Abstract
Growing fish in a recirculating aquaculture system (RAS) involves various stress factors that directly affect their physiological condition. This study aimed to investigate the effect of a chelated organic-mineral additive including Fe, Mn, Cu, Zn, Co, Se and I; on the rearing performance of juvenilecatfish (Clariasgariepinus) under RAS conditions. Four groups of fish (n=50) were formed: a control group (receiving standard feed) and three experimental groups (receiving standard feed with biogenic elements chelate compounds in different concentrations). At the end of the experiment (30 days), the physiological condition of the fish was evaluated by the growth rate, internal organs condition, blood serum biochemical indices and histological examination of the middle intestine. The survival rate of fish in the experimental groups was 96-98%. According to the results, the absolute increase was 14,30% in group III, 11,13% in group II and 6,71% in group I, compared to the control. However, the use of chelated compounds in high concentrations can cause necrosis and erosion of the apical part of the villi (groups II and III). Blood biochemical analysis of fish (group II and III) receiving medium and high concentrations of chelated compounds showed high ALT activity, which was 23,02% (p<0,05) and 45,19% (p<0,05) higher compared to control, respectively. Mineral-chelate compounds, of the studied composition, at a concentration of 0,5 g/kg positively affect the histological structure of the midgut of Clarias gariepinus. This dosage of the investigated feed additive can be recommended for the practical application of rearing Clariasgariepinus in closed water installations.
Keywords:
industrial aquaculture; chelating compounds; blood biochemical parameters; histological studies; biological/fisheries parameters; Clarias gariepinus
Resumo
Este estudo teve como objetivo investigar o efeito de um aditivo orgânico-mineral quelatado que inclui Fe, Mn, Cu, Zn, Co, Se e I sobre o desempenho de criação de juvenis de bagre-africano (Clarias gariepinus) em condições de RAS. Foram formados quatro grupos de peixes (n=50): um grupo controle (recebendo ração padrão) e três grupos experimentais (recebendo ração padrão com elementos biogênicos compostos quelatos em diferentes concentrações). Ao final do experimento (30 dias), a condição fisiológica dos peixes foi avaliada pela taxa de crescimento, condição dos órgãos internos, índices bioquímicos séricos sanguíneos e exame histológico do intestino médio. A taxa de sobrevivência dos peixes nos grupos experimentais foi de 96-98%. De acordo com os resultados, o aumento absoluto foi de 14,30% no grupo III, 11,13% no grupo II e 6,71% no grupo I, em relação ao controle. No entanto, o uso de compostos quelatados em altas concentrações pode causar necrose e erosão da parte apical das vilosidades (grupos II e III). A análise bioquímica do sangue de peixes (grupo II e III) que receberam concentrações médias e altas de compostos quelatados mostrou alta atividade de ALT, que foi 23,02% (p<0,05) e 45,19% (p<0,05) maior comparado ao controle, respectivamente. Compostos mineral-quelatos, da composição estudada, na concentração de 0,5 g/kg, afetam positivamente a estrutura histológica do intestino médio de Clarias gariepinus. Esta dosagem do aditivo alimentar investigado pode ser recomendada para a aplicação prática de criação de Clarias gariepinus em instalações de água fechadas.
Palavras-chave:
aquicultura industrial; compostos quelantes; parâmetros bioquímicos sanguíneos; estudos histológicos; parâmetros biológicos/pescarias; Clarias gariepinus
1. Introduction
The issue of providing the population with fresh fish available for people with different income levels is becoming more critical nowadays (Ye et al., 2017YE, Y., BARANGE, M., BEVERIDGE, M., GARIBALDI, L., GUTIERREZ, N., ANGANUZZI, A. and TACONET, M., 2017. FAO’s statistic data and sustainability of fisheries and aquaculture: comments on Pauly and Zeller. Marine Policy, vol. 81, pp. 401-405. http://dx.doi.org/10.1016/j.marpol.2017.03.012.
http://dx.doi.org/10.1016/j.marpol.2017....
; FAO, 2013FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS – FAO, 2013. Regional principles for responsible aquaculture in the central Asia and Caucasus region. Bishkek: FAO. FAO Fisheries and Aquaculture Circular No. 1078.). Industrial aquaculture technologies allow increasing the automation of production processes, reduce the seasonality of fish production, expand the boundaries of the geographic location of aquaculture facilities, increase net production, and provide import substitution (Sklyarov et al., 2013SKLYAROV, V.Y., BONDARENKO, L.G., KOVALENKO, Y.I., PETRASHOV, V.I., KASHIRIN, A.V. and CHERNYKH, E.N., 2013. Aquaculture on the South of Russia, prospects for development. VNIRO Proceedings, vol. 150, pp. 50-56.; Kumar et al., 2018KUMAR, G., ENGLE, C. and TUCKER, C., 2018. Factors driving aquaculture technology adoption. Journal of the World Aquaculture Society, vol. 49, no. 3, pp. 447-476. http://dx.doi.org/10.1111/jwas.12514.
http://dx.doi.org/10.1111/jwas.12514...
). Thus, aquaculture is one of the main developing sectors of the fishery sector of the world economy. The positive dynamics in the field of aquaculture has become especially noticeable in recent years. In 2019, the production of aquaculture products amounted to 286 thousand tons, increasing the annual growth by 20%, and in the first half of 2020, the increase was 168.5 thousand tons providing the best indicator for all reporting periods (FAO, 2013FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS – FAO, 2013. Regional principles for responsible aquaculture in the central Asia and Caucasus region. Bishkek: FAO. FAO Fisheries and Aquaculture Circular No. 1078.).
Aquaculture is based on the principle of biodiversity preservation and the prevention of negative environmental impacts (Nikiforov-Nikishin et al., 2019NIKIFOROV-NIKISHIN, A.L., SHATOKHIN, M.V., PONOMAREV, A.K., GOLOVACHEVA, N.A., BORODIN, A.L., ZAITSEVA, N.A. and LARIONOVA, A.A., 2019. The influence of anthropogenic factors on the ecological status of the spawning grounds of lake Senezh. Ecology, vol. 28, no. 107, pp. 341-348.). Therefore, today, all over the world, the development of aquaculture in the cultivation of valuable fish species is in evidence (Tidwell and Allan, 2001TIDWELL, J.H. and ALLAN, G.L., 2001. Fish as food: aquaculture’s contribution. Ecological and economic impacts and contributions of fish farming and capture fisheries. EMBO Reports, vol. 2, no. 11, pp. 958-963. http://dx.doi.org/10.1093/embo-reports/kve236. PMid:11713181.
http://dx.doi.org/10.1093/embo-reports/k...
). Catfish (Clarias gariepinus) is a popular object culture-based fishery (Putra et al., 2017PUTRA, I., RUSLIADI, R., FAUZI, M., TANG, U.M. and MUCHLISIN, Z.A., 2017. Growth performance and feed utilization of African catfish Clarias gariepinus fed a commercial diet reared in the biofloc system enhanced with probiotic. F1000 Research, vol. 6, p. 1545. http://dx.doi.org/10.12688/f1000research.12438.1. PMid:28944046.
http://dx.doi.org/10.12688/f1000research...
). This fish species has several advantages, such as high growth rate, stress tolerance, and disease resistance, which explains its commercial value throughout the world (Muchlisin et al., 2015MUCHLISIN, Z.A., NADIAH, W.N., NADIYA, N., FADLI, N., HENDRI, A., KHALIL, M. and SITI-AZIZAH, M.N., 2015. Exploration of natural cryoprotectants for cryopreservation of African catfish, Clariasgariepinus, Burchell 1822 (Pisces: Clariidae) spermatozoa. Czech Journal of Animal Science, vol. 60, no. 1, pp. 10-15. http://dx.doi.org/10.17221/7906-CJAS.
http://dx.doi.org/10.17221/7906-CJAS...
). Catfish fillet is rich in essential omega-3 fatty acids and meets the requirements for high-quality food (Putra et al., 2017PUTRA, I., RUSLIADI, R., FAUZI, M., TANG, U.M. and MUCHLISIN, Z.A., 2017. Growth performance and feed utilization of African catfish Clarias gariepinus fed a commercial diet reared in the biofloc system enhanced with probiotic. F1000 Research, vol. 6, p. 1545. http://dx.doi.org/10.12688/f1000research.12438.1. PMid:28944046.
http://dx.doi.org/10.12688/f1000research...
