Abstract
Trend of biofuel production from microalgal triacylglycerols is enhancing, because this substrate is a good sustainable and advantageous alternative to oil and gas fuel. In the present study, indigenous micro algal isolates were screened from water (n=30) and soil (n=30) samples collected from three districts of Punjab, Pakistan to evaluate their biofuel production potential. The samples were inoculated on BG – 11 agar medium plates by incubating at room temperature of 25°C providing 1000 lux for 16h light cycle followed by 8h of dark cycle for 15 d. Water samples were found to be rich in microalgae and 65.33% microalgae (49 isolates) were isolated from Faisalabad district. On the basis of microscopic morphology microalgal isolates (n=180) were selected and subjected to lipid detection by Nile red staining assay. Nile red positive isolates (n=23) were processed for biochemical (lipid, protein and carbohydrates) characterization. AIN63 isolate showed higher lipids (17.4%) content as detected by micro vanillin assay. Algal isolate AIN128 showed best protein contents (42.91%) detected by Bradford assay and AIN172 isolate showed higher carbohydrate contents (73.83%) as detected by anthrone assay. The selected algal isolates were also analyzed by Fourier transform infrared (FTIR) spectroscopy for confirmation of carbohydrate, protein and lipid analysis. These indigenous algae have the potential for in-vitro biofuel production from agricultural waste.
Keywords:
microalgae; biofuel; Fourier transform infrared; biomass; Nile red staining
Resumo
A tendência de produção de biocombustíveis a partir de triacilgliceróis de microalgas está aumentando, porque esse substrato é uma boa alternativa sustentável e vantajosa ao combustível de petróleo e gás. No presente estudo, isolados de microalgas indígenas foram selecionados de amostras de água (n = 30) e solo (n = 30) coletadas em três distritos de Punjab, Paquistão, para avaliar seu potencial de produção de biocombustíveis. As amostras foram inoculadas em placas de meio BG – 11 agar por incubação em temperatura ambiente de 25°C proporcionando 1000 lux por 16h de ciclo claro seguido de 8h de escuro por 15 dias. As amostras de água foram ricas em microalgas e 65.33% de microalgas (49 isolados) foram isolados do distrito de Faisalabad. Com base na morfologia microscópica, isolados de microalgas (n = 180) foram selecionados e submetidos à detecção de lipídios por ensaio de coloração com vermelho do Nilo. Isolados positivos para vermelho do Nilo (n = 23) foram processados para caracterização bioquímica (lipídica, proteica e de carboidratos). O isolado AIN63 apresentou maior teor de lipídios (17.4%) conforme detectado pelo ensaio de microvanilina. O isolado de alga AIN128 apresentou os melhores teores de proteína (42.91%) detectados pelo ensaio de Bradford, e o isolado AIN172 apresentou maiores teores de carboidratos (73.83%), conforme detectado pelo ensaio de anthrone. Os isolados de algas selecionados também foram analisados por espectroscopia de infravermelho com transformada de Fourier (FTIR) para confirmação da análise de carboidratos, proteínas e lipídios. Essas algas nativas têm potencial para a produção de biocombustíveis in vitro a partir de resíduos agrícolas.
Palavras-chave:
microalgas; biocombustível; infravermelho com transformada de Fourier; biomassa; coloração com vermelho do Nilo
1. Introduction
Microalgae are photosynthetic microorganisms that are able to use the sun energy and carbon dioxide into biomass. These are prokaryotic or eukaryotic microorganisms; green algae (Chlorophyta), diatoms (Bacillariophyta) and Cyanobacteria (Cyanophyceae) are examples of eukaryotic and prokaryotic microalgae, respectively (Dolganyuk et al., 2020DOLGANYUK, V., BELOVA, D., BABICH, O., PROSEKOV, A., IVANOVA, S., KATSEROV, D., PATYUKOV, N. and SUKHIKH, S., 2020. Microalgae: a promising source of valuable bioproducts. Biomolecules, vol. 10, no. 8, p. 1153. http://dx.doi.org/10.3390/biom10081153. PMid:32781745.
http://dx.doi.org/10.3390/biom10081153...
). Microalgae exist in all ecosystems, representing a big variety of species living in a wide range of environmental conditions. It was estimated that more than 50 000 species exist, but only a limited number of around 30 000, have been studied and analyzed (Tan et al., 2020TAN, J.S., LEE, S.Y., CHEW, K.W., LAM, M.K., LIM, J.W., HO, S.H. and SHOW, P.L., 2020. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered, vol. 11, no. 1, pp. 116-129. http://dx.doi.org/10.1080/21655979.2020.1711626. PMid:31909681.
http://dx.doi.org/10.1080/21655979.2020....
). The ability of algae to survive or proliferate over a wide range of environmental conditions is due to tremendous diversity and unusual pattern of lipid metabolism in response to changes in environmental condition (Sharma et al., 2020SHARMA, J., KUMAR, V., KUMAR, S.S., MALYAN, S.K., MATHIMANI, T., BISHNOI, N.R. and PUGAZHENDHI, A., 2020. Microalgal consortia for municipal wastewater treatment – Lipid augmentation and fatty acid profiling for biodiesel production. Journal of Photochemistry and Photobiology. B, Biology, vol. 202, pp. 111638. http://dx.doi.org/10.1016/j.jphotobiol.2019.111638. PMid:31733613.
http://dx.doi.org/10.1016/j.jphotobiol.2...
). The lipids include neutral lipids, polar lipids, wax esters, sterols and hydrocarbons and prenyl derivatives such as tocopherols, carotenoids, terpenes, quinines and phytylated pyrrole derivatives such as the chlorophylls (Laskin et al., 2003LASKIN, A.I., BENNETT, J.W. and GADD, G.M., 2003. Advances in applied microbiology. San Diego: Academic Press, vol. 53.).
In developing countries, demand for energy is increasing day by day, due to this competition for energy resources is becoming tough. To maintain the supply of suitable energy source is the toughest challenge of present era. The supply of clean energy can be attributed to stability of world, prosperity of world’s economic and quality of life (Yao et al., 2019YAO, S., LYU, S., AN, Y., LU, J., GJERMANSEN, C. and SCHRAMM, A., 2019. Microalgae-bacteria symbiosis in microalgal growth and biofuel production: a review. Journal of Applied Microbiology, vol. 126, no. 2, pp. 359-368. http://dx.doi.org/10.1111/jam.14095. PMid:30168644.
http://dx.doi.org/10.1111/jam.14095...
). Due to crisis of energy availability of clean energy has become an emerging problem worldwide to be resolved (Khoo et al., 2020KHOO, K.S., CHEW, K.W., YEW, G.Y., LEONG, W.H., CHAI, Y.H., SHOW, P.L. and CHEN, W.H., 2020. Recent advances in downstream processing of microalgae lipid recovery for biofuel production. Bioresource Technology, vol. 304, p. 122996. http://dx.doi.org/10.1016/j.biortech.2020.122996. PMid:32115347.
http://dx.doi.org/10.1016/j.biortech.202...
). Several countries especially those who have limited natural resources of fuel are trying to find new energy resources to cope up energy crisis. Intense attention has been paid to the production of biofuels from various sources. Along with threat of depletion of natural resources these also contribute to emission of noxious gases which are responsible for climatic changes disturbing the natural ecosystem (Perera et al., 2019PERERA, F., ASHRAFI, A., KINNEY, P. and MILLS, D., 2019. Towards a fuller assessment of benefits to children’s health of reducing air pollution and mitigating climate change due to fossil fuel combustion. Environmental Research, vol. 172, pp. 55-72. http://dx.doi.org/10.1016/j.envres.2018.12.016. PMid:30771627.
http://dx.doi.org/10.1016/j.envres.2018....
). Internal combustion engines or transportation industry utilizing fossil fuels are major contributor in greenhouse gases emissions. So, there is appalling need of time to produce clean energy (from zero emission source) using new techniques for electric as well as thermal energy production.
