Abstracts
This work provides new information on agelasid sponges found on the continental shelf off northern Brazil. Agelas sceptrum (Lamarck, 1815) and Agelas wiedenmayeri Alcolado, 1984 have their first record for the Brazilian coast. Agelas dispar Duchassaing & Michelotti, 1864 and Agelas schmidti Wilson, 1902, previously recorded from Brazil, are cited for the first time off the mouth of the Amazon River.
Porifera; Agelas; off Amazon River mouth; Brazil; taxonomy
Este trabalho fornece novas informações sobre esponjas agelasidas encontradas na costa norte da plataforma continental brasileira. Agelas sceptrum (Lamarck, 1815) e Agelas wiedenmayeri Alcolado, 1984 têm seu primeiro registro para a costa brasileira. Agelas dispar Duchassaing & Michelotti, 1864 e Agelas schmidti Wilson, 1902, registradas anteriormente na costa brasileira, são citadas pela primeira vez ao largo da desembocadura do Rio Amazonas.
Porifera; Agelas; Desembocadura do Rio Amazonas; Brasil; Taxonomia
ARTICLES
New records of the genus Agelas Duchassaing & Michelotti, 1864 (Porifera, Agelasida) off the Amazon River mouth, Brazil, Southwestern Atlantic
Novos registros do gênero Agelas Duchassaing & Michelotti, 1864 (Porifera, Agelasida) ao largo da desembocadura do Rio Amazonas, Brasil, Atlântico Sudoeste
Beatriz MothesI, 1 1 Corresponding author: Beatriz Mothes, e-mail: bmothes@fbz.rs.gov.br ; Maurício CamposI; Cléa LernerI; João Luís CarraroII; Fernando José Parra-VelandiaIII
IMuseu de Ciências Naturais MCN, Fundação Zoobotânica do Rio Grande do Sul, Rua Dr. Salvador França 1427, Bairro Jardim Botânico, CEP 90690-000, Porto Alegre, RS, Brazil, http://www.fzb.rs.gov.br/museu
IIPrograma de Pós-Graduação em Ecologia, Universidade Federal do Rio Grande Do Sul UFRGS Av. Bento Gonçalves 9500 prédio 43435, Bairro Agronomia, CEP 91501- 970, Porto Alegre, RS, Brazil, www.ufrgs.br/ecologia
IIIDepartamento de Biologia y Centro de Estudios en Ciencias del Mar, Instituto de Investigaciones Marinas INVEMAR, Universidad Nacional de Colombia Cerro Punta de Betín, Apartado aéreo 1016 y 873, Santa Marta, Colombia
ABSTRACT
This work provides new information on agelasid sponges found on the continental shelf off northern Brazil. Agelas sceptrum (Lamarck, 1815) and Agelas wiedenmayeri Alcolado, 1984 have their first record for the Brazilian coast. Agelas dispar Duchassaing & Michelotti, 1864 and Agelas schmidti Wilson, 1902, previously recorded from Brazil, are cited for the first time off the mouth of the Amazon River.
Keywords: Porifera, Agelas, off Amazon River mouth, Brazil, taxonomy.
RESUMO
Este trabalho fornece novas informações sobre esponjas agelasidas encontradas na costa norte da plataforma continental brasileira. Agelas sceptrum (Lamarck, 1815) e Agelas wiedenmayeri Alcolado, 1984 têm seu primeiro registro para a costa brasileira. Agelas dispar Duchassaing & Michelotti, 1864 e Agelas schmidti Wilson, 1902, registradas anteriormente na costa brasileira, são citadas pela primeira vez ao largo da desembocadura do Rio Amazonas.
Palavras-chave: Porifera, Agelas, Desembocadura do Rio Amazonas, Brasil, Taxonomia.
Introduction
The taxonomy of the genus Agelas is difficult to elucidate because of the small number of comparative morphological characters. The presence of only one kind of spicule (acanthostyles; acanthoxeas sometimes present) and wide variability in color and habit characterize a sponge group with possible intra-specific variation.