).
However, fish farming in recirculating aquaculture systems (RAS) is associated with many stress factors that directly affect its physiological state (Khrustalev, 2011KHRUSTALEV, E.I., 2011. Evaluation of the growth potential of channel and sharptooth catfish, substantiating polycyclic growing technologies. Fisheries, vol. 1, pp. 25-29.). The reason for the unprofitable production of pond fish maybe not only the absence of an economic integration system (Kozlov and Golovacheva, 2018KOZLOV, A.V. and GOLOVACHEVA, N.A., 2018. Establishing a model for profitable food production based on unprofitable fish farms. Delta Journal of Science, vol. 2, pp. 28-31.), but also the mass death of fish from infectious diseases (Roberts, 2012ROBERTS, R.J., 2012. Fish pathology. 4th ed. Ames: Wiley-Blackwell. http://dx.doi.org/10.1002/9781118222942.
http://dx.doi.org/10.1002/9781118222942...
; Yukhimenko et al., 2015YUKHIMENKO, L.N., BYCHKOVA, L.I. and DRUZHININA, A.A., 2015. Causative agents of bacterial hemorrhagic septicemia (BGS) of fish, microflora of water and feed, which has epidemiological significance. Far East Journal of Infectious Pathology, vol. 26, no. 26, pp. 43-46.). The quality of the diet is considered one of the important factors in determining the ability of fish to resist disease (Davis, 2015DAVIS, A.D., 2015. Feed and feeding practices in aquaculture. Cambridge: Woodhead Publishing.). The most expensive part of the technology of cultivating catfish is the feeding process (Pereira et al., 2020PEREIRA, S.A., JESUS, G.F.A., PEREIRA, G.V., SILVA, B.C., SÁ, L.S., MARTINS, M.L. and MOURIÑO, J.L.P., 2020. The chelating mineral on organic acid salts modulates the dynamics and richness of the intestinal microbiota of a silver catfish Rhamdia quelen. Current Microbiology, vol. 77, no. 8, pp. 1483-1495. http://dx.doi.org/10.1007/s00284-020-01962-z. PMid:32236647.
http://dx.doi.org/10.1007/s00284-020-019...
; Craig and Helfrich, 2014CRAIG, S. and HELFRICH, L., 2014. Understanding fish nutrition, feeds, and feeding. Virginia Cooperative Extension, vol. 269, pp. 1-6.). Many fish farms compose feeds using regional feed resources applying standard premixes (Hamid et al., 2016HAMID, S.N.I.N., ABDULLAH, M.F., ZAKARIA, Z., YUSOF, S.J.H.M. and ABDULLAH, R., 2016. Formulation of fish feed with optimum protein-bound lysine for African catfish (Clarias Gariepinus) fingerlings. Procedia Engineering, vol. 148, pp. 361-369. http://dx.doi.org/10.1016/j.proeng.2016.06.468.
http://dx.doi.org/10.1016/j.proeng.2016....
; Adéyèmi et al., 2020ADÉYÈMI, A.D., KAYODÉ, A.P.P., CHABI, I.B., ODOUARO, O.B.O., NOUT, M.J.R. and LINNEMANN, A.R., 2020. Screening local feed ingredients of Benin, West Africa, for fish feed formulation. Aquaculture Reports, vol. 17, p. 100386. http://dx.doi.org/10.1016/j.aqrep.2020.100386.
http://dx.doi.org/10.1016/j.aqrep.2020.1...
). Still, the use of unbalanced, low-quality feed can cause diseases of the digestive system and lead to economic losses for the enterprise. Deficiency or excess of macro-and microelements in the diet of fish can affect not only the rate of its growth but also the quality of fish fillets (Lall, 2000LALL, S.P., 2000. Nutrition and health of fish. In: Memorias del V Simposium Internacional de Nutrición Acuícola, 19-22 November 2000, Mérida, Mexico. Monterrey, Mexico: Avances en Nutrición Acuícola, pp. 13-23.). Despite the abundance of recipes and feeding technologies, the development and implementation of new recipes for growing fish in industrial aquaculture, including clary catfish, are relevant (Hamid et al., 2016HAMID, S.N.I.N., ABDULLAH, M.F., ZAKARIA, Z., YUSOF, S.J.H.M. and ABDULLAH, R., 2016. Formulation of fish feed with optimum protein-bound lysine for African catfish (Clarias Gariepinus) fingerlings. Procedia Engineering, vol. 148, pp. 361-369. http://dx.doi.org/10.1016/j.proeng.2016.06.468.
http://dx.doi.org/10.1016/j.proeng.2016....
; Ahmad and Ibrahim, 2016AHMAD, M.K. and IBRAHIM, S., 2016. Local fish meal formulation: its principles, prospect sand problems in fishery industry. International Journal of Fisheries and Aquatic Studies, vol. 4, no. 1, pp. 276-279.). To correct the composition of finished feed, complex organomineral chelating compounds of metals (Fe, Mg, Zn, Se, I, Cu) are often used as additives (Apines et al., 2003APINES, M.J.S., SATOH, S., KIRON, V., WATANABE, T. and FUJITA, S., 2003. Bioavailability and tissue distribution of amino acidchelated trace elements in rainbow trout Oncorhynchus mykiss. Fisheries Science, vol. 69, no. 4, pp. 722-730. http://dx.doi.org/10.1046/j.1444-2906.2003.00679.x.
http://dx.doi.org/10.1046/j.1444-2906.20...
; Hernández et al., 2012HERNÁNDEZ, A.J., SATOH, S. and KIRON, V., 2012. Supplementation of citric acid and amino acid chelated trace elements in low‐fish meal diet for rainbow trout affect growth and phosphorus utilization. Journal of the World Aquaculture Society, vol. 43, no. 5, pp. 688-696. http://dx.doi.org/10.1111/j.1749-7345.2012.00589.x.
http://dx.doi.org/10.1111/j.1749-7345.20...
; Pereira et al., 2020PEREIRA, S.A., JESUS, G.F.A., PEREIRA, G.V., SILVA, B.C., SÁ, L.S., MARTINS, M.L. and MOURIÑO, J.L.P., 2020. The chelating mineral on organic acid salts modulates the dynamics and richness of the intestinal microbiota of a silver catfish Rhamdia quelen. Current Microbiology, vol. 77, no. 8, pp. 1483-1495. http://dx.doi.org/10.1007/s00284-020-01962-z. PMid:32236647.
http://dx.doi.org/10.1007/s00284-020-019...
). These microelements have a direct effect on the immune function of intestinal cells and digestibility (Prabhu et al., 2016PRABHU, P.A.J., SCHRAMA, J.W. and KAUSHIK, S.J., 2016. Mineral requirements of fish: a systematic review. Reviews in Aquaculture, vol. 8, no. 2, pp. 172-219. http://dx.doi.org/10.1111/raq.12090.
http://dx.doi.org/10.1111/raq.12090...
). An analysis of the literature data and our preliminary work made it possible to establish the required level of the microelements described above, which presumably do not have a negative effect on fish health. (Simakov et al., 2020SIMAKOV, G., NIKIFOROV-NIKISHIN, A.L., NIKIFOROV-NIKISHIN, D.L., BEKETOV, S.V., KOCHETKOV, N.I. and KLIMOV, V.A., 2020. Histological changes in the liver, intestines and kidneys of Clarias gariepinus when using feed with chelated compounds. International Journal of Pharmaceutical Research, vol. 12, no. 3, pp. 2380-2391.).
This study is aimed to investigate the effect of various concentrations of chelated organic-mineral additive on the rearing performance of juvenile catfish (Clarias gariepinus) under RAS conditions. To determine the effectiveness of using chelate additives and establish reliably safe concentrations, the physiological state of fish was assessed by the intensity of growth, the state of internal organs, biochemical parameters of blood serum, and histological studies of the middle part of the intestine.