In the present study, third generation biofuel from microalgae gains importance because they are easy to grow indoors and outdoors, have faster growth rate and have ability to produce 20 times more bio-oil then traditional crops such as wood, barley straw, and miscanthus (Chowdhury and Loganathan, 2019CHOWDHURY, H. and LOGANATHAN, B., 2019. Third-generation biofuels from microalgae: a review. Current Opinion in Green and Sustainable Chemistry, vol. 20, pp. 39-44. http://dx.doi.org/10.1016/j.cogsc.2019.09.003.
http://dx.doi.org/10.1016/j.cogsc.2019.0...
). In spite of all these advantages of microalgae several hurdles of developmental and applied research must be overcome for commercial production of biofuel from algae (Das et al., 2021DAS, P.K., RANI, J., RAWAT, S. and KUMAR, S., 2021. Microalgal co-cultivation for biofuel production and bioremediation: current status and benefits. BioEnergy Research, vol. 1, pp. 1-26.). One of the main challenges for biofuel production is selection of suitable micro algal species that maintains a steady growth rate despite of changing environmental conditions. Diverse fatty acid profile due to changing environmental conditions and competition among different species of microalgae is also a barrier for production of quality biofuels from microalgae. Most microalgae are rich in polyunsaturated fatty acids having more than four, five and six double bonds. The European Union posed restriction on biodiesel made from alternative oils having 4 and above double bonds of fatty acids due to higher iodine value (Chowdhury and Loganathan, 2019CHOWDHURY, H. and LOGANATHAN, B., 2019. Third-generation biofuels from microalgae: a review. Current Opinion in Green and Sustainable Chemistry, vol. 20, pp. 39-44. http://dx.doi.org/10.1016/j.cogsc.2019.09.003.
http://dx.doi.org/10.1016/j.cogsc.2019.0...
).
Maintenance of the purity of microalgal culture from other microalgae and harmful bacteria in open systems still requires more research and techniques. Another challenge is the recovery of biomass from open or close systems and oil extraction from microalgae. It is a high-cost bearing area which makes biofuels from microalgae more costly then Petro products. Present research has been designed to isolate the microalgae from different soil and water samples. The isolates were screened for lipid production and biochemical profiling for protein, carbohydrates and lipids.
2. Materials and Methods
2.1. Isolation and purification of microalgae
The total number of samples (n=60) of pond water (n=30) and soil (n=30) were collected in sterile containers from three districts (Lahore, Faisalabad and Sheikhupura) of Punjab, Pakistan during the year between 2017 and 2018 and then transferred to Postgraduate Research Laboratory of Institute of Microbiology, University of Veterinary and Animal Sciences (UVAS), Lahore, Pakistan. Blue green 11 (BG- 11) medium was utilized for the isolation, growth and maintenance of microalgae (Pandey et al., 2019PANDEY, M.K., DASGUPTA, C.N., MISHRA, S., SRIVASTAVA, M., GUPTA, V.K., SUSEELA, M.R. and RAMTEKE, P.W., 2019. Bioprospecting microalgae from natural algal bloom for sustainable biomass and biodiesel production. Applied Microbiology and Biotechnology, vol. 103, no. 13, pp. 5447-5458. http://dx.doi.org/10.1007/s00253-019-09856-2. PMid:31101944.
http://dx.doi.org/10.1007/s00253-019-098...
). Water and soil samples were processed according to a previously conducted study. Water sample was 10-fold serially diluted in sterile PBS. To prepare soil suspension, 1 g soil was suspended in 10 mL phosphate buffer saline (PBS) and 10-fold serially diluted. For isolation of microalgae, 1 ml from serially diluted water and soil samples were inoculated on BG-11 medium plates (Park et al., 2019PARK, S., NGUYEN, T.H.T. and JIN, E., 2019. Improving lipid production by strain development in microalgae: strategies, challenges and perspectives. Bioresource Technology, vol. 292, p. 121953. http://dx.doi.org/10.1016/j.biortech.2019.121953. PMid:31405625.
http://dx.doi.org/10.1016/j.biortech.201...
). Processed samples were incubated at room temperature (25°C) under fluorescent light (1000 Lux) for 16 h light cycle and 8 h dark cycle till the growth of microalgal colonies. For purification of microalgae, streak plate method was used and individual colonies from each petri plates were further streaked on BG-11 agar plates.
2.2. Morphological identification of microalgae
Morphological identification was performed on the basis of cultural and microscopic characteristics (El-Sheekh et al., 2020EL-SHEEKH, M., ABU-FADDAN, M., ABO-SHADY, A., NASSAR, M.Z.A. and LABIB, W., 2020. Molecular identification, biomass, and biochemical composition of the marine chlorophyte Chlorella sp. MF1 isolated from Suez Bay. Journal of Genetic Engineering and Biotechnology, vol. 18, no. 1, p. 27. http://dx.doi.org/10.1186/s43141-020-00044-8. PMid:32648005.
http://dx.doi.org/10.1186/s43141-020-000...
). A drop of water sample was placed on clean microscopic glass slide and observed under inverted microscope both at 100X and 400X objectives. Characters of algal cells including color, basal body, arrangement, shape and pattern of cells were observed and images were captured through camera.
2.3. Preliminary screening of lipid containing algae
Lipid containing algae were screened through Nile red fluorescence assay. Single colony of micro algal cells was picked and suspended in 400 µL distilled water containing 25% dimethyl sulfoxide (DMSO). Suspension was heated in a microwave oven for 1 min and 20 µL of Nile red solution (200 µg/mL in acetone) was added to cell suspension. After the addition of Nile red solution, the mixture was re-heated for 1 min and incubated in dark for 10 min. After incubation cells were observed under 490 nm excitation /580 nm emission filter in a fluorescent microscope at 100X and 400X magnifications (Shim et al., 2016SHIM, W.J., SONG, Y.K., HONG, S.H. and JANG, M., 2016. Identification and quantification of microplastics using Nile Red staining. Marine Pollution Bulletin, vol. 113, no. 1-2, pp. 469-476. http://dx.doi.org/10.1016/j.marpolbul.2016.10.049. PMid:28340965.
http://dx.doi.org/10.1016/j.marpolbul.20...
).
2.4. Biomass production
For biochemical profiling, n=23 micro algae were grown in 1liter BG - 11 broth medium. Single purified colony was picked from each plate and inoculated to each medium containing flask, respectively. Flasks were placed under fluorescent light (16 h light and 8 h dark cycles) at room temperature and were bubbled with filtered clean air with the help of air pump for 1 min with an interval of 5 min in order to prevent settling of microalgae and to provide fresh supply of oxygen and carbon dioxide. After 21 d of incubation, optical density (OD) was recorded at 650 nm. Dry weight of selected species was recorded by centrifugation of 500 mL of culture from each flask at 10000 rpm for 10 min and resultant pellet were oven dried in crucible at 70ᵒC. After that the weight of dried pellets was recorded (Brémond et al., 2018BRÉMOND, U., BUYER, R., STEYER, J.-P., BERNET, N. and CARRERE, H., 2018. Biological pretreatments of biomass for improving biogas production: an overview from lab scale to full-scale. Renewable & Sustainable Energy Reviews, vol. 90, pp. 583-604. http://dx.doi.org/10.1016/j.rser.2018.03.103.
http://dx.doi.org/10.1016/j.rser.2018.03...
).
2.5. Biochemical analysis
To evaluate the biochemical profile, isolates were subjected to quantification of carbohydrates, protein and lipids. Carbohydrates were extracted and quantified through anthrone assay (Ludwig and Goldberg, 1956LUDWIG, T.G. and GOLDBERG, J.V., 1956. The anthrone method for the determination of carbohydrates in foods and in oral rinsing. Journal of Dental Research, vol. 35, no. 1, pp. 90-94. http://dx.doi.org/10.1177/00220345560350012301. PMid:13286391.
http://dx.doi.org/10.1177/00220345560350...
