Abstract:
The aim of this study was to evaluate the influence of soil use on the morphological diversity of springtails and to verify the relationship of these organisms with soil physical, chemical, and microbiological properties. Samples were collected in the Southern region of Santa Catarina, involving three municipalities: Orleans, Lauro Müller, and Siderópolis, in the land use systems (LUS) of native forest (NF), Eucalyptus plantation (EP), pasture (PA), integrated crop-livestock (ICL), and no-tillage (NT). Soil samples to determine edaphic properties and pitfall traps were collected in winter and summer, in the same areas. The collected springtails were counted and morphotyped and the data were analyzed through abundance, Shannon-Wiener diversity (H') and Margalef index, Simpson dominance index (D), Pielou evenness index (J), morphotype richness, and Principal Components Analysis (PCA). Springtails morphotypes were influenced by the management conditions of each system, especially the impact of LUS on these organisms depends on the intensity of the forest/agricultural practices used, including crop rotation and soil preparation.
Keywords:
Collembola morphotypes; bioindicators; soil quality; edaphic mesofauna
Resumo:
O objetivo deste estudo foi avaliar a influência do uso do solo na diversidade morfológica de colêmbolos e verificar a relação destes organismos com os atributos físicos, químicos e microbiológicos do solo. Foram coletadas amostras na região Sul de Santa Catarina, envolvendo três municípios: Orleans, Lauro Müller e Siderópolis, nos sistemas de uso do solo (SUS) de floresta nativa (FN), reflorestamento de eucalipto (RE), pastagem perene (PA), integração lavoura-pecuária (ILP) e plantio direto (PD). As coletas de solo para determinação dos atributos edáficos e a instalação de armadilhas de queda, foram realizadas no inverno e verão, nos mesmos pontos. Os colêmbolos coletados foram contados e morfotipados e os dados foram analisados por meio da abundância, diversidade de Shannon-Wiener (H') e Margalef, índice de dominância de Simpson (D), uniformidade de Pielou (J), riqueza de morfotipos e Análise de Componentes Principais (ACP). Os morfotipos de Collembola foram influenciados pelas condições de manejo de cada sistema, sobretudo o impacto dos SUS nesses organismos depende da intensidade das práticas florestais/agrícolas utilizadas, incluindo rotações culturais e preparo do solo.
Palavras-chave:
Morfotipos de Collembola; bioindicadores; qualidade do solo; mesofauna edáfica
Introduction
Springtails are small arthropods of the edaphic mesofauna, belonging to the Collembola Class. They participate in the control of fungi biomass in the soil, also acting as its dispersers, besides serving as food for several other animals and mainly act indirectly in the decomposition of organic matter (Berude et al. 2015BERUDE, M., GALOTE, J.K. B., PINTO, P.H. & AMARAL, A.A. 2015. A mesofauna do solo e sua importância como bioindicadora. Encicl. Biosf. 11:14-28.). The abundance and diversity of springtails in agricultural and forest systems depend on different biotic and abiotic factors, but in general, the factors may be related to climate (time of year), pH, release of certain ions and humidity, besides the appearance of substances that make up pesticides and/or metals (Cassagne et al. 2003CASSAGNE, N., GERS, C. & GAUQUELIN, T. 2003. Relationships between Collembola, soil chemistry and humus types in forest stands. Biol. Fertil. Soils 37: 355-361., 2004CASSAGNE, N., BAL-SERIN, M.C., GERS, C & GAUQUELIN, T. 2004. Changes in humus properties and collembolan communities following the replanting of beech forests with spruce. Pedobiologia 48: 267-276. , Oliveira Filho & Baretta 2016OLIVEIRA FILHO, L.C.I. & BARETTA, D. 2016. Por que devemos nos importar com os colêmbolos edáficos? Sci. Agrar. 17(2):21-40.).
In addition, they show a vertical distribution along the layers of the soil, being able to be found three life forms (eco-morphological groups), based on their degree of adaptation to the soil, separated according to morphological characteristics (traits), being: epigeous, those more adapted to the plant litter; hemiedaphic are the intermediates that live between the 5 cm of the soil surface; and the edaphic ones, more adapted to the soil, that lives below 5 cm of the surface (Oliveira Filho & Baretta 2016OLIVEIRA FILHO, L.C.I., KLAUBERG FILHO, O., BARETTA, D., TANAKA, C.A.S. & SOUSA. J.P. 2016. Collembola community structure as a tool to assess land use effects on soil quality. Rev. Bras. Cienc. Solo 40:1-18.). This classification can be adopted when a rapid assessment on biodiversity is required since it requires less identification time (practicality), less specific knowledge in taxonomy and materials, when compared to identification at family, gender and species levels (Reis et al. 2016REIS, F., CARVALHO, F., SILVA, P.M., MENDES, S., SANTOS, S.A.P. & SOUSA, J.P. 2016. The use of a functional approach as surrogate of Collembola species richness in European perennial crops and forests. Ecol. Indic. 61:676-682.).
In this sense, because they present different life strategies in the vertical stratification of the soil and because of their sensitivity to environmental changes, the springtails are being used as bioindicators of soil quality (Baretta et al. 2014BARETTA, D., BARTZ, M .L. C., FACHINI, I., ANSELMI, R., ZORTÉA, T., BARETTA, C. R. D. M. 2014. Soil fauna and its relation with environmental variables in soil management systems. Rev. Ciênc. Agron 45(5): 871-879., Rieff et al. 2016RIEFF, G.G., NATAL-DA-LUZ, T., SOUSA, J.P., WALLAU, M.O., HAHN, L., SACCOL DE SÁ, E.L. 2016. Collembolans and mites communities as a tool for assessing soil quality: effect of eucalyptus plantations on soil mesofauna biodiversity. Curr. Sci. 110(4):713-719,), since they are sensitive to changes in soil and in vegetal cover, caused by anthropic activities or by natural causes (Oliveira Filho & Baretta 2016OLIVEIRA FILHO, L.C.I. & BARETTA, D. 2016. Por que devemos nos importar com os colêmbolos edáficos? Sci. Agrar. 17(2):21-40.). In addition, they show sensitivity to temperature changes (Rieff et al. 2014RIEFF, G.G., NATAL-DA-LUZ, T., SOUSA, J.P. & SÁ, E.L.S. 2014. Diversity of springtails and mites of a native forest in southern Brazil: relationship with the indices of temperature and precipitation in the native environment. Int. J. Emerg. Technol. Adv. Eng. 4(9):684-692.), precipitation and humidity (Oliveira Filho & Baretta 2016OLIVEIRA FILHO, L.C.I. & BARETTA, D. 2016. Por que devemos nos importar com os colêmbolos edáficos? Sci. Agrar. 17(2):21-40.) and pesticide application (Lima & Silva et al. 2017LIMA e SILVA, C., BRENNAN, N., BROUWER, J.M., COMMANDEUR, D., VERWEIJ, R.A. & VAN GESTEL, C.A.A. 2017. Comparative toxicity of imidacloprid and thiacloprid to different species of soil invertebrates. Ecotoxicology 26:555-564.).
