroxide concentrations (H2O2) for the two principal components (PC1 and PC2).
The two-dimensional projections of the effects of treatments and variables in the first and second principal components (PC1 and PC2) are presented in Figure 2A-2B. In the first principal component (PC1), a process possibly characterized by the effect of the interaction between ECw and H2O2 concentrations was identified: the correlation coefficients between CRH, CRV, CRD, % IEL, RWC, WSD, Chl a, Chl b, Chl t, and Car were higher than 0.70 (Table 2).
Two-dimensional projection of the scores of the principal components for the factors electrical conductivity of irrigation water (S) and concentrations of hydrogen peroxide (H) (A) and the variables analyzed (B) in the two principal components (PC1 and PC2).
In principal component 1, it is possible to note that soursop plants grown with ECw of 1.6 dS m-1 and sprayed with an H2O2 concentration of 10 μM (S2H2) stood out from those of the other treatments, as they had the highest values (Table 2) of CRH (1.64 m), CRV (0.44 m3), CRD (1.47 m), RWC (80.25%), Chl a (1,824 mg g-1 FM), Chl b (752.54 mg g-1 FM), Chl t (2,576.54 mg g-1 FM), and Car (648.57 mg g-1 FM).
When comparing the results obtained in plants of the S2H2 treatment to those obtained in plants of the S2H1 treatment, there were increments of 15.49, 46.67, 14.84, 10.62, 22.63, 29.92, 24.67, and 13.96% in CRH, CRV, CRD, RWC, Chl a, Chl b, Chl t, and Car, respectively, showing the beneficial effect of the foliar application of H2O2 at concentration of 10 μM. Hydrogen peroxide is a reactive oxygen species (ROS) that, when applied at low concentrations, helps acclimatize to salt stress, due to metabolic changes that are responsible for increasing its tolerance to stress, thus enabling the use of waters with higher concentrations of salts (Andrade et al., 2019ANDRADE, E.M.G., LIMA, G.S., LIMA, V.L.A., SILVA, S.S., GHEYI, H.R. and SILVA, A.A.R., 2019. Gas exchanges and growth of passion fruit under saline water irrigation and H2O2 application. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 23, no. 12, pp. 945-951. http://dx.doi.org/10.1590/1807-1929/agriambi.v23n12p945-951.
http://dx.doi.org/10.1590/1807-1929/agri...
; Silva et al., 2022SILVA, A.A.R., SOUSA, P.F.N., LIMA, G.S., SOARES, L.A.A., GHEYI, H.R. and AZEVEDO, C.A.V., 2022. Hydrogen peroxide reduces the effect of salt stress on growth and postharvest quality of hydroponic mini watermelon. Water, Air, and Soil Pollution, vol. 233, no. 6, p. 198. http://dx.doi.org/10.1007/s11270-022-05669-8.
http://dx.doi.org/10.1007/s11270-022-056...
).
In addition, H2O2 can stimulate activities of antioxidant enzymes, promoting less degradation of pigments and an increase in photosynthetic activity, which reflects in the greater growth and development of plants (Ashraf et al., 2015ASHRAF, M.A., RASHEED, R., HUSSAIN, I., IQBAL, M., HAIDER, M.Z., PARVEEN, S. and SAJID, M.A., 2015. Hydrogen peroxide modulates antioxidant system and nutrient relation in maize (Zea mays L.) under water-deficit conditions. Archives of Agronomy and Soil Science, vol. 61, no. 4, pp. 507-523. http://dx.doi.org/10.1080/03650340.2014.938644.
http://dx.doi.org/10.1080/03650340.2014....
).
In a study evaluating morphophysiological changes in soursop (Annona muricata L.) under irrigation with saline water (1.6 to 4.0 dS m-1) and H2O2 in the post-grafting stage, Veloso et al. (2020)VELOSO, L.L.S.A., LIMA, G.S., AZEVEDO, C.A.V., NOBRE, R.G., SILVA, A.A.R., CAPITULINO, J.D., GHEYI, H.R. and BONIFÁCIO, B.F., 2020. Physiological changes and growth of soursop plants under irrigation with saline water and H2O2 in post-grafting phase. Semina: Ciências Agrárias, vol. 41, no. 6, suppl. 2, pp. 3023-3038. http://dx.doi.org/10.5433/1679-0359.2020v41n6Supl2p3023.
http://dx.doi.org/10.5433/1679-0359.2020...
also found a beneficial effect of H2O2 (20 μM) on photosynthetic pigments; in addition, foliar application of H2O2 promoted increment in photosynthesis and reduction in electrolyte leakage in the leaf blade.