Members of Agelas are typically found in tropical waters, with their highest diversity in the Caribbean Sea, followed by the Indo-Pacific Ocean and the Mediterranean Sea. In the Caribbean they are important components of reef communities. Sponges of this genus have potential economic value due to their pronounced bioactive properties (Assmann et al. 2001). Records of Agelas from the Brazilian coast were given by Johnson (1971), Boury-Esnault (1973), Hechtel (1976), Solé-Cava et al. (1981), Collette & Rützler (1977), Muricy & Moraes (1998) and Muricy et al. (2006). Our results provided two new records for the Brazilian coast and new occurrences of Agelas dispar Duchassaing & Michelotti, 1864 and Agelas schmidti Wilson, 1902 for the area. With the new records, the southern limits of distribution of Agelas sceptrum (Lamarck, 1815), Agelas schmidti Wilson, 1902 and Agelas wiedenmayeri Alcolado, 1984 are extended to the southwestern Atlantic. This study contributes to knowledge of the diversity of sponges of one of the least-studied areas off the Brazilian coast.
Material and Methods
The samples were dredged from depths between 25 and 100 m, off the states of Pará to Maranhão (Figura 1), during several oceanographic expeditions carried out to inventory the biological resources and to characterize the environment [e.g., Diretoria de Hidrografia e Navegação da Marinha do Brasil (DHNM - Comissão Pesca Norte I), Superintendência de Desenvolvimento do Nordeste (SUDENE - Comissão Maranhão), and Programa de Avaliação do Potencial de Recursos Vivos da Zona Econômica Exclusiva (REVIZEE - Score Norte II, III and IV)].
Spicule mounts and thick sections were made according to methods of Mothes-De-Moraes (1978) and Mothes et al. (2004a), respectively. Preparations for Scanning Electron Microscopy (SEM) study followed the procedures described in Silva & Mothes (1996). Spicule measurements comprised minimum, mean, and maximum sizes in micrometers (mµ), from n = 50 spicules except when indicated. When N < 20 only the minimum and maximum are indicated. The microspines were included in the spicule width measurements. Measurements of fibres and meshes are of the diameter. The samples were preserved in 96° ethyl alcohol and deposited in the collection of the Museu de Ciências Naturais - Porifera Collection, Fundação Zoobotânica do Rio Grande do Sul, Porto Alegre, RS, Brazil (MCNPOR).
Abbreviations used: ZMA, Zoölogisch Museum Amsterdam, Amsterdam, Netherlands; INVEMAR, Instituto de Investigaciones Marinas y Costeras, Santa Marta, Colombia; INV-POR, Porifera collection, Instituto de Investigaciones Marinas de Punta Betín, Colombia; IdO, Instituto de Oceanología de la Academia de Ciencias de Cuba, La Habana, Cuba; MCNPOR, Porifera collection, Museu de Ciências Naturais da Fundação Zoobotânica do Rio Grande do Sul, Brazil.
Characterization of the study area
The study area is located off the northern coast of Brazil, comprising a geographical area from the Oiapoque River, state of Amapá, to the Parnaíba River, state of Piauí (sampling was concentrated off the Amazon River mouth, from Pará to Maranhão State). In this region the continental shelf is wide, especially near the Amazon River where it extends up to 150 miles, with a slight slope at the north and an abrupt slope at the northeast (Coutinho & Morais 1970). The ocean current system in the Amazon Gulf is complex (Mothes et al. 2004b). The region lies in the equatorial zone, with warm surface waters (~27° C) and salinities of 36-38% (Ottman 1959, Vannucci 1964). The surface sediments of the continental margin from Cabo Orange to the Parnaíba Delta are composed of terrestrial sediments on the inner and middle shelf, which change to biodetritic sediment toward the shelf break (Martins & Coutinho 1981).