2. Materials and Methods
2.1. Experimental design
The study was carried out in the period from May to July 2020 based on the aquaculture center of the Institute of Biotechnology and Fisheries of the K.G. Razumovsky Moscow State University of Technology and Management, in compliance with all applicable international, national, and/or institutional recommendations for the care of animals and their use. The object of the study was juvenile African Sharptooth catfish (Clarias gariepinus) with an average weight of 20.51–20.60 g and and age of 3 months. As part of the experiment of studying the mineral supplement effect, 4 groups of fish were formed: one control and three experimental groups (I, II, and III) (Table 1). Experimental studies were carried out under conditions of recirculating aquaculture systems (RAS) in 0.5 m3 aquariums equipped with mechanical and biological filtration systems. The experimental groups were kept under the following conditions: 26-27 C, Ph 7-7.5, O2 5-6 mg/l.
The main diet (MD) was based on a standard composition feed intended for rearing juvenile catfish (Table 2). The control group received standard feed without additives. Experimental groups (I, II, III) received standard feed with the addition of chelated compounds of biogenic elements in various concentrations (Table 3).
The composition and concentration of the investigated chelating compounds of microelements and their distribution by groups.
The fish were fed manually, 2 times a day. A single portion of feed was determined by the method of its complete consumption by fish within 10 minutes. The daily feed rate comprised 6% of the fish biomass. The diet was recalculated every 10 days based on the total biomass of individuals, separately for each group. Throughout the experimental study (30 days), the fish were kept at the same stocking density. The fish rearing process was accompanied by the control of the hydrochemical parameters of the RAS basins (Privezentsev, 1972PRIVEZENTSEV, Y.A., 1972. Hydrochemistry of freshwater bodies. Moscow: Food Industry, 119 p.). Water temperature, pH values, oxygen, ammonium nitrogen, nitrites, and nitrates were measured every 3 days. Determination of nitrites was carried out under GOST 33045-2014 “Water. Methods for the determination of nitrogen-containing substances” (method B) (NBSM, 2016NATIONAL BODY FOR STANDARDS AND METROLOGY – NBSM, 2016. Water. Methods for determination of nitrogen-containing matters. GOST 33045-2014. Yerevan: NBSM.). Water quality control was carried out by means of the following laboratory instruments: a laboratory combined pH electrode of general-purpose ESK 10303 (OOO “Izmeritelnaya Tekhnika”, Russia) to a tolerance of ± 0.2 pH at 200C (measurement method according to the electrode datasheet); ionomer Expert-001 (“Econix-Expert” LLC, Russia), to a tolerance of ± 0.02 pX, ± 1.5 mV; ion-selective electrode NH4 Alice (OOO “Measuring equipment”, Russia), to a tolerance of ± 6 mV at 200C (measurement method according to the electrode datasheet); spectrophotometer PF 5400VI (OOO Ekrokhim, Russia) with an absolute tolerance of ± 0.5%; high-precision oximeter HI 9147 (HANNA Instruments, USA), with O2 tolerance ± 1.0% of full scale and temperature ± 0.2 °C.
2.2. Feed additives production
The introduction of mineral chelate additives into the feed mass was carried out in process of calculating the diet in the form of an aqueous solution. The choice of the concentrations of the studied feed additives was based on the results of the author's research (Simakov et al., 2020SIMAKOV, G., NIKIFOROV-NIKISHIN, A.L., NIKIFOROV-NIKISHIN, D.L., BEKETOV, S.V., KOCHETKOV, N.I. and KLIMOV, V.A., 2020. Histological changes in the liver, intestines and kidneys of Clarias gariepinus when using feed with chelated compounds. International Journal of Pharmaceutical Research, vol. 12, no. 3, pp. 2380-2391.). Coppens PRE GROWER (Coppens, Netherlands) was used as the base feed. Under sterile conditions, an aqueous solution of chelating compounds was diluted in distilled water to certain experimental concentrations (0.5, 1.0, and 2.0 g/kg), according to standard methods (Sklyarov, 2008SKLYAROV, V.Y., 2008. Feeds and fish feeding in aquaculture. Moscow: VNIRO Publishing.; Davis, 2015DAVIS, A.D., 2015. Feed and feeding practices in aquaculture. Cambridge: Woodhead Publishing.). By spraying, an aqueous solution of chelating compounds was applied to the granulated feed, after which the granules were dried in a desiccator (Binder FDL 115, Germany) to an initial moisture content of 60-65%. The amount of prepared feed was calculated for three days of feeding and was stored in the refrigerator.
2.3. Ranging, biochemical parameters and histological studies
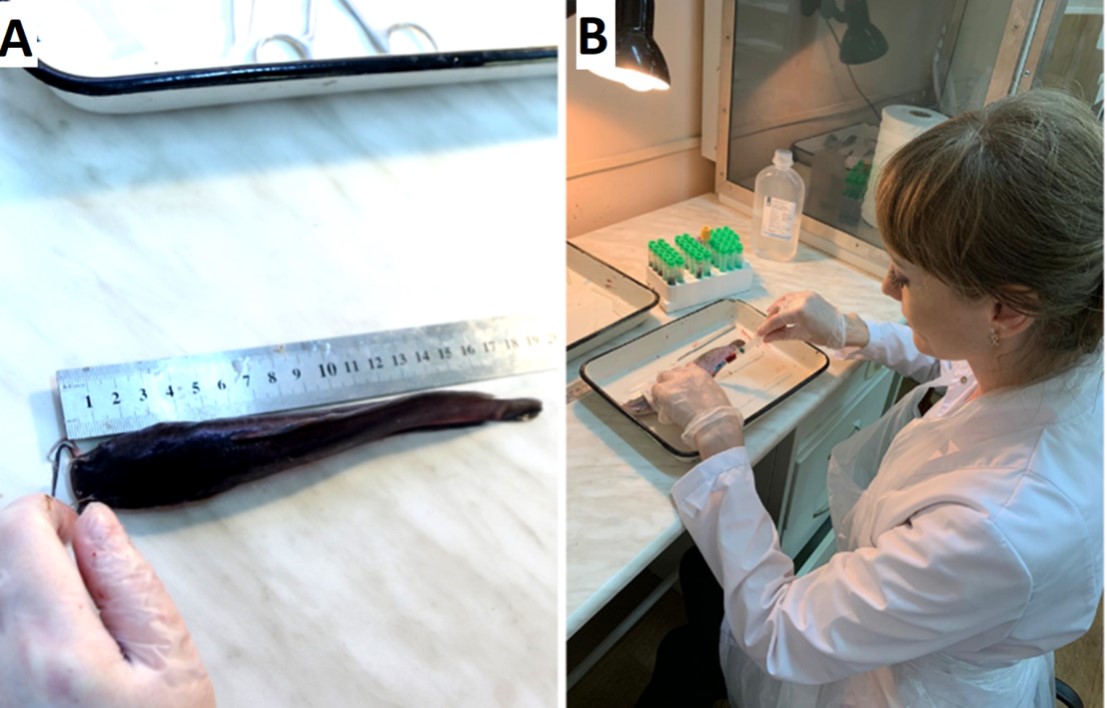
Control over the state and development of fish was determined by the rate of body size and muscle mass increase. Weighing and measurement of fish were carried out according to the methodological recommendations (Vlasov, 2009VLASOV, V.A., 2009. Growth and development of the African catfish (Clarias gariepinus Burchell) depending on the conditions of feeding and maintenance. News of the Timiryazev Agricultural Academy, vol. 3, pp. 148-156.; Fattalahi and Vlasov, 2008FATTALAHI, M. and VLASOV, V.A., 2008. Growth of the African catfish (Clarias gariepinus) in a closed water installation (RAS). Fish farming and fisheries, vol. 1., pp. 42-53.) (Figure 1A). Determination of the live weight of fish was carried out utilizing a laboratory electronic scale VM-2202 (Vesta, Russia) to a tolerance of ± 0.01 g. Biochemical parameters were investigated through the tail vein blood sampling at the end of the experiment (Figure 1B). Biochemical studies of blood serum were performed following the recommended protocol (Pronina et al., 2010PRONINA, G.I., MASLOVA, N.I. and PETRUSHIN, A.B., 2010. Methods for assessing breeding groups of common catfish using physiological, biochemical and immunological indicators: methodological guidelines. Moscow: Feather.) on an automatic analyzer Labio 200 (Mindray, China) using reagents supplied by Biocon (India).