It is also verified in PC1 that plants cultivated with ECw of 3.2 dS m-1 and subjected to a concentration of 30 μM (S4H4) had the highest values of % IEL (32.18%) and WSD (49.79%). The increase in the percentage of electrolyte leakage and water saturation deficit in plants subjected to higher H2O2 concentration (30 µM) may occur because, at high concentration, hydrogen peroxide can intensify the deleterious effects of salt stress, causing changes in plant metabolism due to the restriction of photosynthetic processes. Under salt stress conditions, photosynthetic processes can be affected directly, by stomatal restriction, reduced transpiration, and consequently, low CO2 availability, or indirectly, by the imbalance between the production and removal of reactive oxygen species (ROS) produced during the photosynthetic process, causing oxidative stress (Carvalho et al., 2011CARVALHO, F.E.L., LOBO, A.K.M., BONIFACIO, A., MARTINS, M.O., LIMA NETO, M.C. and SILVEIRA, J.A.G., 2011. Aclimatação ao estresse salino em plantas de arroz induzida pelo pré-tratamento com H2O2. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 15, no. 4, pp. 416-423. http://dx.doi.org/10.1590/S1415-43662011000400014.
http://dx.doi.org/10.1590/S1415-43662011...
; Silva et al., 2021aSILVA, A.A.R., LIMA, G.S., AZEVEDO, C.A.V., GHEYI, H.R., SOUZA, A.R. and FERNANDES, P.D., 2021a. Salicylic acid relieves the effect of saline stress on soursop morphysiology. Ciência e Agrotecnologia, vol. 45, no. 1, p. e007021. http://dx.doi.org/10.1590/1413-7054202145007021.
http://dx.doi.org/10.1590/1413-705420214...
).
For principal component 2 (PC2), it can be observed that soursop plants irrigated with ECw of 1.6 dS m-1 and subjected to spraying with H2O2 at a concentration of 10 μM (S2H2), obtained the highest values of SD (27.27 mm) and VVI (2.76), corresponding to increments of 12.69% (3.07 mm) in SD and 17.44% (0.41) in VVI compared to plants irrigated with the same ECw (1.6 dS m-1) and without application of H2O2 (S2H1). On the other hand, plants cultivated with ECw of 3.2 dS m-1 and without H2O2 application (S4H1) had the lowest values of SD (21.9 mm) and VVI (2.21).
Similar results were obtained in a study conducted by Silva et al. (2021a)SILVA, A.A.R., LIMA, G.S., AZEVEDO, C.A.V., GHEYI, H.R., SOUZA, A.R. and FERNANDES, P.D., 2021a. Salicylic acid relieves the effect of saline stress on soursop morphysiology. Ciência e Agrotecnologia, vol. 45, no. 1, p. e007021. http://dx.doi.org/10.1590/1413-7054202145007021.
http://dx.doi.org/10.1590/1413-705420214...
, who evaluated the effect of salt stress caused by irrigation water (0.8 to 4.0 dS m-1) on soursop morphophysiology at 480 days after transplanting and found that irrigation with ECw above 1.7 dS m-1 reduced soursop growth, with the lowest values of SD (30.09 mm) and VVI (2.42) obtained in plants subjected to ECw of 4.0 dS m-1.
The decrease in soursop SD and VVI reflects the effect of the osmotic potential of the soil solution caused by the high concentrations of salts, which hinders the absorption of water and nutrients by plants and, consequently, causes loss of turgor pressure in the cells, which compromises plant growth (Lima et al., 2021LIMA, G.S., SOARES, M.G.S., SOARES, L.A.A., GHEYI, H.R., PINHEIRO, F.W.A. and SILVA, J.B., 2021. Potassium and irrigation water salinity on the formation of sour passion fruit seedlings. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 25, no. 6, pp. 393-401. http://dx.doi.org/10.1590/1807-1929/agriambi.v25n6p393-401.
http://dx.doi.org/10.1590/1807-1929/agri...