Even with such importance, there is still a lack of studies in Brazil that deal with the biological quality of the soil. In Santa Catarina, studies with this theme are even more incipient, especially in the case of springtails as bioindicators (Oliveira Filho et al. 2016OLIVEIRA FILHO, L.C.I. & BARETTA, D. 2016. Por que devemos nos importar com os colêmbolos edáficos? Sci. Agrar. 17(2):21-40., Santos et al. 2018SANTOS, M.A.B., OLIVEIRA FILHO, L.C.I., POMPEO, P.N., ORTIZ, D.C., MAFRA, Á.L., KLAUBERG FILHO, O., BARETTA, D. 2018. Morphological diversity of springtails in land use systems. Rev. Bras. Cienc. Solo 42:e0170277.). Studies with this type of approach allow a better understanding of how intensive management practices may be harmful to biological diversity and, consequently, how they impact several ecosystem processes, such as nutrient cycling and organic matter decomposition. Hence, less intensive management practices can ensure equilibrium in the soil and in the environment as a whole, such as that found in more conservative agricultural systems and native forests, for example.
Thus, to study the diversity of springtails present in different land use systems allows to evaluate which system conserves better the biological quality of the soil. Therefore, the objective of this study was to evaluate the effect of soil use intensity on the morphological diversity of springtails, as well as their relationship with soil chemical, physical and microbiological properties, in the southern region of Santa Catarina, using the technique of morphotyping as an alternative to traditional taxonomy.
Material and Methods
1. Study area
The study was carried out in the municipalities of Orleans, Lauro Müller, and Siderópolis, in the southern region of the state of Santa Catarina (SC) (Table 1). The climate of the southern region is characterized by Köppen as mesothermal moist, with hot summer (Cfa). The soils found were classified as Argissolo Vermelho-Amarelo (Typic Hapludult), derived from siltstone, formation in Rio Bonito, Lauro Müller, and Siderópolis; Argissolo Vermelho-Amarelo (Typic Hapludult), derived from granite, intrusive suite, in Orleans.
Characteristics of land use systems, native forest (NF), Eucalyptus plantation (EP), pasture (PA), integrated crop-livestock (ICL) and no-tillage (NT) in the municipalities of Orleans, Lauro Müller and Siderópolis, in the southern region of Santa Catarina.
The land use systems (LUS) studied include: native forest (NF), Eucalyptus plantation (EP), pasture (PA), integrated crop-livestock (ICL), and no-tillage (NT), with the purpose of establishing a gradient of anthropogenic intervention, considering less interference in NF and greater interference in NT. The selection of the areas in each of the three municipalities sought similar environmental conditions that allowed the comparison between the systems, such as similar management history, geographic characteristics and even soil type. Information on the characteristics and history of the areas are shown in Table 1.
2. Experimental design and sampling
Samples were collected at two distinct seasons, winter (July 2011) and summer (January 2011). Sampling in each treatment (LUS) occurred in a sample grid of 3×3 points, with spacing between each point of 30 m (to avoid autocorrelation) and 20 m of border, totaling 1 ha for each area, same scheme used by Oliveira Filho et al. (2016)OLIVEIRA FILHO, L.C.I. & BARETTA, D. 2016. Por que devemos nos importar com os colêmbolos edáficos? Sci. Agrar. 17(2):21-40. and Santos et al. (2018)SANTOS, M.A.B., OLIVEIRA FILHO, L.C.I., POMPEO, P.N., ORTIZ, D.C., MAFRA, Á.L., KLAUBERG FILHO, O., BARETTA, D. 2018. Morphological diversity of springtails in land use systems. Rev. Bras. Cienc. Solo 42:e0170277.. This sampling would result in 270 samples in total, as it was considered three municipalities, in which five LUS were analyzed, and in each LUS nine sample points, in two periods (winter and summer). However, for the analyzes of this study, only the first five sample points cataloged (totaling 150 samples) were considered, because the number of springtails per sample was very high and, therefore, it was considered that five samples would already be representative of the community.
For the evaluation of soil chemical and microbiological properties, fifteen subsamples were collected around approximately 0.10 to 0.20 m from each of the sampling grid points in the 0.00-0.20 m layer to form a representative composite sample. For the soil physical properties, samples were collected with structures preserved in steel cylinders, 5 cm in diameter and 5 cm in height (undeformed).
3. Sampling and morphotyping of springtails
The springtails were captured by pitfall traps containing a solution of approximately 200 mL of water with detergent (3:1 ratio), installed at each of the sample points and maintained in the field for 72 hours. Afterward, they were collected, sorted, separated and fixed in absolute alcohol (99.5%) for preservation. Specimens of this survey are housed at the Collection of the Universidade do Estado de Santa Catarina.
