Abstract
Any solid, unprotected, and undefended surface in the aquatic environment will be fouled. Fouling, on the other hand, can affect a wide range of species that can tolerate some epibiosis. Several others, on the other hand, aggressively keep the epibionts off their body surface (antifouling). Antifouling defenses are built into marine plants like seaweed and seagrass. They do have a distinctive surface structure with tightly packed needle-like peaks and antifouling coverings, which may hinder settling bacteria's ability to cling. Chemical antifouling resistance is most probably a biological reaction to epibiosis' ecological drawbacks, especially for organisms capable of performing photosynthesis. The goal of this study was to see how effective natural compounds derived from littoral seaweeds were in preventing fouling. The brown mussel, an important fouling organism, was evaluated in laboratory bioassays against fifty-one populations' crude organic extracts including fort-two macroalgae species. Antifouling activity, exhibited a distinct phylogenetic pattern, with red macroalgae having the largest share of active species, subsequently brown macroalgae. Antifouling action in green seaweeds has never been significant. Seven species showed some level of induced antifouling defense. Our findings appear to back up previous findings about secondary metabolite synthesis in seaweeds, indicating that in the hunt for novel antifoulants, researchers should concentrate their efforts on tropical red macroalgae.
Keywords:
secondary metabolites; marine chemical ecology; epibiosis; biofouling; antifoulants
Resumo
Qualquer superfície sólida, desprotegida e indefesa no ambiente aquático será contaminada. A incrustação, por outro lado, pode afetar uma ampla gama de espécies que podem tolerar alguma epibiose. Vários outros, por outro lado, mantêm agressivamente os epibiontes fora de sua superfície corporal (anti-incrustante). As defesas anti-incrustantes são construídas em plantas marinhas como algas marinhas e ervas marinhas. Elas têm uma estrutura de superfície distinta com picos semelhantes a agulhas bem compactadas e coberturas anti-incrustantes, o que pode dificultar a capacidade de fixação das bactérias. A resistência química anti-incrustante é provavelmente uma reação biológica às desvantagens ecológicas da epibiose, especialmente para organismos capazes de realizar fotossíntese. O objetivo deste estudo foi verificar a eficácia dos compostos naturais derivados de algas marinhas do litoral na prevenção da incrustação. O mexilhão-marrom, importante organismo incrustante, foi avaliado em bioensaios de laboratório contra extratos orgânicos brutos de 51 populações, incluindo duas espécies de macroalgas. A atividade anti-incrustante exibiu um padrão filogenético distinto, com macroalgas vermelhas tendo a maior participação de espécies ativas, posteriormente macroalgas marrons. A ação anti-incrustante em algas verdes nunca foi significativa. Sete espécies apresentaram algum nível de defesa anti-incrustante induzida. Nossas descobertas parecem corroborar descobertas anteriores sobre a síntese de metabólitos secundários em algas marinhas, indicando que, na busca por novos anti-incrustantes, os pesquisadores devem concentrar seus esforços em macroalgas vermelhas tropicais.
Palavras-chave:
metabólitos secundários; ecologia química marinha; epibiose; bioincrustação; anti-incrustantes
1. Introduction
Biofouling is one of the most significant obstacles to effective and long-term output in marine aquaculture. In marine environments, biofouling is a common occurrence (Santos and Mayoral, 2006SANTOS, A. and MAYORAL, E., 2006. Bioerosive structures of sclerozoan foraminifera from the Lower Pliocene of southern Spain: a contribution to the palaeoecology of marine hard substrate communities. Palaeontology, vol. 49, no. 4, pp. 719-732. http://dx.doi.org/10.1111/j.1475-4983.2006.00560.x.
http://dx.doi.org/10.1111/j.1475-4983.20...
; Zatoń et al., 2018ZATOŃ, M., ZAPALSKI, M.K., BERKOWSKI, B. and WRZOŁEK, T., 2018. Cryptic encrusting communities in a Middle Devonian mesophotic paleoenvironment of the Holy Cross Mountains, Poland. Palaeogeography, Palaeoclimatology, Palaeoecology, vol. 501, pp. 82-91. http://dx.doi.org/10.1016/j.palaeo.2018.04.015.
http://dx.doi.org/10.1016/j.palaeo.2018....
). Seaweeds are one of the most prolific biomass providers in the ocean. Marine algae, particularly brown algae, have long been known to be a significant source of biogenic chemicals having antifouling potential which might be perfect substitutes for tributyltin. Marine organisms that serve as substrata, such as crustaceans and seaweeds could be harmed due to overgrowth by fouling (epibiosis). On living organisms, fouling can have both indirect and direct consequences. Because they are sessile and limited to the photic zone, where circumstances for fouling growth and the development are ideal, seaweeds ought to be particularly vulnerable to fouling (Rocha et al., 2018ROCHA, D.H., SECA, A.M. and PINTO, D.C., 2018. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Marine Drugs, vol. 16, no. 11, pp. 410. http://dx.doi.org/10.3390/md16110410. PMid:30373208.
http://dx.doi.org/10.3390/md16110410...
; Rosa et al., 2019ROSA, G.P., TAVARES, W.R., SOUSA, P.M.C., PAGÈS, A.K., SECA, A.M.L. and PINTO, D.C.G.A., 2019. Seaweed secondary metabolites with beneficial health effects: an overview of successes in in vivo studies and clinical trials. Marine Drugs, vol. 18, no. 1, pp. 8. http://dx.doi.org/10.3390/md18010008. PMid:31861879.
http://dx.doi.org/10.3390/md18010008...
