Abstract:
A total of 1,471 specimens of 16 species of flatfishes (Pleuronectiformes) were caught during 48 sampling campaigns between July 2005 and June 2007 at ten stations in Guanabara Bay, Rio de Janeiro, Brazil. Paralichthyidae was the dominant family, with Etropus crossotus as the dominant species. The outer stations, especially those on the western side of the lower estuary, were distinguished as a result of their higher abundance of flatfishes and number of species. The spatial distribution of E. crossotus and its population structure indicate that this species is an estuarine resident despite the apparent reduction in its area of occupation within the estuarine complex. Among the other species, nine were classified as marine stragglers (Achirus declivis, Bothus ocellatus, Cyclopsetta chittendeni, Etropus longimanus, Paralichthys orbignyanus, P. patagonicus, Syacium micrurum, Symphurus diomedeanus and Trinectes paulistanus) and three as estuarine opportunists (Bothus robinsi, Citharichthys macrops and Syacium papillosum); another three could not be classified due to the small number of captures or lack of previous data (S. tessellatus, A. lineatus and C. spilopterus).
Keywords:
biodiversity; community; estuarine fishes; flatfishes; Paralichthyidae
Resumo:
Um total de 1.471 espécimes de 16 espécies de linguados (Pleuronectiformes) foram capturados durante 48 campanhas de amostragem entre julho de 2005 e junho de 2007 em dez estações na Baía de Guanabara, Rio de Janeiro, Brasil. Paralichthyidae foi a família dominante, com Etropus crossotus como a espécie dominante. As estações externas, especialmente aquelas no lado ocidental do baixo estuário, foram distinguidas como resultado de sua maior abundância de linguados e número de espécies. A distribuição espacial de E. crossotus e sua estrutura populacional indicam que esta espécie é uma residente estuarina, apesar da aparente redução em sua área de ocupação dentro do complexo estuarino. Dentre as outras espécies, nove foram classificadas como migrantes marinhos (Achirus declivis, Bothus ocellatus, Cyclopsetta chittendeni, Etropus longimanus, Paralichthys orbignyanus, P. patagonicus, Syacium micrurum, Symphurus diomedeanus e Trinectes paulistanus) e três como oportunistas estuarinos (Bothus robinsi, Citharichthys macrops e Syacium papillosum); outras três não puderam ser classificados devido ao pequeno número de capturas ou falta de dados prévios (S. tessellatus, A. lineatus e C. spilopterus).
Palavras-chave:
biodiversidade; comunidade; peixes estuarinos; linguados; Paralichthyidae
Introduction
The species of Pleuronectiformes, commonly known as flatfishes, can be easily identified by their asymmetrically compressed body, being usually pigmented on the side with eyes and depigmented on the blind side. They are demersal fishes with a carnivorous diet and have no swim bladder (Nelson et al. 2016NELSON, J.S. 2006. Fishes of the World. 4a. Ed. 624 pp. John Wiley & Sons, Inc., NY.). Pleuronectiformes is composed of 772 living species, primarily marine, distributed among 129 genera and 14 families (Nelson et al. 2016NELSON, J.S. 2006. Fishes of the World. 4a. Ed. 624 pp. John Wiley & Sons, Inc., NY.). In Brazilian waters, 56 marine species have been recorded and are distributed in 20 genera and five families (Menezes et al. 2003MENEZES, N.A.; BUCKUP, P.A.; FIGUEIREDO, J.L. & MOURA, R.L. 2003. Catálogo das espécies de peixes marinhos do Brasil. MZUSP, São Paulo.).
Despite the economic importance of Pleuronectiformes, their interactions with the varied ecosystems in which they are found are poorly understood. This is evident in environments such as estuaries, essential ecosystems for fish populations that are used as breeding and feeding grounds for several species, many of commercial value (Silva-Júnior et al. 2012SILVA-JÚNIOR, D.R.; GOMES, V.S.; LINDE-ARIAS, A.R. & VIANNA, M. 2012. Metallothionein in the pond perch Diplectrum radiale (Teleostei) as a biomarker of pollution in Guanabara bay estuary, Brazil. J Braz Soc Ecotoxicol 7 (1): 83-88., Blaber 2013BLABER, S.J.M. 2013. Fish and fisheries in tropical estuaries: The last 10 years. Estuar Coast Shelf Sci 135: 57-65.). Pleuronectiformes contains species of high abundance in estuarine ecosystems (Vieira et al. 1998VIEIRA, J.P.; CASTELLO, J.P. & PEREIRA, L.E. 1998. Ictiofauna In. SEELIGER, U.; ODEBRECHT, C. & CASTELLO, J.P. (eds.). Os ecossistemas costeiro e marinho do extremo sul do Brasil. Rio Grande, Ecoscientia., Chaves et al. 2003CHAVES, P.T.C.; COVA-GRANDO, G. & CALLUF, C. 2003. Ictiofauna demersal numa região de plataforma continental do sul do Brasil submetida à pesca camaroneira. Acta Biol Parana 32 (1,2,3,4): 69-82.), and these species undergo part or all of their reproductive cycle within these environments (Allen & Baltz 1997ALLEN, R.L. & BALTZ, D.M. 1997. Distribution and microhabitat use by flatfishes in a Louisiana estuary. Env Biol Fish 50: 85-103., Vieira et al. 1998VIEIRA, J.P.; CASTELLO, J.P. & PEREIRA, L.E. 1998. Ictiofauna In. SEELIGER, U.; ODEBRECHT, C. & CASTELLO, J.P. (eds.). Os ecossistemas costeiro e marinho do extremo sul do Brasil. Rio Grande, Ecoscientia., West et al. 2003WEST, J.M.; WILLIAMS, G.D.; MADON, S.P. & ZEDLER, J.B. 2003. Integrating spatial and temporal variability into the analysis of fish food web linkages in Tijuana Estuary. Environ Biol Fish 67: 297-309., Chaves & Bouchereau 2004CHAVES, P.T.C. & BOUCHEREAU, J.-L. 2004. Trophic organization and functioning of fish populations in the bay of Guaratuba, Brazil, on the basis of a trophic contribution factor. Acta Adriat 45 (1): 83-94.). Furthermore, flatfishes play an important role in the trophic chain of estuaries by acting as top or second-order predators (West et al. 2003WEST, J.M.; WILLIAMS, G.D.; MADON, S.P. & ZEDLER, J.B. 2003. Integrating spatial and temporal variability into the analysis of fish food web linkages in Tijuana Estuary. Environ Biol Fish 67: 297-309., Bouchereau & Chaves 2003BOUCHEREAU, J.L. & CHAVES, P.T.C. 2003. Ichthyofauna in the ecological organisation of a South-West Atlantic mangrove ecosystem: The bay of Guaratuba, South East Brazil. Vie et Millieu 53 (2-3): 103-110., Chaves & Bouchereau 2004CHAVES, P.T.C. & BOUCHEREAU, J.-L. 2004. Trophic organization and functioning of fish populations in the bay of Guaratuba, Brazil, on the basis of a trophic contribution factor. Acta Adriat 45 (1): 83-94.).
Despite the ecological and socioeconomic importance of estuaries, they experience constant processes of degradation caused by human activities. However, studies on the biology of estuarine fishes have attracted the attention of researchers (e.g., Silva-Júnior et al. 2012SILVA-JÚNIOR, D.R.; GOMES, V.S.; LINDE-ARIAS, A.R. & VIANNA, M. 2012. Metallothionein in the pond perch Diplectrum radiale (Teleostei) as a biomarker of pollution in Guanabara bay estuary, Brazil. J Braz Soc Ecotoxicol 7 (1): 83-88., Bodin et al. 2014BODIN, N.; TAPIE, N.; LE MÉNACH, K.; CHASSOT, E.; ELIE, P.; ROCHARD, E. & BUDZINSKI, H. 2014. PCB contamination in fish community from the Gironde Estuary (France): Blast from the past. Chemosphere 98: 66-72.). Some studies have explored the interaction between flatfishes and the estuary environment, both tropical and temperate (Martinho et al. 2010MARTINHO, F.; DOLBETH, M.; VEGAS, I.; BAPTISTA, J.; CABRAL, H.N. & PARDAL, M.A. 2010. Does the flatfish community of the Mondego estuary (Portugal) reflect environmental changes? J Appl Ichthyol 26: 843-852., Oliveira & Favaro 2011OLIVEIRA, E.C. & FAVARO, L.F. 2011. Reproductive biology of the flatfish Etropus crossotus (Pleuronectiformes: Paralichthyidae) in the Paranaguá Estuarine Complex, Paraná State, subtropical region of Brazil. Neotrop Ichthyol 9 (4): 795-805.), despite only a few being from estuaries located in the western South Atlantic coast.
In the tropical estuary of Guanabara Bay (Rio de Janeiro State, Brazil), the harvest of flatfishes is associated with intense fishing as bycatch of shrimp trawl fisheries (Jablonski et al. 2006JABLONSKI, S.; AZEVEDO, A.F. & MOREIRA, L.H.A. 2006. Fisheries and Conflicts in Guanabara bay, Rio de Janeiro, Brazil. Braz Arch Biol Technol 49 (1): 79-91.). However, very little information is available on the fish fauna of this estuary (Rodrigues et al. 2007RODRIGUES, C.; LAVRADO, H.P.; FALCÃO, A.P.C. & SILVA, S.H.G. 2007. Distribuição da ictiofauna capturada em arrastos de fundo na Baía de Guanabara - Rio de Janeiro, Brasil. Arq Mus Nac 65 (2): 199-210.; Andrade-Tubino et al. 2009ANDRADE-TUBINO, M.F.; FIORE-CORREIA, L.B. & VIANNA, M. 2009. Morphometrics and length structure of Micropogonias furnieri (Desmarest, 1823) (Perciformes, Sciaenidae) in Guanabara bay, State of Rio de Janeiro, Brazil. Bol Inst Pesca 35: 239-246.; Rosenfelder et al. 2012ROSENFELDER, N.; LEHNERT, K.; KAFFARNIK, S.; TORRES, J.P.M.; VIANNA, M.; VETTER, W. 2012. Thorough analysis of polyhalogenated compounds in ray liver samples off the coast of Rio de Janeiro, Brazil. Environ Sci Pollut R 19: 379-389.; Silva-Junior et al. 2012; Franco et al. 2014FRANCO, A.C.S.; BROTTO, D.S.; ZEE, D.M.W. & SANTOS, L.N. 2014. Reproductive biology of Cetengraulis edentulus (Cuvier, 1829), the major fishery resource in Guanabara Bay, Brazil. Neotrop Ichthyol 12 (4): 819-826.). The scarcity of information about the flatfish community of Guanabara Bay, a tropical estuary with a history of environmental degradation, demands a thorough assessment of the fish community. Continuous efforts have been undertaken to understand the ecological processes and patterns that drive the local ichthyofauna, leading to relevant contributions to the comprehension of composition and abundance of Tetraodontiformes (Andrade et al. 2015ANDRADE, A.C.; SANTOS, S.R.; VERANI, J.R. & VIANNA, M. 2016. Guild composition and habitat use by Tetraodontiformes (Teleostei, Acanthopterygii) in a south-western Atlantic tropical estuary. J Mar Biol Assoc UK 96(6): 1251-1264., Santos et al. 2015SANTOS, S.R.; ANDRADE, A.C.; VERANI, J.R. & VIANNA, M. 2015. Population explosion of the burrfish Chilomycterus spinosus spinosus (Diodontidae, Tetraodontiformes) in a eutrophic tropical estuary. Mar Biol Res 11(9): 955-964.) and Gerreidae (Côrrea & Vianna 2016), as well as the population biology of Genidens genidens (Silva-Júnior et al. 2013SILVA-JUNIOR, D.R.; CARVALHO, D.M.T. & VIANNA, M. 2013. The catfish Genidens genidens (Cuvier, 1829) as a potential sentinel species in Brazilian estuarine waters. J Appl Ichthyol 29:1297-303.) and the whitemouth croaker (Micropogonias furnieri) (Mulato et al. 2015MULATO, I.P.; CORRÊA, B. & VIANNA, M. 2015. Distribuição espaço-temporal de Micropogonias furnieri (Perciformes, Sciaenidae) em um estuário tropical no sudeste do Brasil. Bol Inst Pesca 41 (1): 1-18.).