).
In the present study, it was found that foliar application of H2O2 at a concentration of 10 μM promoted a significant increase in the growth, chlorophyll synthesis, and relative water content of soursop irrigated with ECw of 1.6 dS m-1. In addition, H2O2 (10 μM) reduced water saturation deficit and percentage of electrolyte leakage in plants irrigated with ECw of 1.6 dS m-1. The beneficial effects of applying H2O2 at a concentration of (10 μM) are related to the ability of this ROS to influence the tolerance of plants to salt stress, caused by increased synthesis of metabolites (El-Mogy et al., 2018EL-MOGY, M.M., GARCHERY, C. and STEVENS, R., 2018. Irrigation with salt water affects growth, yield, fruit quality, storability and marker-gene expression in cherry tomato. Acta Agriculturae Scandinavica, vol. 68, no. 1, pp. 727-737.; Nazir et al., 2019NAZIR, F., HUSSAIN, A. and FARIDUDDIN, Q., 2019. Interactive role of epibrassinolide and hydrogen peroxide in regulating stomatal physiology, root morphology, photosynthetic and growth traits in Solanum lycopersicum L. under nickel stress. Environmental and Experimental Botany, vol. 162, no. 24, pp. 479-495. http://dx.doi.org/10.1016/j.envexpbot.2019.03.021.
http://dx.doi.org/10.1016/j.envexpbot.20...
).
Therefore, the efficiency of H2O2 in attenuating the damage caused by salinity on the variables analyzed is evident. Possibly, the stress signaling function performed by H2O2 may have triggered the activation of antioxidant enzymes that persist in plants to alleviate oxidative damage, leading to improvements in physiological attributes for plant growth under stress (Dito and Gadallah, 2019DITO, S. and GADALLAH, M., 2019. Hydrogen peroxide supplementation relieves the deleterious effects of cadmium on photosynthetic pigments and oxidative stress and improves the growth, yield and quality of pods in pea plants (Pisum sativum L.). Acta Physiologiae Plantarum, vol. 41, no. 113, pp. 2-12.).
4. Conclusions
Hydrogen peroxide at a concentration of 10 µM increases growth, chlorophyll synthesis, and relative water content in the leaves and consequently reduces the leaf water saturation deficit of soursop irrigated with ECw up to 1.6 dS m-1.
Hydrogen peroxide concentration of 30 μM intensifies the deleterious effects of salt stress on the percentage of intercellular electrolyte leakage and photosynthetic pigments of soursop, 270 days after transplanting.
Acknowledgements
The authors gratefully acknowledge the Post-Graduate Agricultural Engineering Program of Universidade Federal de Campina Grande for use of infrastructure facilities. To the National Council for Scientific Development and Technology (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
References
- ANDRADE, E.M.G., LIMA, G.S., LIMA, V.L.A., SILVA, S.S., GHEYI, H.R. and SILVA, A.A.R., 2019. Gas exchanges and growth of passion fruit under saline water irrigation and H2O2 application. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 23, no. 12, pp. 945-951. http://dx.doi.org/10.1590/1807-1929/agriambi.v23n12p945-951
» http://dx.doi.org/10.1590/1807-1929/agriambi.v23n12p945-951 - ARNON, D.I., 1949. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiology, vol. 24, no. 1, pp. 1-15. http://dx.doi.org/10.1104/pp.24.1.1 PMid:16654194.