).
For host seaweeds, only a few examples are losses to consumers lured by appetizing epibionts, increased algal tissue losses and drag during storms, and reduced reproduction and growth (Machado et al., 2019MACHADO, G.B., FERREIRA, A.P. and LEITE, F.P., 2019. Testing the importance of predation refuge vs. food quality in determining the use of macroalgal hosts by a generalist marine mesograzer. Marine Biology, vol. 166, no. 5, pp. 1-12. http://dx.doi.org/10.1007/s00227-019-3502-8.
http://dx.doi.org/10.1007/s00227-019-350...
; Bué et al., 2020BUÉ, M., SMALE, D.A., NATANNI, G., MARSHALL, H. and MOORE, P.J., 2020. Multiple-scale interactions structure macroinvertebrate assemblages associated with kelp understory algae. Diversity & Distributions, vol. 26, no. 11, pp. 1551-1565. http://dx.doi.org/10.1111/ddi.13140.
http://dx.doi.org/10.1111/ddi.13140...
). Epibionts may also induce nutritional depletion and a decrease in photosynthetic light. Epiphytes on other seaweeds and bryozoans on kelps, are some of the examples that seaweeds might benefit from epibiosis as a form of protection or concealment (Baumgartner, 2021BAUMGARTNER, V., 2021 [viewed 2022 April 24]. Bryozoan settlement varies by kelp species [online]. Available from: https://digital.lib.washington.edu/researchworks/handle/1773/47159.
https://digital.lib.washington.edu/resea...
). Nonetheless, in a phenomenon known as the “shared doom” effect, by luring consumers either indirectly or directly, epibiosis appears to be deleterious to host seaweeds (Herstoff and Iyengar, 2011HERSTOFF, E.M. and IYENGAR, E.V., 2011. Individuals of Crepidula adunca (Mollusca, Gastropoda) avoid shared doom through host specificity. Journal of Experimental Marine Biology and Ecology, vol. 406, no. 1–2, pp. 79-86. http://dx.doi.org/10.1016/j.jembe.2011.06.007.
http://dx.doi.org/10.1016/j.jembe.2011.0...
; Emerson et al., 2012EMERSON, S.E., BROWNS, J.S., WHELAN, C.J. and SCHMIDT, K.A., 2012. Scale-dependent neighborhood effects: shared doom and associational refuge. Oecologia, vol. 168, no. 3, pp. 659-670. http://dx.doi.org/10.1007/s00442-011-2144-4. PMid:21987268.
http://dx.doi.org/10.1007/s00442-011-214...
). Epibiosis' ecological drawbacks, notably in photosynthetic organisms, have triggered an antifouling chemical defense which is most likely an evolutionary reaction (Leonard et al., 2017LEONARD, K., HEWITT, C.L., CAMPBELL, M.L., PRIMO, C. and MILLER, S.D., 2017. Epibiotic pressure contributes to biofouling invader success. Scientific Reports, vol. 7, no. 1, pp. 5173. http://dx.doi.org/10.1038/s41598-017-05470-2. PMid:28701736.
http://dx.doi.org/10.1038/s41598-017-054...
; Wang et al., 2018WANG, S., WEINBERGER, F. and LENZ, M., 2018. Fluctuations in the strength of chemical antifouling defenses in a red macroalga in response to variations in epibiont colonization pressure. Marine Biology, vol. 165, no. 6, pp. 1-14. http://dx.doi.org/10.1007/s00227-018-3365-4.
http://dx.doi.org/10.1007/s00227-018-336...
; Paul, 2019PAUL, V.J., 2019. Ecological roles of marine natural products. Ithaca: Cornell University Press. Chemical defenses of benthic marine invertebrates, pp. 164–188.).
A total of 3600 secondary metabolites are produced by marine macroalgae, the majority of which have yet to be identified ecological functions (El-Beltagi et al., 2019EL-BELTAGI, H.S., MOHAMED, H.I. and EL-ENAIN, M.M.A., 2019. Role of secondary metabolites from seaweeds in the context of plant development and crop production. In: L. PEREIRA, K. BAHCEVANDZIEV and N. H. HOSHI, eds. Seaweeds as plant fertilizer, agricultural biostimulants and animal fodder. Boca Raton: CRC Press, pp. 64–79. http://dx.doi.org/10.1201/9780429487156-4.
http://dx.doi.org/10.1201/9780429487156-...
). Although an increasing amount of data suggests to multiple/other activities, like antifouling or epibiosis resistance, which boost their adaptive value, the major ecological job of seaweed secondary metabolites is commonly assumed to be protection against herbivores. Nonetheless, just a few research have looked into the antifouling properties of these chemicals (Salama et al., 2018SALAMA, A.J., SATHEESH, S. and BALQADI, A.A., 2018. Antifouling activities of methanolic extracts of three macroalgal species from the Red Sea. Journal of Applied Phycology, vol. 30, no. 3, pp. 1943-1953. http://dx.doi.org/10.1007/s10811-017-1345-6.
http://dx.doi.org/10.1007/s10811-017-134...