The fish was further dissected for the study of the pathological, epizootic state and the selection of tissues for histological examination. Tissue samples were taken from the mid-intestine of five individuals from each group. The tissue was fixed in a 4% formalin solution for 14 hours. The preparation of histological specimens was carried out following the generally approved methodology (Humason, 1962HUMASON, G.L., 1962. Animal tissue techniques. San Francisco: W.H. Freeman. http://dx.doi.org/10.5962/bhl.title.5890.
http://dx.doi.org/10.5962/bhl.title.5890...
). Histological sections were stained with hematoxylin and eosin (H&E). Histological images were obtained at 40 ×, 100 ×, and 400 × magnifications using an Olympus BX53 light microscope (Olympus Corporation, Japan) with a Carl Zeiss ERc 5s eyepiece attachment (Zeiss, Germany) and ZEN lite software (Zeiss, Germany).
2.4. Statistical analysis
The research results were processed by conventional methods of biological statistics (Jain et al., 2007JAIN, A.K., FLYNN, P. and ROSS, A.A., 2007. Handbook of biometrics. New York: Springer.) in the STATISTICA v.10.0 software package (StatSoft Inc., USA) and Microsoft Excel 2019 (Microsoft, USA). All parameters were expressed as mean values and standard deviation. Comparative analysis between groups was performed using analysis of variance (ANOVA) and Dunnett's post-Hawk test to compare multiple groups with a control group. A p-value <0.05 was taken as statistically significant.
3. Results
3.1. Hydrochemical water parameters
The hydrochemical water parameters were optimal for the fish species under consideration and did not exceed the permissible limits for RAS (Table 4). The average water temperature was 24.75 ± 1.07 оС. The average content of dissolved oxygen 4.83 ± 0.29 mg/l, the pH value remained at the level of 7.05 ± 0.36 and corresponded to the standard values. The nitrite content was 0.17 ± 0.02 mg/l, which is normal for this fish species (<1 mg/l). In the experimental study, the content of nitrates in water did not exceed optimal values and was at the level of 18.05 ± 0.44 mg/l.
Indicators of the hydrochemical state of the RAS aquatic environment (mean value ± standard deviation) when growing the African sharptooth catfish.
3.2. Biological/fisheries parameters
All selected specimens of experimental fish were active, with elastic muscles. The skin was normal, the cornea transparent. The gill filaments of a scarlet-red color, full-blooded, laid parallel. Biological/fisheries parameters of juvenile catfish are presented in Table 5. During the experiment, the body length of fish in group III was higher than in control by 5.31%, in group II by 3.88%, and in group I by 0, 73%. Weight gain was observed in all groups. However, the best indicators of the juvenile's growth rate were noted in the third group (III), where a 2 g/kg concentration of feed containing the mineral supplement was used. In this group, the increase in fish body weight was 16.33% higher than in the control group (p <0.05). The increase in the concentration of chelates had a significant effect on the indicators of the relative growth of fish in comparison with other experimental groups. In experimental groups I and II, the increase was 8.28% (p <0.05) and 14.91% (p <0.05), respectively, compared with the control. A similar trend was observed when evaluating other mass characteristics. The specific growth rate of fish of the II and III experimental groups with the use of the mineral supplement was 4.96% and 4.97%, respectively. The survival capacity of fish in the experimental groups was at the level of 96-98%, which is 4-6% higher than in the control.
Influence of various concentrations of mineral additives on biological/fisheries parameters of juvenile catfish. Values are presented as mean ± standard deviation.
3.3. Histological study of the middle intestinal segment
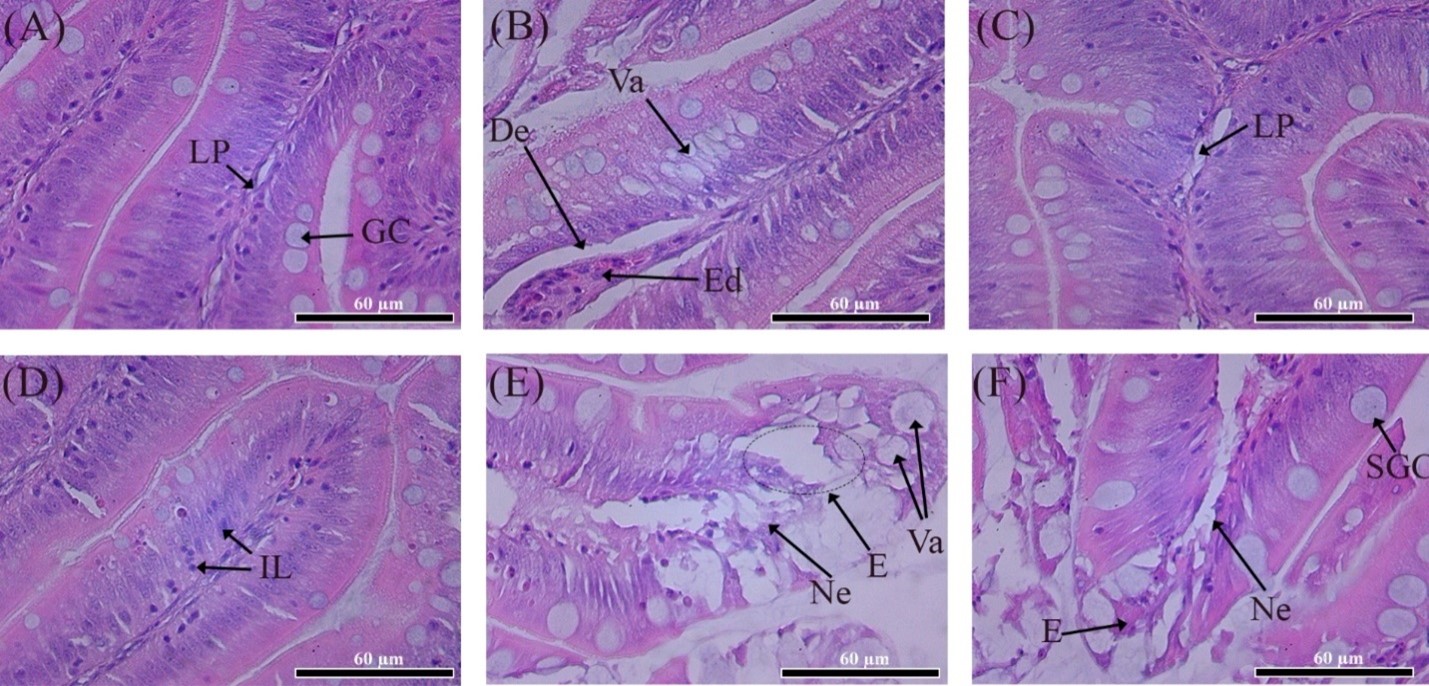
Histological examination of the middle part of the intestine of C. gariepinus showed the presence of minor pathological abnormalities in the experimental groups (Figure 2). In the control group, the intestinal structure was close to normal. In all investigated mid-intestinal specimens, the lamina propria, goblet cells, and villous epithelium could be distinguished (Figure 3A). Closer to the dorsal part, a separate edema phenomenon was observed at the tips of the villi. Most often, this pattern was accompanied by desquamation of the epithelium and small absorbing vacuoles (Figure 3B).
Histological images of the middle intestine of the studied groups of C. Gariepinus (H&E staining): (A) (B) - Control group; (C) (D) - Group I; (E) - Group II; (F) - Group III. Legend: LP - lamina propria of the mucous membrane; GC - goblet cell; Va - vacuolization; De - desquamation; Ed - edema; IL - lymphocyte infiltration; E - erosion; Ne - necrosis; SGC - swollen goblet cell. Scale (bottom right) - 60µm.
Internal organs of Clarias gariepinus. A, B, C - Control group; D, E, F - Experimental group I. Legend: Gi - gills; In - intestine; GB - gallbladder; Ki - mesonephric kidney; Sp - spleen; Li - liver.
In group I, there were no pathological abnormalities, typical for the control group. None of the investigated specimens showed edema of the own mucous membrane or desquamation of the epithelium. Some villi showed a slight increase in the lamina propria (Figure 3C) and lymphocyte infiltration of the prismatic epithelium of the villi (Figure 3D).
A significant deviation from the norm was observed in the intestinal structure of groups II and III (Figure 3E-3F). In most of the samples examined, the phenomena of necrosis and erosion of the apical part of the villi were observed, in some cases accompanied by the phenomena of vacuolization (Figure 3E). In these groups, goblet cells of unusual size were present with apparently inhibited secretion (Figure 3F).