The continental shelf off the state of Maranhão has a sandy bottom, which increases at northwest from the mouth of the Parnaíba River, with the highest content off Pará. Only the sandy and biodetritic facies are quantitatively important; the presence of mud is limited to isolated spots, due to the influence of the coastal rivers. Organogenic bottoms cover an extensive area of the Brazilian continental shelf between the states of Pará and Rio de Janeiro, with a calcareous-algae facies which is mainly comprised of living algae, which form isolated reefs of various sizes. At the northern limit of the shelf, living calcareous algae are dominant, with large numbers and many forms, mainly encrusting algae (Luna 1979). The abundant presence of hard substratum off the mouth of the Amazon River was also corroborated by Collette & Rützler (1977).
Results
Agelasidae
1. Agelas sceptrum (Lamarck, 1815)
Alcyonium sceptrum Lamarck, 1815:163 (type locality: unknown).
Agelas sceptrum; Van Soest & Stentoft 1988:100; Lehnert 1993:50, figs. 23,87-88; Lehnert & Van Soest 1998:81; Lehnert & Van Soest 1999:155.
Further synonymy: see Zea (1987).
Material examined: BRAZIL, Maranhão: 00° 51' 44" S and 44° 21' 24" W, 100 m, unregistered substratum, coll R/V "Almirante Saldanha",13.XI.1997, REVIZEE - II (MCNPOR 3842); 00° 22' 00" N and 44° 52' 00" W, 72 m, unregistered substratum, coll R/V "Almirante Saldanha", 18.VII.2001, REVIZEE - IV (MCNPOR 5347).
Description: (Figure 2) Ramose sponge. Surface irregular with meandering invaginations, hispid to the touch. Round oscules slightly aligned (the greater oscule observed has 0.3 cm), some obstructed by a fine translucent membrane. Pores not observed. Preserved material very elastic, extremely difficult to cut; coloration brown. Dimensions of the largest specimen (MCNPOR 5347), in cm: 33 length, 2.0 width and 1.0 diameter.
Skeleton: (Figure 3) Ectosome consisting of a fine, very characteristic layer of the genus, made by brushes of spicules, which protrudes in the surface. Choanosome with primary ascending echinated fibres, multispicularly cored by 08-12 spicules per cross section, interconnected by secondary fibres, uncored or rarely cored by one spicule; the fibre system usually present rounded or polygonal meshes.
Spicules: (Figure 4) Acanthostyles slightly curved or sometimes straight; Acanthoxeas rare. Dimensions in Table 1.
Remarks: The specific identification was based on slides of Agelas sceptrum (Lamarck, 1815), collected on Pear Tree Bottom, Discovery Bay, Jamaica (INV-POR 1005). Spicules of the studied material are very similar to the Caribbean samples. In regard to the mesh sizes, the examined specimens shown a smaller size than the registered for Caribbean material (Lehnert & Van Soest 1999). This difference can be related to the contracted fibres in fixed specimens.
Geographic distribution: Tropical Western Atlantic: from the Archipelago of the Bahamas to Colombia (Zea 1987); Brazil: Maranhão (first record).
Bathymetric distribution: From 12 m, Isla Tesoro, Colombia (Zea 1987) to 150 m, Barbados (Lewis 1965).
2. Agelas dispar Duchassaing & Michelotti, 1864
Agelas dispar Duchassaing & Michelotti, 1864:76 (type locality: St. Martin, Caribbean); Boury-Esnault 1973:285, fig. 45, pl. 1, fig. 8; Solé-Cava et al. 1981:131 fig. 7; Zea 1987:207, fig.75, pl. 12, fig. 8; Lehnert 1993:50, figs. 22, 79, 80-82; Muricy & Moraes 1998: 215; Lehnert & Van Soest 1998:81; Lehnert &.Van Soest 1999:154; Muricy et al. 2006:116
Further synonymy: see Wiedenmayer (1977) and Zea (1987).