). Amongst the primary issues now confronting marine technology are fouling of ship hulls, thus different antifouling paints such as organotin-based paints like tributyltin have been used in the past, until they were shown to be hazardous to many marine organisms lately (Birchenough et al., 2002BIRCHENOUGH, A.C., EVANS, S.M., MOSS, C. and WELCH, R., 2002. Re-colonisation and recovery of populations of dogwhelks Nucella lapillus (L.) on shores formerly subject to severe TBT contamination. Marine Pollution Bulletin, vol. 44, no. 7, pp. 652-659. http://dx.doi.org/10.1016/S0025-326X(01)00308-3. PMid:12222888.
http://dx.doi.org/10.1016/S0025-326X(01)...
; Voulvoulis et al., 2002VOULVOULIS, N., SCRIMSHAW, M.D. and LESTER, J.N., 2002. Comparative environmental assessment of biocides used in antifouling paints. Chemosphere, vol. 47, no. 7, pp. 789-795. http://dx.doi.org/10.1016/S0045-6535(01)00336-8. PMid:12079074.
http://dx.doi.org/10.1016/S0045-6535(01)...
).
Many governments have banned or restricted the use of these substances and in January 2008, the international maritime organization banned them all (Arai et al., 2009ARAI, T., HARINO, H., OHJI, M. and LANGSTON, W.J., 2009. Ecotoxicology of antifouling biocides. Tokyo: Springer. http://dx.doi.org/10.1007/978-4-431-85709-9.
http://dx.doi.org/10.1007/978-4-431-8570...
). Currently, eighteen distinct booster biocides are at least utilized as antifouling paint alternatives, however they could represent a risk to the aquatic ecosystem as has been demonstrated recently (Barletta et al., 2018BARLETTA, M., AVERSA, C., PIZZI, E., PUOPOLO, M. and VESCO, S., 2018. Design, development and first validation of “biocide-free” anti-fouling coatings. Progress in Organic Coatings, vol. 123, pp. 35-46. http://dx.doi.org/10.1016/j.porgcoat.2018.06.007.
http://dx.doi.org/10.1016/j.porgcoat.201...
; Manoj et al., 2018MANOJ, S., MAHESH, S. and SRIKANTH, N., 2018. Review of biofouling paints on the marine vessel. In: Asian Conference on Energy, Power and Transportation Electrification (ACEPT), 2018, Singapore. New York: IEEE, p. 1-6. http://dx.doi.org/10.1109/ACEPT.2018.8610796.
http://dx.doi.org/10.1109/ACEPT.2018.861...
; Raf et al., 2018RAF, M., DE BAERE KRIS, P.G. and REMKE, W., 2018. Development of a test platform for anti-fouling coatings. In: International Symposium on Corrosion and Fouling. Antwerpen: Antwerp Maritime Academy, p. 132.; Nwuzor et al., 2021NWUZOR, I.C., IDUMAH, C.I., NWANONENYI, S.C. and EZEANI, O.E., 2021. Emerging trends in self-polishing anti-fouling coatings for marine environment. Safety in Extreme Environments, vol. 3, no. 1, pp. 9-25. http://dx.doi.org/10.1007/s42797-021-00031-3.
http://dx.doi.org/10.1007/s42797-021-000...
). As a result, new ecologically friendly antifoulants are in high demand (Ciriminna et al., 2015CIRIMINNA, R., BRIGHT, F.V. and PAGLIARO, M., 2015. Ecofriendly antifouling marine coatings. ACS Sustainable Chemistry & Engineering, vol. 3, no. 4, pp. 559-565. http://dx.doi.org/10.1021/sc500845n.
http://dx.doi.org/10.1021/sc500845n...
; Anisimov et al., 2019ANISIMOV, A.V., MIKHAILOVA, M.A. and UVAROVA, E.A., 2019. Modern approaches to the development of marine antifouling coatings. Inorganic Materials: Applied Research, vol. 10, no. 6, pp. 1384-1389. http://dx.doi.org/10.1134/S2075113319060029.
http://dx.doi.org/10.1134/S2075113319060...
; Koning et al., 2020KONING, J.T., BOLLMANN, U.E. and BESTER, K., 2020. The occurrence of modern organic antifouling biocides in Danish marinas. Marine Pollution Bulletin, vol. 158, pp. 111402. http://dx.doi.org/10.1016/j.marpolbul.2020.111402. PMid:32753187.
http://dx.doi.org/10.1016/j.marpolbul.20...
). For this purpose, several scientists are attempting to use chemical defense mechanisms found in marine life (Ma et al., 2017MA, C., ZHANG, W., ZHANG, G. and QIAN, P., 2017. Environmentally friendly antifouling coatings based on biodegradable polymer and natural antifoulant. ACS Sustainable Chemistry & Engineering, vol. 5, no. 7, pp. 6304-6309. http://dx.doi.org/10.1021/acssuschemeng.7b01385.
http://dx.doi.org/10.1021/acssuschemeng....
; Gu et al., 2020GU, Y., YU, L., MOU, J., WU, D., XU, M., ZHOU, P. and REN, Y., 2020. Research strategies to develop environmentally friendly marine antifouling coatings. Marine Drugs, vol. 18, no. 7, pp. 371. http://dx.doi.org/10.3390/md18070371. PMid:32708476.
http://dx.doi.org/10.3390/md18070371...