). Oven dried microalgae (10 mg) was washed thrice with normal saline. Washed pellet was suspended in 0.2 mL distilled water and vortexed. A volume of 0.4 mL KOH (40% w/v) was added and suspension was heated at 90ᵒC for 1 h. Cold ethanol (1.2 mL) was poured in the mixture and placed overnight at -20ᵒC. Sample was then centrifuged at 6 000rpm for 5 min and supernatant was discarded. Pellet obtained was then re-suspended in 1.5 mL H2O. OD values were recorded with the help of an ELISA reader. Exact quantity of carbohydrates was estimated by locating the absorbance value of sample on a prepared standard curve.
Total proteins were extracted by alkaline extraction assay and measured by Bradford assay (Kielkopf et al., 2020KIELKOPF, C.L., BAUER, W. and URBATSCH, I.L., 2020. Bradford assay for determining protein concentration. Cold Spring Harbor Protocols, vol. 2020, no. 4, p. pdb-prot102269. http://dx.doi.org/10.1101/pdb.prot102269. PMid:32238597.
http://dx.doi.org/10.1101/pdb.prot102269...
). Oven dried microalgae (10mg) was washed three times with normal saline in order to remove residual salts. Washed pellet was suspended in 1 mL of 0.5N NaOH. The mixture was incubated at 80ᵒC for 10 min with occasional stirring. After incubation mixture was cooled at room temperature and centrifuged at 10000 rpm for 5 min. Supernatant was transferred to a new tube. The procedure was repeated thrice. The final step was to heat at 100ᵒC for 10 min in order to complete extraction of residual protein. All three extracts were pooled into tube for further analyses. Protein contents were estimated by Bradford assay (Kruger, 2009KRUGER, N.J., 2009. The Bradford method for protein quantitation. In: J.M. WALKER, ed. The protein protocols handbook. New York: Humana Totowa, pp. 17-24. http://dx.doi.org/10.1007/978-1-59745-198-7_4.
http://dx.doi.org/10.1007/978-1-59745-19...
). OD values were recorded and proteins were quantified by locating the absorbance value of sample on a prepared standard curve.
A single step method was used for lipid extraction (Grace et al., 2020GRACE, C.E.E., LAKSHMI, P.K., MEENAKSHI, S., VAIDYANATHAN, S., SRISUDHA, S. and MARY, M.B., 2020. Biomolecular transitions and lipid accumulation in green microalgae monitored by FTIR and Raman analysis. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, vol. 224, p. 117382. http://dx.doi.org/10.1016/j.saa.2019.117382. PMid:31357053.
http://dx.doi.org/10.1016/j.saa.2019.117...
). Oven dried microalgae (10 mg) was washed thrice with normal saline. Washed pellet was suspended in chloroform/methanol solution (2:1 v/v). The mixture was vortexed in order to completely dissolve cells into chloroform/methanol. Later NaCl water solution (0.73%) was added in order to produce a 2:1:0.7 system of chloroform/methanol/NaCl (v/v). Lower layer was transferred to new tube for lipid estimation. Total lipid content in microalgae was estimated by colorimetric micro-vanillin method (Micro vanilline assay) following standard protocol (Byreddy et al., 2016BYREDDY, A.R., GUPTA, A., BARROW, C.J. and PURI, M., 2016. A quick colorimetric method for total lipid quantification in microalgae. Journal of Microbiological Methods, vol. 125, pp. 28-32. http://dx.doi.org/10.1016/j.mimet.2016.04.002. PMid:27050419.
http://dx.doi.org/10.1016/j.mimet.2016.0...
). A volume of 100 µL sample was added into each well of microtiter plate. Solvent was evaporated at 90ᵒC for about 10 min. Concentrated sulfuric acid (100 µL) was added into each well. Later micro titration plate was incubated at 90ᵒC for 10 min in an oven and cooled at 4°C for two min. Background absorbance was measured at 540 nm, using a microplate reader. Vanillin–phosphoric acid reagent 50µL (0.2 mg vanillin per mL of 17% phosphoric acid) was added and micro-titration plate was incubated for 10 min at room temperature for color development. After 10 min the plate was read at 540 nm and OD values were recorded and lipids were quantified by locating the absorbance value of sample on a prepared standard curve.
2.6. FTIR analysis
FTIR analysis of microalgae was conducted (Al-Alawi et al., 2004AL-ALAWI, A., VAN DE VOORT, F.R. and SEDMAN, J., 2004. New FTIR method for the determination of FFA in oils. Journal of the American Oil Chemists’ Society, vol. 81, no. 5, pp. 441-446. http://dx.doi.org/10.1007/s11746-004-0920-9.
http://dx.doi.org/10.1007/s11746-004-092...
). A volume of 1.5 milliliter culture of microalgae (growth at day 15) was centrifuged at 6000 rpm. The supernatant was discarded and pellet was washed thrice with normal saline in order to completely remove the salts. Final pellet was oven dried and used for FTIR scan.
3. Results
3.1. Distribution of microalgae
Total 180 isolates were recovered from water and soil samples, all samples including water and soil (60/60, 100%) were positive for algal growth. Serial dilutions along with spread plate method were successful for isolation of large number of microalgae.
Algal isolates (n=180) were recovered on the basis of difference in color, shape and texture. Total 65 (36.11%) microalgae isolates were isolated from Sheikhupura district from which 23 (35.38%) were from soil and 42 (64.62%) were from water. While from Faisalabad district 75 (41.67%) isolates were obtained out of which 26 (34.67%) were from soil and 49 (65.33%) from water. From Lahore out of 40 (22.22%) isolates, 15(37.5%) isolates were from soil and 25 (62.5%) isolates were from water samples. The highest number of isolates (n=75) were collected from Faisalabad district, while the lowest number of isolates (n=40) were obtained from Lahore district. Out of 180 isolates, 64 isolates (35.55%) were from soil and 116 (64.45%) isolates were from water origin. Data is presented in (Table 1). Water samples were found to be rich in microalgae. Maximum numbers of water isolates were from khanpur, Chak 239 RB, Muhammad Wala and Chak 108 RB (5%) respectively and lowest number of isolates was from Janjatey, Mohlanwal, Shahpur Kanjaran, UVAS and Kot Noor Muhammad (1%) respectively and data shown in (Figure 1). From water samples, maximum number of diverse algae were found in Jallo park (28%), second highest were from Kajlay (14%) and Chak 78 GB (west).
Soil samples showed a smaller number of algae isolates, maximum number of soil isolates were from Khanpur (8%) and Kajlay (6%), respectively, while Feroze wattwan, Nankana I, Sadonana, Chung Mohlanwal-1, Shahpur Kanjran, Janjatey, Mohlanwal-II, Kot Noor Muhammad and UVAS-2 exhibited lower number of algae isolates (2%) (Figure 2). Maximum number of diverse algae were found in soil samples from Jallo Park (34%), Sharq pur, Muhammad Wala, Kajlay and Chak 78 GB (west) (17%), respectively.
3.2. Microscopic morphology and preliminary screening of lipid contents
Upon treating with Nile red dye out of 180 isolates only 23 isolates revealed yellow fluorescence after observing under florescent microscope (Table 2, Figure 3). Nile red fluorescence of highly lipid producing strains (highest lipid producer AIN-63 and second highest produce AIN 48) was shown in Figure 4.
Location, source, morphology, biomass and percent macromolecules in dried biomass of selected algal isolates (n=23).
Selected microalgae isolates at 400X under light Microscope (A) AIN01, (B) AIN09, (C) AIN 124, (D) AIN63.
Light Microscopy (400X) of algal Isolates (a) AIN163, (b) AIN124, (c) AIN 95, (d) AIN09 (up) and Fluorescence microscopy 400X (down) of selected algal isolates, (e) AIN163, (f) AIN124, (g) AIN095, (h) AIN 09 after Nile red staining. Yellow fluorescence of lipids at 400X under excitation wavelength of 490 nm and emission wavelength of 580 nm.
3.3. Biomass and biochemical analysis of algal biomass
Highest biomass was produced by strain AIN79 as 1505 mg/L while AIN 172 was second highest with biomass production of 1405 mg/L. Lipid, protein and carbohydrate composition analysis of selected algae isolates (n=23) was performed.