3.4. Biochemical blood serum analysis
The results of the fish blood biochemical studies showed that in the blood serum of experimental groups I, II, and III, the level of crude protein was increased by 57.33% (p <0.05), 67.77% (p <0.05), and 80, 21% (p <0.05), respectively, compared with control (Table 6). The albumin level in catfish of groups I, II, and III was 58.22% (p <0.05), 58.79% (p <0.05), and 81.51% (p <0.05) higher, respectively, compared to the control group. The level of alanine aminotransferase (ALT) in the first experimental group was similar to the values of the control group. In the second and third experimental groups, the ALT level was 23.02% (p <0.05) and 45.19% (p <0.05) higher than in the control, respectively.
The glucose level in the II and III experimental groups was 55.31% and 24.84% higher than in the control group, respectively. However, a statistically significant difference compared to the control was noted only in the second group (p <0.05).
3.5. Pathological studies
After studying the fish-biological and biochemical parameters of the blood of juvenile catfish, the fish were dissected to investigate the effect of the mineral supplement on the development of pathological changes (Figure 3). When examining the skin of catfish from the control group, local erosive skin areas were observed on the right and left sides of the body. In some fish, slight reddening of the anus and hemorrhages in the tissue of the anal fin was observed. The gills of the fish in the control group were pale, light red in color (Figure 3A). The liver was not enlarged, with sharp, even edges, loose, unevenly colored, some areas with punctate hemorrhages. The bile was yellow (Figure 3B). The internal fat content was at the level of 2-3 points. The spleen was reduced, pinkish in color, the mesenteric vessels were injected. The kidney was loose, in 2% of the fish, it was enlarged and filled with blood (Figure 3C). No mucus was found in the inner lining of the intestines. Slight catarrhal inflammation of the posterior intestine was found.
When examining the skin of the catfish of the first experimental group, no erosions were observed. However, some fish showed slight reddening of the anus and minor hemorrhages in the pelvic fins. The gills were light red in color. The liver was not enlarged, slightly loose (90%) or elastic (10%), with clear, even edges, light brown in color; hemorrhages were occasionally observed and mesenteric vessels were injected (Figure 3F). The internal fat content was at the level of 1-4 points. The spleen was voluminous, dark cherry color. The gallbladder contents were green (Figure 3D). The kidneys were enlarged, dense, filled with blood, and had a darker brown color compared to controls (Figure 3E). The middle part of the intestine was flabby, the mucous membrane was moist. There was a slight catarrhal inflammation of the back of the intestine.
In catfish of the II experimental group, skin erosion was not observed. The gills were light red in color. The liver was light brown in color, elastic, dense structure, not enlarged, with sharp, even edges. The spleen was dark cherry color, voluminous, not enlarged. The internal fat content was at the level of 1-4 points. The contents of the gallbladder varied from light yellow to dark green. The kidney was not enlarged, slightly friable, partially filled with blood. The middle part of the intestine was flabby, the mucous membrane was moist, with a minimum amount of mucus.
The catfish of the III experimental group showed no ulcerative lesions of the skin. The gills were red. The liver was elastic, with clear, even edges, light brown in color. The spleen was dark cherry color, voluminous, not enlarged. The internal fat content was 3-4 points. The gallbladder was filled with green bile. The kidney was not enlarged, filled with blood. The middle section of the intestine was denser, without catarrhal inflammation, without contents, the mucous membrane was moist.
Microscopic examination of scrapings from the surface of the body and gills of fish in all studied groups revealed no ectoparasites.
4. Discussion
Rearing in a recirculating aquaculture system (RAS) is associated with many stress factors that directly affect the physiological state of fish (Fattalahi and Vlasov, 2008FATTALAHI, M. and VLASOV, V.A., 2008. Growth of the African catfish (Clarias gariepinus) in a closed water installation (RAS). Fish farming and fisheries, vol. 1., pp. 42-53.; Roberts, 2012ROBERTS, R.J., 2012. Fish pathology. 4th ed. Ames: Wiley-Blackwell. http://dx.doi.org/10.1002/9781118222942.
http://dx.doi.org/10.1002/9781118222942...
). The use of unbalanced, low-quality feed causes diseases of the digestive system of fish, and also leads to economic losses of the enterprise (Lall, 2000LALL, S.P., 2000. Nutrition and health of fish. In: Memorias del V Simposium Internacional de Nutrición Acuícola, 19-22 November 2000, Mérida, Mexico. Monterrey, Mexico: Avances en Nutrición Acuícola, pp. 13-23.). The issues of complete feeding of fish, as well as the effect of feed on its physiological state, are under study in many countries today (Vlasov, 2012VLASOV, V.A., 2012. What kind of feed is better absorbed by the sharptooth catfish. Compound feed, vol. 5, pp. 67-69.; Craig and Helfrich, 2014CRAIG, S. and HELFRICH, L., 2014. Understanding fish nutrition, feeds, and feeding. Virginia Cooperative Extension, vol. 269, pp. 1-6.; Ringø et al., 2016RINGØ, E., ZHOU, Z., VECINO, J.L.G., WADSWORTH, S., ROMERO, J., KROGDAHL, Å., OLSEN, R.E., DIMITROGLOU, A., FOEY, A., DAVIES, S., OWEN, M., LAUZON, H.L., MARTINSEN, L.L., SCHRYVER, P., BOSSIER, P., SPERSTAD, S. and MERRIFIELD, D.L., 2016. Effect of dietary components on the gut microbiota of aquatic animals. A never‐ending story? Aquaculture Nutrition, vol. 22, no. 2, pp. 219-282. http://dx.doi.org/10.1111/anu.12346.
http://dx.doi.org/10.1111/anu.12346...
). This investigation deals with the effect of a chelated mineral supplement on the efficiency of rearing juveniles of Clarias gariepinus catfish under RAS conditions.
All conditions necessary for maintaining health and growth must be created for fish reared in RAS. The temperature of the water, the content of oxygen and other gases, the purity of the water, the pH level, the presence of microorganisms and pathogens are considered critical parameters (Boyd, 2017BOYD, C.E., 2017. General relationship between water quality and aquaculture performance in ponds. In: G. JENEY, ed. Fish diseases: prevention and control strategies. London: Academic Press, pp. 147-166. http://dx.doi.org/10.1016/B978-0-12-804564-0.00006-5.
http://dx.doi.org/10.1016/B978-0-12-8045...
). High concentrations of nitrites and nitrates can have a toxic impact and affect the physiological state of the fish organism (Svobodová et al., 1993SVOBODOVÁ, Z., LLOYD, R., MÁCHOVÁ, J. and VYKUSOVÁ, B., 1993. Water quality and fish health. Rome: FAO. EIFAC Technical Paper 54.). Throughout the experiment, the hydrochemical parameters of the RAS were optimal for Clarias gariepinus and did not exceed the norms permissible for the recirculating aquaculture systems.
The physiological state of fish was assessed by the intensity of growth, survival capability, the state of internal organs, as well as biochemical parameters of blood serum. Analyzing the data of the main biological/fisheries parameters, it can be noted that the use of the mineral supplement “Chelavit” in the diet of clary catfish facilitates their growth. Adding the mineral supplement to the fish diet in an amount from 0.5 to 2.0 g / kg of feed, led to the fish final weight increase. The effectiveness of the chelate additive was most reflected in the growth rate of fish in experimental groups II and III, where the absolute growth was 16.33% and 14.91%, respectively, compared with the control. Other authors also observed an improvement in the growth of tagged fish (Xiphophorous helleri) and lamellar fish (X. maculatus) when Fe was added to the diet, which indicates its nutritional value (Kasozi et al., 2019KASOZI, N., TANDLICH, R., FICK, M., KAISER, H. and WILHELMI, B., 2019. Iron supplementation and management in aquaponic systems: a review. Aquaculture Reports, vol. 15, p. 100221. http://dx.doi.org/10.1016/j.aqrep.2019.100221.
http://dx.doi.org/10.1016/j.aqrep.2019.1...
; Roeder and Roeder, 1966ROEDER, M. and ROEDER, R.H., 1966. Effect of iron on the growth rate of fishes. The Journal of Nutrition, vol. 90, no. 1, pp. 86-90. http://dx.doi.org/10.1093/jn/90.1.86. PMid:5918854.
http://dx.doi.org/10.1093/jn/90.1.86...