Then, the springtails were counted and separated in different morphotypes, using a stereoscopic microscope with an increase of up to 50 times. The separation of springtails in morphotypes followed the methodology used in the studies of Oliveira Filho et al. (2016)OLIVEIRA FILHO, L.C.I. & BARETTA, D. 2016. Por que devemos nos importar com os colêmbolos edáficos? Sci. Agrar. 17(2):21-40. and Santos et al. (2018)SANTOS, M.A.B., OLIVEIRA FILHO, L.C.I., POMPEO, P.N., ORTIZ, D.C., MAFRA, Á.L., KLAUBERG FILHO, O., BARETTA, D. 2018. Morphological diversity of springtails in land use systems. Rev. Bras. Cienc. Solo 42:e0170277.. This methodology is based on the springtails adaptation to the soil, evaluated according to the EMI (Eco-morphological index), which is based on the observation of five morphological traits for each springtail: presence or absence of ocelli, bristles and/or scales, pigmentation, antenna length, and furcula (Reis et al. 2016REIS, F., CARVALHO, F., SILVA, P.M., MENDES, S., SANTOS, S.A.P. & SOUSA, J.P. 2016. The use of a functional approach as surrogate of Collembola species richness in European perennial crops and forests. Ecol. Indic. 61:676-682.). To each of these traits was assigned a partial EMI value, and the sum of these values (total EMI) indicates a greater or lesser adaptation of the springtail to the soil. The higher the value of the total EMI, the greater its adaptation to the soil and the lower the dispersion power of the organism, and vice versa. This index can vary between 0 and 20 (Oliveira Filho et al. 2016OLIVEIRA FILHO, L.C.I. & BARETTA, D. 2016. Por que devemos nos importar com os colêmbolos edáficos? Sci. Agrar. 17(2):21-40., Santos et al. 2018SANTOS, M.A.B., OLIVEIRA FILHO, L.C.I., POMPEO, P.N., ORTIZ, D.C., MAFRA, Á.L., KLAUBERG FILHO, O., BARETTA, D. 2018. Morphological diversity of springtails in land use systems. Rev. Bras. Cienc. Solo 42:e0170277.).
Thus, for each different combination of the five traits a morphotype (morphological form) was assigned, allowing them to be separated into three eco-morphological groups, being: edaphic (with life in soil), which encompasses morphotypes with total EMI value ranging from 14 to 20; hemiedaphic, with values between 8 to 12, and epigeous (litter inhabitants), those with values between 0 to 6. Each combination of morphological characteristics that correspond to a given morphotype can be found in Santos et al. (2018)SANTOS, M.A.B., OLIVEIRA FILHO, L.C.I., POMPEO, P.N., ORTIZ, D.C., MAFRA, Á.L., KLAUBERG FILHO, O., BARETTA, D. 2018. Morphological diversity of springtails in land use systems. Rev. Bras. Cienc. Solo 42:e0170277..
It is worth mentioning that the use of morphotypes to study the community of Collembola make it possible to understand both the functional role of organisms and the influence of habitat alterations, and is able to provide reliable data that resemble species richness (Reis et al. 2016)REIS, F., CARVALHO, F., SILVA, P.M., MENDES, S., SANTOS, S.A.P. & SOUSA, J.P. 2016. The use of a functional approach as surrogate of Collembola species richness in European perennial crops and forests. Ecol. Indic. 61:676-682.. The nomenclature of the morphotypes, used in the present study, refers to the eco-morphological group to which they belong, so Ed refers to the edaphic, H to the hemiedaphic, and Ep to the epigeous.
4. Chemical, physical and microbiological analyzes of the soil
The determination of the chemical properties occurred according to the methodologies of Tedesco et al. (1995)TEDESCO, M.J., GIANELLO, C., BISSANI, C.A., BOHNEN, H. & VOLKWEISS, S.J. 1995. Análise de solo, plantas e outros materiais. 2 ed. Universidade Federal do Rio Grande do Sul, Porto Alegre, p.174., being: pH in water, Ca2+, Mg2+, Al3+, potential acidity (H+Al), Mg/K, organic matter (OM), and C/N ratio. The soil physical properties such as total porosity (TP), microporosity (Micro), macroporosity (Macro), and biopores (Bio) were determined according to Claessen (1997)CLAESSEN, M.E.C. 1997. Manual de métodos de análise de solo. 2 ed. Embrapa Solos, Rio de Janeiro. methodologies. Soil volumetric moisture was determined in the laboratory with samples dried in an oven at 105 °C for 24 hours. For the properties related to soil carbon dynamics, the carbon of the microbial biomass (MBC) was determined by the fumigation-extraction method (Vance et al. 1987VANCE, E.D., BROOKS, P.C. & JENKINSON, D.S. 1987. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703-707.) and the microbial activity determined by microbial basal respiration (MBR) (Alef & Nannipieri 1995ALEF, K. & NANNIPIERI, P. 1995. Methods in applied soil microbiology and biochemistry. London, p.576.). With the results of MBR and MBC, the metabolic quotient (qCO2) was calculated (Tótola & Chaer 2002TÓTOLA, M.R. & CHAER, G.M. 2002. Microrganismos e processos microbiológicos como indicadores da qualidade do solo. In Tópicos em Ciência do Solo (V.H. Alvarez, C.E.G.R Schaefer, N.F. Barros, J.W.V. Melo, L.M. Costa eds) Sociedade Brasileira de Ciência do Solo, Viçosa. 2:195-276.). Total organic carbon (TOC) was determined by dry combustion by the CNHS Vario EL Cube Elemental Analyzer. Fresh soil samples were used to quantify microbiological properties and the results were expressed on dry soil.
5. Data analysis
The analyzes were performed at LUS level, using the values of three municipalities (true replicates of LUS) and five samples per municipality (n=3×5=15). The Collembola morphotypes were analyzed by abundance, Shannon-Wiener (H'), Margalef diversity index, Simpson dominance index (D), Pielou evenness index (J), and morphotype richness for each LUS, using the PAST 3.0 Software.
In order to compare the results among the LUSs in each age, we used variance analysis (ANOVA) of the main effects using the Newman-Keuls post-hoc test (p<0.05), and for the variables that did not meet the normality assumptions and homogeneity, even after the data transformation, the Kruskal-Wallis non-parametric test was used, both tests using the program STATISTICA version 7.
In addition, the abundance of morphotypes was subjected to Detrended Correspondence Analysis (DCA), to obtain the value of the gradient length, which presented a result lower than three (≤3), meaning that the data showed a linear response, recommending the user Principal Component Analysis (PCA).