3.4. Quantification of carbohydrates
Among the 23 cultivated strains, the isolate AIN172 showed highest carbohydrate contents (73.83%) while AIN79, AIN164 and AIN145 were the second, third and fourth highest carbohydrate producer 69.96%, 63.64% and 63.42%, respectively. Carbohydrate contents (percentage dry weight) of algal isolates are presented in Table 2.
3.5. Quantification of proteins
Out of 23 cultivated strains, the isolate AIN128 strain showed highest protein content (42.91%) while isolates AIN24, AIN163 and AIN143 were the second, third and fourth highest protein producer 25.96%, 25.63% and 24.96%, respectively. Protein contents (percentage dry weight) of algal isolates are presented in Table 2.
3.6. Quantification of lipids
In comparison to 23 cultivated strains, the isolate AIN63 strain showed highest lipid contents (17.4%) while isolates AIN68, AIN48 and AIN128 were the second, third and fourth highest oil producer (16.6%, 13.28% and 12.46%, respectively). Lipid contents (percentage dry weight) of algal isolates are presented in Table 2.
3.7. FTIR analysis
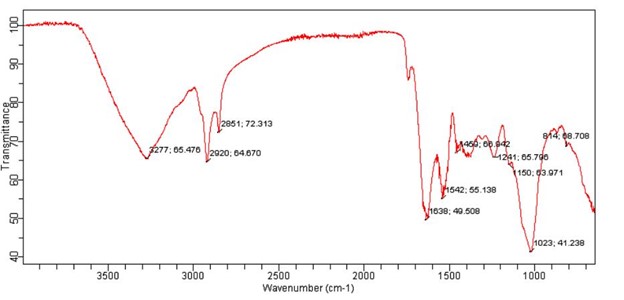
FTIR analysis of selected microalgae was performed in order to validate the lipid, carbohydrates and protein contents. Represented picture of FTIR analyses of strain (AIN63) is shown in Figure 5. The peak in area of wave number 3029- 3639 (cm-1) range indicates the protein stretching bonds, the peak in the area of wave number 2809-1709 (cm-1) indicated the stretching of lipids and carbohydrates, peak in the area of wave number 1583-1709 (cm-1) indicated the presence of protein amide 1 bonds, peak in the area of wave number 1481 to 1585 (cm-1) indicates the presence of amide-II bands, while poly saccharide is indicated in peak range of wave number 980-1072 (cm-1).
4. Discussion
Out of n=60 samples; water (n=30) and soil (n=30), 180 microalgal isolates were obtained from three districts (Lahore, Sheikhupura and Faisalabad) of Punjab, Pakistan. Micropipette washing and spread plate techniques were applied to obtain pure isolates of microalgae. For water sample collection, water bodies containing greenish tinge were selected. The micropipette washing technique is not a wide spread technique and not ideal for isolation of large number of microalgae because during suction step microalgae usually fail to be sucked by micropipette hence no growth was observed. Micropipette washing technique unethical for isolation of large number of isolates (Haoujar et al., 2020HAOUJAR, I., CACCIOLA, F., MANCHADO, M., ABRINI, J., HAOUJAR, M., CHEBBAKI, K., OTERI, M., RIGANO, F., MANGRAVITI, D., MONDELLO, L., ESSAFI, A., CHAIRI, H. and SENHAJI, N.S., 2020. Isolation of microalgae from Mediterranean seawater and production of lipids in the cultivated species. Foods, vol. 9, no. 11, p. 1601. http://dx.doi.org/10.3390/foods9111601. PMid:33158015.
http://dx.doi.org/10.3390/foods9111601...
); however, spread plate method for isolation of microalgae is convenient and less laborious to isolate large number of microalgae. Blue green 11 (BG- 11) medium was utilized for the isolation of microalgae; different media were analyzed by researcher for isolation of microalgae i.e., Bolds Basic medium, F/2 and K medium. After spreading the samples on BG - 11 agar plates, different microalgal colonies were appeared on agar plates, microalgae isolates purified on BG- 11 agar plates. Previous studies also reported the use of similar methodology for isolation of various microalgae like Botryrococcus, Chlorella, and Scenedesmus from pond and fresh water (Aketo et al., 2020AKETO, T., HOSHIKAWA, Y., NOJIMA, D., YABU, Y., MAEDA, Y., YOSHINO, T., TAKANO, H. and TANAKA, T., 2020. Selection and characterization of microalgae with potential for nutrient removal from municipal wastewater and simultaneous lipid production. Journal of Bioscience and Bioengineering, vol. 129, no. 5, pp. 565-572. http://dx.doi.org/10.1016/j.jbiosc.2019.12.004. PMid:31974048.
http://dx.doi.org/10.1016/j.jbiosc.2019....
). Botryococcus was isolated from soil and fresh water samples by Kajikawa et al. (2019)KAJIKAWA, M., YAMAUCHI, M., SHINKAWA, H., TANAKA, M., HATANO, K., NISHIMURA, Y., KATO, M. and FUKUZAWA, H., 2019. Isolation and characterization of chlamydomonas autophagy-related mutants in nutrient-deficient conditions. Plant & Cell Physiology, vol. 60, no. 1, pp. 126-138. http://dx.doi.org/10.1093/pcp/pcy193. PMid:30295899.
http://dx.doi.org/10.1093/pcp/pcy193...
. Algal species can be isolated and cultivated in lab scale cultures and are ubiquitous in nature (Naghshbandi et al., 2019NAGHSHBANDI, M.P., TABATABAEI, M., AGHBASHLO, M., AFTAB, M.N. and IQBAL, I., 2019. Metabolic engineering of microalgae for biofuel production. In: K. SPILLING, ed. Biofuels from algae: methods and protcols. New York: Humana, vol. 1980, pp. 153-172. http://dx.doi.org/10.1007/7651_2018_205. PMid:30666564.
http://dx.doi.org/10.1007/7651_2018_205...
). Microalgae can be preliminary identified by microscopy (Abdelaziz et al., 2014ABDELAZIZ, A.E.M., LEITE, G.B., BELHAJ, M.A. and HALLENBECK, P.C., 2014. Screening microalgae native to Quebec for wastewater treatment and biodiesel production. Bioresource Technology, vol. 157, pp. 140-148. http://dx.doi.org/10.1016/j.biortech.2014.01.114. PMid:24549235.
http://dx.doi.org/10.1016/j.biortech.201...
; Selvarajan et al., 2015SELVARAJAN, R., FELFÖLDI, T., TAUBER, T., SANNIYASI, E., SIBANDA, T. and TEKERE, M., 2015. Screening and Evaluation of Some Green Algal Strains (Chlorophyceae) Isolated from Freshwater and Soda Lakes for Biofuel Production. Energies, vol. 8, no. 7, pp. 7502-7521. http://dx.doi.org/10.3390/en8077502.
http://dx.doi.org/10.3390/en8077502...
). Microscopic characteristics for identification of microalgae includes as unicellular or multicellular, occurs singly or in the form of clusters, shape may be circular, elliptical or elongated, pyrenoids may be present, chloroplast is cup shaped, circular or griddle shaped, mucilagenous sheet present or absent (Selvarajan et al., 2015SELVARAJAN, R., FELFÖLDI, T., TAUBER, T., SANNIYASI, E., SIBANDA, T. and TEKERE, M., 2015. Screening and Evaluation of Some Green Algal Strains (Chlorophyceae) Isolated from Freshwater and Soda Lakes for Biofuel Production. Energies, vol. 8, no. 7, pp. 7502-7521. http://dx.doi.org/10.3390/en8077502.
http://dx.doi.org/10.3390/en8077502...