Material examined: BRAZIL Pará: 00° 26'00" S - 47° 35' 05" W, 24-25 m, sandy substratum, coll. R/V "Almirante Saldanha", 21.XI.1968, Comissão Pesca Norte I, (MCNPOR 1872); Maranhão: 01° 55' 00" S and 42° 44' 00" W, 63 m, biodetritic substratum, coll. Fishing boat "IV SUDENE", 18.II.1973, Comissão Maranhão, (MCNPOR 1848).
Description: (Figure 5) Round-shaped fragment. Surface microconulose, smooth to the touch. Oscules round irregularly distributed on the surface (the largest oscule observed 0.3 cm), some covered by pinacoderm. Pores not observed. Preserved material compressible difficult to cut; coloration brown. Dimensions of the largest specimen (MCNPOR 1872), in cm: length 4.0, width 2.0 and diameter 1.0.
Skeleton: (Figure 6) Ectosome consisting of fine dermic membrane supported by brushes of spicules disposed in primary fibres. Choanosome with ascending echinated primary fibres cored by 01-05 spicules per cross section; secondary fibres commonly uncored, interconnecting primary fibres and forming rectangular or rounded meshes.
Spicules: (Figure 7) Acanthostyles straight or slightly curved. Dimensions in Table 2.
Remarks: The studied specimens were identified based on a comparative analysis with the schizoholotype's slides of Agelas dispar Duchassaing & Michelotti, 1864 (MCNPOR 2635) (Holotype ZMAPOR 607, Saint Martin) and material collected in Carrie Bow Cay, Belize (INV-POR 953). Spicules of the schizoholotype are similar to those of the studied material while those of Belize are much more robust. In the studied material acanthoxeas have not been observed. The occurrence of such spicules in Agelas seems to be rare. Spicules more robust had been recorded by Solé-Cava et al. (1981) in specimens collected more to the south in the Brazilian coast, in the infralittoral of Guarapari, Espirito Santo.
Geographic distribution: Tropical Western Atlantic: From Bahamas to Colombia (Wiedenmayer 1977, Zea 1987). Brazil: Pará and Maranhão (first records); Fernando de Noronha; Pernambuco and Bahia (Boury-Esnault 1973); Espírito Santo (Solé-Cava et al. 1981, Muricy et al. 2006); Rio de Janeiro, Brazil (Muricy et al. 2006).
Bathymetric distribution: From 1 m, Rosário Islands, Colombia (Zea 1987) to 270 m, Rio de Janeiro, Brazil (Muricy et al. 2006).
3. Agelas schmidti Wilson, 1902
Agelas schmidti Wilson, 1902:398 (type locality: Virgin Islands, Caribbean); Zea, 1987:210, fig. 9, 76; Van Soest & Stentoft 1988:102, fig. 50; Lehnert & Van Soest 1998:81; Lehnert &.Van Soest 1999:156; Muricy et al. 2006:116 [non A. schmidti Wiedenmayer, 1977 = A. wiedenmayeri Alcolado, 1984].
Material examined: BRAZIL, Maranhão: 02° 14.21' 49" S and 42° 00.12' 20" W, 72 m, unregistered substratum, 06.XII.1997 REVIZEE - II (MCNPOR 3789); 02° 12" 00" S - 41° 48' 00" W, 56 m, biodetritic substratum, coll. Fishing boat "IV SUDENE",15.II.1973 Comissão Maranhão, (MCNPOR 1833).
Description: (Figure 8) Sponge massive-lobate, irregular, apparently repent. Surface slightly microconulose, smooth to the touch. Oscules randomly distributed all over the surface, some of them linearly positioned and/or forming areas with more concentration (largest oscule observed 0.4 cm), rarely obstructed by the pinacoderm. Pores not observed. Preserved material compressible, elastic, difficult to cut; variable coloration: rose, dark-yellow or purple. Dimensions of a branch of the largest specimen (MCNPOR 3789), in cm: length 10.5, width 3.0 and diameter 1.6.