The present study aims to determine the specific composition and abundance of flatfishes in Guanabara Bay. The data collected from all sampled species are compared to the data in the literature in order to classify the use of the estuarine complex and identify temporal and spatial changes in richness and evenness. Previous studies of other taxonomic fish groups occupying a large variety of niches in Guanabara Bay have shown a high diversity when compared to other Brazilian estuaries, but with a distinct predominance of one species over all others from each group (Andrade et al. 2015ANDRADE, A.C.; SANTOS, S.R.; VERANI, J.R. & VIANNA, M. 2016. Guild composition and habitat use by Tetraodontiformes (Teleostei, Acanthopterygii) in a south-western Atlantic tropical estuary. J Mar Biol Assoc UK 96(6): 1251-1264., Santos et al. 2015SANTOS, S.R.; ANDRADE, A.C.; VERANI, J.R. & VIANNA, M. 2015. Population explosion of the burrfish Chilomycterus spinosus spinosus (Diodontidae, Tetraodontiformes) in a eutrophic tropical estuary. Mar Biol Res 11(9): 955-964., Silva-Júnior et al. 2013SILVA-JUNIOR, D.R.; CARVALHO, D.M.T. & VIANNA, M. 2013. The catfish Genidens genidens (Cuvier, 1829) as a potential sentinel species in Brazilian estuarine waters. J Appl Ichthyol 29:1297-303., Silva-Júnior et al. 2016SILVA-JUNIOR. D.R.; PARANHOS, R. & VIANNA, M. 2016. Spatial patterns of distribution and the influence of seasonal and abiotic factors on demersal ichthyofauna in an estuarine tropical bay. J Fish Biol 89: 821-846.).
Materials and Methods
1. Study area
The tropical estuary of Guanabara Bay is located on the western South Atlantic coast (22º40'-23°00'S and 043º00'-043º20'W). The Guanabara Bay is considered to be a large estuarine complex, covering approximately 324 km2 and with an estimated water volume of 1.87 × 109 m3. It is supplied by a drainage basin that extends over 4,000 km2 and includes 35 rivers. The opening of the bay is 1,500 m wide and is divided into two channels by Cotunduba Island. The estuary is located in the metropolitan region of the city of Rio de Janeiro, the fourth largest city in Latin America, with over 11 million inhabitants (Forstall et al. 2009FORSTALL, R.L.; GREENE, R.P. & PICK, J.B. 2009. Which are the largest? Why lists of major urban areas vary so greatly. Tijdschr Econ Soc Geogr 100 (3): 277-297.). The tidal regime is semidiurnal, with a maximum height of 1.4 m. The depths range from less than 1 m to over 30 m in the central channel. The bay suffers from continuous and extensive land reclamation (recent and historical), silting, solid waste and sewage disposal (Carreira et al. 2004CARREIRA R.S.; WAGENER A.L.R. & READMAN, J.W. 2004. Sterols as markers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): space-time variations. Estuar Coast Shelf Sci 60: 587-598.), intense industrial activity (Soares-Gomes et al. 2016SOARES-GOMES, A.; GAMA, B.A.P.; NETO, J.A.B.; FREIRE, D.G.; CORDEIRO, R.C.; MACHADO, W.; BERNARDES, M.C.; COUTINHO, R.; THOMPSON, F.L. & PEREIRA, R.C. 2016. An environmental overview of Guanabara Bay, Rio de Janeiro. Regional Studies in Marine Science 8(2): 319-330.), unplanned urban growth with continual deficiencies in sanitation (Costa et al. 2018COSTA, L.A.A.; PESSOA, D.M.M. & CARREIRA, R.S. 2018. Chemical and biological indicators of sewage river input to an urban tropical estuary (Guanabara Bay, Brazil). Ecol Indic 90: 513-518.), and pollution by heavy metals (Neto et al. 2006NETO, J.A.B.; GINGELE, F.X.; LEIPE, T. & BREHME, I. 2006. Spatial distribution of heavy metals in surficial sediments from Guanabara Bay: Rio de Janeiro, Brazil. Environ Geol 49: 1051-1063.) and petroleum and its by-products (Soares-Gomes et al. 2010SOARES-GOMES, A.; NEVES, R.L.; AUCÉLIO, R.; VAN DER VEN, P.H.; PITOMBO, F.B.; MENDES, C.L.T. & ZIOLLI, R.L. 2010. Changes and variations of polycyclic aromatic hydrocarbon concentrations in fish, barnacles and crabs following an oil spill in a mangrove of Guanabara Bay, Southeast Brazil. Mar Poll Bull 60: 1359-1363.). The relevance of such impacts increases as a result of the importance of this ecosystem to the inhabitants of the metropolitan region of Rio de Janeiro because of its various uses, such as fishing, shipping, port activities, recreation, and tourism.
The sampling stations were selected on the basis of Mayr et al. (1989)MAYR, L.M.; PARANHOS, R.; TENENBAUM, D.R.; NOGUEIRA, C.R.; BONECKER, A.C.T.; VILLAC, M.C. & BONECKER, S.L.C. 1989. Hydrobiological Characterization of Guanabara bay. In: Coastlines of Brazil (Ed. NEVES, C.). American Society of Civil Engineers, NY., who characterized the hydrobiology of Guanabara Bay and divided it into five areas according to the water quality. Sampling was conducted at ten stations, two in each of the five predefined areas (Figure 1):
Map of Guanabara Bay, an estuary in the southeastern Brazil. The map shows all ten sampling stations divided into five areas (1 to 5) and distributed from the upper estuary to the lower estuary. The limits of the Guapimirim Environmental Protected Area are also indicated.
Area 1 - The upper estuary, north of Governador Island. This region is severely impacted by pollution from domestic sewage, industrial waste and garbage dumps. This area also suffers from impaired circulation caused by historical land reclamation, which reduces the removal of pollutants by coastal waters (Carreira et al. 2004CARREIRA R.S.; WAGENER A.L.R. & READMAN, J.W. 2004. Sterols as markers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): space-time variations. Estuar Coast Shelf Sci 60: 587-598.; Silva Jr. et al. 2016). It is distinguished by muddy sediment and low salinities (27.0-29.5) and temperatures between 24.0 and 25.9°C at the bottom (Mayr et al. 1989MAYR, L.M.; PARANHOS, R.; TENENBAUM, D.R.; NOGUEIRA, C.R.; BONECKER, A.C.T.; VILLAC, M.C. & BONECKER, S.L.C. 1989. Hydrobiological Characterization of Guanabara bay. In: Coastlines of Brazil (Ed. NEVES, C.). American Society of Civil Engineers, NY.).
Area 2 - The upper estuary near Paquetá Island. This area is characterised by (i) the discharge of the least polluted rivers, (ii) the presence of a large extent of the remaining original mangrove coverage, which is protected under a Federal Conservation Unit (EPA of Guapimirim), and (iii) the influence of the central channel, a main source of less polluted waters and the origin of the salt wedge, which favours a less degraded environment than that found in Area 1 (Carreira et al. 2004CARREIRA R.S.; WAGENER A.L.R. & READMAN, J.W. 2004. Sterols as markers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): space-time variations. Estuar Coast Shelf Sci 60: 587-598.). This portion of the estuary is characterised by muddy sediment and waters with salinities and temperatures at the bottom ranging from 27.0 to 29.5 and 22.5 to 23.9°C, respectively (Mayr et al. 1989MAYR, L.M.; PARANHOS, R.; TENENBAUM, D.R.; NOGUEIRA, C.R.; BONECKER, A.C.T.; VILLAC, M.C. & BONECKER, S.L.C. 1989. Hydrobiological Characterization of Guanabara bay. In: Coastlines of Brazil (Ed. NEVES, C.). American Society of Civil Engineers, NY.).
Area 3 - The middle estuary, which is directly influenced by the central channel and is an area with a greater exchange of water and better environmental conditions than the upper estuary (Carreira et al. 2004CARREIRA R.S.; WAGENER A.L.R. & READMAN, J.W. 2004. Sterols as markers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): space-time variations. Estuar Coast Shelf Sci 60: 587-598.). The substrate is composed of muddy sediments and fine sand, and the waters have high salinity, above 30.0, and bottom temperatures below 25°C (Mayr et al. 1989MAYR, L.M.; PARANHOS, R.; TENENBAUM, D.R.; NOGUEIRA, C.R.; BONECKER, A.C.T.; VILLAC, M.C. & BONECKER, S.L.C. 1989. Hydrobiological Characterization of Guanabara bay. In: Coastlines of Brazil (Ed. NEVES, C.). American Society of Civil Engineers, NY.).
Area 4 - As the most exposed portion of the bay, the lower estuary is strongly influenced by the coastal waters and marine conditions. The stations are located near the estuary mouth, and this area is subjected to contributions of raw sewage from the cities of Rio de Janeiro (4.2) and Niterói (4.1) (Figure 1) (Silva Jr. et al. 2016). Fine or medium sand covers the bottom of this area, while water in the region has high salinity (always > 32.0) and low temperatures (always < 25°C), closer to the conditions found in the adjacent coastal waters (Mayr et al. 1989MAYR, L.M.; PARANHOS, R.; TENENBAUM, D.R.; NOGUEIRA, C.R.; BONECKER, A.C.T.; VILLAC, M.C. & BONECKER, S.L.C. 1989. Hydrobiological Characterization of Guanabara bay. In: Coastlines of Brazil (Ed. NEVES, C.). American Society of Civil Engineers, NY.).
Area 5 - South of Governador Island. This area is subjected to high intake of the sewage produced on the island. Despite these conditions, the close proximity to the central channel allows relatively good water circulation and the dilution of pollutants (Carreira et al. 2004CARREIRA R.S.; WAGENER A.L.R. & READMAN, J.W. 2004. Sterols as markers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): space-time variations. Estuar Coast Shelf Sci 60: 587-598.; Silva Jr. et al. 2016). The waters found in this area are characterised by salinities ranging from 29.5 to 32.0 and temperatures similar to those in Area 2. The substrate is composed of muddy sediment (Mayr et al. 1989MAYR, L.M.; PARANHOS, R.; TENENBAUM, D.R.; NOGUEIRA, C.R.; BONECKER, A.C.T.; VILLAC, M.C. & BONECKER, S.L.C. 1989. Hydrobiological Characterization of Guanabara bay. In: Coastlines of Brazil (Ed. NEVES, C.). American Society of Civil Engineers, NY.).
2. Biological sampling
Specimens were collected every two weeks between July 2005 and June 2007 at ten stations (two per area), for a total of 48 campaigns and 480 hauls. The campaigns were organised in seasons: winter (July, August and September), spring (October, November and December), summer (January, February and March), and autumn (April, May and June). Samplings were conducted with a commercial fishing boat. The wooden trawler was 9.5 m long and had a 36 hp motor. The fishing gear included a 7.0 m-long net with a 14 m ground rope and mesh of 18 mm. Each trawl lasted 30 min at a constant speed of 1.5 knots. The depth, temperature and salinity were measured at the bottom using a YSI multiparameter.