).
Histological studies were performed to determine the effect of chelating supplements at various concentrations on the histological structure of the intestine of Clarias gariepinus. The results of the study allow concluding that high concentrations of chelate-mineral compounds in the feed (1 and 2 g/kg) can pose an adverse effect on the histological structure of the intestine of the catfish. Pathological changes such as necrosis and erosion of the upper part of the villi, accompanied by vacuolization and desquamation of the prismatic epithelium, are typical for the fish receiving feed of unbalanced composition. Other authors have noted similar pathologies in the intestines of Oncorhynchus mykiss when using inorganic salts as a source of trace elements (Apines et al., 2003APINES, M.J.S., SATOH, S., KIRON, V., WATANABE, T. and FUJITA, S., 2003. Bioavailability and tissue distribution of amino acidchelated trace elements in rainbow trout Oncorhynchus mykiss. Fisheries Science, vol. 69, no. 4, pp. 722-730. http://dx.doi.org/10.1046/j.1444-2906.2003.00679.x.
http://dx.doi.org/10.1046/j.1444-2906.20...
; Roberts, 2012ROBERTS, R.J., 2012. Fish pathology. 4th ed. Ames: Wiley-Blackwell. http://dx.doi.org/10.1002/9781118222942.
http://dx.doi.org/10.1002/9781118222942...
). It was also noted that necrosis of the villous epithelium can be caused by high concentrations of Cu salts (Al-Bairuty et al., 2013AL-BAIRUTY, G.A., SHAW, B.J., HANDY, R.D. and HENRY, T.B., 2013. Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquatic Toxicology, vol. 126, pp. 104-115. http://dx.doi.org/10.1016/j.aquatox.2012.10.005. PMid:23174144.
http://dx.doi.org/10.1016/j.aquatox.2012...
). However, in this experiment, in group III, receiving a dose of 2 g/kg, the estimated dose of Cu chelate was 0.01 g/kg. Copper in this amount could not lead to the pathological abnormalities described above, since it did not exceed biologically active doses (Prabhu et al., 2016PRABHU, P.A.J., SCHRAMA, J.W. and KAUSHIK, S.J., 2016. Mineral requirements of fish: a systematic review. Reviews in Aquaculture, vol. 8, no. 2, pp. 172-219. http://dx.doi.org/10.1111/raq.12090.
http://dx.doi.org/10.1111/raq.12090...
). Most likely, pathological abnormalities in groups II and III were caused by Zn and Mn chelates. Since chelated compounds of biogenic metals are more bioavailable (Apines-Amar et al., 2004APINES-AMAR, M.J.S., SATOH, S., CAIPANG, C.M.A., KIRON, V., WATANABE, T. and AOKI, T., 2004. Amino acid-chelate: a better source of Zn, Mn and Cu for rainbow trout, Oncorhynchus mykiss. Aquaculture, vol. 240, no. 1-4, pp. 345-358. http://dx.doi.org/10.1016/j.aquaculture.2004.01.032.
http://dx.doi.org/10.1016/j.aquaculture....
), their deposition can have a significant effect on metabolic processes in tissues, resulting in atrophic changes (Roberts, 2012ROBERTS, R.J., 2012. Fish pathology. 4th ed. Ames: Wiley-Blackwell. http://dx.doi.org/10.1002/9781118222942.
http://dx.doi.org/10.1002/9781118222942...
).
Biochemical blood analysis is one of the important key aspects when studying the effect of various feed additives on the physiological state of fish and the quality of the final product. The data of biochemical parameters enable quantitative and qualitative determination of the state of protein and carbohydrate metabolism in biological fluids of the body, to study the nature of changes and certain physiological states of these parameters in pathology or behavior deviation. In the blood serum of all experimental groups, the level of total protein was increased, which points to the higher level of metabolic processes in the body of sharptooth catfish consuming the mineral supplement. This may be due to a large number of catalysts, transfer proteins, and immune protection proteins (Wiegertjes et al., 1996WIEGERTJES, G.F., STET, R.M.J., PARMENTIER, H.K. and VAN MUISWINKEL, W.B., 1996. Immunogenetics of disease resistance in fish: a comparative approach. Developmental and Comparative Immunology, vol. 20, no. 6, pp. 365-381. http://dx.doi.org/10.1016/S0145-305X(96)00032-8. PMid:9040980.
http://dx.doi.org/10.1016/S0145-305X(96)...
; Peyghan et al., 2014PEYGHAN, R., KHADJEH, G.H. and ENAYATI, A., 2014. Effect of water salinity on total protein and electrophoretic pattern of serum proteins of grass carp, Ctenopharyngodon idella. Veterinary Research Forum, vol. 5, no. 3, pp. 225-229. PMid:25568723.). Albumin, which performs a transfer function, is a protein reservoir and is consumed primarily during prolonged starvation (Moman et al., 2020MOMAN, R.N., GUPTA, N. and VARACALLO, M., 2020. Physiology, Albumin [online]. Treasure Island: StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459198/
https://www.ncbi.nlm.nih.gov/books/NBK45...
). The albumin level in catfish of all experimental groups was significantly higher than in the control group. This may indicate that more transfer and metabolic processes took place in the organism of the catfish of the experimental groups (Januar et al., 2015JANUAR, H.I., FAJARNINGSIH, N.D., ZILDA, D.S., BRAMANDITO, A. and WRIGHT, A.D., 2015. Concentration of fish serum albumin (FSA) in the aqueous extract of Indonesian Perciformes fishes’ muscle tissue. Natural Product Research, vol. 29, no. 23, pp. 2230-2232. http://dx.doi.org/10.1080/14786419.2014.1003298. PMid:25611089.
http://dx.doi.org/10.1080/14786419.2014....
).
Alanine aminotransferase (ALT) is one of the main aminotransferases in the body. Aminotransferases are enzymes that catalyze the intermolecular transfer of an amino group from accompanying amino acids to form new keto and amino acids. In this respect, alanine aminotransferase plays one of the core roles in protein metabolism, carrying out the deamination of amino acids (Moriles and Azer, 2021MORILES, K.E. and AZER, S.A., 2021. Alanine Amino Transferase [online]. Treasure Island: StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559278/
https://www.ncbi.nlm.nih.gov/books/NBK55...
). It is found in large quantities in the liver, heart, and skeletal muscles. However, an increase in the level of ALT in the blood serum indicates a pathology (Rastiannasab et al., 2016RASTIANNASAB, A., AFSHARMANESH, S., RAHIMI, R. and SHARIFIAN, I., 2016. Alternations in the liver enzymatic activity of Common carp, Cyprinuscarpio in response to parasites, Dactylogyrus spp. and Gyrodactylusspp. Journal of Parasitic Diseases, vol. 40, no. 4, pp. 1146-1149. http://dx.doi.org/10.1007/s12639-014-0638-9. PMid:27876903.
http://dx.doi.org/10.1007/s12639-014-063...
). The highest ALT activity was noted in the Clary catfish of the III experimental group, which received the maximum concentration of chelate-mineral compounds. Other studies have shown that sublethal zinc concentrations affect hematological changes and liver dysfunctions in fish, increasing the activity of ALT and aminotransferases (Younis et al., 2012YOUNIS, E.M., ABDEL-WARITH, A.A. and AL-ASGAH, N.A., 2012. Hematological and enzymatic responses of Nile tilapia Oreochromisniloticus during short and long term sublethal exposure to zinc. African Journal of Biotechnology, vol. 11, no. 19, pp. 4442-4446. http://dx.doi.org/10.5897/AJB11.3987.
http://dx.doi.org/10.5897/AJB11.3987...
; Nasri et al., 2020NASRI, F., HEYDARNEJAD, M.S. and NEMATOLLAHI, A., 2020. Toxicity of zinc at sublethal exposure to rainbow trout (Oncorhynchus mykiss). CES Medicina Veterinaria y Zootecnia, vol. 15, no. 1, pp. 9-21. http://dx.doi.org/10.21615/cesmvz.15.1.1.
http://dx.doi.org/10.21615/cesmvz.15.1.1...
). The ALT content in fish in the first group (with the minimum concentration of chelates) was at the same level as in the control group.