; Tian et al., 2020TIAN, L., YIN, Y., JIN, H., BING, W., JIN, E., ZHAO, J. and REN, L., 2020. Novel marine antifouling coatings inspired by corals. Materials Today Chemistry, vol. 17, pp. 100294. http://dx.doi.org/10.1016/j.mtchem.2020.100294.
http://dx.doi.org/10.1016/j.mtchem.2020....
). Natural product antifoulants have been identified as a possible substitute to commercial antifoulants (Pan et al., 2019PAN, J., XIE, Q., CHIANG, H., PENG, Q., QIAN, P.-Y., MA, C. and ZHANG, G., 2019. “From the nature for the nature”: an eco-friendly antifouling coating consisting of poly (lactic acid)-based polyurethane and natural antifoulant. ACS Sustainable Chemistry & Engineering, vol. 8, no. 3, pp. 1671-1678. http://dx.doi.org/10.1021/acssuschemeng.9b06917.
http://dx.doi.org/10.1021/acssuschemeng....
). The main objective of this study which is part of a larger, continuing task aimed at identifying marine organisms (marine invertebrates and algae) with the capacity to generate antifouling secondary metabolites is to evaluate the potential of natural product antifoulants derived from seaweeds along the coast of the southwestern Atlantic.
2. Materials and Methods
Many creatures, such as marine seaweeds, ascidians, corals, sponges and others, are thought to generate abundant natural anti-fouling chemicals capable of removing fouling organisms. In this investigation, seaweeds were hand-collected in the subtidal zone's shallow depths. In isothermic boxes, they were delivered to the research facility and were carefully rinsed in saltwater, cleansed of related biota, sorted, and biovolume measured. After that, macroalgae were detected and either freeze-dried to constant dry weight or dried at room temperature. Biomass was the primary criterion for selecting seaweed species, which ought to be adequate for field and laboratory testing, as well as substance purification, if necessary. By soaking dried algae in a combination of dichloromethane and methanol or pure dichloromethane, crude, whole-algal extracts or purified components from fifty-one seaweed assemblages including forty-two species were generated. In a room temperature of rotary evaporator, the mixture was filtered and solvents were removed under decreased pressure. Triplicate extracts of each species were made. Prior to use in bioassays, the remaining was kept at -10oC and weighed.
Seaweeds were stressed before being extracted, with the results compared to control macroalgae to see if defensive induction occurred. The induction of herbivore was carried out similarly as reported in previous studies (Jormalainen and Honkanen, 2008JORMALAINEN, V. and HONKANEN, T., 2008. Macroalgal chemical defenses and their roles in structuring temperate marine communities. In: C.D. AMSLER, ed. Algal chemical ecology. Berlin: Springer, pp. 57–89. http://dx.doi.org/10.1007/978-3-540-74181-7_3.
http://dx.doi.org/10.1007/978-3-540-7418...
; Molis et al., 2008MOLIS, M., KÖRNER, J., KO, Y.W. and KIM, J.H., 2008. Specificity of inducible seaweed anti-herbivory defences depends on identity of macroalgae and herbivores. Marine Ecology Progress Series, vol. 354, pp. 97-105. http://dx.doi.org/10.3354/meps07255.
http://dx.doi.org/10.3354/meps07255...
), with stressed macroalgae being cultivated in the lab for a week and then subjected to amphipod grazing before being extracted. With the exception of herbivores, control macroalgae were cultivated under the identical circumstances. Similarly, at the same time a Cryptonemia seminervis (Red seaweed) was identified both without and with the epibionts, and they were evaluated to see if the existence of epibionts elicited a defensive reaction. The brown mussel was used to evaluate pure chemicals and extracts in laboratory bioassays (Figure 1) (Galvao et al., 2015GALVAO, P., LONGO, R., TORRES, J.P.M. and MALM, O., 2015. Estimating the potential production of the brown mussel Perna perna (Linnaeus, 1758) reared in three tropical bays by different methods of condition indices. Journal of Marine Biology, vol. 2015, pp. 948053. http://dx.doi.org/10.1155/2015/948053.
http://dx.doi.org/10.1155/2015/948053...
; Cortez et al., 2018CORTEZ, F.S., SOUZA, L.S., GUIMARÃES, L.L., ALMEIDA, J.E., PUSCEDDU, F.H., MARANHO, L.A., MOTA, L.G., NOBRE, C.R., MORENO, B.B. and ABESSA, D.M.S., 2018. Ecotoxicological effects of losartan on the brown mussel Perna perna and its occurrence in seawater from Santos Bay (Brazil). The Science of the Total Environment, vol. 637-638, pp. 1363-1371. http://dx.doi.org/10.1016/j.scitotenv.2018.05.069. PMid:29801229.
http://dx.doi.org/10.1016/j.scitotenv.20...
; Lins and Rocha, 2020LINS, D.M. and ROCHA, R.M., 2020. Cultivated brown mussel (Perna perna) size is reduced through the impact of three invasive fouling species in southern Brazil. Aquatic Invasions, vol. 15, no. 1, pp. 114-126. http://dx.doi.org/10.3391/ai.2020.15.1.08.
http://dx.doi.org/10.3391/ai.2020.15.1.0...
).