The abundance of morphotypes was used as response variable (effect) and the soil physical, chemical, and microbiological properties were used as explanatory environmental variables in the PCAs. The collinear explanatory variables were verified by the variance inflation factor and by forward selection interventions, performing continuous Redundancy Analysis (RDA) based on Monte-Carlo test permutations for each type of variable, the explanatory variables that presented collinearity were withdrawn and those that best explained the variation of the data (p≤0.05) were selected. Thus, it was possible to choose a minimum set of significant physical, chemical and microbiological variables, which best explain the variation of Collembola morphotypes in each collection season. Finally, only the significant RDA variables were later used in the PCAs as explanatory environmental variables, which clarified the changes observed for the morphotypes. For the accomplishment of the multivariate analysis (DCA, RDA, and PCA), the statistical software CANOCO version 4.5 was used.
Results
1. Abundance, richness, and diversity of morphotypes
Eighteen Collembola morphotypes were found in the studied LUS, regardless the collection season, of which two belong to the edaphic (Ed) eco-morphological group, 10 to the hemiedaphic (H), and six to the epigeous (Ep). In the summer, 22,248 springtails were distributed in 18 morphotypes and 24,376 springtails in the winter, distributed in 12 morphotypes, totaling 46,624 springtails. The morphological group that presented the greatest representativity was the hemiedaphic with 46,002 individuals (98.67% of the total abundance) followed by the epigeous with 448 organisms (0.96%) and with the smaller number of edaphic representatives, with 174 springtails (0.37%). The most representative morphotypes were: H48 (48.47%), H32 (37.44%), and H50 (9.90%). While the least expressive, considering the abundance equal to and less than 15 individuals, were the morphotypes H35, H57, and Ep24 (0.002%).
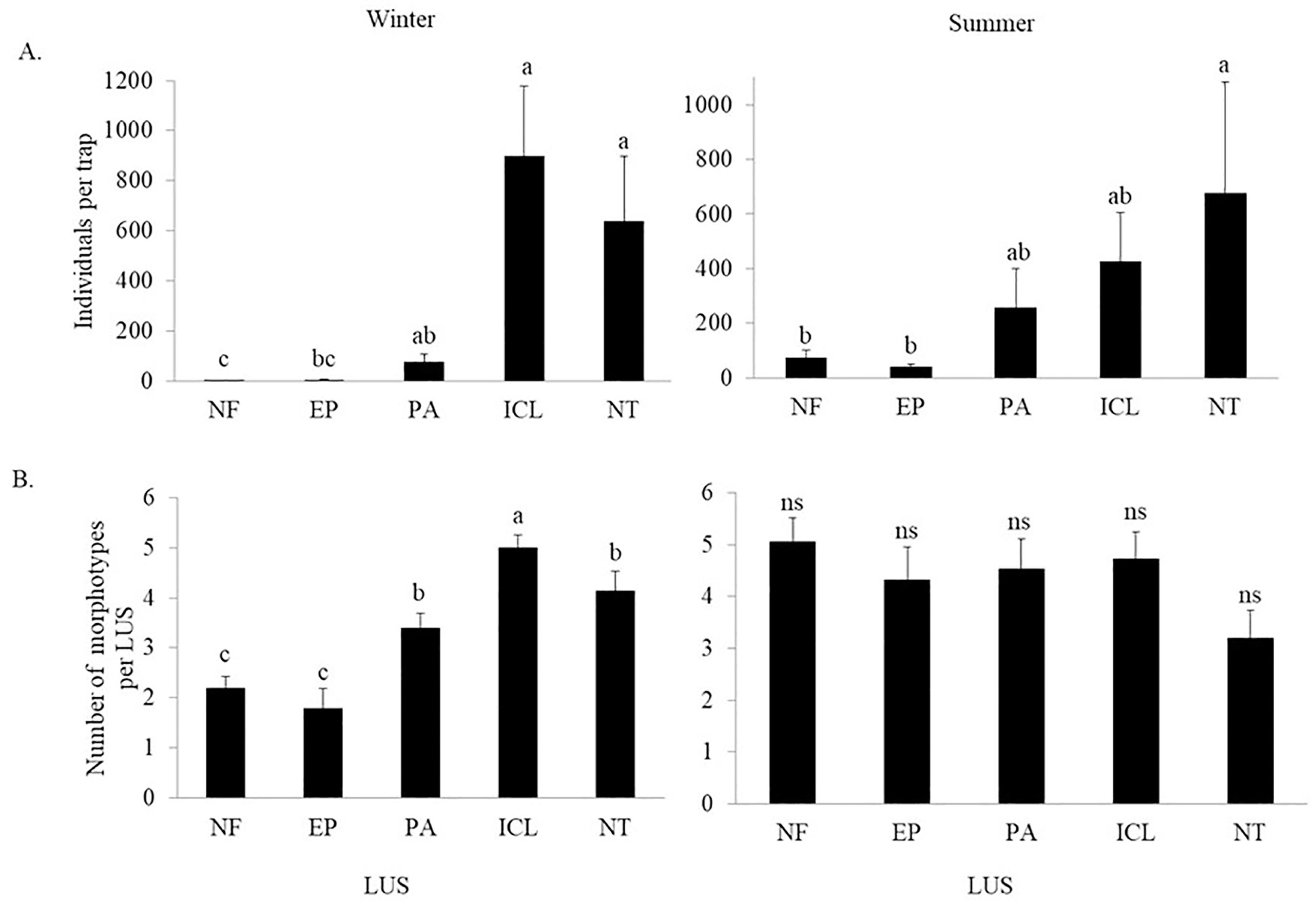
The total abundance of springtails in the winter showed a difference (p<0.05) between the LUS (Figure 1A), in which the ICL presented the greatest abundance of springtails, without, however, differing from NT and PA. Meanwhile, NF presented the lowest abundance of springtails, not differing from EP, and ER being equal to PA. In the summer, NT presented the greatest abundance of springtails, differing from the other LUS. For the richness of morphotypes found in winter (Figure 1B), the ICL system presented the highest richness value, differing from of the other LUS.
Springtails average abundance [individuals per trap] (A) and richness of morphotypes [number of morphotypes per LUS] (B), in the southern region of Santa Catarina, in native forest (NF), Eucalyptus plantation (EP), pasture (PA), integrated crop-livestock (ICL) and no-tillage (NT), in winter and summer. ns: not significant. The summer means followed by the same letter in the bars do not differ from each other by Newman-Keuls test (p<0.05; n=15). * Winter means values followed by the same letter in the bars do not differ from each other by the Kruskal-Wallis test of multiple comparisons (p<0.05, n=15).