Skeleton: (Figure 9) Ectosome similar to the previous species, formed by an organic dermis supported by brushes of choanosomal primary fibres with approximately 11 spicules. Choanosome with primary fibres cored by several spicules, connected by uncored secondary fibres or with a great number of echinating spicules in the inner portion of the rounded or polygonal meshes.
Spicules: (Figure 10) Acanthostyles: straight, some slightly curved; acanthoxeas rare. Dimensions in Table 3.
Remarks: The identification of the studied material is based on the slides of Agelas schmidti Wilson, 1902, from Santa Marta, Colombia (INV-POR 989). Acanthostyles of Caribbean specimens are slightly larger (Zea 1987; Van Soest & Stentoft 1988). However, the number of verticilles and thorns are similar to those of the studied material.
Geographic distribution: Tropical Western Atlantic: from Virgin Islands to Barbados (Van Soest & Stentoft 1988). Brazil: Maranhão (first record); Espírito Santo and Rio de Janeiro, Brazil (Muricy et al. 2006).
Bathymetric distribution. From 6 m, Cartagena, Colombia (Zea 1987) to 153 m, Paynes Bay, Barbados (Van Soest & Stentoft 1988).
4. Agelas wiedenmayeri Alcolado, 1984
Agelas wiedenmayeri Alcolado, 1984:11, figs 7-B-C, 8B (type locality: Barlovento, Havana, Cuba).
Agelas schmidti; Wiedenmayer 1977:129, fig. 137, lam. 27, fig. 1. non Agelas schmidti Wilson, 1902 (a valid species)
Material examined: BRAZIL, Maranhão: 01° 34' 36" S and 3° 15' 57" W, 60 m, unregistered substratum, 17.VI.1999 REVIZEE III (MCNPOR 4808); 00° 35' 12" S - 43° 20' 55" W, 94 m, unregistered substratum, 14.VI.1999 REVIZEE III (MCNPOR 4978).
Description: (Figure 11) Sponge ramose slightly conical at the apex. Dimensions of the largest specimen (MCNPOR 4978), length 53; width 3.0 and diameter 2.5 cm. Surface irregular, smooth to the touch. Circular openings, oscules or pores (diameter: 0.2-0.4 cm) randomly distributed on the surface; rarely in a linear pattern, some of them obstructed by the pinacoderm. Preserved material very compressible, however difficult to cut; external coloration dark purple, with some brown areas.
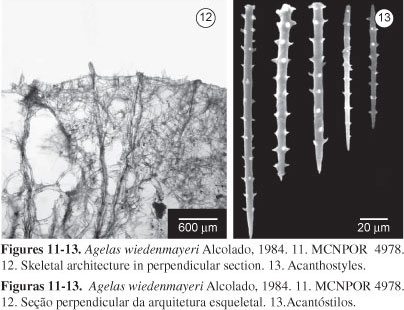
Skeleton: (Figure 12) Ectosome as a thin layer, without specialization, supported by spicule brushes which protrude from the choanosome. Choanosome formed by multispicular primary fibres, up to 8 spicules per section cross, some spicules in echinating arrangement. At the surface, spicules protrude in brushes; primary fibres connected by secondary fibres, cored by few spicules, in general bearing echinating spicules, forming rounded or polygonal meshes. Echinating spicules of secondary fibres, near to the sponge surface, also protrude in small brushes with 2-3 spicules; some regions bear isolated protruding spicules, regularly organized, with the appearance of a discrete palisade.
Spicules: (Figure 13) Acanthostyles: straight or slightly curved to bent; acanthoxeas rare. Dimensions in Table 4.
Remarks: Identification of the studied material was based on the material of Agelas wiedenmayeri Alcolado, 1984 (Holotype IdO-409, schizoholotype deposited in MCNPOR 7375), and on INV-POR 978, San Andres Island, Colombia. Caribbean specimens have thicker spicules and more thorns per verticil.