In an ecological framework, mussels are important fouling organisms since they are typically observed lodged on seaweeds. Throughout low tide, juvenile mussels were retrieved from Itaipu's rocky coast, and placed in a 400-liter activated carbon, protein skimming, and biologically filtered recirculating laboratory aquarium for twelve hours at constant aeration, salinity, and temperature. Following that, the mussels were disaggregated with care by severing the byssal threads, and those which were chosen to participate in the research were demonstrating substratum probing activity. A process, which was adapted from Ina et al. (1989)INA, K., TAKASAWA, R., YAGI, A., YAMASHITA, N., ETOH, H. and SAKATA, K., 1989. An improved assay method for antifouling substances using the blue mussel, Mytilus edulis. Agricultural and Biological Chemistry, vol. 53, no. 12, pp. 3319-3321. and Goto et al. (1992)GOTO, R., KADO, R., MURAMOTO, K. and KAMIYA, H., 1992. Fatty acids as antifoulants in a marine sponge. Biofouling, vol. 6, no. 1, pp. 61-68. http://dx.doi.org/10.1080/08927019209386210.
http://dx.doi.org/10.1080/08927019209386...
, was used to assess antifouling activity.
To validate laboratory findings, antifouling effects of extracts/purified chemicals were investigated using more ecologically relevant field testing. The approach used has been tweaked, and it offers several benefits over standard field experiments. To gently diffuse into the water, compounds or extracts are first mixed into a gel (at seaweed's normal volumetric concentrations), much like they do in a live organism. To help retain the gel surface's physical qualities, the extracts are spread throughout the gel. As a result, variations in larval formation could only be linked to the extracts' chemical qualities. Eventually, foulers settle on the test gel under natural diffusion and flow conditions, in the presence of a natural source of algal spores and larvae. In sterile polystyrene Petri dishes, macroalgal extracts were combined with phytagel. In the controls, only solvent was introduced to the phytagel. A solution of 35 ml distilled water heated to boiling point and 1.52 g phytagel was used to make the gel plates. Then, while 0.5 ml of methanol was added to the mixture it was stirred vigorously, then poured into the Petri dishes for hardening. The compound/extract supplied to each 35 ml gel was the same as a 35 ml fresh seaweed extract (the levels of natural metabolite should remain stable). To permit extract diffusion via just one side of the test plates, gels were retained in circular molds (Petri dishes). In comparison to the prior methods, this would theoretically lower diffusion rates by half (Henrikson and Pawlik, 1995HENRIKSON, A.A. and PAWLIK, J.R., 1995. A new antifouling assay method: results from field experiments using extracts of four marine organisms. Journal of Experimental Marine Biology and Ecology, vol. 194, no. 2, pp. 157-165. http://dx.doi.org/10.1016/0022-0981(95)00088-7.
http://dx.doi.org/10.1016/0022-0981(95)0...
).
Between treatments, mussel byssal thread attachment data were compared by employing one-way analyses of variance (ANOVA). Dunnett's post hoc test was used to compare treatments and controls if ANOVA revealed major findings. Non-parametric tests of Kruskal-Wallis were used when the variance homogeneity and normality assumptions did not fulfilled, even after data transformations. When p<0.05, significance differences were considered.
3. Results and Discussion
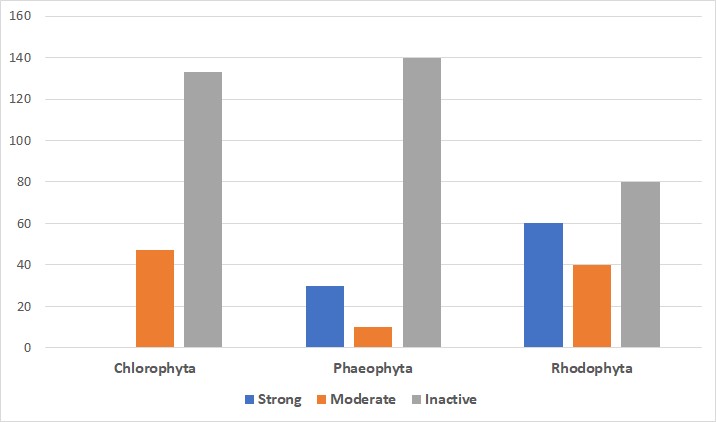
Biofouling will occur on any hard substrate that is immersed in water. The main film, primary colonizers (diatoms and bacteria), secondary colonizers (spores of protozoa and seaweeds), and tertiary colonizers (invertebrates like tunicates, mussels, and barnacles) are all components of biofouling. In this study, under various conditions, a total of 68 pure chemicals or extracts were evaluated from 42 seaweed species. Among them, 24 species belonged to the Rhodophyta family (Red algae), 9 to the Phaeophyta family (Brown algae), and 9 to the Chlorophyta family (Green algae). All of the findings were reported as mussel byssal thread attachment mean percentage inhibition with relation to the corresponding controls. Green macroalgae demonstrated substantial antifouling activity. Brown macroalgae results prevented fouling, and they did so considerably. One purified component and two extracts from one of them, demonstrated potent stimulant action (i.e., mussel attachment was stimulated rather than inhibited). Red algae were the most common kind of algae studied, and antifouling activity was seen in the majority of them (Table 1), with more than half of them having substantial fouling inhibition (Figure 2). The volume of active extracts in red algae was significantly higher (fouling inhibition that is moderate or strong) than in brown algae. Antifouling action in green seaweeds has never been significant (Figure 2). Following herbivore damage (HD)/ non-damaged algae (ND), some degree of induction was shown in 7 of the 9 examined species of seaweed for induced antifouling defense. Rhodophyta (Gracilaria cearensis) and Chlorophyta (Codium decorticatum) were shown to have significant defensive inductions.