For the Shannon diversity index (H'), in winter, it varied only between EP and ICL, where EP presented the lowest value and ICL the largest. For the Pielou index (J), the NF showed the highest value and did not differ only from the ER, while the other LUSs studied were similar to each other. The Margalef diversity was higher in NF, but it did not differ from PA, while ICL and NT were similar and the lowest value was in EP (Table 2).
Values of Shannon (H’) and Margalef diversity indexes, Pielou evenness index (J), Simpson dominance index (D) in native forest systems (NF), Eucalyptus plantation (EP), perennial pasture (PA), integrated crop-livestock (ICL), and no-tillage (NT), in winter and summer in the southern region of Santa Catarina.
In summer, for the H' index, the NT system was different from all studied LUSs, presenting the lowest value of diversity, while the other LUSs did not present significant differences. For the Margalef diversity, the NT had the lowest value, while the highest value was observed in the NF (Table 2).
2. Analysis of community composition
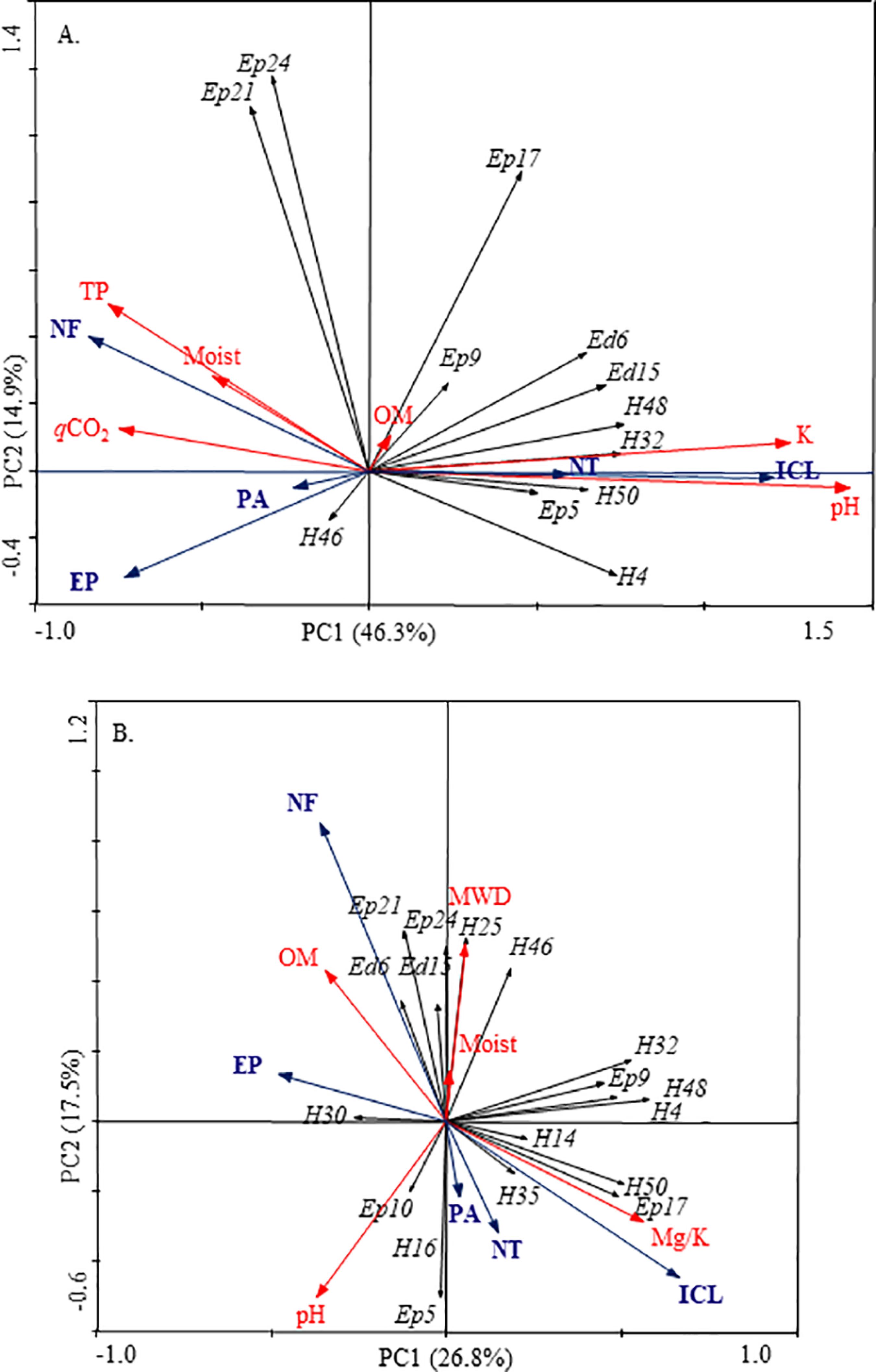
The PCAs for the abundance of springtails morphotypes, both in winter (Figure 2A) and in summer (Figure 2B), showed differences between LUS. In winter (Figure 2A), the total variability of springtails morphotype abundance was explained in 46.3% by principal component 1 (PC1) and 14.9% by PC2, totaling 61.3%. It is observed through the PCA a clear separation of LUS referring to the intensity of soil use, where NF was separated from the other LUS, the EP and PA were close and far from NT and ICL, which were related to each other.
The relationship between the principal components 1 (PC1) and 2 (PC2) of the Principal Component Analysis (PCA), the Collembola morphotypes (black arrows) and their relation to the land use systems (blue arrows) and the environmental variables (red arrows) used as explanatory variables, in winter (A) and summer (B), in the southern region of Santa Catarina. NF: native forest; EP: Eucalyptus plantation; ICL: integrated crop-livestock; PA: pasture; NT: no-tillage; Ed: edaphic springtail; H: hemiedaphic springtail; Ep: epigeous springtail; K: potassium; pH: potential hydrogen; OM: organic matter; qCO2: metabolic quotient; TP: total porosity; Moist: soil moisture; Mg/K: magnesium/potassium ratio; MWD: mean weight diameter of soil aggregates.