Geographic distribution: Tropical western Atlantic: Bahamas (Wiedenmayer 1977); Cuba (Alcolado 1984); Belize; San Andres Islands, Rosario Islands and Santa Marta (Colombia) (F.J. Parra unpublished data); Brazil: Maranhão (new record).
Bathymetric distribution: From 13 m, Barlovento, Cuba (Alcolado 1984) to 94 m, Maranhão, Brazil (new record).
General Remarks
The spicule widths in the material studied are smaller than in Caribbean species. This difference may be influenced by dissolved silica in the environment. As Jones (1979) noted, silica availability is one of the factors that affects the growth of spicules. The Brazilian species were collected off the coast, where the amounts of silica tend to be smaller, which would result in narrower spicules. Zea (1987) also observed small spicules in samples collected off Colombian islands, in contrast to those present in closed areas influenced by discharges of the great rivers on the continental coast of Colombia and Central America. At these locations we find larger spicules, because in these areas the dissolved silica content is higher. Similarly, wider spicules of A. dispar were reported by Solé-Cava et al. (1981), working with material collected at Guaraparí, state of Espírito Santo, southeastern Brazilian coast (128-167-188 / 8.5-11.3-18.1 µm); these wider spicules probably resulted from the higher silica quantities.
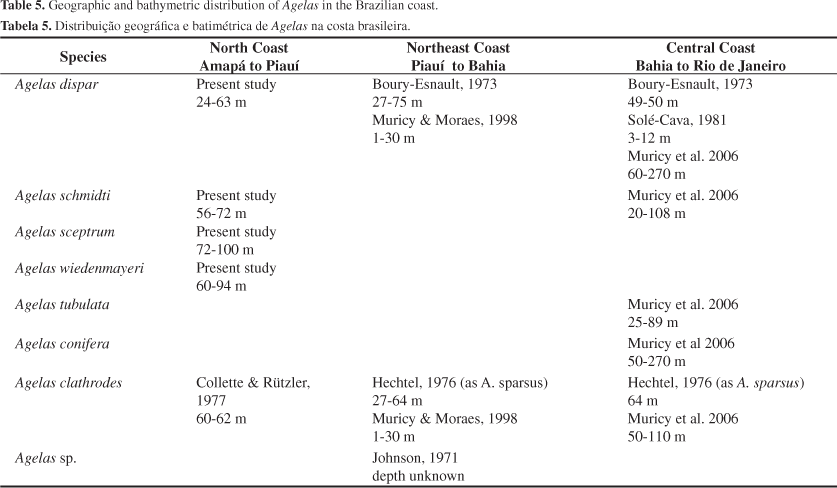
Substrata and depth range, where the species were collected, are also more diverse compared to observations for Caribbean specimens. In the Caribbean, most species of Agelas occur in coral reefs and in shallow waters, up to 50 m (Wiendenmayer 1977; Alcolado 1984; Zea 1987; Lehnert & Van Soest 1998, 1999), except for A. schmidti, which is common in deeper waters, around 100-153 m (Van Soest & Stentoft 1988). Off the Brazilian coast there are records from 50 m down to 270 m, such as for A. dispar and A. conifera (Schmidt, 1870) (Table 5). In the Caribbean the environment is characterized by coral reefs, where sponges occur up to edge of the reef slope (Zea 1987). In the deeper waters off the Brazilian coast, there is a bioclastic granule bottom composed of calcareous algae, which creates free spaces for the sponges to attach. This kind of bottom occurs over the middle and outer shelf off Brazil, most commonly deeper than 50 m (Diaz 2000). The richness of species of Agelas in the northern and central region is practically the same (Table 5), except for Caribbean species such as A. wiedenmayeri and A. sceptrum, which until recently were not recorded for the Brazilian central region (Muricy et al. 2006). Agelas conifera and A. tubulata Lehnert & Van Soest, 1996 (have not yet been recorded off the north Brazilian coast (Collette & Rützler 1997; present study).