All flatfishes caught were identified, and the total length (TL, cm) and total weight (TW, g) were recorded for each individual. Identification was based on the literature (Gutherz 1967GUTHERZ, E.J. 1967. Field Guide to the flatfishes of the family Bothidae in the western North Atlantic. United States Fish and Wildlife Service Circular 263: 1-47., Figueiredo & Menezes 2000FIGUEIREDO, J.L. & MENEZES, N.A. 2000. Manual de peixes marinhos do sudeste do Brasil: VI. Teleostei (5). Museu de Zoologia USP. São Paulo., Carpenter 2002CARPENTER, K.E. 2002. The living marine resources of the Western Central Atlantic. Volume 3: Bony fishes part 2 (Opistognathidae to Molidae), sea turtles and marine mammals. FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5. 1375-2127. Roma.). The original descriptions of Citharichthys spilopterus (Günther, 1862) and Citharichthys arenaceus (Evermann & Marsh, 1902EVERMANN, B.W. & MARSH, M.C. 1902. The Fishes of Porto Rico. In: Investigations of the Aquatic Resources and Fisheries of Porto Rico by the United States Fish Comission Steamer Fish Hawk: 49-350.) and the voucher specimens from the Ichthyological Collection of the Museu Nacional / Universidade Federal do Rio de Janeiro (MNRJ) were consulted in order to check for precise identification of specimens. The following lots were examined: C. arenaceus - MNRJ 18234, MNRJ 30616 and MNRJ 30768; C. spilopterus - MNRJ 9135, MNRJ 25695, MNRJ 29412, MNRJ 30628 and MNRJ 30672. A voucher specimen of C. spilopterus from this study was deposited in the Ichthyological Collection of the MNRJ.
The catch per unit effort (CPUE), based on the number of individuals (ind./h) and weight (g/h), and the seasonal and spatial distribution were calculated. The evaluation of the distribution of species per station was based on absolute frequencies. The effectiveness of the sampling design and the number of campaigns was determined based on the stabilisation of the sample-based rarefaction curve (Gotelli & Colwell 2010GOTELLI, N.J. & COLWELL, R.K. 2010. Estimating species richness. In Biological diversity: frontiers in measurement and assessment (Magurran, A.E., McGill, B.J., ed), pp. 39-54 Oxford, UK: Oxford University Press). Furthermore, for each station, Margalef's richness and Shannon's diversity and evenness were calculated using the natural logarithm (Magurran 2004MAGURRAN, A.E. 2004. Measuring biological diversity. Oxford, UK. Blackwell Science Ltda.).
A principal component analysis (PCA) was performed in XLSTAT statistical software for Excel using Pearson's r as an index of similarity. In addition, a hierarchical cluster analysis (HCA) was conducted to identify the associations among the different areas of the tropical estuary. UPGMA was employed to group species and areas and the Bray-Curtis dissimilarity index was calculated with the program PAST 3.08 (Hammer et al. 2001HAMMER, Ø.; HARPER, D.A.T. & RYAN, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Paleontol Electronica 4 (1): 9pp.). We used the Bray-Curtis similarity index expressed as 1-B. A cophenetic correlation coefficient, a measure of the accuracy of the dendrogram using the matrix of distances, was also calculated for each index (Valentin 2012VALENTIN, J.L. 2012. Ecologia numérica. Uma introdução à análise multivariada de dados ecológicos. 2nd edition. Interciência, Rio de Janeiro.). Multivariate clustering analysis and ordination were applied using the total number of individuals per species as descriptors of the ten sampling points. The normality of the data was verified using Shapiro-Wilk's test. Given the non-normal distribution of the data, the Wilcoxon signed-rank test was chosen and conducted using PAST 3.08 and IBM SPSS Statistics 22. The results were considered significant at the 95% level.
When possible, each species was classified in a functional group and according to its use of the estuary, based on observations of this study and the available literature (Allen & Baltz 1997ALLEN, R.L. & BALTZ, D.M. 1997. Distribution and microhabitat use by flatfishes in a Louisiana estuary. Env Biol Fish 50: 85-103., Reichert 1998REICHERT, M.J.M. 1998. Etropus crossotus, an annual flatfish species; age and growth of the fringed flounder in South Carolina. J Sea Res 40: 323-332., Chaves & Serenato 1998CHAVES, P.T.C. & SERENATO, A. 1998. Diversidade de dietas na assembléia de linguados (Teleostei, Pleuronectiformes) do manguezal da Baía de Guaratuba, Paraná, Brasil. Rev Bras Oceanogr 46 (1): 61-68., Walsh et al. 1999WALSH, H.J.; PETERS, D.S. & CYRUS, D.P. 1999. Habitat utilization by small flatfishes in a North Carolina estuary. Estuaries 22: 803-813., Reichert 2003REICHERT, M.J.M. 2003. Diet, consumption, and growth of juvenile fringed flounder (Etropus crossotus); a test of the 'maximum growth/optimum food hypothesis' in a subtropical nursery area. J Sea Res 50: 97-116., Chaves & Bouchereau 2004CHAVES, P.T.C. & BOUCHEREAU, J.-L. 2004. Trophic organization and functioning of fish populations in the bay of Guaratuba, Brazil, on the basis of a trophic contribution factor. Acta Adriat 45 (1): 83-94., Dias et al. 2005DIAS, J.F.; FIADI, C.B.; SILBIGER, H.L.N. & SOARES, L.S.H. 2005. Reproductive and population dynamics of the bay whiff Citharichthys spilopterus Günther, 1862 (Pleuronectiformes: Paralichthyidae) in the Mamanguá inlet, Rio de Janeiro, Brazil. Neotrop Ichthyol 3 (3): 411-419.). The functional group classification followed Elliott et al. (2007)ELLIOTT, M.; WHITFIELD, A.K.; POTTER, I.C.; BLABER, S.J.M.; CYRUS, D.P.; NORDLIE, F.G. & HARRISON, T.D. 2007. The guild approach to categorizing estuarine fish assemblages: a global review. Fish Fish 8: 241-268..
Results
1. Species composition, abundance and spatial distribution
The trawl survey resulted in the capture of 1,471 flatfishes belonging to 16 species, nine genera, and four families, with a total weight of 31.1 kg. There was a clear predominance of Paralichthyidae in terms of both number of individuals and richness, followed by Achiridae, Bothidae and Cynoglossidae (Table 1). The most abundant species was Etropus crossotus, with 49.7% of all specimens caught and occurring in all samples, followed by Symphurus tessellatus, with 23.7% of all specimens caught and occurring in 97.9% of the samples (Table 1). The collector's curve suggests that the number of samples was sufficient to provide a reasonably accurate inventory of the flatfish assemblage in Guanabara Bay, as it was stabilised on the 16th campaign, with just one additional species added in the following campaigns (Figure 2). Only three species were distributed across all five areas of the estuary: E. crossotus, S. tessellatus and C. spilopterus. Other species of Pleuronectiformes were rare (less than 10 specimens) or had restricted distributions (Table 2). Five species showed a frequency of occurrence that was greater than 50% in all studied months: E. crossotus, S. tessellatus, Citharichthys macrops, Syacium papillosum and C. spilopterus. While Bothus robinsi was captured in 42% of the samples, the other species were present in fewer than 20%.
Number of individuals (N), frequency of occurrence (%), and amplitude of the length (TL) and weight (TW) of the pleuronectiform species captured between July 2005 and June 2007 in Guanabara Bay, south-eastern Brazil.
Sample-based rarefaction curve of species of Pleuronectiformes collected during each campaign conducted between July 2005 and June 2007 in Guanabara Bay, southeastern Brazil.
Abundance per sampling station of all Pleuronectiform species collected between July 2005 and June 2007, in five areas of Guanabara Bay, Brazil (ER – Estuarine Resident, MO – Marine estuarine opportunist, MS – Marine straggler).
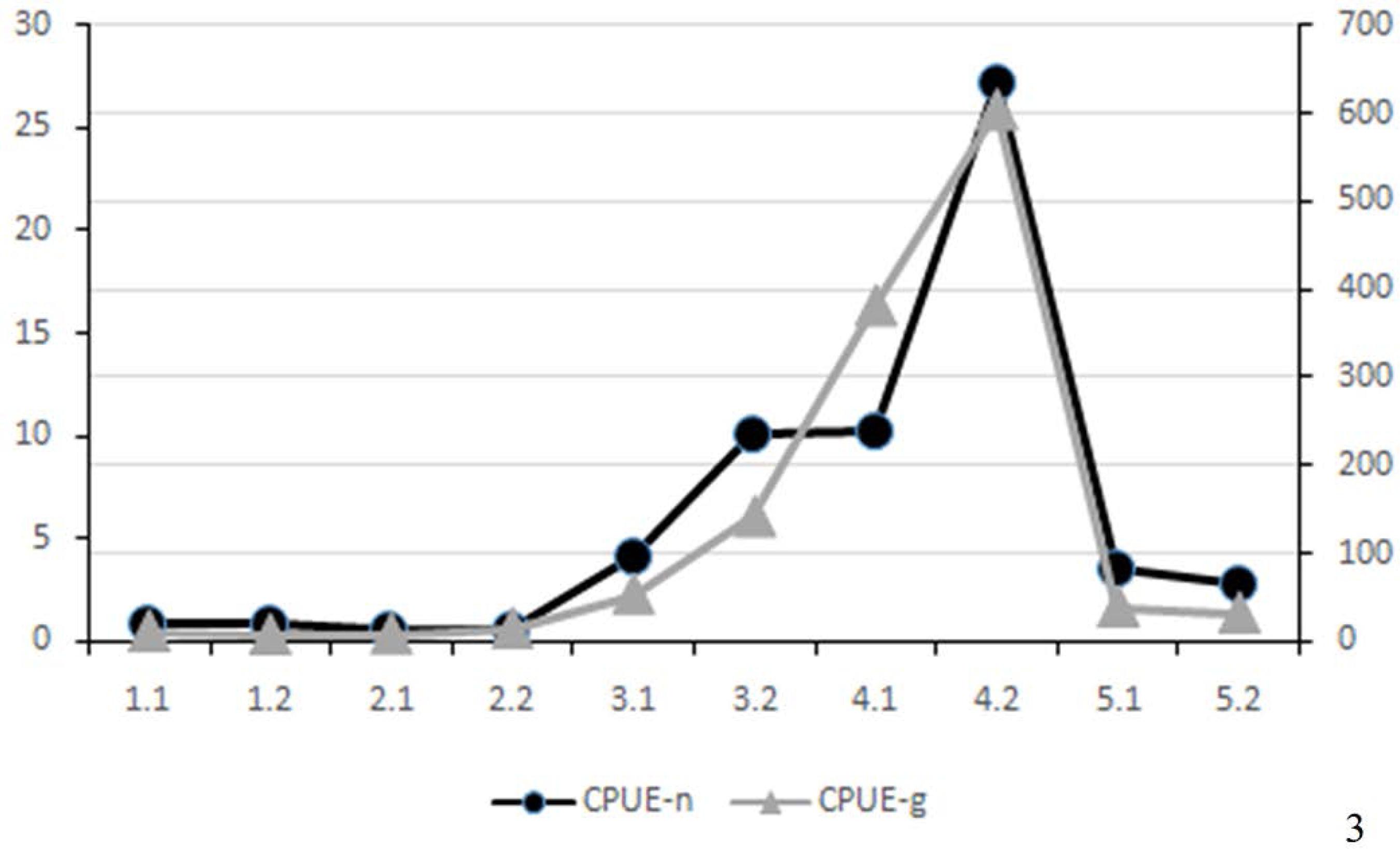
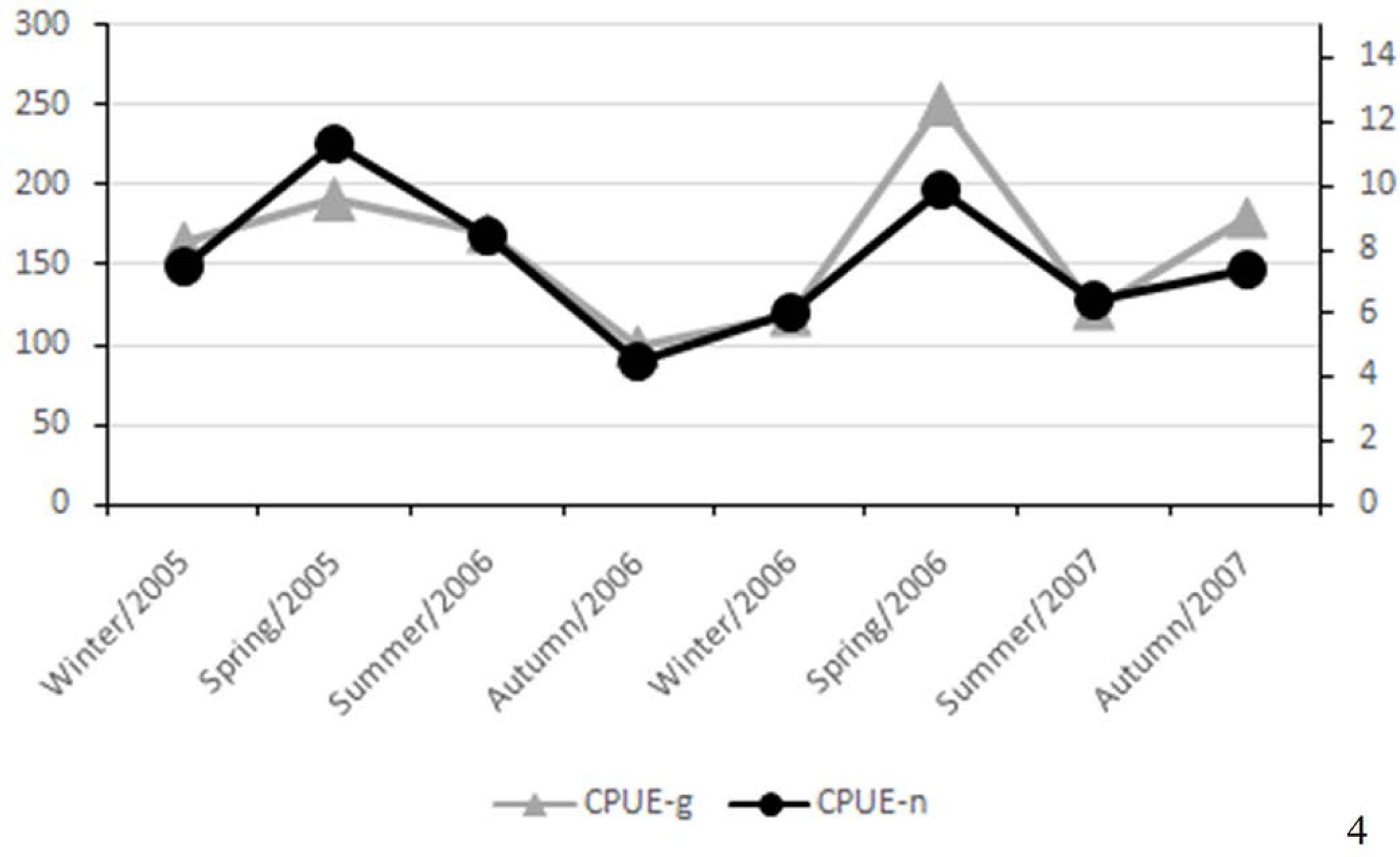