Evaluation of the pathological parameters of the catfish revealed insignificant differences in all experimental groups compared with the control. Nevertheless, the internal organs of all the studied groups born no visible anatomical and physiological changes that could lead to the death of the fish.
5. Conclusion
This article presents the results of the influence of a mineral-organic supplement in various concentrations on the growth rate, survival capability, the state of internal organs, biochemical parameters of blood serum, as well as on the morphometric parameters of the mid-intestine of juvenile Clarias gariepinus. Studies have shown that the use of chelated compounds in high concentrations can cause necrosis and erosion of the apex of the villi. Biochemical analysis of the blood of fish that received medium and high concentrations of chelate compounds showed a high ALT activity, which indicates a possible pathological process.
The results of all the conducted analyzes enable concluding that the mineral-chelate compounds of the studied composition, at a concentration of 0.5 g/kg, have a positive effect on the histological structure of the middle part of the intestine of the sharptooth catfish. This dose of the investigated feed additive can be recommended for practical use when growing Clarias gariepinus in recirculating aquaculture systems.
References
- ADÉYÈMI, A.D., KAYODÉ, A.P.P., CHABI, I.B., ODOUARO, O.B.O., NOUT, M.J.R. and LINNEMANN, A.R., 2020. Screening local feed ingredients of Benin, West Africa, for fish feed formulation. Aquaculture Reports, vol. 17, p. 100386. http://dx.doi.org/10.1016/j.aqrep.2020.100386
» http://dx.doi.org/10.1016/j.aqrep.2020.100386 - AHMAD, M.K. and IBRAHIM, S., 2016. Local fish meal formulation: its principles, prospect sand problems in fishery industry. International Journal of Fisheries and Aquatic Studies, vol. 4, no. 1, pp. 276-279.
- AL-BAIRUTY, G.A., SHAW, B.J., HANDY, R.D. and HENRY, T.B., 2013. Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquatic Toxicology, vol. 126, pp. 104-115. http://dx.doi.org/10.1016/j.aquatox.2012.10.005 PMid:23174144.
» http://dx.doi.org/10.1016/j.aquatox.2012.10.005 - APINES, M.J.S., SATOH, S., KIRON, V., WATANABE, T. and FUJITA, S., 2003. Bioavailability and tissue distribution of amino acidchelated trace elements in rainbow trout Oncorhynchus mykiss. Fisheries Science, vol. 69, no. 4, pp. 722-730. http://dx.doi.org/10.1046/j.1444-2906.2003.00679.x
» http://dx.doi.org/10.1046/j.1444-2906.2003.00679.x - APINES-AMAR, M.J.S., SATOH, S., CAIPANG, C.M.A., KIRON, V., WATANABE, T. and AOKI, T., 2004. Amino acid-chelate: a better source of Zn, Mn and Cu for rainbow trout, Oncorhynchus mykiss. Aquaculture, vol. 240, no. 1-4, pp. 345-358. http://dx.doi.org/10.1016/j.aquaculture.2004.01.032
» http://dx.doi.org/10.1016/j.aquaculture.2004.01.032 - BOYD, C.E., 2017. General relationship between water quality and aquaculture performance in ponds. In: G. JENEY, ed. Fish diseases: prevention and control strategies London: Academic Press, pp. 147-166. http://dx.doi.org/10.1016/B978-0-12-804564-0.00006-5
» http://dx.doi.org/10.1016/B978-0-12-804564-0.00006-5 - CRAIG, S. and HELFRICH, L., 2014. Understanding fish nutrition, feeds, and feeding. Virginia Cooperative Extension, vol. 269, pp. 1-6.
- DAVIS, A.D., 2015. Feed and feeding practices in aquaculture Cambridge: Woodhead Publishing.
- FATTALAHI, M. and VLASOV, V.A., 2008. Growth of the African catfish (Clarias gariepinus) in a closed water installation (RAS). Fish farming and fisheries, vol. 1., pp. 42-53.
- FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS – FAO, 2013. Regional principles for responsible aquaculture in the central Asia and Caucasus region Bishkek: FAO. FAO Fisheries and Aquaculture Circular No. 1078.
- HAMID, S.N.I.N., ABDULLAH, M.F., ZAKARIA, Z., YUSOF, S.J.H.M. and ABDULLAH, R., 2016. Formulation of fish feed with optimum protein-bound lysine for African catfish (Clarias Gariepinus) fingerlings. Procedia Engineering, vol. 148, pp. 361-369. http://dx.doi.org/10.1016/j.proeng.2016.06.468
» http://dx.doi.org/10.1016/j.proeng.2016.06.468 - HERNÁNDEZ, A.J., SATOH, S. and KIRON, V., 2012. Supplementation of citric acid and amino acid chelated trace elements in low‐fish meal diet for rainbow trout affect growth and phosphorus utilization. Journal of the World Aquaculture Society, vol. 43, no. 5, pp. 688-696. http://dx.doi.org/10.1111/j.1749-7345.2012.00589.x
» http://dx.doi.org/10.1111/j.1749-7345.2012.00589.x - HUMASON, G.L., 1962. Animal tissue techniques San Francisco: W.H. Freeman. http://dx.doi.org/10.5962/bhl.title.5890
» http://dx.doi.org/10.5962/bhl.title.5890 - JAIN, A.K., FLYNN, P. and ROSS, A.A., 2007. Handbook of biometrics New York: Springer.
- JANUAR, H.I., FAJARNINGSIH, N.D., ZILDA, D.S., BRAMANDITO, A. and WRIGHT, A.D., 2015. Concentration of fish serum albumin (FSA) in the aqueous extract of Indonesian Perciformes fishes’ muscle tissue. Natural Product Research, vol. 29, no. 23, pp. 2230-2232. http://dx.doi.org/10.1080/14786419.2014.1003298 PMid:25611089.
» http://dx.doi.org/10.1080/14786419.2014.1003298 - KASOZI, N., TANDLICH, R., FICK, M., KAISER, H. and WILHELMI, B., 2019. Iron supplementation and management in aquaponic systems: a review. Aquaculture Reports, vol. 15, p. 100221. http://dx.doi.org/10.1016/j.aqrep.2019.100221
» http://dx.doi.org/10.1016/j.aqrep.2019.100221 - KHRUSTALEV, E.I., 2011. Evaluation of the growth potential of channel and sharptooth catfish, substantiating polycyclic growing technologies. Fisheries, vol. 1, pp. 25-29.
- KOZLOV, A.V. and GOLOVACHEVA, N.A., 2018. Establishing a model for profitable food production based on unprofitable fish farms. Delta Journal of Science, vol. 2, pp. 28-31.
- KUMAR, G., ENGLE, C. and TUCKER, C., 2018. Factors driving aquaculture technology adoption. Journal of the World Aquaculture Society, vol. 49, no. 3, pp. 447-476. http://dx.doi.org/10.1111/jwas.12514
» http://dx.doi.org/10.1111/jwas.12514 - LALL, S.P., 2000. Nutrition and health of fish. In: Memorias del V Simposium Internacional de Nutrición Acuícola, 19-22 November 2000, Mérida, Mexico. Monterrey, Mexico: Avances en Nutrición Acuícola, pp. 13-23.
- MOMAN, R.N., GUPTA, N. and VARACALLO, M., 2020. Physiology, Albumin [online]. Treasure Island: StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459198/
» https://www.ncbi.nlm.nih.gov/books/NBK459198/ - MORILES, K.E. and AZER, S.A., 2021. Alanine Amino Transferase [online]. Treasure Island: StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559278/
» https://www.ncbi.nlm.nih.gov/books/NBK559278/ - MUCHLISIN, Z.A., NADIAH, W.N., NADIYA, N., FADLI, N., HENDRI, A., KHALIL, M. and SITI-AZIZAH, M.N., 2015. Exploration of natural cryoprotectants for cryopreservation of African catfish, Clariasgariepinus, Burchell 1822 (Pisces: Clariidae) spermatozoa. Czech Journal of Animal Science, vol. 60, no. 1, pp. 10-15. http://dx.doi.org/10.17221/7906-CJAS
» http://dx.doi.org/10.17221/7906-CJAS - NASRI, F., HEYDARNEJAD, M.S. and NEMATOLLAHI, A., 2020. Toxicity of zinc at sublethal exposure to rainbow trout (Oncorhynchus mykiss). CES Medicina Veterinaria y Zootecnia, vol. 15, no. 1, pp. 9-21. http://dx.doi.org/10.21615/cesmvz.15.1.1
» http://dx.doi.org/10.21615/cesmvz.15.1.1 - NATIONAL BODY FOR STANDARDS AND METROLOGY – NBSM, 2016. Water. Methods for determination of nitrogen-containing matters. GOST 33045-2014 Yerevan: NBSM.