To corroborate laboratory results, 13 ecologically relevant field experiments were carried out. Brown macroalgae provided three replies (according to the laboratory data, one stimulates fouling, one has antifouling activity, and one is inert) while red macroalgae (three of them had substantial antifouling action, whereas the other seven were inert) provided 10.
4. Conclusion
The presence of biofouling in marine creatures is undeniable. Barnacles, for example, may adhere to whales, while the blue mussel Mytilus edulis feeds on filamentous algae mostly. Certain marine seaweed species are extremely good at defending themselves against biofouling. In the current study, fouling resistance investigations of sixty-eight pure chemicals or extracts from seaweeds demonstrated some level of fouling inhibition in 26 cases. Sixteen macroalgal extracts, the bulk of which were from red seaweeds, were shown to have strong antifouling activity. Three instances, showed a considerable enhancement of mussel attachment. Fouling inhibition was seen in four out of eleven instances, three of which were rhodophyceans, in field studies to back up laboratory findings. These findings show that antifouling chemical defense is prevalent among macroalgae, at least in the littoral. The fact that none of the test organisms died indicates that fouling inhibition is non-toxic (death statistics aren't shown). Considering the imbalanced quantity of macroalgae evaluated across locations, towards lower latitudes no latitudinal tendency of enhanced antifouling activity, in which it's thought that the fouling pressure is stronger, appears to exist. The idea that tropical seaweeds were more guarded than temperate equivalents was frequently proposed in previous studies, particularly in defense against herbivores, although it has also been contested. According to the previous studies, this is the first investigation to look at a latitudinal gradient in antifouling activity.
References
- ANISIMOV, A.V., MIKHAILOVA, M.A. and UVAROVA, E.A., 2019. Modern approaches to the development of marine antifouling coatings. Inorganic Materials: Applied Research, vol. 10, no. 6, pp. 1384-1389. http://dx.doi.org/10.1134/S2075113319060029
» http://dx.doi.org/10.1134/S2075113319060029 - ARAI, T., HARINO, H., OHJI, M. and LANGSTON, W.J., 2009. Ecotoxicology of antifouling biocides. Tokyo: Springer. http://dx.doi.org/10.1007/978-4-431-85709-9
» http://dx.doi.org/10.1007/978-4-431-85709-9 - BARLETTA, M., AVERSA, C., PIZZI, E., PUOPOLO, M. and VESCO, S., 2018. Design, development and first validation of “biocide-free” anti-fouling coatings. Progress in Organic Coatings, vol. 123, pp. 35-46. http://dx.doi.org/10.1016/j.porgcoat.2018.06.007
» http://dx.doi.org/10.1016/j.porgcoat.2018.06.007 - BAUMGARTNER, V., 2021 [viewed 2022 April 24]. Bryozoan settlement varies by kelp species [online]. Available from: https://digital.lib.washington.edu/researchworks/handle/1773/47159
» https://digital.lib.washington.edu/researchworks/handle/1773/47159 - BIRCHENOUGH, A.C., EVANS, S.M., MOSS, C. and WELCH, R., 2002. Re-colonisation and recovery of populations of dogwhelks Nucella lapillus (L.) on shores formerly subject to severe TBT contamination. Marine Pollution Bulletin, vol. 44, no. 7, pp. 652-659. http://dx.doi.org/10.1016/S0025-326X(01)00308-3 PMid:12222888.
» http://dx.doi.org/10.1016/S0025-326X(01)00308-3 - BUÉ, M., SMALE, D.A., NATANNI, G., MARSHALL, H. and MOORE, P.J., 2020. Multiple-scale interactions structure macroinvertebrate assemblages associated with kelp understory algae. Diversity & Distributions, vol. 26, no. 11, pp. 1551-1565. http://dx.doi.org/10.1111/ddi.13140
» http://dx.doi.org/10.1111/ddi.13140 - CIRIMINNA, R., BRIGHT, F.V. and PAGLIARO, M., 2015. Ecofriendly antifouling marine coatings. ACS Sustainable Chemistry & Engineering, vol. 3, no. 4, pp. 559-565. http://dx.doi.org/10.1021/sc500845n
» http://dx.doi.org/10.1021/sc500845n - CORTEZ, F.S., SOUZA, L.S., GUIMARÃES, L.L., ALMEIDA, J.E., PUSCEDDU, F.H., MARANHO, L.A., MOTA, L.G., NOBRE, C.R., MORENO, B.B. and ABESSA, D.M.S., 2018. Ecotoxicological effects of losartan on the brown mussel Perna perna and its occurrence in seawater from Santos Bay (Brazil). The Science of the Total Environment, vol. 637-638, pp. 1363-1371. http://dx.doi.org/10.1016/j.scitotenv.2018.05.069 PMid:29801229.