The spatial analysis of the CPUE indicated a clear distinction between the lowest values found in the upper estuary (1.1, 1.2, 2.1 and 2.2) and the highest values in the lower estuary (3.2, 4.1 and 4.2). The middle estuary (3.1, 5.1 and 5.2) indicated CPUE values slightly above those found in the upper estuary (Figure 3). The seasonal variation in the CPUE revealed that the highest values in terms of number of individuals and TW were found during spring. While the difference in CPUE-n values between the first and second years showed a slightly larger number of individuals captured in the first year, there was a clear difference in CPUE-g, with larger values found in the second year. This indicates that the specimens caught during the first year, especially during spring, were smaller than those captured in the second year (Figure 4).
Spatial variation in CPUE in terms of the number and total weight of specimens of Pleuronectiformes captured in Guanabara Bay, an estuary in the southeastern Brazil, between July 2005 and June 2007.
Seasonal variation in CPUE in terms of number and total weight of specimens of Pleuronectiformes captured in Guanabara Bay, an estuary in the southeastern Brazil, between July 2005 and June 2007.
The analysis of the CPUE values and percentage of individuals per sampling station showed that station 4.2, located on the western side of the lower estuary, was the locality where most of the pleuronectiforms (44.4%) were captured, with the abundance and biomass being far greater than those recorded in other stations (Figures 3 and 4). While a significant difference was found in terms of the total length for the total catch of Pleuronectiformes between the two years of the study (Wilcoxon, p<0.001), the data reflect a significant change in size (Wilcoxon, p<0.001) and mass (Wilcoxon, p=0.006) for Syacium papillosum in the second year, with means of 11.7 cm TL and 28.0 g TW for the first year, increasing to 17.2 cm TL and 60.6 g TW in the second year.
2. Richness, evenness and diversity
A total of 15 species was represented among specimens collected in the first year, while 16 were recorded in the second year (Table 3). Margalef's species richness was 2.06 when considering both sampled years; specifically, 2.09 in the first year and 2.31 in the second year. The highest richness was obtained during the summer of 2006, with a value of 2.26 (13 species), and the lowest was recorded in the winter of 2006, with a value of 1.21 (7 species). The sampling station with the highest richness was 4.2, with a value of 2.16 (15 species), and the lowest richness values were found at stations 5.2 and 5.1, with values of 0.47 and 0.45, respectively (3 species each).
Seasonal and spatial variation in the values of richness, diversity and evenness of Pleuronectiform species collected between July 2005 and June 2007, in five areas of Guanabara Bay, Brazil (standard deviation in parenthesis).
The diversity value obtained for the two years was 1.55; 1.44 in the first year and 1.60 in the second. The period with the highest diversity value (1.83) was autumn of 2006, and the lowest value (1.14) occurred in the winter of the same year. The station with the highest diversity was 1.1 (1.58), and the lowest value was found at 5.2 (0.70) (Table 3). The evenness obtained for the first and second years was 0.53 and 0.58, respectively, whereas the value for both years was 0.56. The period with the highest value of evenness was autumn of 2006, with a value of 0.76, and the lowest occurred during the spring of 2005, with a value of 0.52. The stations with the highest values of evenness were 1.1 and 2.2, with values of 0.88 and 0.87, respectively. The lowest values were recorded at stations 3.2 (0.35) and 4.2 (0.47) (Table 3).
3. Principal component analysis (PCA) and hierarchical clustering (HCA)
The PCA (Figure 5) helped to characterise the sampled area using the correlations between different values of abundance among species. The factorial plan 1-2 explains 72% of the variability (50% and 22% for axes 1 and 2, respectively). Stations 3.2 and 4.2 have a characteristic distribution and are isolated in the graph. To a lesser extent, station 4.1 was also isolated from the other stations. Three sampling stations could be defined based on their distinct groups of species: 4.2 was distinguished because of the dominance of E. crossotus and the presence of Paralichthys patagonicus, Bothus ocellatus, B. robinsi, Etropus longimanus and Syacium micrurum, species that occurred only or primarily at this site. Station 3.2 was segregated by the dominance of S. tessellatus and the exclusive presence of Symphurus diomedeanus and Cyclopsetta chittendeni. Lastly, station 4.1 showed a dominance of C. macrops and S. papillosum and a low frequency of S. tessellatus.
Principal component analysis (PCA) of the Pleuronectiformes species captured in Guanabara Bay, an estuary in the southeastern Brazil, between July 2005 and June 2007.
The HCA (Figure 6) identified three large groups. The first was composed of areas 3 and 5, representing the middle estuary; the second was composed of area 4 in the lower estuary; and areas 1 and 2 were associated with the third group, representing the upper estuary. When considering the more closely related stations, six other associations were identified. Station 3.1 and area 5 (5.2 and 5.1) shared a similarity greater than 0.80. Area 1 (1.1 and 1.2) had a similarity of 0.65, and area 2 (2.1 and 2.2) scored 0.70. The remaining stations, 3.2, 4.1 and 4.2, were isolated from the others. The cophenetic correlation coefficient for this analysis was 0.91.
Hierarchical cluster analysis (HCA) of species of Pleuronectiformes recorded in Guanabara Bay, an estuary in the southeastern Brazil, between July 2005 and June 2007, showing the three main identified groups.
Discussion
1. Composition
The sampling strategy was based on a trawl net due to the low selectivity of this fishing gear, thus enabling a fairly accurate characterisation of the diversity of demersal fish associated with unconsolidated substrates (Vianna & Almeida 2005VIANNA, M. & ALMEIDA, T. 2005. Bony fish bycatch in the southern Brazil pink shrimp (Farfantepenaeus brasiliensis and F. paulensis) fishery. Braz Arch Biol Technol 48 (4): 611-623.). The same sampling design was used in a previous study by Rodrigues et al. (2007)RODRIGUES, C.; LAVRADO, H.P.; FALCÃO, A.P.C. & SILVA, S.H.G. 2007. Distribuição da ictiofauna capturada em arrastos de fundo na Baía de Guanabara - Rio de Janeiro, Brasil. Arq Mus Nac 65 (2): 199-210., who identified only six species (all at low abundance) of pleuronectiforms in Guanabara Bay. The results presented here, a total of 16 flatfish species, indicate that the Richness of Pleuronectiformes in the Guanabara Bay was not properly assessed by earlier studies. The specific composition and predominance of Paralichthyidae in Guanabara Bay was similar to the diversity of flatfishes found in other tropical estuaries from South-eastern Brazil. Andreatta et al. (2002)ANDREATA, J.V.; MEURER, B.C.; BAPTISTA, M.G.S.; MANZANO, F.V.; TEIXEIRA, D.E.; LONGO, M.M. & FRERET, N.V. 2002. Composição da assembléia de peixes da Baía da Ribeira, Angra dos Reis, Rio de Janeiro, Brasil. Rev Bras Zool 19 (4): 1139-1146. identified 11 pleuronectiform species in Ribeira Bay (22º59'S / 44º22'W), while Mendonça & Araújo (2002)MENDONÇA, P. & ARAÚJO, F.G. 2002. Composição das populações de linguados (Osteichthyes, Pleuronectiformes) da Baía de Sepetiba, Rio de Janeiro, Brasil. Rev Bras Zool 19 (2): 339-347. found 14 species in Sepetiba Bay (22º59'S / 44º22'W); in both studies, Paralichthyidae was the most speciose family, with eight and nine species, respectively. Further north, in the bay of Vitória (20º17'S / 40º16'W), seven Paralichthyidae species were recorded among a total of 14 flatfishes (Chagas et al. 2006CHAGAS, L.P.; JOYEUX, J.-C. & FONSECA, F.R. 2006. Small-scale spatial changes in the estuarine fish: subtidal assemblages in tropical Brazil. J Mar Biol Assoc UK 86(4): 861-875.; Catelani et al. 2014CATELANI, PA; PETRY, A.C.; DARIO, F.D.; SANTOS, V.L.M. & MINCARONE, M.M. 2014. Fish composition (Teleostei) of the estuarine region of the Macaé River, southeastern Brazil. Check List 10(4): 927-935.).