The EP and PA systems seem close, but the EP was not related to any specific morphotype, whereas the PA was related to a hemiedaphic morphotype (H46). On the other hand, the NT and ICL systems, which were close and similar, were the LUS that related to most of the morphotypes (Ed6, Ed15, H48, H32, H50, H4, Ep9, Ep5, and Ep17), distributed in all the eco-morphological groups. Native forest in winter was related to only two epigeous morphotypes (Ep24 and Ep21).
The environmental variables used in the PCA help to explain the distribution of morphotypes in each LUS, through the association that they showed after being projected a posteriori in the analysis. Thus, in winter, TP, qCO2, and moisture contribute to explain the abundance of morphotypes in the NF. While K and pH explain the morphotypes associated with NT and ICL. The OM variable was very close to the ordering center, not being associated with any LUS, but it was closer to the Ep9 morphotype. For LUS, EP, and PA, they did not have any variables that contributed to the explanation of the springtails morphotypes abundance (Figure 2A).
In summer (Figure 2B), the total variability of the springtails morphotype abundance data was explained in 26.8% by PC1, and 17.5% by PC2, totaling 44.3%. Native forest and EP seem close, but EP was related to only one hemiedaphic morphotype (H30), whereas, NF showed a relation with six morphotypes (Ed6, Ed15, H25, H46, Ep21, and Ep24), distributed among all eco-morphological groups. Next, PA and NT seem to be close, but little related to the abundance of springtails (H16, Ep10, and Ep5); while ICL, even with proximity to NT and PA, has a relation with more morphotypes, total of eight (H32, H48, H4, H14, H35, H50, Ep17, and Ep9) belonging to the group of hemiedaphic and epigeous (Figure 2B).
The properties OM, MWD, and moisture explained the occurrence of morphotypes in the NF in the summer. Meanwhile, the Mg/K ratio explains the morphotypes associated with ICL, and pH was the variable that best contributed to explain the morphotypes associated with PA and NT. Eucalyptus plantation had no variable explaining the abundance of springtails morphotypes (Figure 2B).
Discussion
1. Abundance, richness, and diversity of morphotypes
Normally, due to the quality of the environment, the diversity of plant species and the microclimate established, it is expected an increased abundance of organisms in the areas of NF. On the other hand, the greater abundance in NT and ICL may be related to the good management adopted in these systems, with the maintenance of cultural residues on the soil surface, improving the microclimate for edaphic fauna (Bartz et al. 2013BARTZ, M.L.C., PASINI, A. & BROWN, G.G. 2013. Earthworms as soil quality indicators in Brazilian no-tillage systems. Appl. Soil Ecol. 69:39-48.). In addition to the maintenance of cultural residues, which can be observed in the history of NT and ICL (Table 1), there is also crop rotation between winter and summer, which favors more diversified habitat and with different food offerings.
Above all, some studies indicate that the application of exogenous substances, organic fertilizers, increases the activity of microorganisms (Zhang et al. 2014ZHANG, B., CHANG, L.; NI, Z., CALLAHAM, M. A., SUN, X. & WU, D. 2014. Effects of land use changes on winter-active Collembola in Sanjiang Plain of China. Appl. Soil Ecol. 83:51-58.), which is a food source for many springtails and mites. From the history of the LUS (Table 1), it can be observed that the ICL system located in Orleans had poultry litter applied, which consequently provides a greater amount of organic matter, influencing the microbial biomass that serves as food for the springtails (Berude et al. 2015BERUDE, M., GALOTE, J.K. B., PINTO, P.H. & AMARAL, A.A. 2015. A mesofauna do solo e sua importância como bioindicadora. Encicl. Biosf. 11:14-28.), favoring the development of its population.
Another determinant factor for the abundance of organisms in the LUS may be the presence of natural predators, which may have contributed to the fact that the NF did not present, in any of the stations, higher values of springtails abundance, possibly because they had a higher performance or more predators that in other LUS. In the study by Machado et al. (2015)MACHADO, D.L., PEREIRA, M.G., CORREIA, M.E. F., DINIZ, A.R. & MENEZES, C.E.G. 2015. Fauna edáfica na dinâmica sucessional da Mata Atlântica em Floresta Estacional Semidecidual no Rio Paraíba do Sul, RJ. Cienc. Florest. 25:91-106., when analyzing the community of edaphic organisms in forests with different stages of succession (initial stage, secondary in medium stage, and secondary in advanced stage), verified a higher number of Collembola (Entomobryomorpha) in sites with initial and medium stage of succession than in advanced stages, one of the possible explanations was that in these areas there was no expressive participation of predatory groups, which could control the populations of Collembola.
Low values of the abundance of springtails in Eucalyptus plantation, in two seasons, may be related to the plant material provided by this species, since the Eucalyptus plantations are biologically poor for providing low nutritional quality material, which consequently implies food resources of low quality in this system. In the study by Martins et al. (2013)MARTINS, C., NATAL-DA-LUZ, T., SOUSA, J.P., GONÇALVES, M.J., SALGUEIRO, L. & CANHOTO, C. 2013. Effects of essential oils from Eucalyptus globulus leaves on soil organisms involved in leaf degradation. Plos One 8(4):1-7., when analyzing the effect of the essential oils present in the degradation of E. globulus leaves, using a reproduction and leakage test with springtails of Folsomia candida species, verified that the eucalyptus oils affected both the reproduction and the feeding preference of the springtails.