» http://dx.doi.org/10.1016/j.scitotenv.2018.05.069 - EL-BELTAGI, H.S., MOHAMED, H.I. and EL-ENAIN, M.M.A., 2019. Role of secondary metabolites from seaweeds in the context of plant development and crop production. In: L. PEREIRA, K. BAHCEVANDZIEV and N. H. HOSHI, eds. Seaweeds as plant fertilizer, agricultural biostimulants and animal fodder. Boca Raton: CRC Press, pp. 64–79. http://dx.doi.org/10.1201/9780429487156-4
» http://dx.doi.org/10.1201/9780429487156-4 - EMERSON, S.E., BROWNS, J.S., WHELAN, C.J. and SCHMIDT, K.A., 2012. Scale-dependent neighborhood effects: shared doom and associational refuge. Oecologia, vol. 168, no. 3, pp. 659-670. http://dx.doi.org/10.1007/s00442-011-2144-4 PMid:21987268.
» http://dx.doi.org/10.1007/s00442-011-2144-4 - GALVAO, P., LONGO, R., TORRES, J.P.M. and MALM, O., 2015. Estimating the potential production of the brown mussel Perna perna (Linnaeus, 1758) reared in three tropical bays by different methods of condition indices. Journal of Marine Biology, vol. 2015, pp. 948053. http://dx.doi.org/10.1155/2015/948053
» http://dx.doi.org/10.1155/2015/948053 - GOTO, R., KADO, R., MURAMOTO, K. and KAMIYA, H., 1992. Fatty acids as antifoulants in a marine sponge. Biofouling, vol. 6, no. 1, pp. 61-68. http://dx.doi.org/10.1080/08927019209386210
» http://dx.doi.org/10.1080/08927019209386210 - GU, Y., YU, L., MOU, J., WU, D., XU, M., ZHOU, P. and REN, Y., 2020. Research strategies to develop environmentally friendly marine antifouling coatings. Marine Drugs, vol. 18, no. 7, pp. 371. http://dx.doi.org/10.3390/md18070371 PMid:32708476.
» http://dx.doi.org/10.3390/md18070371 - HENRIKSON, A.A. and PAWLIK, J.R., 1995. A new antifouling assay method: results from field experiments using extracts of four marine organisms. Journal of Experimental Marine Biology and Ecology, vol. 194, no. 2, pp. 157-165. http://dx.doi.org/10.1016/0022-0981(95)00088-7
» http://dx.doi.org/10.1016/0022-0981(95)00088-7 - HERSTOFF, E.M. and IYENGAR, E.V., 2011. Individuals of Crepidula adunca (Mollusca, Gastropoda) avoid shared doom through host specificity. Journal of Experimental Marine Biology and Ecology, vol. 406, no. 1–2, pp. 79-86. http://dx.doi.org/10.1016/j.jembe.2011.06.007
» http://dx.doi.org/10.1016/j.jembe.2011.06.007 - INA, K., TAKASAWA, R., YAGI, A., YAMASHITA, N., ETOH, H. and SAKATA, K., 1989. An improved assay method for antifouling substances using the blue mussel, Mytilus edulis. Agricultural and Biological Chemistry, vol. 53, no. 12, pp. 3319-3321.
- JORMALAINEN, V. and HONKANEN, T., 2008. Macroalgal chemical defenses and their roles in structuring temperate marine communities. In: C.D. AMSLER, ed. Algal chemical ecology. Berlin: Springer, pp. 57–89. http://dx.doi.org/10.1007/978-3-540-74181-7_3
» http://dx.doi.org/10.1007/978-3-540-74181-7_3 - KONING, J.T., BOLLMANN, U.E. and BESTER, K., 2020. The occurrence of modern organic antifouling biocides in Danish marinas. Marine Pollution Bulletin, vol. 158, pp. 111402. http://dx.doi.org/10.1016/j.marpolbul.2020.111402 PMid:32753187.
» http://dx.doi.org/10.1016/j.marpolbul.2020.111402 - LEONARD, K., HEWITT, C.L., CAMPBELL, M.L., PRIMO, C. and MILLER, S.D., 2017. Epibiotic pressure contributes to biofouling invader success. Scientific Reports, vol. 7, no. 1, pp. 5173. http://dx.doi.org/10.1038/s41598-017-05470-2 PMid:28701736.