Guanabara Bay is an open ecosystem that experiences the periodic influence of different water masses. Nevertheless, even with the stabilisation of the species rarefaction curve, the addition of another species, Etropus longimanus, in the 37th sampling campaign was not unexpected. Different water masses allows the presence of species associated with coastal waters and colder and deeper water masses. Consequently, it is likely that other species may be recorded in the future. A thorough analysis of the abiotic data for the same samples but extended to all fish community can be seen in Silva Jr. et al. (2016). The comparison of the species composition among estuaries showed an interesting difference. The flatfish Citharichthys arenaceus was the only species absent in this study but present in other tropical estuaries along the Rio de Janeiro coast. This inconsistency may be due to difficulties associated with the taxonomy of the species. There are problems in distinguishing C. arenaceus and Citharichthys spilopterus, which have overlapping morphometric and meristic characters (Carpenter 2002CARPENTER, K.E. 2002. The living marine resources of the Western Central Atlantic. Volume 3: Bony fishes part 2 (Opistognathidae to Molidae), sea turtles and marine mammals. FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5. 1375-2127. Roma.). There is a lack of comparative studies between both species (Figueiredo & Menezes 2000FIGUEIREDO, J.L. & MENEZES, N.A. 2000. Manual de peixes marinhos do sudeste do Brasil: VI. Teleostei (5). Museu de Zoologia USP. São Paulo.). The comparison of the specimens captured in Guanabara Bay in this study with many specimens of both species from the Ichthyological Collection of the MNRJ allowed us to conclude that the specimens previously identified as C. arenaceus in Guanabara Bay corresponded to C. spilopterus. No significant change in total length or total weight was identified between the first and second years of the study, except in Syacium papillosum.
2. Abundance
The decline in abundance of species of Pleuronectiformes collected between the two years was a direct reflection of the strong decrease in the capture of E. crossotus. The highest captures occurred during springtime due to various factors. The peak in the spring of 2005 was a consequence of the increased abundance of E. crossotus, which is in contrast to the peak during the spring of 2006, characterised by increased captures of other species of flatfishes despite the low abundance of E. crossotus. The lowest capture rate for all flatfishes occurred on the autumn of 2006. There was no marked seasonality among the pleuronectiform species in Guanabara Bay. However, Barletta et al. (2003)BARLETTA, M.; BARLETTA-BERGAN, A.; SAINT-PAUL, U. & HUBOLD, G. 2003. Seasonal changes in density, biomass and diversity of estuarine fishes in tidal mangrove creeks of the lower Caeté Estuary (northern Brazilian coast, east Amazon). Mar Ecol Prog Ser 256: 217-228. reported an increase in the abundance of species in the rainy season and identified it as a pattern that is common in tropical estuaries.
The high abundance of flatfishes in the lower estuary (4.2) was due to the elevated capture of specimens of E. crossotus along with the presence of species associated with coastal waters, such as Bothus ocellatus, B. robinsi, Citharichthys macrops, Syacium micrurum and S. papillosum (Figueiredo & Menezes 2000FIGUEIREDO, J.L. & MENEZES, N.A. 2000. Manual de peixes marinhos do sudeste do Brasil: VI. Teleostei (5). Museu de Zoologia USP. São Paulo.). This behaviour was not recorded on the other side of the bay mouth (4.1), presumably due to the different environmental conditions. Originally, it was expected that the two sampling stations would be similar in terms of their fish assemblages due to their location at the entrance of the bay and the predominant marine environmental conditions (high salinity and sandy bottom). The source for this unexpected difference may be the disposal of organic matter through an outfall located close to the sampling station. Banks covered in algae and bryozoans are indicative of the disposal of organic matter in wastewater and were recorded during sampling at station 4.1 but not at 4.2.
Even though the central channel (3.2) had the same abundance in terms of the number of specimens as the lower estuary station (4.1), the recorded biomass was lower. This result reflects the dominance of Symphurus tessellatus, a species that is smaller than the more abundant species at station 4.1 (E. crossotus, S. papillosum and C. macrops). The highest abundance of S. tessellatus found in the central channel may be a consequence of the characteristics of the area, especially the muddy bottom. This species naturally inhabits areas with such characteristics (Figueiredo & Menezes 2000FIGUEIREDO, J.L. & MENEZES, N.A. 2000. Manual de peixes marinhos do sudeste do Brasil: VI. Teleostei (5). Museu de Zoologia USP. São Paulo.), which contrasts with the sandy bottom found at the entrance of the bay.
3. Richness
Seasonally, lower values of richness were recorded in the winter and spring of both sampling years, with the lowest values found during the winter. Conversely, the highest values of richness occurred in the rainy season, during the summer and autumn. This seasonal variation in richness was consistent with the pattern observed by Barletta et al. (2003)BARLETTA, M.; BARLETTA-BERGAN, A.; SAINT-PAUL, U. & HUBOLD, G. 2003. Seasonal changes in density, biomass and diversity of estuarine fishes in tidal mangrove creeks of the lower Caeté Estuary (northern Brazilian coast, east Amazon). Mar Ecol Prog Ser 256: 217-228., with more species found in the rainy season. Results, however, differ from those presented by Vendel et al. (2002)VENDEL, A.L.; SPACH, H.L.; LOPES, S.G. & SANTOS, C. 2002. Structure and Dynamics of Fish Assemblages in a Tidal Creek Environment. Braz Arch Biol Technol 45 (3): 365-373., which reported larger values of fish richness in the autumn and winter in a Brazilian subtropical estuary. In another subtropical estuary from South-eastern Brazil, Pinheiros Bay (Paraná), Schwarz-Junior et al. (2006)SCHWARZ-JUNIOR, R.; FRANCO, A.C.N.P.; SARPEDONTI, V.; PICHLER, H.A. & QUEIROZ, G.M.L.N. 2006. Composição e estrutura da ictiofauna demersal na Baía dos Pinheiros, Paraná. Braz J Aquat Sci Technol 10 (1): 27-39. found no seasonal pattern in the richness of the fish fauna.
Spatially, the number of species followed the same pattern observed for the abundance, with considerably higher values detected in the western part of the estuary mouth (4.2) than in any of the other sampling stations, including the central channel (3.2) and the eastern part of the lower estuary (4.1). However, Margalef's richness, which considers the number of individuals in the sample, showed a different picture; the western lower estuary was the richest, followed by stations 1.1 and 2.2. This result is a consequence of the low abundance and relatively high number of species in the upper estuary. An increase in the number of species in the outermost regions of the estuary, in addition to an increased presence of marine species, has been observed in Guanabara Bay (Rodrigues et al. 2007RODRIGUES, C.; LAVRADO, H.P.; FALCÃO, A.P.C. & SILVA, S.H.G. 2007. Distribuição da ictiofauna capturada em arrastos de fundo na Baía de Guanabara - Rio de Janeiro, Brasil. Arq Mus Nac 65 (2): 199-210.), Sepetiba Bay (Araújo et al. 1998ARAÚJO, F.G.; CRUZ-FILHO, A.G.; AZEVEDO, M.C. & SANTOS, A.C.A. 1998. Estrutura da comunidade de peixes demersais da Baía de Sepetiba, RJ. Rev Bras Biol 58 (3): 417-430.), both in the State of Rio de Janeiro, and Guaratuba Bay, in the State of Paraná (Bouchereau & Chaves 2003CHAVES, P.T.C.; COVA-GRANDO, G. & CALLUF, C. 2003. Ictiofauna demersal numa região de plataforma continental do sul do Brasil submetida à pesca camaroneira. Acta Biol Parana 32 (1,2,3,4): 69-82.) and appears to be a trend in tropical and subtropical estuaries (Blaber 2000BLABER, S.J.M. 2000. Fish and Aquatic Resources Series, Vol 7: Tropical Estuarine Fishes: Ecology, Exploitation and Conservation. Oxford, UK. Blackwell Science Ltd.; Blaber 2013BLABER, S.J.M. 2013. Fish and fisheries in tropical estuaries: The last 10 years. Estuar Coast Shelf Sci 135: 57-65.; Catelani et al. 2014CATELANI, PA; PETRY, A.C.; DARIO, F.D.; SANTOS, V.L.M. & MINCARONE, M.M. 2014. Fish composition (Teleostei) of the estuarine region of the Macaé River, southeastern Brazil. Check List 10(4): 927-935.). A two-way ANOVA based on fish metrics (CPUE, richness, diversity and equitability) was conducted by Silva Jr. et al. (2016) based on the same two years of sampling but including all fishes caught. While changes in abundance, diversity and equitability were independently associated with differences in area and season, shifts in richness were exclusively related to spatial variability. The greatest diversity and abundance values for Pleuronectiformes were found in the lower estuary and the central channel, which is in accordance with what was reported for other trophic fish groups (Silva Jr. et al. 2016). No seasonal pattern was observed in the variation of the diversity and evenness indices. The lack of patterns in these indices was also recorded by Vendel et al. (2002)VENDEL, A.L.; SPACH, H.L.; LOPES, S.G. & SANTOS, C. 2002. Structure and Dynamics of Fish Assemblages in a Tidal Creek Environment. Braz Arch Biol Technol 45 (3): 365-373. and Spach et al. (2003)SPACH, H.L.; SANTOS, C. & GODEFROID, R.S. 2003. Padrões temporais na assembléia de peixes na gamboa do Sucuriú, Baía de Paranaguá, Brasil. Rev Bras Zool 20 (4): 591-600. in two estuaries in Paranaguá Bay, by Schwarz-Junior et al. (2006)SCHWARZ-JUNIOR, R.; FRANCO, A.C.N.P.; SARPEDONTI, V.; PICHLER, H.A. & QUEIROZ, G.M.L.N. 2006. Composição e estrutura da ictiofauna demersal na Baía dos Pinheiros, Paraná. Braz J Aquat Sci Technol 10 (1): 27-39. in Pinheiros Bay and by Chagas et al. (2006)CHAGAS, L.P.; JOYEUX, J.-C. & FONSECA, F.R. 2006. Small-scale spatial changes in the estuarine fish: subtidal assemblages in tropical Brazil. J Mar Biol Assoc UK 86(4): 861-875. in Vitória Bay.