» http://dx.doi.org/10.1104/pp.24.1.1 - ASHRAF, M.A., RASHEED, R., HUSSAIN, I., IQBAL, M., HAIDER, M.Z., PARVEEN, S. and SAJID, M.A., 2015. Hydrogen peroxide modulates antioxidant system and nutrient relation in maize (Zea mays L.) under water-deficit conditions. Archives of Agronomy and Soil Science, vol. 61, no. 4, pp. 507-523. http://dx.doi.org/10.1080/03650340.2014.938644
» http://dx.doi.org/10.1080/03650340.2014.938644 - BORDIGNON, R., MEDINA FILHO, H.P., SIQUEIRA, W.J. and PIO, R.M., 2003. Características da laranjeira Valência sobre clones e híbridos de porta-enxertos tolerantes à tristeza. Bragantia, vol. 62, no. 3, pp. 381-395. http://dx.doi.org/10.1590/S0006-87052003000300005
» http://dx.doi.org/10.1590/S0006-87052003000300005 - CALIMAN, M.E., SILVA, D.C., PEREIRA, J. and BEZERRA, J.L., 2021. Biologia e patogenicidade de Prillieuxina winteriana em pomares de gravioleira na Bahia. Summa Phytopathologica, vol. 47, no. 1, pp. 27-33. http://dx.doi.org/10.1590/0100-5405/182617
» http://dx.doi.org/10.1590/0100-5405/182617 - CARVALHO, F.E.L., LOBO, A.K.M., BONIFACIO, A., MARTINS, M.O., LIMA NETO, M.C. and SILVEIRA, J.A.G., 2011. Aclimatação ao estresse salino em plantas de arroz induzida pelo pré-tratamento com H2O2 Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 15, no. 4, pp. 416-423. http://dx.doi.org/10.1590/S1415-43662011000400014
» http://dx.doi.org/10.1590/S1415-43662011000400014 - CAVALCANTI, F.J.A., 2008. Recomendação de adubação para o estado de Pernambuco: 2a. aproximação 3rd ed. Recife: Instituto Agronômico de Pernambuco, 212 p.
- DITO, S. and GADALLAH, M., 2019. Hydrogen peroxide supplementation relieves the deleterious effects of cadmium on photosynthetic pigments and oxidative stress and improves the growth, yield and quality of pods in pea plants (Pisum sativum L.). Acta Physiologiae Plantarum, vol. 41, no. 113, pp. 2-12.
- EL-MOGY, M.M., GARCHERY, C. and STEVENS, R., 2018. Irrigation with salt water affects growth, yield, fruit quality, storability and marker-gene expression in cherry tomato. Acta Agriculturae Scandinavica, vol. 68, no. 1, pp. 727-737.
- FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS – FAO, 2021 [viewed 5 December 2021]. FAOSTAT [online]. Available from: https://www.fao.org/faostat/en/#data/QCL/visualize
» https://www.fao.org/faostat/en/#data/QCL/visualize - GOHARI, G., ALAVI, Z., ESFANDIARI, E., PANAHIRAD, S., HAJIHOSEINLOU, S. and FOTOPOULOS, V., 2020. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiologia Plantarum, vol. 168, no. 2, pp. 361-373. PMid:31433490.
- GOVAERTS, B., SAYRE, K.D., LICHTER, K., DENDOOVEN, L. and DECKERS, J., 2007. Influence of permanent raised bed planting and residue management on physical and chemical soil quality in rain fed maize/wheat systems. Plant and Soil, vol. 291, no. 2, pp. 39-54. http://dx.doi.org/10.1007/s11104-006-9172-6
» http://dx.doi.org/10.1007/s11104-006-9172-6 - HAIR, F.J., BLACK, W.C., BABIN, B.J., ANDERSON, R.E. and TATHAM, R.L., 2009. Análise multivariada de dados 6th ed. Porto Alegre: The Bookman, 688 p.
- HOSSAIN, M.A., BHATTACHARJEE, S., ARMIN, S.M., QIAN, P., XIN, W., LI, H.Y., BURRITT, D.J., FUJITA, M. and TRAN, L.S.P., 2015. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Frontiers in Plant Science, vol. 6, no. 1, p. 420. http://dx.doi.org/10.3389/fpls.2015.00420 PMid:26136756.
» http://dx.doi.org/10.3389/fpls.2015.00420 - HOTELLING, H., EISENHART, C., HASTAY, M.W. and WALLIS, W.A., 1947. Multivariate quality control: techniques of statistical analysis New York: John Wiley & Sons, 73 p.