). Selected isolates belong to class chlorophyceae, which includes Chlorella, Scenedesmus, Dunaliella, Botryococcus, Ankistrodesmus, Graesiella, Chroococcus, Selenastrum and Haematococcus (Sánchez-Bayo et al., 2020). The Nile red is a vital stain for intracellular visualization of lipids by fluorescent microscopy and selection criteria for microalgae was based on presence of algal colonies after 15 d of incubation and presence of florescence (Nile red assay), Nile red dye binds with the lipids (triglycerides) and upon excitation at 490 nm and emission at 580 nm this dye emits yellow fluorescence for lipids and red fluorescence for chlorophyll. The Nile red staining presented linear relationship with lipids present in microalgae (Wang et al., 2020WANG, L., CHEN, L. and WU, S., 2020. Microalgae Cultivation Using Screened Liquid Dairy Manure Applying Different Folds of Dilution: Nutrient Reduction Analysis with Emphasis on Phosphorus Removal. Applied Biochemistry and Biotechnology, vol. 192, no. 2, pp. 381-391. http://dx.doi.org/10.1007/s12010-020-03316-8. PMid:32385813.
http://dx.doi.org/10.1007/s12010-020-033...
). Present study selection criteria were in agreement to a previously conducted study (presence of colonies at 15 d and confirmation of lipids by Nile red fluorescent dye) for selection of microalgae for further studies (Hossain and Mahlia, 2019HOSSAIN, N. and MAHLIA, T.M.I., 2019. Progress in physicochemical parameters of microalgae cultivation for biofuel production. Critical Reviews in Biotechnology, vol. 39, no. 6, pp. 835-859. http://dx.doi.org/10.1080/07388551.2019.1624945. PMid:31185749.
http://dx.doi.org/10.1080/07388551.2019....
; Raghuvanshi et al., 2018RAGHUVANSHI, S., BHAKAR, V., CHAVA, R. and SANGWAN, K.S., 2018. Comparative study using life cycle approach for the biodiesel production from microalgae grown in wastewater and fresh water. Procedia CIRP, vol. 69, pp. 568-572. http://dx.doi.org/10.1016/j.procir.2017.11.030.
http://dx.doi.org/10.1016/j.procir.2017....
).
The biomass production of selected isolates is presented in Table 2. Higher biomass yield was obtained from AIN79 (Scenedesmus) 1505 mg/L or 71 mg/L/D. Third and fourth highest producers were AIN99 (Scenedesmus) and AIN161 (Scenedesmus) with production capacity of 1252.5 mg/L or 69.6 mg/L/D and 1137 mg/L or 54.14 mg/L/D respectively. Chu (2017)CHU, W.L., 2017. Strategies to enhance production of microalgal biomass and lipids for biofuel feedstock. European Journal of Phycology, vol. 52, no. 4, pp. 419-437. http://dx.doi.org/10.1080/09670262.2017.1379100.
http://dx.doi.org/10.1080/09670262.2017....
reported that Scenedesmus biomass yield was high as compared to Chlorella. In another study highest biomass yield of Scenedesmus 3880 mg/L was reported (Wang et al., 2020WANG, L., CHEN, L. and WU, S., 2020. Microalgae Cultivation Using Screened Liquid Dairy Manure Applying Different Folds of Dilution: Nutrient Reduction Analysis with Emphasis on Phosphorus Removal. Applied Biochemistry and Biotechnology, vol. 192, no. 2, pp. 381-391. http://dx.doi.org/10.1007/s12010-020-03316-8. PMid:32385813.
http://dx.doi.org/10.1007/s12010-020-033...
). Second highest biomass production was obtained by isolate AIN 172 (Chlorella) 1419.5 mg/L or 67.5 mg/L/D. The biomass yield of Chlorella vulgaris is 188 mg/L/D and 57.87g m-2d-1 respectively has been reported by Jain et al. (2017)JAIN, P., ARORA, N., MEHTANI, J., PRUTHI, V. and MAJUMDER, C.B., 2017. Pretreated algal bloom as a substantial nutrient source for microalgae cultivation for biodiesel production. Bioresource Technology, vol. 242, pp. 152-160. http://dx.doi.org/10.1016/j.biortech.2017.03.156. PMid:28389041.
http://dx.doi.org/10.1016/j.biortech.201...
. Lower biomass was produced by AIN 145 (Chroococcus) 19.8 mg/L/D. Chlorophyta and Chlorella biomass production range of 4 – 13 g.m-2d-1 and 7 – 21 g.m-2d-1 was reported by Hase et al. (2000)HASE, R., OIKAWA, H., SASAO, C., MORITA, M. and WATANABE, Y., 2000. Photosynthetic production of microalgal biomass in a raceway system under greenhouse conditions in Sendai city. Journal of Bioscience and Bioengineering, vol. 89, no. 2, pp. 157-163. http://dx.doi.org/10.1016/S1389-1723(00)88730-7. PMid:16232719.
http://dx.doi.org/10.1016/S1389-1723(00)...
. Biomass production difference was observed due to the differences in light intensities in closed photo-reactor and in raceway ponds.
The selected microalgae isolates were subjected to biochemical characterization on the basis of total carbohydrates, protein and lipids content. Carbohydrates are usually present in the form of monosaccharides, oligosaccharides and poly saccharides and can be converted into starch and glycogen Reference. For quantification of carbohydrates, extraction was performed following the hot alkaline extraction method and analyzed by anthrone assay (Liberton et al., 2019LIBERTON, M., BANDYOPADHYAY, A. and PAKRASI, H.B., 2019. Enhanced nitrogen fixation in a glgX-deficient strain of Cyanothece sp. Strain ATCC 51142, a unicellular nitrogen-fixing cyanobacterium. Applied and Environmental Microbiology, vol. 85, no. 7, p. 02887-18 . http://dx.doi.org/10.1128/AEM.02887-18. PMid:30709817.
http://dx.doi.org/10.1128/AEM.02887-18...
). Selvarajan and his coworkers adopted the similar methodology for detection and quantification of carbohydrates (Selvarajan et al., 2015SELVARAJAN, R., FELFÖLDI, T., TAUBER, T., SANNIYASI, E., SIBANDA, T. and TEKERE, M., 2015. Screening and Evaluation of Some Green Algal Strains (Chlorophyceae) Isolated from Freshwater and Soda Lakes for Biofuel Production. Energies, vol. 8, no. 7, pp. 7502-7521. http://dx.doi.org/10.3390/en8077502.
http://dx.doi.org/10.3390/en8077502...
). Higher carbohydrate contents (73.83%) were found in strain AIN172 (Chlorella) while AIN79 (Scenedesmus), AIN164 (Scenedesmus) and AIN145 (Chroococcus) were the second, third and fourth higher carbohydrate producer (69.96%, 63.64% and 63.42%, respectively). Xu and his collegues reported variation in carbohydrate percentages of Scenedesmus from 10 to 52%. The variation in the percentages of carbohydrate, between and within genus is because of the difference of growth phases of microalgae (Xu et al., 2020XU, M., XUE, Z., SUN, S., ZHAO, C., LIU, J., LIU, J. and ZHAO, Y., 2020. Co-culturing microalgae with endophytic bacteria increases nutrient removal efficiency for biogas purification. Bioresource Technology, vol. 314, pp. 123766. http://dx.doi.org/10.1016/j.biortech.2020.123766. PMid:32645575.
http://dx.doi.org/10.1016/j.biortech.202...
). In the present study, the carbohydrate contents were observed higher in species; this could be due to environmental and nutritional factors. George et al., 2014 and Watson et al., 2021 also mentioned the similar findings. The economic impact of high starch content is that, it can be readily converted into ethanol by fermentation and into biogas by anaerobic digestion (Xu et al., 2020XU, M., XUE, Z., SUN, S., ZHAO, C., LIU, J., LIU, J. and ZHAO, Y., 2020. Co-culturing microalgae with endophytic bacteria increases nutrient removal efficiency for biogas purification. Bioresource Technology, vol. 314, pp. 123766. http://dx.doi.org/10.1016/j.biortech.2020.123766. PMid:32645575.
http://dx.doi.org/10.1016/j.biortech.202...
).
Proteins are important component of micro algal biomass. Microalgae are rich in protein and being explored as source of single cell protein like Chlorella and Spirulina (Nasseri et al., 2011). Protein from selected microalgal isolates was extracted by alkaline extraction method and extracted proteins were quantified by Bradford assay. Another study also used the similar procedure for protein quantification (Tang et al., 2020TANG, D.Y.Y., KHOO, K.S., CHEW, K.W., TAO, Y., HO, S.H. and SHOW, P.L., 2020. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresource Technology, vol. 304, pp. 122997. http://dx.doi.org/10.1016/j.biortech.2020.122997. PMid:32094007.
http://dx.doi.org/10.1016/j.biortech.202...