Acknowledgments
The authors are grateful to: Dr. Sven Zea (INV), Dr. Pedro Alcolado, (IdO) and Dr. Rob Van Soest (ZMA) for the loan of comparative material and for the valuable commentaries; M. Sc. Marlúcia Maria Ferreira Correia (Laboratório de Hidrobiologia, Universidade Federal do Maranhão, Brazil) and Prof. José Audísio Costa Luna (Universidade Federal de Pernambuco, Brazil) for the concession of the samples from respectively REVIZEE-Norte and "Comissão Norte" and "Comissão Maranhão"; Prof. Francisco José Kiss (Universidade Federal do Rio Grande do Sul, Brazil), for making the SEM photos, and two anonymous reviewers for their insightful suggestions which significantly improved the manuscript. We also thank FAPERGS, CAPES and CNPq (Brazil) for research grants. F. Parra-Velandia was founded by grants from Colombian Science Fund-COLCIENCIAS (1101-09-11241), from the Netherlands Foundation for the Advancement of Tropical Research WOTRO (WB 82-261), and matching funds from Universidad Nacional de Colombia - División de Investigaciones Sede Bogotá (DIB) and Universiteit van Amsterdam-IBED.
Data Received 11/01/07
Revised 27/07/07
Published 01/09/07
ISSN 1676-0603.
- ALCOLADO, P.M. 1984. Nuevas especies de esponjas encontradas en Cuba. Poeyana 271:11-12.
- ASSMANN, M., VAN SOEST, R.W.M. & KÖCH, M. 2001. Description of Agelas cerebrum, a new species and re-description of A. dilatata (Porifera). Proc. biol. Soc. Wash. 114(2):359-366.
- BOURY-ESNAULT, N. 1973. Campagne de la "Calypso" au large des côtes Atlantiques de L'Amérique du Sud (1961-1962). I. Résultats scientifiques des Campagnes de la "Calypso" 10(29):263-295.
- COLLETTE, B. & RÜTZLER, K. 1977. Reef fishes over sponge bottoms off the mouth of the Amazon River. Proc. Third Int. Coral Reef Symp. 1:305-310.
- COUTINHO, P.N. & MORAIS, J.O. 1970. Distribuición de los sedimentos en la plataforma continental norte-nordeste del Brasil. Arq. Cienc. Mar 10(1):79-90.
- DIAZ, G.T.M. 2000. Granulados bioclásticos - Algas calcárias. Rev. Bras. Geof. 18(3):307-318.
- DUCHASSAING DE FONBRESSIN, P. & MICHELOTTI, G. 1864. Spongiaires de la mer Caraïbe. Nat. Verh. Holl. Maatsch. Wetensch. Haarlem 21(2):1-124.
- HECHTEL, G.J. 1976. Zoogeography of Brazilian marine Demospongiae. In Aspects of Sponge Biology (F.W. Harrison & R.R. Cowden, eds). Academic Press, New York, p. 237-260.
- JOHNSON, M.F. 1971. Some marine sponges of northeast Brazil. Arq. Cienc. Mar 11(2):103-116.
- JONES, W.C. 1979. The microstructure and genesis of sponge biominerals. In Biologie des Spongiaires (C. Lévi & N. Boury-Esnault, eds). Colloques Internationaux du C.N.R.S. 291, Paris, p. 425-447.
- LAMARCK, J.B. 1815. Suite des polypiers empâtés. Mém. Mus. Hist. Nat. 1:69-80, 162-168, 432-458.
- LEHNERT, H. 1993. The sponges from Cozumel (Mexico). Inventory, critical comparison of taxonomic characters and description of a new species. Acta biol. Benrodis 5:35-127.
- LEHNERT, H. & VAN SOEST, R.W.M. 1998. Shallow water sponges of Jamaica. Beaufortia 48(5):71-103.
- LEHNERT, H. & VAN SOEST, R.W.M. 1999. More north Jamaica deep fore-reef sponges. Beaufortia 49(12):141-169.