- KAISER, H.F., 1960. The application of electronic computers to factor analysis. Educational and Psychological Measurement, vol. 20, no. 1, pp. 141-151. http://dx.doi.org/10.1177/001316446002000116
» http://dx.doi.org/10.1177/001316446002000116 - LEMOS, E.E.P., 2014. A produção de anonáceas no Brasil. Revista Brasileira de Fruticultura, vol. 36, Spe 1, pp. 77-85. http://dx.doi.org/10.1590/S0100-29452014000500009
» http://dx.doi.org/10.1590/S0100-29452014000500009 - LIMA, G.S., GHEYI, H.R., NOBRE, R.G., SOARES, L.A.A., XAVIER, D.A. and SANTOS JUNIOR, J.A., 2015. Water relations and gas exchange in castor bean irrigated with saline water of distinct cationic nature. African Journal of Agricultural Research, vol. 10, no. 13, pp. 1581-1594. http://dx.doi.org/10.5897/AJAR2015.9606
» http://dx.doi.org/10.5897/AJAR2015.9606 - LIMA, G.S., SOARES, M.G.S., SOARES, L.A.A., GHEYI, H.R., PINHEIRO, F.W.A. and SILVA, J.B., 2021. Potassium and irrigation water salinity on the formation of sour passion fruit seedlings. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 25, no. 6, pp. 393-401. http://dx.doi.org/10.1590/1807-1929/agriambi.v25n6p393-401
» http://dx.doi.org/10.1590/1807-1929/agriambi.v25n6p393-401 - NAZIR, F., HUSSAIN, A. and FARIDUDDIN, Q., 2019. Interactive role of epibrassinolide and hydrogen peroxide in regulating stomatal physiology, root morphology, photosynthetic and growth traits in Solanum lycopersicum L. under nickel stress. Environmental and Experimental Botany, vol. 162, no. 24, pp. 479-495. http://dx.doi.org/10.1016/j.envexpbot.2019.03.021
» http://dx.doi.org/10.1016/j.envexpbot.2019.03.021 - PORTELLA, C.R., MARINHO, C.S., AMARAL, B.D., CARVALHO, W.S.G., CAMPOS, G.S., SILVA, M.P.S.D. and SOUSA, M.C.D., 2016. Desempenho de cultivares de citros enxertadas sobre o trifoliateiro ‘flying Dragon’e limoeiro ‘cravo’ em fase de formação do pomar. Bragantia, vol. 75, no. 1, pp. 70-75. http://dx.doi.org/10.1590/1678-4499.267
» http://dx.doi.org/10.1590/1678-4499.267 - RICHARDS, L.A., 1954. Diagnosis and improvement of saline and alkali soils. Washington: U.S. Department of Agriculture, 160 p.
- SÁ, F.V.S., OLIVEIRA, F.S., TORRES, S.B., PAIVA, E.P., NOGUEIRA, N.W., SARMENTO, E.C.S. and MELO, A.S., 2022. Hydric and saline stress on Phaseolus lunatus L. seeds. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 82, no. 1, p. e233550. http://dx.doi.org/10.1590/1519-6984.233550 PMid:34133547.
» http://dx.doi.org/10.1590/1519-6984.233550 - SCOTTI-CAMPOS, P., PHAM-THI, A.-T., SEMEDO, J.N., PAIS, I.P., RAMALHO, J.C. and MATOS, M.C., 2013. Physiological responses and membrane integrity in three Vigna genotypes with contrasting drought tolerance. Emirates Journal of Food and Agriculture, vol. 25, no. 12, pp. 1002-1013. http://dx.doi.org/10.9755/ejfa.v25i12.16733
» http://dx.doi.org/10.9755/ejfa.v25i12.16733 - SILVA, A.A.R., LIMA, G.S., AZEVEDO, C.A.V., GHEYI, H.R., SOUZA, A.R. and FERNANDES, P.D., 2021a. Salicylic acid relieves the effect of saline stress on soursop morphysiology. Ciência e Agrotecnologia, vol. 45, no. 1, p. e007021. http://dx.doi.org/10.1590/1413-7054202145007021