; Gui et al., 2021GUI, J., CHEN, S., LUO, G., WU, Z., FAN, Y., YAO, L. and XU, H., 2021. Nutrient deficiency and an algicidal bacterium improved the lipid profiles of a novel promising oleaginous dinoflagellate, Prorocentrum donghaiense, for biodiesel production. Applied and Environmental Microbiology, vol. 87, no. 19, p. e01159-21. http://dx.doi.org/10.1128/AEM.01159-21. PMid:34319787.
http://dx.doi.org/10.1128/AEM.01159-21...
). Among the 23 cultivated strains, the isolate AIN128 (Scenedesmus) strain showed higher protein content (42.91%) while isolates AIN24 (Botryrococcus), AIN163 (Selenastrum) and AIN143 (Chlorococcum) were the second, third and fourth highest protein producer (25.96%, 25.63% and 24.96%, respectively). The percentages of proteins vary in Scenedesmus from 3.37% to 56%, Selenastrum from 3.42 to 7.29%, Chlorococcum from 5.59 to 21.27%. The variation in the percentages of protein, between and within genus might be due to the difference of growth phase of microalgae. In the current study, the protein contents were high in microalgae species because of the nutritional and environmental factors. George and his colleagues as well Watson and his co-workers reported the variation in protein contents of microalgae due to environment and nutritional factors (George et al., 2014GEORGE, B., PANCHA, I., DESAI, C., CHOKSHI, K., PALIWAL, C., GHOSH, T. and MISHRA, S., 2014. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus – a potential strain for bio-fuel production. Bioresource Technology, vol. 171, pp. 367-374. http://dx.doi.org/10.1016/j.biortech.2014.08.086. PMid:25218209.
http://dx.doi.org/10.1016/j.biortech.201...
; Watson et al., 2021WATSON, J., SWOBODA, M., AIERZHATI, A., WANG, T., SI, B. and ZHANG, Y., 2021. Biocrude oil from algal bloom microalgae: a novel integration of biological and thermochemical techniques. Environmental Science & Technology, vol. 55, no. 3, pp. 1973-1983. http://dx.doi.org/10.1021/acs.est.0c05924. PMid:33434016.
http://dx.doi.org/10.1021/acs.est.0c0592...
).
Lipids are the chief constituents of microalgae that play vital role for production of biofuel from microalgae. The lipid contents of microalgae are the most desirable character of microalgae by which they are chosen as potential candidate for biofuel production (Ling et al., 2019LING, Y., SUN, L.P., WANG, S.Y., LIN, C.S.K., SUN, Z. and ZHOU, Z.G., 2019. Cultivation of oleaginous microalga Scenedesmus obliquus coupled with wastewater treatment for enhanced biomass and lipid production. Biochemical Engineering Journal, vol. 148, pp. 162-169. http://dx.doi.org/10.1016/j.bej.2019.05.012.
http://dx.doi.org/10.1016/j.bej.2019.05....
). However, the challenge of isolation of microalgae with high lipid contents is still not yet achieved. For extraction of lipids solvent extraction method were utilized and then lipids are detected by Micro vanillin assay. Among the 23 cultivated strains, the isolate AIN63 (Scenedesmus) strain showed higher lipid contents (17.4%) while isolates AIN68 (Scenedesmus), AIN48 (Ankistodesmus) and AIN128 (Scenedesmus) were the second, third and fourth higher lipid producer (16.6%, 13.28% and 12.46% respectively). The lipid percentages of Scenedesmus and Ankistodesmus were 6.41 to 41%, and 11.48 to 30.79%, respectively (Karpagam et al., 2021KARPAGAM, R., ABINAYA, N. and GNANAM, R., 2021. Assortment of native microalgae for improved biomass and lipid production on employing vegetable waste as a frugal cultivation approach for biodiesel application. Current Microbiology, vol. 78, no. 10, pp. 3770-3781. http://dx.doi.org/10.1007/s00284-021-02643-1. PMid:34487210.
http://dx.doi.org/10.1007/s00284-021-026...
). The variation in the percentages of lipids, between and within genus is because of the difference of growth phase of microalgae. In the present study, the lipid contents were less in concentration at various species as compared to literature; this is due environmental and nutritional factors. Jin and his colleagues as well Watson and his co-workers reported the variation in lipid contents of microalgae due to environment and nutritional factors (Jin et al., 2020JIN, H., ZHANG, H., ZHOU, Z., LI, K., HOU, G., XU, Q., CHUAI, W., ZHANG, C., HAN, D. and HU, Q., 2020. Ultrahigh‐cell‐density heterotrophic cultivation of the unicellular green microalga Scenedesmus acuminatus and application of the cells to photoautotrophic culture enhance biomass and lipid production. Biotechnology and Bioengineering, vol. 117, no. 1, pp. 96-108. http://dx.doi.org/10.1002/bit.27190. PMid:31612991.
http://dx.doi.org/10.1002/bit.27190...
; Watson et al., 2021WATSON, J., SWOBODA, M., AIERZHATI, A., WANG, T., SI, B. and ZHANG, Y., 2021. Biocrude oil from algal bloom microalgae: a novel integration of biological and thermochemical techniques. Environmental Science & Technology, vol. 55, no. 3, pp. 1973-1983. http://dx.doi.org/10.1021/acs.est.0c05924. PMid:33434016.
http://dx.doi.org/10.1021/acs.est.0c0592...
).
It was concluded that soil and water in different areas of Punjab are rich in algal diversity. Algae from this environment have good lipid accumulation capacity as 17.4% was maximum reported concentration. In conclusion, it could be suggested that Nile red staining is a good method for initial screening of potential algal candidate for biofuel production. The indigenous algae can be successfully grown in-vitro and further experimentation leading to growth optimization is required to select good candidate for biofuel production as lipid accumulation is a major bottleneck in biofuel production from microalgae and AIN63 strain showed highest lipid contents (17.4%). The selected candidate can be optimized for production at local ponds by utilizing agricultural waste and can help community to produce green fuel at cheaper costs.
Acknowledgements
The authors are thankful to Institute of Microbiology, University of Veterinary and Animal Sciences, Lahore, Pakistan, for conducting this research and laboratory facility.
References
- ABDELAZIZ, A.E.M., LEITE, G.B., BELHAJ, M.A. and HALLENBECK, P.C., 2014. Screening microalgae native to Quebec for wastewater treatment and biodiesel production. Bioresource Technology, vol. 157, pp. 140-148. http://dx.doi.org/10.1016/j.biortech.2014.01.114 PMid:24549235.
» http://dx.doi.org/10.1016/j.biortech.2014.01.114 - AKETO, T., HOSHIKAWA, Y., NOJIMA, D., YABU, Y., MAEDA, Y., YOSHINO, T., TAKANO, H. and TANAKA, T., 2020. Selection and characterization of microalgae with potential for nutrient removal from municipal wastewater and simultaneous lipid production. Journal of Bioscience and Bioengineering, vol. 129, no. 5, pp. 565-572. http://dx.doi.org/10.1016/j.jbiosc.2019.12.004 PMid:31974048.
» http://dx.doi.org/10.1016/j.jbiosc.2019.12.004 - AL-ALAWI, A., VAN DE VOORT, F.R. and SEDMAN, J., 2004. New FTIR method for the determination of FFA in oils. Journal of the American Oil Chemists’ Society, vol. 81, no. 5, pp. 441-446. http://dx.doi.org/10.1007/s11746-004-0920-9
» http://dx.doi.org/10.1007/s11746-004-0920-9 - BRÉMOND, U., BUYER, R., STEYER, J.-P., BERNET, N. and CARRERE, H., 2018. Biological pretreatments of biomass for improving biogas production: an overview from lab scale to full-scale. Renewable & Sustainable Energy Reviews, vol. 90, pp. 583-604. http://dx.doi.org/10.1016/j.rser.2018.03.103
» http://dx.doi.org/10.1016/j.rser.2018.03.103 - BYREDDY, A.R., GUPTA, A., BARROW, C.J. and PURI, M., 2016. A quick colorimetric method for total lipid quantification in microalgae. Journal of Microbiological Methods, vol. 125, pp. 28-32. http://dx.doi.org/10.1016/j.mimet.2016.04.002 PMid:27050419.