4. Principal component analysis and hierarchical cluster analysis
Eutrophic environments commonly present conditions of hypoxia or anoxia in their deeper layers, which do not mix with the upper layers due to the formation of a cline (Breitburg 2002BREITBURG, D. 2002. Effects of Hypoxia, and the Balance between Hypoxia and Enrichment, on Coastal Fishes and Fisheries. Estuaries 25 (4b): 767-781.). Intense eutrophication and consequent hypoxia are reported to occur in large portions of Guanabara Bay (Ribeiro & Kjerfve 2002RIBEIRO, C.H.A. & KJERFVE, B. 2002. Anthropogenic influence on the water quality in Guanabara Bay, Rio de Janeiro, Brazil. Reg Environ Change 3:13-19.; Aguiar et al. 2011AGUIAR, V.M.C.; NETO, JAB & RANGEL, C.M. 2011. Eutrophication and hypoxia in four streams discharging in Guanabara Bay, RJ, Brazil, a case study. Mar Poll Bull 62 (8): 1915-1919.). While the lower estuary is characterized by greater exposure to less polluted coastal waters, the upper portions of the bay, especially the western and northwestern sectors, receive the majority of the drainage from metropolitan Rio de Janeiro (Ribeiro & Kjerfve 2002RIBEIRO, C.H.A. & KJERFVE, B. 2002. Anthropogenic influence on the water quality in Guanabara Bay, Rio de Janeiro, Brazil. Reg Environ Change 3:13-19.). The existing facilities for sewage treatment and waste disposal are insufficient to halt the environmental degradation. Ribeiro & Kjerfve (2002)RIBEIRO, C.H.A. & KJERFVE, B. 2002. Anthropogenic influence on the water quality in Guanabara Bay, Rio de Janeiro, Brazil. Reg Environ Change 3:13-19. estimated that in order to achieve pre-1950 conditions, it would be necessary to properly treat 80-90% of all domestic and industrial sewage, far from the 15% that is presently treated. The data presented in this study therefore address flatfish populations that have been exposed to a long history of human impacts in an already naturally stressed environment.
The PCA analysis separated the three stations (3.2, 4.1 and 4.2) that had the most diverse environmental features, while the remaining seven stations were grouped in a single cluster. At these three stations, the number of species was higher than at the others. Coincidentally, these are the stations with the best estuarine environmental conditions, where the dissolution of the organic load is higher due to the action of the tidal regime. The isolation of the western (4.2) and eastern (4.1) sides of the lower estuary and central channel (3.2) was expected based on the field observations. The hierarchical cluster analysis showed some similarity, albeit small, between 4.1 and 4.2 and grouped areas 1 and 2 and 3.1 and 5. This result indicated that the composition of Pleuronectiformes varied between four zones: the upper estuary, above Governador and Paquetá islands, represented by areas 1 and 2; the middle of the estuary, formed by stations 5 and 3.1; the lower estuary, indicating the difference between the eastern (4.1) and western (4.2) sides of the estuary mouth; and the central channel (3.2), which is variable in size according to rainfall. The spatial distribution observed for the pleuronectiform species follows the pattern recorded by Rodrigues et al. (2007)RODRIGUES, C.; LAVRADO, H.P.; FALCÃO, A.P.C. & SILVA, S.H.G. 2007. Distribuição da ictiofauna capturada em arrastos de fundo na Baía de Guanabara - Rio de Janeiro, Brasil. Arq Mus Nac 65 (2): 199-210. for the entire demersal fish assemblage of Guanabara Bay. Patterns of distribution influenced by local rainfall are supported by data from other species in the same estuary. This is the case with the abundance and distribution of the pink shrimp Farfantepenaeus brasiliensis (Gomes et al. 2013GOMES, A.P.P.; KEUNECKE, K.A.; SILVA-JÚNIOR, D.M. & VIANNA, M. 2013. Modulating reproduction of Penaeidae shrimps: ecological responses of two sympatric species (Decapoda: Dendrobranchiata) on south-eastern Brazilian coast. J Mar Biol Assoc UK 93 (3): 733-740.) and the blue crab Callinectes ornatus (Keunecke et al. 2012KEUNECKE, K.A.; D'INCAO, F.; VERANI, J.R. & VIANNA, M. 2012. Reproductive strategies of two sympatric swimming crabs Callinectes danae and Callinectes ornatus (Crustacea: Portunidae) in a estuarine system, south-eastern Brazil. J Mar Biol Assoc UK 92 (2): 343-347.) with differences according to environmental conditions, among them the seasonal spawning peaks in the first and distinctive patterns of sexual distribution in the latter.
5. Use of the estuary
Despite the recent review of the classification of estuarine fish guilds (Potter et al. 2013POTTER, I.C.; TWEEDLEY, J.R.; ELLIOT, M. & WHITFIELD, A.K. 2013. The ways in which fish use estuaries: a refinement and expansion of the guild approach. Fish Fish 16 (2): 230-239.), due to the scarcity of data on the fish community of the Guanabara Bay, this study follows Elliot et al. (2007). The most abundant species, Etropus crossotus, was classified as an estuarine resident in this study and in Guaratuba Bay, which is a subtropical estuary in southern Brazil (Chaves & Bouchereau 2004CHAVES, P.T.C. & BOUCHEREAU, J.-L. 2004. Trophic organization and functioning of fish populations in the bay of Guaratuba, Brazil, on the basis of a trophic contribution factor. Acta Adriat 45 (1): 83-94.). This close relationship between E. crossotus and the estuarine environment is supported by the present study due to its regular presence throughout the sampling campaigns, high relative abundance, various size classes and wide distribution in the estuary. Sánchez-Gil et al. (2008)SÁNCHEZ-GIL, P.; YÁÑEZ-ARANCIBIA, A.; TAPIA, M.; DAY, J.W.; WILSON, C.A. & COWAN JR., J.H. 2008. Ecological and biological strategies of Etropus crossotus and Citharichthys spilopterus (Pleuronectiformes:Paralichthyidae) related to the estuarine plume, Southern Gulf of Mexico. J Sea Res 59: 173-185. considered E. crossotus to be an estuarine species but found recruitment in the estuarine plume on the inner continental shelf off an estuary of the southern Gulf of Mexico. The reduction in the abundance of E. crossotus between the first and second years was responsible for the observed decrease in the whole flatfish assemblage. This decrease in catch should be investigated through continuous monitoring of the area; it may indicate an early depletion of the population or only a natural fluctuation.
The reduction in the abundance of E. crossotus, despite the lack of a seasonal pattern, may be associated with its life cycle. This reduction coincides with the presence of larger individuals prior to the recruitment observed in the second year. It should be noted, however, that no population data are available for the species in coastal areas besides studies on bycatch of the shrimp fishery, where E. crossotus was the most abundant species among the pleuronectiforms (Vianna & Almeida 2005VIANNA, M. & ALMEIDA, T. 2005. Bony fish bycatch in the southern Brazil pink shrimp (Farfantepenaeus brasiliensis and F. paulensis) fishery. Braz Arch Biol Technol 48 (4): 611-623.), indicating that the estuarine resident Etropus crossotus may also explore areas outside of the estuary. Differences in rainfall patterns may affect the living conditions and the annual cycle of flatfish populations. Increased freshwater inflow in the rainy season, usually with a high content of organic matter, deteriorates the environmental conditions in the bay (Valentin et al. 1999VALENTIN, J.L.; TENENBAUM, D.R.; BONECKER, A.C.T.; BONECKER, S.L.C.; NOGUEIRA, C.R.; PARANHOS, R. & VILLAC, M.C. 1999. Caractéristiques hydrobiologiques de la Baie de Guanabara (Rio de Janeiro, Brésil). J Oceanogr Rech 24 (1): 33-41.) and increases the size of the area under hypoxia, forcing individuals of E. crossotus to move to areas with better conditions in the estuary and adjacent coastal areas (the species was found up to 40 m deep) (Vianna & Almeida 2005VIANNA, M. & ALMEIDA, T. 2005. Bony fish bycatch in the southern Brazil pink shrimp (Farfantepenaeus brasiliensis and F. paulensis) fishery. Braz Arch Biol Technol 48 (4): 611-623.).
The wide size range and presence of larvae (Castro et al. 2005CASTRO, M.S.; BONECKER, A.C.T. & VALENTIN, J.L. 2005. Seasonal variation fish larvae at the entrance of Guanabara bay, Brazil. Braz Arch Biol Technol 48 (1): 121-128.) suggest that E. crossotus completes its life cycle in the estuary. A similar pattern was described for the south-eastern United States, where it was observed that the species development had a strong dependency on a nursery area with plenty of resources (Reichert 1998REICHERT, M.J.M. 1998. Etropus crossotus, an annual flatfish species; age and growth of the fringed flounder in South Carolina. J Sea Res 40: 323-332.). Other groups of species were also observed: Marine stragglers, characterised by rare species, were represented only in low abundance and did not show dependence on estuaries in the literature. This group includes the majority of the collected species: A. declivis, B. ocellatus, C. chittendeni, E. longimanus, P. orbignyanus, P. patagonicus, S. micrurum, S. diomedeanus and T. paulistanus. Marine estuarine opportunist species are present in varying abundance, are restricted to the lower estuary and include smaller, most likely young, individuals. This group is composed of B. robinsi, C. macrops and S. papillosum. Three other species, two rare and one abundant (S. tessellatus, A. lineatus and C. spilopterus), are indicated as estuarine residents by Chaves & Bouchereau (2004)CHAVES, P.T.C. & BOUCHEREAU, J.-L. 2004. Trophic organization and functioning of fish populations in the bay of Guaratuba, Brazil, on the basis of a trophic contribution factor. Acta Adriat 45 (1): 83-94.; however, in Guanabara Bay, the data led to a different classification or precluded any determination.
The tonguefish S. tessellatus was represented mostly by adults and a few small, most likely young, specimens. However, the population of S. tessellatus may have been subsampled since young individuals of this species can escape through the mesh of the net. Achirus lineatus and C. spilopterus had low capture rates in Guanabara Bay; hence, the ability to classify these two species was hindered. However, both are commonly found in Brazilian estuaries and were considered as estuarine residents in previous studies (Allen & Baltz 1997ALLEN, R.L. & BALTZ, D.M. 1997. Distribution and microhabitat use by flatfishes in a Louisiana estuary. Env Biol Fish 50: 85-103., Chaves & Serenato 1998CHAVES, P.T.C. & SERENATO, A. 1998. Diversidade de dietas na assembléia de linguados (Teleostei, Pleuronectiformes) do manguezal da Baía de Guaratuba, Paraná, Brasil. Rev Bras Oceanogr 46 (1): 61-68., Mendonça & Araújo 2002MENDONÇA, P. & ARAÚJO, F.G. 2002. Composição das populações de linguados (Osteichthyes, Pleuronectiformes) da Baía de Sepetiba, Rio de Janeiro, Brasil. Rev Bras Zool 19 (2): 339-347., Chaves & Bouchereau 2004CHAVES, P.T.C. & BOUCHEREAU, J.-L. 2004. Trophic organization and functioning of fish populations in the bay of Guaratuba, Brazil, on the basis of a trophic contribution factor. Acta Adriat 45 (1): 83-94., Chaves & Bouchereau 2004CHAVES, P.T.C. & BOUCHEREAU, J.-L. 2004. Trophic organization and functioning of fish populations in the bay of Guaratuba, Brazil, on the basis of a trophic contribution factor. Acta Adriat 45 (1): 83-94., Chagas et al. 2006CHAGAS, L.P.; JOYEUX, J.-C. & FONSECA, F.R. 2006. Small-scale spatial changes in the estuarine fish: subtidal assemblages in tropical Brazil. J Mar Biol Assoc UK 86(4): 861-875., Schwarz-Junior et al. 2006SCHWARZ-JUNIOR, R.; FRANCO, A.C.N.P.; SARPEDONTI, V.; PICHLER, H.A. & QUEIROZ, G.M.L.N. 2006. Composição e estrutura da ictiofauna demersal na Baía dos Pinheiros, Paraná. Braz J Aquat Sci Technol 10 (1): 27-39.). The conclusion that A. lineatus as a resident estuarine species is also supported by the presence of larvae in the Guanabara Bay (Castro 1998CASTRO, M.S. 1998. Variação temporal das larvas de peixes em um ponto fixo da Entrada da Baía de Guanabara, RJ (Brasil). Dissertação apresentada na Universidade Federal Fluminense, Niterói.). The results presented here revealed a relatively high diversity of Pleuronectiformes in the Guanabara Bay estuary. This diversity included species of flatfishes at different niches as indicated by different trophic levels and their distinct morphologies, which has already been indicated in other estuaries, including in Brazil (Guedes & Araújo 2008GUEDES, A.P.P.; ARAÚJO, F.G. 2018. Trophic resource partitioning among five flatfish species (Actinopterygii: Pleuronectiformes) in a tropical bay in south-eastern Brail. J Fish Biol 72(4): 1035-1054.; Russo et al. 2008RUSSO, T.; PULCINI, D.; O'LEARY, Á; CATAUDELLA, S. & MARIANI, S. 2008. Relationship between body shape and trophic niche segregation in two closely related sympatric fishes. J Fish Biol 73(4): 809-828.; Lima et al. 2018LIMA, C.S.S.; CLARK, F.J.K.; SALES, N.S. & PESSANHA, A. 2018. Strategies of resource partitioning between two sympatric puffer fishes in a tropical hypersaline estuary, Brazil. Environ Biol Fish: 1-15. https://doi.org/10.1007/s10641-018-0729-z.
https://doi.org/10.1007/s10641-018-0729-...