» http://dx.doi.org/10.1590/1413-7054202145007021 - SILVA, A.A.R., LIMA, G.S., AZEVEDO, C.A.V., GHEYI, H.R., SOUZA, L.P. and VELOSO, L.L.S.A., 2019. Gas exchanges and growth of passion fruit seedlings under salt stress and hydrogen peroxide. Pesquisa Agropecuária Tropical, vol. 49, no. 1, p. e55671. http://dx.doi.org/10.1590/1983-40632019v4955671
» http://dx.doi.org/10.1590/1983-40632019v4955671 - SILVA, A.A.R., LIMA, G.S., AZEVEDO, C.A.V., VELOSO, L.L.S.A. and GHEYI, H.R., 2020. Salicylic acid as an attenuator of salt stress in soursop. Revista Caatinga, vol. 33, no. 4, pp. 1092-1101. http://dx.doi.org/10.1590/1983-21252020v33n424rc
» http://dx.doi.org/10.1590/1983-21252020v33n424rc - SILVA, A.A.R., SOUSA, P.F.N., LIMA, G.S., SOARES, L.A.A., GHEYI, H.R. and AZEVEDO, C.A.V., 2022. Hydrogen peroxide reduces the effect of salt stress on growth and postharvest quality of hydroponic mini watermelon. Water, Air, and Soil Pollution, vol. 233, no. 6, p. 198. http://dx.doi.org/10.1007/s11270-022-05669-8
» http://dx.doi.org/10.1007/s11270-022-05669-8 - SILVA, A.A.R., VELOSO, L.L.S.A., LIMA, G.S., AZEVEDO, C.A.V., GHEYI, H.R. and FERNANDES, P.D., 2021b. Hydrogen peroxide in the acclimation of yellow passion fruit seedlings to salt stress. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 25, no. 2, pp. 116-123. http://dx.doi.org/10.1590/1807-1929/agriambi.v25n2p116-123
» http://dx.doi.org/10.1590/1807-1929/agriambi.v25n2p116-123 - SOUSA, V.F.O., SANTOS, A.S., SALES, W.S., SILVA, A.J., GOMES, F.A.L., DIAS, T.J., GONÇALVES-NETO, A.C., FARAZ, A., SANTOS, J.P.O., SANTOS, G.L., CRUZ, J.M.F.L., SILVA, L.D.R. and ARAÚJO, J.R.E.S., 2024. Exogenous application of salicylic acid induces salinity tolerance in eggplant seedlings. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 84, no. 1, p. e257739. PMid:35081218.
- STATSOFT INC., 2004. Statistica: data analysis software system. Version 7 [software]. Available from: www.statsoft.com.
- TATAGIBA, S.D., MORAES, G.A.B.K., NASCIMENTO, K.J.T. and PELOSO, A.F., 2014. Limitações fotossintéticas em folhas de plantas de tomateiro submetidas a crescentes concentrações salinas. Engenharia na Agricultura, vol. 22, no. 2, pp. 138-149. http://dx.doi.org/10.13083/reveng.v22i2.488
» http://dx.doi.org/10.13083/reveng.v22i2.488 - TEIXEIRA, P.C., DONAGEMMA, G.K., FONTANA, A. and TEIXEIRA, W.G., 2017. Manual de métodos de análise de solo 3rd ed. Brasília: Embrapa Solos, 212 p.
- VELOSO, L.L.S.A., AZEVEDO, C.A.V., SILVA, A.A.R., LIMA, G.S., GHEYI, H.R., NÓBREGA, R.A., PINHEIRO, F.W.A. and LUCENA, R.C.M., 2019. Effects of saline water and exogenous application of hydrogen peroxide (H2O2) on soursop (Annona muricata L.) at vegetative stage. Australian Journal of Crop Science, vol. 13, no. 3, pp. 472-479. http://dx.doi.org/10.21475/ajcs.19.13.03.p1583
» http://dx.doi.org/10.21475/ajcs.19.13.03.p1583 - VELOSO, L.L.S.A., LIMA, G.S., AZEVEDO, C.A.V., NOBRE, R.G., SILVA, A.A.R., CAPITULINO, J.D., GHEYI, H.R. and BONIFÁCIO, B.F., 2020. Physiological changes and growth of soursop plants under irrigation with saline water and H2O2 in post-grafting phase. Semina: Ciências Agrárias, vol. 41, no. 6, suppl. 2, pp. 3023-3038. http://dx.doi.org/10.5433/1679-0359.2020v41n6Supl2p3023
» http://dx.doi.org/10.5433/1679-0359.2020v41n6Supl2p3023
Publication Dates
-
Publication in this collection
01 July 2022 -
Date of issue
2024
History
-
Received
18 Feb 2022 -
Accepted
13 June 2022