» http://dx.doi.org/10.1016/j.mimet.2016.04.002 - CHOWDHURY, H. and LOGANATHAN, B., 2019. Third-generation biofuels from microalgae: a review. Current Opinion in Green and Sustainable Chemistry, vol. 20, pp. 39-44. http://dx.doi.org/10.1016/j.cogsc.2019.09.003
» http://dx.doi.org/10.1016/j.cogsc.2019.09.003 - CHU, W.L., 2017. Strategies to enhance production of microalgal biomass and lipids for biofuel feedstock. European Journal of Phycology, vol. 52, no. 4, pp. 419-437. http://dx.doi.org/10.1080/09670262.2017.1379100
» http://dx.doi.org/10.1080/09670262.2017.1379100 - DAS, P.K., RANI, J., RAWAT, S. and KUMAR, S., 2021. Microalgal co-cultivation for biofuel production and bioremediation: current status and benefits. BioEnergy Research, vol. 1, pp. 1-26.
- DOLGANYUK, V., BELOVA, D., BABICH, O., PROSEKOV, A., IVANOVA, S., KATSEROV, D., PATYUKOV, N. and SUKHIKH, S., 2020. Microalgae: a promising source of valuable bioproducts. Biomolecules, vol. 10, no. 8, p. 1153. http://dx.doi.org/10.3390/biom10081153 PMid:32781745.
» http://dx.doi.org/10.3390/biom10081153 - EL-SHEEKH, M., ABU-FADDAN, M., ABO-SHADY, A., NASSAR, M.Z.A. and LABIB, W., 2020. Molecular identification, biomass, and biochemical composition of the marine chlorophyte Chlorella sp. MF1 isolated from Suez Bay. Journal of Genetic Engineering and Biotechnology, vol. 18, no. 1, p. 27. http://dx.doi.org/10.1186/s43141-020-00044-8 PMid:32648005.
» http://dx.doi.org/10.1186/s43141-020-00044-8 - GEORGE, B., PANCHA, I., DESAI, C., CHOKSHI, K., PALIWAL, C., GHOSH, T. and MISHRA, S., 2014. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus – a potential strain for bio-fuel production. Bioresource Technology, vol. 171, pp. 367-374. http://dx.doi.org/10.1016/j.biortech.2014.08.086 PMid:25218209.
» http://dx.doi.org/10.1016/j.biortech.2014.08.086 - GRACE, C.E.E., LAKSHMI, P.K., MEENAKSHI, S., VAIDYANATHAN, S., SRISUDHA, S. and MARY, M.B., 2020. Biomolecular transitions and lipid accumulation in green microalgae monitored by FTIR and Raman analysis. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, vol. 224, p. 117382. http://dx.doi.org/10.1016/j.saa.2019.117382 PMid:31357053.
» http://dx.doi.org/10.1016/j.saa.2019.117382 - GUI, J., CHEN, S., LUO, G., WU, Z., FAN, Y., YAO, L. and XU, H., 2021. Nutrient deficiency and an algicidal bacterium improved the lipid profiles of a novel promising oleaginous dinoflagellate, Prorocentrum donghaiense, for biodiesel production. Applied and Environmental Microbiology, vol. 87, no. 19, p. e01159-21. http://dx.doi.org/10.1128/AEM.01159-21 PMid:34319787.
» http://dx.doi.org/10.1128/AEM.01159-21 - HAOUJAR, I., CACCIOLA, F., MANCHADO, M., ABRINI, J., HAOUJAR, M., CHEBBAKI, K., OTERI, M., RIGANO, F., MANGRAVITI, D., MONDELLO, L., ESSAFI, A., CHAIRI, H. and SENHAJI, N.S., 2020. Isolation of microalgae from Mediterranean seawater and production of lipids in the cultivated species. Foods, vol. 9, no. 11, p. 1601. http://dx.doi.org/10.3390/foods9111601 PMid:33158015.
» http://dx.doi.org/10.3390/foods9111601 - HASE, R., OIKAWA, H., SASAO, C., MORITA, M. and WATANABE, Y., 2000. Photosynthetic production of microalgal biomass in a raceway system under greenhouse conditions in Sendai city. Journal of Bioscience and Bioengineering, vol. 89, no. 2, pp. 157-163. http://dx.doi.org/10.1016/S1389-1723(00)88730-7 PMid:16232719.
» http://dx.doi.org/10.1016/S1389-1723(00)88730-7 - HOSSAIN, N. and MAHLIA, T.M.I., 2019. Progress in physicochemical parameters of microalgae cultivation for biofuel production. Critical Reviews in Biotechnology, vol. 39, no. 6, pp. 835-859. http://dx.doi.org/10.1080/07388551.2019.1624945 PMid:31185749.
» http://dx.doi.org/10.1080/07388551.2019.1624945 - JAIN, P., ARORA, N., MEHTANI, J., PRUTHI, V. and MAJUMDER, C.B., 2017. Pretreated algal bloom as a substantial nutrient source for microalgae cultivation for biodiesel production. Bioresource Technology, vol. 242, pp. 152-160. http://dx.doi.org/10.1016/j.biortech.2017.03.156 PMid:28389041.
» http://dx.doi.org/10.1016/j.biortech.2017.03.156 - JIN, H., ZHANG, H., ZHOU, Z., LI, K., HOU, G., XU, Q., CHUAI, W., ZHANG, C., HAN, D. and HU, Q., 2020. Ultrahigh‐cell‐density heterotrophic cultivation of the unicellular green microalga Scenedesmus acuminatus and application of the cells to photoautotrophic culture enhance biomass and lipid production. Biotechnology and Bioengineering, vol. 117, no. 1, pp. 96-108. http://dx.doi.org/10.1002/bit.27190 PMid:31612991.
» http://dx.doi.org/10.1002/bit.27190 - KAJIKAWA, M., YAMAUCHI, M., SHINKAWA, H., TANAKA, M., HATANO, K., NISHIMURA, Y., KATO, M. and FUKUZAWA, H., 2019. Isolation and characterization of chlamydomonas autophagy-related mutants in nutrient-deficient conditions. Plant & Cell Physiology, vol. 60, no. 1, pp. 126-138. http://dx.doi.org/10.1093/pcp/pcy193 PMid:30295899.
» http://dx.doi.org/10.1093/pcp/pcy193 - KARPAGAM, R., ABINAYA, N. and GNANAM, R., 2021. Assortment of native microalgae for improved biomass and lipid production on employing vegetable waste as a frugal cultivation approach for biodiesel application. Current Microbiology, vol. 78, no. 10, pp. 3770-3781. http://dx.doi.org/10.1007/s00284-021-02643-1 PMid:34487210.
» http://dx.doi.org/10.1007/s00284-021-02643-1 - KHOO, K.S., CHEW, K.W., YEW, G.Y., LEONG, W.H., CHAI, Y.H., SHOW, P.L. and CHEN, W.H., 2020. Recent advances in downstream processing of microalgae lipid recovery for biofuel production. Bioresource Technology, vol. 304, p. 122996. http://dx.doi.org/10.1016/j.biortech.2020.122996 PMid:32115347.
» http://dx.doi.org/10.1016/j.biortech.2020.122996 - KIELKOPF, C.L., BAUER, W. and URBATSCH, I.L., 2020. Bradford assay for determining protein concentration. Cold Spring Harbor Protocols, vol. 2020, no. 4, p. pdb-prot102269. http://dx.doi.org/10.1101/pdb.prot102269 PMid:32238597.