). The pattern observed in this study is similar to the reports for other fish groups, in which a high diversity of species was accompanied by the biomass being predominantly represented by a single species: Etropus crossotus for Pleuronectiformes, as revealed by this study, Chilomycterus spinosus for Tetraodontiformes (Andrade et al. 2016ANDRADE, A.C.; SANTOS, S.R.; VERANI, J.R. & VIANNA, M. 2016. Guild composition and habitat use by Tetraodontiformes (Teleostei, Acanthopterygii) in a south-western Atlantic tropical estuary. J Mar Biol Assoc UK 96(6): 1251-1264.), Genidens genidens for Siluriformes (Silva Jr. et al. 2013), Eucinostomus argenteus for Gerreidae (Corrêa & Vianna 2016CORRÊA, B. & VIANNA, M. 2016. Spatial and temporal distribution patterns of the silver mojarra Eucinostomus argenteus (Perciformes: Gerreidae) in a tropical semi-enclosed bay. J Fish Biol 89: 641-660.) and Micropogonias furnieri for Sciaenidae (Silva Jr. et al. 2016).
In conclusion, pleuronectiform species have a number of unique characteristics, such as limited swimming ability due to the absence of a swim bladder and demersal habits, which make them especially vulnerable to low-oxygen environments (Tallqvist et al. 1999TALLQVIST, M.; SANDBERG-KILPI, E. & BONSDORFF, E. 1999. Juvenile flounder, Platichthys flesus (L.), under hypoxia: effects on tolerance, ventilation rate and predation efficiency. J Exp Mar Biol Ecol 242: 75-93., Breitburg 2002BREITBURG, D. 2002. Effects of Hypoxia, and the Balance between Hypoxia and Enrichment, on Coastal Fishes and Fisheries. Estuaries 25 (4b): 767-781., Bell & Eggleston 2005BELL, G.W. & EGGLESTON, D.B. 2005. Species-specific responses by blue crabs and fish to chronic and episodic hypoxia. Mar Biol 146: 761-770.). In the present study, the sensitivity to these adverse conditions appears to affect the distribution of flatfishes. The specific composition shows that, in Guanabara Bay, the main species caught are small in size and that while the species with the highest market value are present, Paralichthys spp. and Syacium spp., they are not commercially attractive because of their small size and low abundance. The greatest diversity was found in areas of the lower estuary, while both the middle and upper estuary showed low richness and abundance. New studies are necessary to establish the extent to which flatfish populations are affected by the ongoing environmental degradation, such as urban pollution and dredging, and the effects on the observed distribution patterns.
Acknowledgements
The authors would like to thank all who were involved in this study, especially the BioTecPesca colleagues for their assistance in collecting samples and in the biometric analyses. We thank Dr. Fabio Di Dario and one anonymous reviewer whose comments helped improve and clarify this manuscript. This study was part of the "Environmental Assessment of Guanabara Bay" program, coordinated and funded by CENPES-PETROBRAS, supported by the Long Term Ecological Program - CNPq (403809/2012-6) and FAPERJ (E-26/110.114/2013).
References
- AGUIAR, V.M.C.; NETO, JAB & RANGEL, C.M. 2011. Eutrophication and hypoxia in four streams discharging in Guanabara Bay, RJ, Brazil, a case study. Mar Poll Bull 62 (8): 1915-1919.
- ALLEN, R.L. & BALTZ, D.M. 1997. Distribution and microhabitat use by flatfishes in a Louisiana estuary. Env Biol Fish 50: 85-103.
- ANDRADE, A.C.; SANTOS, S.R.; VERANI, J.R. & VIANNA, M. 2016. Guild composition and habitat use by Tetraodontiformes (Teleostei, Acanthopterygii) in a south-western Atlantic tropical estuary. J Mar Biol Assoc UK 96(6): 1251-1264.
- ANDRADE-TUBINO, M.F.; FIORE-CORREIA, L.B. & VIANNA, M. 2009. Morphometrics and length structure of Micropogonias furnieri (Desmarest, 1823) (Perciformes, Sciaenidae) in Guanabara bay, State of Rio de Janeiro, Brazil. Bol Inst Pesca 35: 239-246.
- ANDREATA, J.V.; MEURER, B.C.; BAPTISTA, M.G.S.; MANZANO, F.V.; TEIXEIRA, D.E.; LONGO, M.M. & FRERET, N.V. 2002. Composição da assembléia de peixes da Baía da Ribeira, Angra dos Reis, Rio de Janeiro, Brasil. Rev Bras Zool 19 (4): 1139-1146.
- ARAÚJO, F.G.; CRUZ-FILHO, A.G.; AZEVEDO, M.C. & SANTOS, A.C.A. 1998. Estrutura da comunidade de peixes demersais da Baía de Sepetiba, RJ. Rev Bras Biol 58 (3): 417-430.
- BARLETTA, M.; BARLETTA-BERGAN, A.; SAINT-PAUL, U. & HUBOLD, G. 2003. Seasonal changes in density, biomass and diversity of estuarine fishes in tidal mangrove creeks of the lower Caeté Estuary (northern Brazilian coast, east Amazon). Mar Ecol Prog Ser 256: 217-228.
- BELL, G.W. & EGGLESTON, D.B. 2005. Species-specific responses by blue crabs and fish to chronic and episodic hypoxia. Mar Biol 146: 761-770.
- BLABER, S.J.M. 2000. Fish and Aquatic Resources Series, Vol 7: Tropical Estuarine Fishes: Ecology, Exploitation and Conservation. Oxford, UK. Blackwell Science Ltd.
- BLABER, S.J.M. 2013. Fish and fisheries in tropical estuaries: The last 10 years. Estuar Coast Shelf Sci 135: 57-65.
- BODIN, N.; TAPIE, N.; LE MÉNACH, K.; CHASSOT, E.; ELIE, P.; ROCHARD, E. & BUDZINSKI, H. 2014. PCB contamination in fish community from the Gironde Estuary (France): Blast from the past. Chemosphere 98: 66-72.
- BOUCHEREAU, J.L. & CHAVES, P.T.C. 2003. Ichthyofauna in the ecological organisation of a South-West Atlantic mangrove ecosystem: The bay of Guaratuba, South East Brazil. Vie et Millieu 53 (2-3): 103-110.
- BREITBURG, D. 2002. Effects of Hypoxia, and the Balance between Hypoxia and Enrichment, on Coastal Fishes and Fisheries. Estuaries 25 (4b): 767-781.
- CARPENTER, K.E. 2002. The living marine resources of the Western Central Atlantic. Volume 3: Bony fishes part 2 (Opistognathidae to Molidae), sea turtles and marine mammals. FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5. 1375-2127. Roma.
- CARREIRA R.S.; WAGENER A.L.R. & READMAN, J.W. 2004. Sterols as markers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): space-time variations. Estuar Coast Shelf Sci 60: 587-598.
- CASTRO, M.S. 1998. Variação temporal das larvas de peixes em um ponto fixo da Entrada da Baía de Guanabara, RJ (Brasil). Dissertação apresentada na Universidade Federal Fluminense, Niterói.
- CASTRO, M.S.; BONECKER, A.C.T. & VALENTIN, J.L. 2005. Seasonal variation fish larvae at the entrance of Guanabara bay, Brazil. Braz Arch Biol Technol 48 (1): 121-128.
- CATELANI, PA; PETRY, A.C.; DARIO, F.D.; SANTOS, V.L.M. & MINCARONE, M.M. 2014. Fish composition (Teleostei) of the estuarine region of the Macaé River, southeastern Brazil. Check List 10(4): 927-935.
- CHAGAS, L.P.; JOYEUX, J.-C. & FONSECA, F.R. 2006. Small-scale spatial changes in the estuarine fish: subtidal assemblages in tropical Brazil. J Mar Biol Assoc UK 86(4): 861-875.
- CHAVES, P.T.C. & BOUCHEREAU, J.-L. 2004. Trophic organization and functioning of fish populations in the bay of Guaratuba, Brazil, on the basis of a trophic contribution factor. Acta Adriat 45 (1): 83-94.
- CHAVES, P.T.C. & SERENATO, A. 1998. Diversidade de dietas na assembléia de linguados (Teleostei, Pleuronectiformes) do manguezal da Baía de Guaratuba, Paraná, Brasil. Rev Bras Oceanogr 46 (1): 61-68.
- CHAVES, P.T.C.; COVA-GRANDO, G. & CALLUF, C. 2003. Ictiofauna demersal numa região de plataforma continental do sul do Brasil submetida à pesca camaroneira. Acta Biol Parana 32 (1,2,3,4): 69-82.
- CORRÊA, B. & VIANNA, M. 2016. Spatial and temporal distribution patterns of the silver mojarra Eucinostomus argenteus (Perciformes: Gerreidae) in a tropical semi-enclosed bay. J Fish Biol 89: 641-660.
- COSTA, L.A.A.; PESSOA, D.M.M. & CARREIRA, R.S. 2018. Chemical and biological indicators of sewage river input to an urban tropical estuary (Guanabara Bay, Brazil). Ecol Indic 90: 513-518.
- DIAS, J.F.; FIADI, C.B.; SILBIGER, H.L.N. & SOARES, L.S.H. 2005. Reproductive and population dynamics of the bay whiff Citharichthys spilopterus Günther, 1862 (Pleuronectiformes: Paralichthyidae) in the Mamanguá inlet, Rio de Janeiro, Brazil. Neotrop Ichthyol 3 (3): 411-419.
- ELLIOTT, M.; WHITFIELD, A.K.; POTTER, I.C.; BLABER, S.J.M.; CYRUS, D.P.; NORDLIE, F.G. & HARRISON, T.D. 2007. The guild approach to categorizing estuarine fish assemblages: a global review. Fish Fish 8: 241-268.
- EVERMANN, B.W. & MARSH, M.C. 1902. The Fishes of Porto Rico. In: Investigations of the Aquatic Resources and Fisheries of Porto Rico by the United States Fish Comission Steamer Fish Hawk: 49-350.
- FIGUEIREDO, J.L. & MENEZES, N.A. 2000. Manual de peixes marinhos do sudeste do Brasil: VI. Teleostei (5). Museu de Zoologia USP. São Paulo.
- FORSTALL, R.L.; GREENE, R.P. & PICK, J.B. 2009. Which are the largest? Why lists of major urban areas vary so greatly. Tijdschr Econ Soc Geogr 100 (3): 277-297.