In the study by Santos et al. (2018)SANTOS, M.A.B., OLIVEIRA FILHO, L.C.I., POMPEO, P.N., ORTIZ, D.C., MAFRA, Á.L., KLAUBERG FILHO, O., BARETTA, D. 2018. Morphological diversity of springtails in land use systems. Rev. Bras. Cienc. Solo 42:e0170277., in the same LUSs in the eastern region of Santa Catarina, they found greater values of richness in the NF and ICL systems in winter, although the ICL did not differ from the NT and PA systems. For the authors, what influences the richness of morphotypes is the functional diversity and the least perturbation in areas of NF, associated to the diversity of the vegetation, providing several trophic and habitat resources for the springtails. These authors brought important points to be observed in the results found in the present study, in which the cultivation system as ICL presented positive results regarding the richness of species of springtails and what was expected was that higher values were found in the NF of according to the intensity of land use. Thus, we can highlight the advantages of a mixed production system, more sustainable than conventional systems, specializing in only one crop. This type of system has as advantages the synergism between the pastures and the annual crops used, which positively affects the physical, chemical and biological properties of the soil (Vilela et al. 2011VILELA, l., MARTHA JUNIOR, G.B., MACEDO, M. C.M., MARCHÃO, R.L., GUIMARÃES JÚNIOR, R., PULROLNIK, K., MACIEL. 2011. Sistemas de integração lavoura-pecuária na região do Cerrado. Pesq. agropec. bras 46(10):1127-1138.). In a study by Tsiafouli et al. (2015)TSIAFOULI, M. A., THÉBAULT, E., SGARDELIS, S.P., RUITER, P.C., VAN DE PUTTEN, W.H., BIRKHOFER, K., HEMERIK, L., VRIES, F.T., BARDGETT, R.D., BRADY, M.V., BJORNLUND, L., JØRGENSEN, H.B., CHRISTENSEN, S., HERTEFELDT. T.D., HOTES, S., GERA HOL, W., FROUZ, J., LIIRI, M., MORTIMER, S.R., SETÄLÄ, H., TZANOPOULOS, J., UTESENY, K., PIŽL, V., STARY, J., WOLTERS, V. & HEDLUND, K. 2015. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change. Biol. 21(2):973-985. analyzing groups of earthworms, springtails, and mites in all regions of Europe, observed that the richness of these groups was negatively influenced by the increase in the intensity of soil use.
In addition, one of the main characteristics of this system is the entry and replenishment of soil organic matter (SOM), reducing its fluctuation, which normally occurs during the grazing period for grazing (SOM increases during grazing period and decreases in the period of grains), thus having a better equilibrium as regards the content of SOM (García-Préchac et al. 2004GARCÍA-PRÉCHAC, F., ERNST, O., SIRI-PRIETO, G., TERRA, J.A. 2004. No-till into crop-pasture rotations in Uruguay. Soil Till Res 77(1):1-13., Almeida et al. 2017ALMEIDA, H. S., TROMBETA, H. W., WELTER, P. D. 2017. Organismos edáficos em sistema de integração lavoura pecuária. J. Agron. Sci. 6:23-40. ). Finally, it favors the presence of organisms of the fauna of the soil, as the springtails, because there are refuges, different alimentary resources and a greater diversity of vegetal species, when compared to conventional crops, that use monoculture and solved of the ground.
The indices addressed in this study did not show the expected pattern of land use intensification (NF<EP<PA<ICL<NT). However, one of the factors that may raise the diversity of Collembola in a given environment, in the case of ICL in winter and NF in summer, is the richness of plant species. Sabais et al. (2011)SABAIS, A.C.W., SCHEU, S. & EISENHAUER, N. 2011. Plant species richness drives the density and diversity of Collembola in temperate grassland. Acta Oecol. 37: 195-202. found that the richness of plant species led to the greater diversity of Collembola species in temperate pastures.
In addition, higher values of H' (winter and summer) and Margalef (winter) in NF were also found by Santos et al. (2018)SANTOS, M.A.B., OLIVEIRA FILHO, L.C.I., POMPEO, P.N., ORTIZ, D.C., MAFRA, Á.L., KLAUBERG FILHO, O., BARETTA, D. 2018. Morphological diversity of springtails in land use systems. Rev. Bras. Cienc. Solo 42:e0170277.. The authors commented that Margalef less than 2.0 indicates areas of low diversity and H' generally varies from 1.5 (low diversity) to 3.5 (high diversity), so, they pointed out that although the results found indicated low diversity, as in the present study, this indicates that this diversity is naturally low in NF and even higher than the other LUS. The factors that may be associated with this scenario are the lower temperature and humidity variations, together with the larger litter deposit in the NF, environment conducive to springtails.
It was found that having high abundance in the system does not necessarily reflect higher values of richness and diversity index. Higher values of springtail abundance, as found in NT in summer (Figure 1A) and higher value in index D (Table 2) may mean higher dominance of one or a few species, which may reflect less diversity and consequently lower resilience to ecosystem services (Baretta et al. 2011BARETTA, D., PIRES, J.C.P., SEGAT, J.C., GEREMIA, E.V., OLIVEIRA FILHO, L.C.I. & ALVES, M.V. 2011. Fauna edáfica e qualidade do solo. In Tópicos Especiais em Ciência do Solo. (O. Klauberg Filho., A.L. Mafra., L.C. Gatiboni eds). Viçosa: Sociedade Brasileira de Ciência do Solo, v.7, p.141-192.).
In the case of ICL, which in winter showed higher abundance (Figure 1A) and the lower value of D index (Table 2), not following the same pattern occurred in the NT above, may be related to the management adopted in the ICL. Eventually one of the disadvantages in these LUS is the use of pesticides for the control of spontaneous plants and pests, which can cause a decline in the diversity of the Collembola community. In fact, some studies, such as Zortéa et al. (2015)ZORTÉA, T., BARETTA, D., SEGAT, J.C., MACCARI, A.P. & BARETTA, C.R.M. 2015. Comportamento de fuga de colêmbolos expostos a solos contaminados com cipermetrina. Sci. Agrar. 16(4):49-58., show that the springtails may be sensitive to some agrochemicals, such as cypermethrin (pyrethroid), which, through the ecotoxicological leakage test, had a negative effect on the behavior of F. candida species. As a result, some species may be more sensitive and others more tolerant, which may determine the dominance of one or a few species in the system.