» http://dx.doi.org/10.1101/pdb.prot102269 - KRUGER, N.J., 2009. The Bradford method for protein quantitation. In: J.M. WALKER, ed. The protein protocols handbook New York: Humana Totowa, pp. 17-24. http://dx.doi.org/10.1007/978-1-59745-198-7_4
» http://dx.doi.org/10.1007/978-1-59745-198-7_4 - LASKIN, A.I., BENNETT, J.W. and GADD, G.M., 2003. Advances in applied microbiology San Diego: Academic Press, vol. 53.
- LIBERTON, M., BANDYOPADHYAY, A. and PAKRASI, H.B., 2019. Enhanced nitrogen fixation in a glgX-deficient strain of Cyanothece sp. Strain ATCC 51142, a unicellular nitrogen-fixing cyanobacterium. Applied and Environmental Microbiology, vol. 85, no. 7, p. 02887-18 . http://dx.doi.org/10.1128/AEM.02887-18 PMid:30709817.
» http://dx.doi.org/10.1128/AEM.02887-18 - LING, Y., SUN, L.P., WANG, S.Y., LIN, C.S.K., SUN, Z. and ZHOU, Z.G., 2019. Cultivation of oleaginous microalga Scenedesmus obliquus coupled with wastewater treatment for enhanced biomass and lipid production. Biochemical Engineering Journal, vol. 148, pp. 162-169. http://dx.doi.org/10.1016/j.bej.2019.05.012
» http://dx.doi.org/10.1016/j.bej.2019.05.012 - LUDWIG, T.G. and GOLDBERG, J.V., 1956. The anthrone method for the determination of carbohydrates in foods and in oral rinsing. Journal of Dental Research, vol. 35, no. 1, pp. 90-94. http://dx.doi.org/10.1177/00220345560350012301 PMid:13286391.
» http://dx.doi.org/10.1177/00220345560350012301 - NAGHSHBANDI, M.P., TABATABAEI, M., AGHBASHLO, M., AFTAB, M.N. and IQBAL, I., 2019. Metabolic engineering of microalgae for biofuel production. In: K. SPILLING, ed. Biofuels from algae: methods and protcols New York: Humana, vol. 1980, pp. 153-172. http://dx.doi.org/10.1007/7651_2018_205 PMid:30666564.
» http://dx.doi.org/10.1007/7651_2018_205 - PANDEY, M.K., DASGUPTA, C.N., MISHRA, S., SRIVASTAVA, M., GUPTA, V.K., SUSEELA, M.R. and RAMTEKE, P.W., 2019. Bioprospecting microalgae from natural algal bloom for sustainable biomass and biodiesel production. Applied Microbiology and Biotechnology, vol. 103, no. 13, pp. 5447-5458. http://dx.doi.org/10.1007/s00253-019-09856-2 PMid:31101944.
» http://dx.doi.org/10.1007/s00253-019-09856-2 - PARK, S., NGUYEN, T.H.T. and JIN, E., 2019. Improving lipid production by strain development in microalgae: strategies, challenges and perspectives. Bioresource Technology, vol. 292, p. 121953. http://dx.doi.org/10.1016/j.biortech.2019.121953 PMid:31405625.
» http://dx.doi.org/10.1016/j.biortech.2019.121953 - PERERA, F., ASHRAFI, A., KINNEY, P. and MILLS, D., 2019. Towards a fuller assessment of benefits to children’s health of reducing air pollution and mitigating climate change due to fossil fuel combustion. Environmental Research, vol. 172, pp. 55-72. http://dx.doi.org/10.1016/j.envres.2018.12.016 PMid:30771627.
» http://dx.doi.org/10.1016/j.envres.2018.12.016 - RAGHUVANSHI, S., BHAKAR, V., CHAVA, R. and SANGWAN, K.S., 2018. Comparative study using life cycle approach for the biodiesel production from microalgae grown in wastewater and fresh water. Procedia CIRP, vol. 69, pp. 568-572. http://dx.doi.org/10.1016/j.procir.2017.11.030
» http://dx.doi.org/10.1016/j.procir.2017.11.030 - SELVARAJAN, R., FELFÖLDI, T., TAUBER, T., SANNIYASI, E., SIBANDA, T. and TEKERE, M., 2015. Screening and Evaluation of Some Green Algal Strains (Chlorophyceae) Isolated from Freshwater and Soda Lakes for Biofuel Production. Energies, vol. 8, no. 7, pp. 7502-7521. http://dx.doi.org/10.3390/en8077502
» http://dx.doi.org/10.3390/en8077502 - SHARMA, J., KUMAR, V., KUMAR, S.S., MALYAN, S.K., MATHIMANI, T., BISHNOI, N.R. and PUGAZHENDHI, A., 2020. Microalgal consortia for municipal wastewater treatment – Lipid augmentation and fatty acid profiling for biodiesel production. Journal of Photochemistry and Photobiology. B, Biology, vol. 202, pp. 111638. http://dx.doi.org/10.1016/j.jphotobiol.2019.111638 PMid:31733613.
» http://dx.doi.org/10.1016/j.jphotobiol.2019.111638 - SHIM, W.J., SONG, Y.K., HONG, S.H. and JANG, M., 2016. Identification and quantification of microplastics using Nile Red staining. Marine Pollution Bulletin, vol. 113, no. 1-2, pp. 469-476. http://dx.doi.org/10.1016/j.marpolbul.2016.10.049 PMid:28340965.
» http://dx.doi.org/10.1016/j.marpolbul.2016.10.049 - TAN, J.S., LEE, S.Y., CHEW, K.W., LAM, M.K., LIM, J.W., HO, S.H. and SHOW, P.L., 2020. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered, vol. 11, no. 1, pp. 116-129. http://dx.doi.org/10.1080/21655979.2020.1711626 PMid:31909681.
» http://dx.doi.org/10.1080/21655979.2020.1711626 - TANG, D.Y.Y., KHOO, K.S., CHEW, K.W., TAO, Y., HO, S.H. and SHOW, P.L., 2020. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresource Technology, vol. 304, pp. 122997. http://dx.doi.org/10.1016/j.biortech.2020.122997 PMid:32094007.
» http://dx.doi.org/10.1016/j.biortech.2020.122997 - WANG, L., CHEN, L. and WU, S., 2020. Microalgae Cultivation Using Screened Liquid Dairy Manure Applying Different Folds of Dilution: Nutrient Reduction Analysis with Emphasis on Phosphorus Removal. Applied Biochemistry and Biotechnology, vol. 192, no. 2, pp. 381-391. http://dx.doi.org/10.1007/s12010-020-03316-8 PMid:32385813.
» http://dx.doi.org/10.1007/s12010-020-03316-8 - WATSON, J., SWOBODA, M., AIERZHATI, A., WANG, T., SI, B. and ZHANG, Y., 2021. Biocrude oil from algal bloom microalgae: a novel integration of biological and thermochemical techniques. Environmental Science & Technology, vol. 55, no. 3, pp. 1973-1983. http://dx.doi.org/10.1021/acs.est.0c05924 PMid:33434016.
» http://dx.doi.org/10.1021/acs.est.0c05924 - XU, M., XUE, Z., SUN, S., ZHAO, C., LIU, J., LIU, J. and ZHAO, Y., 2020. Co-culturing microalgae with endophytic bacteria increases nutrient removal efficiency for biogas purification. Bioresource Technology, vol. 314, pp. 123766. http://dx.doi.org/10.1016/j.biortech.2020.123766 PMid:32645575.
» http://dx.doi.org/10.1016/j.biortech.2020.123766 - YAO, S., LYU, S., AN, Y., LU, J., GJERMANSEN, C. and SCHRAMM, A., 2019. Microalgae-bacteria symbiosis in microalgal growth and biofuel production: a review. Journal of Applied Microbiology, vol. 126, no. 2, pp. 359-368. http://dx.doi.org/10.1111/jam.14095 PMid:30168644.
» http://dx.doi.org/10.1111/jam.14095
Publication Dates
-
Publication in this collection
04 July 2022 -
Date of issue
2024
History
-
Received
04 Mar 2022 -
Accepted
02 June 2022