- FRANCO, A.C.S.; BROTTO, D.S.; ZEE, D.M.W. & SANTOS, L.N. 2014. Reproductive biology of Cetengraulis edentulus (Cuvier, 1829), the major fishery resource in Guanabara Bay, Brazil. Neotrop Ichthyol 12 (4): 819-826.
- GOMES, A.P.P.; KEUNECKE, K.A.; SILVA-JÚNIOR, D.M. & VIANNA, M. 2013. Modulating reproduction of Penaeidae shrimps: ecological responses of two sympatric species (Decapoda: Dendrobranchiata) on south-eastern Brazilian coast. J Mar Biol Assoc UK 93 (3): 733-740.
- GOTELLI, N.J. & COLWELL, R.K. 2010. Estimating species richness. In Biological diversity: frontiers in measurement and assessment (Magurran, A.E., McGill, B.J., ed), pp. 39-54 Oxford, UK: Oxford University Press
- GUEDES, A.P.P.; ARAÚJO, F.G. 2018. Trophic resource partitioning among five flatfish species (Actinopterygii: Pleuronectiformes) in a tropical bay in south-eastern Brail. J Fish Biol 72(4): 1035-1054.
- GUTHERZ, E.J. 1967. Field Guide to the flatfishes of the family Bothidae in the western North Atlantic. United States Fish and Wildlife Service Circular 263: 1-47.
- HAMMER, Ø.; HARPER, D.A.T. & RYAN, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Paleontol Electronica 4 (1): 9pp.
- JABLONSKI, S.; AZEVEDO, A.F. & MOREIRA, L.H.A. 2006. Fisheries and Conflicts in Guanabara bay, Rio de Janeiro, Brazil. Braz Arch Biol Technol 49 (1): 79-91.
- LIMA, C.S.S.; CLARK, F.J.K.; SALES, N.S. & PESSANHA, A. 2018. Strategies of resource partitioning between two sympatric puffer fishes in a tropical hypersaline estuary, Brazil. Environ Biol Fish: 1-15. https://doi.org/10.1007/s10641-018-0729-z
» https://doi.org/10.1007/s10641-018-0729-z - KEUNECKE, K.A.; D'INCAO, F.; VERANI, J.R. & VIANNA, M. 2012. Reproductive strategies of two sympatric swimming crabs Callinectes danae and Callinectes ornatus (Crustacea: Portunidae) in a estuarine system, south-eastern Brazil. J Mar Biol Assoc UK 92 (2): 343-347.
- MAGURRAN, A.E. 2004. Measuring biological diversity. Oxford, UK. Blackwell Science Ltda.
- MARTINHO, F.; DOLBETH, M.; VEGAS, I.; BAPTISTA, J.; CABRAL, H.N. & PARDAL, M.A. 2010. Does the flatfish community of the Mondego estuary (Portugal) reflect environmental changes? J Appl Ichthyol 26: 843-852.
- MAYR, L.M.; PARANHOS, R.; TENENBAUM, D.R.; NOGUEIRA, C.R.; BONECKER, A.C.T.; VILLAC, M.C. & BONECKER, S.L.C. 1989. Hydrobiological Characterization of Guanabara bay. In: Coastlines of Brazil (Ed. NEVES, C.). American Society of Civil Engineers, NY.
- MENDONÇA, P. & ARAÚJO, F.G. 2002. Composição das populações de linguados (Osteichthyes, Pleuronectiformes) da Baía de Sepetiba, Rio de Janeiro, Brasil. Rev Bras Zool 19 (2): 339-347.
- MENEZES, N.A.; BUCKUP, P.A.; FIGUEIREDO, J.L. & MOURA, R.L. 2003. Catálogo das espécies de peixes marinhos do Brasil. MZUSP, São Paulo.
- MULATO, I.P.; CORRÊA, B. & VIANNA, M. 2015. Distribuição espaço-temporal de Micropogonias furnieri (Perciformes, Sciaenidae) em um estuário tropical no sudeste do Brasil. Bol Inst Pesca 41 (1): 1-18.
- NELSON, J.S. 2006. Fishes of the World. 4a. Ed. 624 pp. John Wiley & Sons, Inc., NY.
- NETO, J.A.B.; GINGELE, F.X.; LEIPE, T. & BREHME, I. 2006. Spatial distribution of heavy metals in surficial sediments from Guanabara Bay: Rio de Janeiro, Brazil. Environ Geol 49: 1051-1063.
- OLIVEIRA, E.C. & FAVARO, L.F. 2011. Reproductive biology of the flatfish Etropus crossotus (Pleuronectiformes: Paralichthyidae) in the Paranaguá Estuarine Complex, Paraná State, subtropical region of Brazil. Neotrop Ichthyol 9 (4): 795-805.
- POTTER, I.C.; TWEEDLEY, J.R.; ELLIOT, M. & WHITFIELD, A.K. 2013. The ways in which fish use estuaries: a refinement and expansion of the guild approach. Fish Fish 16 (2): 230-239.
- REICHERT, M.J.M. 1998. Etropus crossotus, an annual flatfish species; age and growth of the fringed flounder in South Carolina. J Sea Res 40: 323-332.
- REICHERT, M.J.M. 2003. Diet, consumption, and growth of juvenile fringed flounder (Etropus crossotus); a test of the 'maximum growth/optimum food hypothesis' in a subtropical nursery area. J Sea Res 50: 97-116.
- RIBEIRO, C.H.A. & KJERFVE, B. 2002. Anthropogenic influence on the water quality in Guanabara Bay, Rio de Janeiro, Brazil. Reg Environ Change 3:13-19.
- RODRIGUES, C.; LAVRADO, H.P.; FALCÃO, A.P.C. & SILVA, S.H.G. 2007. Distribuição da ictiofauna capturada em arrastos de fundo na Baía de Guanabara - Rio de Janeiro, Brasil. Arq Mus Nac 65 (2): 199-210.
- ROSENFELDER, N.; LEHNERT, K.; KAFFARNIK, S.; TORRES, J.P.M.; VIANNA, M.; VETTER, W. 2012. Thorough analysis of polyhalogenated compounds in ray liver samples off the coast of Rio de Janeiro, Brazil. Environ Sci Pollut R 19: 379-389.
- RUSSO, T.; PULCINI, D.; O'LEARY, Á; CATAUDELLA, S. & MARIANI, S. 2008. Relationship between body shape and trophic niche segregation in two closely related sympatric fishes. J Fish Biol 73(4): 809-828.
- SÁNCHEZ-GIL, P.; YÁÑEZ-ARANCIBIA, A.; TAPIA, M.; DAY, J.W.; WILSON, C.A. & COWAN JR., J.H. 2008. Ecological and biological strategies of Etropus crossotus and Citharichthys spilopterus (Pleuronectiformes:Paralichthyidae) related to the estuarine plume, Southern Gulf of Mexico. J Sea Res 59: 173-185.
- SANTOS, S.R.; ANDRADE, A.C.; VERANI, J.R. & VIANNA, M. 2015. Population explosion of the burrfish Chilomycterus spinosus spinosus (Diodontidae, Tetraodontiformes) in a eutrophic tropical estuary. Mar Biol Res 11(9): 955-964.
- SCHWARZ-JUNIOR, R.; FRANCO, A.C.N.P.; SARPEDONTI, V.; PICHLER, H.A. & QUEIROZ, G.M.L.N. 2006. Composição e estrutura da ictiofauna demersal na Baía dos Pinheiros, Paraná. Braz J Aquat Sci Technol 10 (1): 27-39.
- SILVA-JÚNIOR, D.R.; GOMES, V.S.; LINDE-ARIAS, A.R. & VIANNA, M. 2012. Metallothionein in the pond perch Diplectrum radiale (Teleostei) as a biomarker of pollution in Guanabara bay estuary, Brazil. J Braz Soc Ecotoxicol 7 (1): 83-88.
- SILVA-JUNIOR, D.R.; CARVALHO, D.M.T. & VIANNA, M. 2013. The catfish Genidens genidens (Cuvier, 1829) as a potential sentinel species in Brazilian estuarine waters. J Appl Ichthyol 29:1297-303.
- SILVA-JUNIOR. D.R.; PARANHOS, R. & VIANNA, M. 2016. Spatial patterns of distribution and the influence of seasonal and abiotic factors on demersal ichthyofauna in an estuarine tropical bay. J Fish Biol 89: 821-846.
- SOARES-GOMES, A.; GAMA, B.A.P.; NETO, J.A.B.; FREIRE, D.G.; CORDEIRO, R.C.; MACHADO, W.; BERNARDES, M.C.; COUTINHO, R.; THOMPSON, F.L. & PEREIRA, R.C. 2016. An environmental overview of Guanabara Bay, Rio de Janeiro. Regional Studies in Marine Science 8(2): 319-330.
- SOARES-GOMES, A.; NEVES, R.L.; AUCÉLIO, R.; VAN DER VEN, P.H.; PITOMBO, F.B.; MENDES, C.L.T. & ZIOLLI, R.L. 2010. Changes and variations of polycyclic aromatic hydrocarbon concentrations in fish, barnacles and crabs following an oil spill in a mangrove of Guanabara Bay, Southeast Brazil. Mar Poll Bull 60: 1359-1363.
- SPACH, H.L.; SANTOS, C. & GODEFROID, R.S. 2003. Padrões temporais na assembléia de peixes na gamboa do Sucuriú, Baía de Paranaguá, Brasil. Rev Bras Zool 20 (4): 591-600.
- TALLQVIST, M.; SANDBERG-KILPI, E. & BONSDORFF, E. 1999. Juvenile flounder, Platichthys flesus (L.), under hypoxia: effects on tolerance, ventilation rate and predation efficiency. J Exp Mar Biol Ecol 242: 75-93.
- VALENTIN, J.L.; TENENBAUM, D.R.; BONECKER, A.C.T.; BONECKER, S.L.C.; NOGUEIRA, C.R.; PARANHOS, R. & VILLAC, M.C. 1999. Caractéristiques hydrobiologiques de la Baie de Guanabara (Rio de Janeiro, Brésil). J Oceanogr Rech 24 (1): 33-41.
- VALENTIN, J.L. 2012. Ecologia numérica. Uma introdução à análise multivariada de dados ecológicos. 2nd edition. Interciência, Rio de Janeiro.
- VENDEL, A.L.; SPACH, H.L.; LOPES, S.G. & SANTOS, C. 2002. Structure and Dynamics of Fish Assemblages in a Tidal Creek Environment. Braz Arch Biol Technol 45 (3): 365-373.
- VIANNA, M. & ALMEIDA, T. 2005. Bony fish bycatch in the southern Brazil pink shrimp (Farfantepenaeus brasiliensis and F. paulensis) fishery. Braz Arch Biol Technol 48 (4): 611-623.
- VIEIRA, J.P.; CASTELLO, J.P. & PEREIRA, L.E. 1998. Ictiofauna In. SEELIGER, U.; ODEBRECHT, C. & CASTELLO, J.P. (eds.). Os ecossistemas costeiro e marinho do extremo sul do Brasil. Rio Grande, Ecoscientia.
- WALSH, H.J.; PETERS, D.S. & CYRUS, D.P. 1999. Habitat utilization by small flatfishes in a North Carolina estuary. Estuaries 22: 803-813.
- WEST, J.M.; WILLIAMS, G.D.; MADON, S.P. & ZEDLER, J.B. 2003. Integrating spatial and temporal variability into the analysis of fish food web linkages in Tijuana Estuary. Environ Biol Fish 67: 297-309.
Publication Dates
-
Publication in this collection
11 Feb 2019 -
Date of issue
2019
History
-
Received
14 Apr 2018 -
Reviewed
19 Nov 2018 -
Accepted
14 Jan 2019