» http://dx.doi.org/10.1038/s41598-017-05470-2 - LINS, D.M. and ROCHA, R.M., 2020. Cultivated brown mussel (Perna perna) size is reduced through the impact of three invasive fouling species in southern Brazil. Aquatic Invasions, vol. 15, no. 1, pp. 114-126. http://dx.doi.org/10.3391/ai.2020.15.1.08
» http://dx.doi.org/10.3391/ai.2020.15.1.08 - MA, C., ZHANG, W., ZHANG, G. and QIAN, P., 2017. Environmentally friendly antifouling coatings based on biodegradable polymer and natural antifoulant. ACS Sustainable Chemistry & Engineering, vol. 5, no. 7, pp. 6304-6309. http://dx.doi.org/10.1021/acssuschemeng.7b01385
» http://dx.doi.org/10.1021/acssuschemeng.7b01385 - MACHADO, G.B., FERREIRA, A.P. and LEITE, F.P., 2019. Testing the importance of predation refuge vs. food quality in determining the use of macroalgal hosts by a generalist marine mesograzer. Marine Biology, vol. 166, no. 5, pp. 1-12. http://dx.doi.org/10.1007/s00227-019-3502-8
» http://dx.doi.org/10.1007/s00227-019-3502-8 - MANOJ, S., MAHESH, S. and SRIKANTH, N., 2018. Review of biofouling paints on the marine vessel. In: Asian Conference on Energy, Power and Transportation Electrification (ACEPT), 2018, Singapore. New York: IEEE, p. 1-6. http://dx.doi.org/10.1109/ACEPT.2018.8610796
» http://dx.doi.org/10.1109/ACEPT.2018.8610796 - MOLIS, M., KÖRNER, J., KO, Y.W. and KIM, J.H., 2008. Specificity of inducible seaweed anti-herbivory defences depends on identity of macroalgae and herbivores. Marine Ecology Progress Series, vol. 354, pp. 97-105. http://dx.doi.org/10.3354/meps07255
» http://dx.doi.org/10.3354/meps07255 - NWUZOR, I.C., IDUMAH, C.I., NWANONENYI, S.C. and EZEANI, O.E., 2021. Emerging trends in self-polishing anti-fouling coatings for marine environment. Safety in Extreme Environments, vol. 3, no. 1, pp. 9-25. http://dx.doi.org/10.1007/s42797-021-00031-3
» http://dx.doi.org/10.1007/s42797-021-00031-3 - PAN, J., XIE, Q., CHIANG, H., PENG, Q., QIAN, P.-Y., MA, C. and ZHANG, G., 2019. “From the nature for the nature”: an eco-friendly antifouling coating consisting of poly (lactic acid)-based polyurethane and natural antifoulant. ACS Sustainable Chemistry & Engineering, vol. 8, no. 3, pp. 1671-1678. http://dx.doi.org/10.1021/acssuschemeng.9b06917
» http://dx.doi.org/10.1021/acssuschemeng.9b06917 - PAUL, V.J., 2019. Ecological roles of marine natural products Ithaca: Cornell University Press. Chemical defenses of benthic marine invertebrates, pp. 164–188.
- RAF, M., DE BAERE KRIS, P.G. and REMKE, W., 2018. Development of a test platform for anti-fouling coatings. In: International Symposium on Corrosion and Fouling Antwerpen: Antwerp Maritime Academy, p. 132.
- ROCHA, D.H., SECA, A.M. and PINTO, D.C., 2018. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Marine Drugs, vol. 16, no. 11, pp. 410. http://dx.doi.org/10.3390/md16110410 PMid:30373208.
» http://dx.doi.org/10.3390/md16110410 - ROSA, G.P., TAVARES, W.R., SOUSA, P.M.C., PAGÈS, A.K., SECA, A.M.L. and PINTO, D.C.G.A., 2019. Seaweed secondary metabolites with beneficial health effects: an overview of successes in in vivo studies and clinical trials. Marine Drugs, vol. 18, no. 1, pp. 8. http://dx.doi.org/10.3390/md18010008 PMid:31861879.
» http://dx.doi.org/10.3390/md18010008 - SALAMA, A.J., SATHEESH, S. and BALQADI, A.A., 2018. Antifouling activities of methanolic extracts of three macroalgal species from the Red Sea. Journal of Applied Phycology, vol. 30, no. 3, pp. 1943-1953. http://dx.doi.org/10.1007/s10811-017-1345-6
» http://dx.doi.org/10.1007/s10811-017-1345-6 - SANTOS, A. and MAYORAL, E., 2006. Bioerosive structures of sclerozoan foraminifera from the Lower Pliocene of southern Spain: a contribution to the palaeoecology of marine hard substrate communities. Palaeontology, vol. 49, no. 4, pp. 719-732. http://dx.doi.org/10.1111/j.1475-4983.2006.00560.x
» http://dx.doi.org/10.1111/j.1475-4983.2006.00560.x - TIAN, L., YIN, Y., JIN, H., BING, W., JIN, E., ZHAO, J. and REN, L., 2020. Novel marine antifouling coatings inspired by corals. Materials Today Chemistry, vol. 17, pp. 100294. http://dx.doi.org/10.1016/j.mtchem.2020.100294
» http://dx.doi.org/10.1016/j.mtchem.2020.100294 - VOULVOULIS, N., SCRIMSHAW, M.D. and LESTER, J.N., 2002. Comparative environmental assessment of biocides used in antifouling paints. Chemosphere, vol. 47, no. 7, pp. 789-795. http://dx.doi.org/10.1016/S0045-6535(01)00336-8 PMid:12079074.
» http://dx.doi.org/10.1016/S0045-6535(01)00336-8 - WANG, S., WEINBERGER, F. and LENZ, M., 2018. Fluctuations in the strength of chemical antifouling defenses in a red macroalga in response to variations in epibiont colonization pressure. Marine Biology, vol. 165, no. 6, pp. 1-14. http://dx.doi.org/10.1007/s00227-018-3365-4
» http://dx.doi.org/10.1007/s00227-018-3365-4 - ZATOŃ, M., ZAPALSKI, M.K., BERKOWSKI, B. and WRZOŁEK, T., 2018. Cryptic encrusting communities in a Middle Devonian mesophotic paleoenvironment of the Holy Cross Mountains, Poland. Palaeogeography, Palaeoclimatology, Palaeoecology, vol. 501, pp. 82-91. http://dx.doi.org/10.1016/j.palaeo.2018.04.015
» http://dx.doi.org/10.1016/j.palaeo.2018.04.015
Publication Dates
-
Publication in this collection
04 Nov 2022 -
Date of issue
2024
History
-
Received
24 Apr 2022 -
Accepted
05 June 2022