- LEWIS, J.B. 1965. A preliminary description of some marine benthic communities of Barbados, West Indies. Can. J. Zool. 43:1049-1074.
- LUNA, J.A.C. 1979. Plataforma continental do Estado do Maranhão. Operação pesquisador IV. I. Nota sobre a natureza do fundo. Trab. Oceanogr. Univ. Fed. PE 14:7-20.
- MARTINS, L.R. & COUTINHO, P.N. 1981. The Brazilian continental margin. Earth Sci. Rev. 17:87-107.
- MOTHES-DE-MORAES, B. 1978. Esponjas tetraxonidas do litoral sul-brasileiro: II. Material coletado pelo N/Oc. "Prof. W. Besnard" durante o Programa RS. Bol. Inst. Oceanogr. 27(2):57-78.
- MOTHES, B., CAMPOS, M.A., LERNER, C. & FERREIRA-CORREIA, M.M. 2004a. Esponjas (Demospongiae, Halichondrida) da costa do Maranhão, Brasil. Iheringia, Sér. Zool. 94(2):149-154.
- MOTHES, B., CAMPOS, M., LERNER, C., CARRARO, J. L. & VAN SOEST, R. W. M. 2004b. A new species of Biemna Gray, 1867 (Demospongiae, Poecilosclerida) from the north coast of Brazil. Zootaxa 1087:39-44.
- MURICY, G. & MORAES, F.C. 1998. Marine sponges of Pernambuco State, NE Brazil. Rev. Bras. Oceanog. 46(2):213-217.
- MURICY, G., SANTOS, C.P., BAPTISTA, D., LOPES, D.A., PAGNOCELLI, D., MONTEIRO, L.C., OLIVEIRA, M.V., MOREIRA, M.C.F., CARVALHO, M.S., MELÃO, M., KLAUTAU, M., DOMINGUEZ, P.R., COSTA, R.N., SILVANO, R.G., SCHWIENTEK, S., RIBEIRO, S.M., PINHEIRO, U.S. & HAJDU, E. 2006. Filo Porifera. In Biodiversidade Bentônica da região central da Zona Econômica Exclusiva brasileira (H.P. Lavrado & B.L. Ignacio, eds.). Museu Nacional, Rio de Janeiro, p. 109-145.
- OTTMAN, F. 1959. Estudo das amostras do fundo recolhidas pelo N.E. "Almirante Saldanha", na região da embocadura do Rio Amazonas. Trab. Instit. Biol. Marít. Oceanogr. Univ. Recife 1(1):77-106.
- SILVA, C.M.M. & MOTHES, B. 1996. SEM analysis: an important instrument in the study of marine sponges biodiversity. Acta Microsc. 5(B):188-189.
- SOLÉ-CAVA, A.M., KELECOM, A. & KANNENGIESSER, G.J. 1981. Study of some sponges (Porifera, Demospongiae) from the infralitoral of Guarapari, Espírito Santo, Brazil. Iheringia, Sér. Zool. 60:125-150.
- VAN SOEST, R.W.M. & STENTOFT, N. 1988. Barbados deep water sponges. Stud. Fauna Curaçao Caribb. Isl. 70:1-175.
- VANNUCCI, M. 1964. Zoogeografía Marinha do Brasil. Bol. Inst. Biol. Mar. 7:113-121.
- WIEDENMAYER, F. 1977. A monograph of the shallow-water sponges of the Western Bahamas. Experientia, Suppl. 28:1-287.
- WILSON, H.V. 1902. The sponges collected in Porto Rico in 1899 by the U.S. Fish Commission Steatner 'Fish Hawk'. Bull. U.S. Fish. Comm. For 1900 2:375-411.
- ZEA, S. 1987. Esponjas del Caribe Colombiano. INVEMAR, Bogotá.
Publication Dates
-
Publication in this collection
11 Mar 2008 -
Date of issue
2007
History
-
Received
11 Jan 2007 -
Reviewed
27 July 2007 -
Accepted
01 Sept 2007