- NIKIFOROV-NIKISHIN, A.L., SHATOKHIN, M.V., PONOMAREV, A.K., GOLOVACHEVA, N.A., BORODIN, A.L., ZAITSEVA, N.A. and LARIONOVA, A.A., 2019. The influence of anthropogenic factors on the ecological status of the spawning grounds of lake Senezh. Ecology, vol. 28, no. 107, pp. 341-348.
- PEREIRA, S.A., JESUS, G.F.A., PEREIRA, G.V., SILVA, B.C., SÁ, L.S., MARTINS, M.L. and MOURIÑO, J.L.P., 2020. The chelating mineral on organic acid salts modulates the dynamics and richness of the intestinal microbiota of a silver catfish Rhamdia quelen Current Microbiology, vol. 77, no. 8, pp. 1483-1495. http://dx.doi.org/10.1007/s00284-020-01962-z PMid:32236647.
» http://dx.doi.org/10.1007/s00284-020-01962-z - PEYGHAN, R., KHADJEH, G.H. and ENAYATI, A., 2014. Effect of water salinity on total protein and electrophoretic pattern of serum proteins of grass carp, Ctenopharyngodon idella. Veterinary Research Forum, vol. 5, no. 3, pp. 225-229. PMid:25568723.
- PRABHU, P.A.J., SCHRAMA, J.W. and KAUSHIK, S.J., 2016. Mineral requirements of fish: a systematic review. Reviews in Aquaculture, vol. 8, no. 2, pp. 172-219. http://dx.doi.org/10.1111/raq.12090
» http://dx.doi.org/10.1111/raq.12090 - PRIVEZENTSEV, Y.A., 1972. Hydrochemistry of freshwater bodies Moscow: Food Industry, 119 p.
- PRONINA, G.I., MASLOVA, N.I. and PETRUSHIN, A.B., 2010. Methods for assessing breeding groups of common catfish using physiological, biochemical and immunological indicators: methodological guidelines Moscow: Feather.
- PUTRA, I., RUSLIADI, R., FAUZI, M., TANG, U.M. and MUCHLISIN, Z.A., 2017. Growth performance and feed utilization of African catfish Clarias gariepinus fed a commercial diet reared in the biofloc system enhanced with probiotic. F1000 Research, vol. 6, p. 1545. http://dx.doi.org/10.12688/f1000research.12438.1 PMid:28944046.
» http://dx.doi.org/10.12688/f1000research.12438.1 - RASTIANNASAB, A., AFSHARMANESH, S., RAHIMI, R. and SHARIFIAN, I., 2016. Alternations in the liver enzymatic activity of Common carp, Cyprinuscarpio in response to parasites, Dactylogyrus spp. and Gyrodactylusspp. Journal of Parasitic Diseases, vol. 40, no. 4, pp. 1146-1149. http://dx.doi.org/10.1007/s12639-014-0638-9 PMid:27876903.
» http://dx.doi.org/10.1007/s12639-014-0638-9 - RINGØ, E., ZHOU, Z., VECINO, J.L.G., WADSWORTH, S., ROMERO, J., KROGDAHL, Å., OLSEN, R.E., DIMITROGLOU, A., FOEY, A., DAVIES, S., OWEN, M., LAUZON, H.L., MARTINSEN, L.L., SCHRYVER, P., BOSSIER, P., SPERSTAD, S. and MERRIFIELD, D.L., 2016. Effect of dietary components on the gut microbiota of aquatic animals. A never‐ending story? Aquaculture Nutrition, vol. 22, no. 2, pp. 219-282. http://dx.doi.org/10.1111/anu.12346
» http://dx.doi.org/10.1111/anu.12346 - ROBERTS, R.J., 2012. Fish pathology 4th ed. Ames: Wiley-Blackwell. http://dx.doi.org/10.1002/9781118222942
» http://dx.doi.org/10.1002/9781118222942 - ROEDER, M. and ROEDER, R.H., 1966. Effect of iron on the growth rate of fishes. The Journal of Nutrition, vol. 90, no. 1, pp. 86-90. http://dx.doi.org/10.1093/jn/90.1.86 PMid:5918854.
» http://dx.doi.org/10.1093/jn/90.1.86 - SIMAKOV, G., NIKIFOROV-NIKISHIN, A.L., NIKIFOROV-NIKISHIN, D.L., BEKETOV, S.V., KOCHETKOV, N.I. and KLIMOV, V.A., 2020. Histological changes in the liver, intestines and kidneys of Clarias gariepinus when using feed with chelated compounds. International Journal of Pharmaceutical Research, vol. 12, no. 3, pp. 2380-2391.
- SKLYAROV, V.Y., 2008. Feeds and fish feeding in aquaculture Moscow: VNIRO Publishing.
- SKLYAROV, V.Y., BONDARENKO, L.G., KOVALENKO, Y.I., PETRASHOV, V.I., KASHIRIN, A.V. and CHERNYKH, E.N., 2013. Aquaculture on the South of Russia, prospects for development. VNIRO Proceedings, vol. 150, pp. 50-56.
- SVOBODOVÁ, Z., LLOYD, R., MÁCHOVÁ, J. and VYKUSOVÁ, B., 1993. Water quality and fish health Rome: FAO. EIFAC Technical Paper 54.
- TIDWELL, J.H. and ALLAN, G.L., 2001. Fish as food: aquaculture’s contribution. Ecological and economic impacts and contributions of fish farming and capture fisheries. EMBO Reports, vol. 2, no. 11, pp. 958-963. http://dx.doi.org/10.1093/embo-reports/kve236 PMid:11713181.
» http://dx.doi.org/10.1093/embo-reports/kve236 - VLASOV, V.A., 2009. Growth and development of the African catfish (Clarias gariepinus Burchell) depending on the conditions of feeding and maintenance. News of the Timiryazev Agricultural Academy, vol. 3, pp. 148-156.
- VLASOV, V.A., 2012. What kind of feed is better absorbed by the sharptooth catfish. Compound feed, vol. 5, pp. 67-69.
- WIEGERTJES, G.F., STET, R.M.J., PARMENTIER, H.K. and VAN MUISWINKEL, W.B., 1996. Immunogenetics of disease resistance in fish: a comparative approach. Developmental and Comparative Immunology, vol. 20, no. 6, pp. 365-381. http://dx.doi.org/10.1016/S0145-305X(96)00032-8 PMid:9040980.
» http://dx.doi.org/10.1016/S0145-305X(96)00032-8 - YE, Y., BARANGE, M., BEVERIDGE, M., GARIBALDI, L., GUTIERREZ, N., ANGANUZZI, A. and TACONET, M., 2017. FAO’s statistic data and sustainability of fisheries and aquaculture: comments on Pauly and Zeller. Marine Policy, vol. 81, pp. 401-405. http://dx.doi.org/10.1016/j.marpol.2017.03.012
» http://dx.doi.org/10.1016/j.marpol.2017.03.012 - YOUNIS, E.M., ABDEL-WARITH, A.A. and AL-ASGAH, N.A., 2012. Hematological and enzymatic responses of Nile tilapia Oreochromisniloticus during short and long term sublethal exposure to zinc. African Journal of Biotechnology, vol. 11, no. 19, pp. 4442-4446. http://dx.doi.org/10.5897/AJB11.3987
» http://dx.doi.org/10.5897/AJB11.3987 - YUKHIMENKO, L.N., BYCHKOVA, L.I. and DRUZHININA, A.A., 2015. Causative agents of bacterial hemorrhagic septicemia (BGS) of fish, microflora of water and feed, which has epidemiological significance. Far East Journal of Infectious Pathology, vol. 26, no. 26, pp. 43-46.
Publication Dates
-
Publication in this collection
29 Aug 2022 -
Date of issue
2024
History
-
Received
20 June 2022 -
Accepted
26 July 2022