2. Analysis of community composition
The environmental variable TP helps explain the relationship of morphotypes in NF in winter (Figure 2A), and it is influenced by soil management. In most cases, native forests have higher soil TP when compared to areas of reforestation, pasture, and agricultural crops, and this is due to soil conservation and presence of roots in these environments (Loss et al. 2014LOSS, A., COSTA, E.M., PEREIRA, M.G. & BEUTLER, S.J. 2014. Agregação, matéria orgânica leve e carbono mineralizável em agregados do solo. Rev. Fac. Agron. 113(1):1-8.). In addition, TP is a property that impacts the community of springtails, because these organisms seek shelter and move mainly through the porous structures of the soil (Oliveira Filho & Baretta 2016OLIVEIRA FILHO, L.C.I. & BARETTA, D. 2016. Por que devemos nos importar com os colêmbolos edáficos? Sci. Agrar. 17(2):21-40.). Santos et al. (2018)SANTOS, M.A.B., OLIVEIRA FILHO, L.C.I., POMPEO, P.N., ORTIZ, D.C., MAFRA, Á.L., KLAUBERG FILHO, O., BARETTA, D. 2018. Morphological diversity of springtails in land use systems. Rev. Bras. Cienc. Solo 42:e0170277. analyzing Collembola morphotypes in the Eastern region of Santa Catarina, in the same LUSs addressed in this study, also found TP as an explanatory environmental variable in the NF area, although it was in the summer, and they highlighted that the morphotypes of all the eco-morphological groups found associated with NF, are benefited by the best soil TP.
The morphotypes related to NF are also benefited by the explanatory variable moisture. According to Bellini (2016)BELLINI, B.C. 2016. Colêmbolos: uma riqueza microscópica no Semiárido. In Conhecendo os artrópodes do Semiárido 1 ed. (F. Bravo & A.R. Calor eds). Métis Produção Editorial, São Paulo, p.43-53., most of the springtails have intimate affinity and dependence on moist soil, making several species susceptible to drought. Temperature and moisture are factors that influence the ideal habitat and the reproduction and growth rates of individuals, as well as their vertical distribution along the soil profile (Rieff et al. 2014RIEFF, G.G., NATAL-DA-LUZ, T., SOUSA, J.P. & SÁ, E.L.S. 2014. Diversity of springtails and mites of a native forest in southern Brazil: relationship with the indices of temperature and precipitation in the native environment. Int. J. Emerg. Technol. Adv. Eng. 4(9):684-692.).
In LUS, NT and ICL, pH was the environmental variable that helped explain the abundance of Collembola morphotypes, and it was the LUS that related to most of the morphotypes (Ed6, Ed15, H48, H32, H50, H4, Ep17, Ep9, and Ep5). In these systems, often the pH of the soil is corrected, making it higher than compared to native forest areas, where the soil largely has acidic pH. In addition, the ICL area in Orleans (Table 1) received an application of poultry litter, in which there is a presence of lime, which, when reacted in the soil, results in elevated pH. Besides, pH is also a factor that influences the presence of Collembola species. Antoniolli et al. (2013)ANTONIOLLI, Z., REDIN, M., SOUZA, E.L. & POCOJESKI, E. 2013. Metais pesados, agrotóxicos e combustíveis: efeito na população de colêmbolos no solo. Cienc. Rural 43(6):992-998. found in their study that the decrease of pH (greater acidity), makes the development of springtail in the soil harder. Thus, it explains the presence of most of the Collembola morphotypes found in the LUS, NT, and ICL, which were favored with the soil pH conditions found there.
Regarding the results found in summer (Figure 2B), the environmental variable OM helps to explain the abundance of springtail morphotypes in the NF, considering that some springtail morphotypes are influenced by the quality and quantity of OM in the soil, since they feed on decaying material, that is, they participate in the decomposition of OM and in the nutrient cycling (Berude et al. 2015BERUDE, M., GALOTE, J.K. B., PINTO, P.H. & AMARAL, A.A. 2015. A mesofauna do solo e sua importância como bioindicadora. Encicl. Biosf. 11:14-28.). The relationship verified by PCA (Figure 2B) between NF, OM and some springtail morphotypes (especially Ed6, Ed15, H57, H46, H25, and Ep21) were also found in the Santos et al. (2018)SANTOS, M.A.B., OLIVEIRA FILHO, L.C.I., POMPEO, P.N., ORTIZ, D.C., MAFRA, Á.L., KLAUBERG FILHO, O., BARETTA, D. 2018. Morphological diversity of springtails in land use systems. Rev. Bras. Cienc. Solo 42:e0170277. study in Eastern Santa Catarina, which reinforces that there is a close relationship between the springtails and the organic material deposited in the soil.
In addition to the greater amount of OM offered by the diversity of plant species in the NF, the greater number of roots favors the aggregation of the soil. The mean weight diameter of soil aggregates (MWD) is a measure of soil aggregate stability, which indicates soil resistance to erosion. Thus, soil aggregation is important to favor the presence of springtails, since the soil structure and the habitable porous space are essential in the abundance of edaphic springtails. A habitable porous space for springtails and other edaphic organisms is the one that favors the development of all its morphological structures (Oliveira Filho & Baretta 2016OLIVEIRA FILHO, L.C.I. & BARETTA, D. 2016. Por que devemos nos importar com os colêmbolos edáficos? Sci. Agrar. 17(2):21-40.).
In the NF, in addition to OM and MWD, moisture was another variable that influenced some morphotypes of springtails, mainly morphotypes Ed6 and Ed15, part of the edaphic eco-morphological group, most influenced by this environmental variable because they live below 5 cm of soil (Figure 2B). Forest environments provide refuge for the permanence of species more sensitive to the variation of moisture since they present forest cover that protects the soil of high temperatures and loss of moisture.
Still, in the summer, the pH was the environmental variable that appeared associated with the springtails in the PA and NT systems, which were close and related only to the Ep10, H16, and Ep5 morphotypes. However, as mentioned earlier, the springtails may have better development and reproduction in soils with the pH closer to neutrality. Nevertheless, the relationship of the springtails with pH is somewhat complex, since there are different morphotypes, and that they have different capacities and limitations.
Conclusion
The impact of land use systems (LUS) on the morphological diversity of Collembola depends on the intensity of the forest/agricultural practices used, including crop rotation, plant material, and soil preparation.
The LUS that retained the greatest morphological diversity of Collembola were the ICL in the winter and the NF in the summer. The variation of the results between the collection seasons was important to highlight the relevance of analyzing different seasons, considering that food resources and properties such as temperature and soil moisture can be different and interfere in the organism's response.
Environmental variables explained that the presence of springtails morphotypes in summer, regardless of LUS, were OM, Mg/K, pH, MWD, and soil moisture, while in the winter were K, pH, OM, qCO2, TP, and soil moisture.
The morphotyping technique, used as an alternative to the traditional taxonomy, proved to be a good tool to study the biological quality of the soil, together with the diversity indexes and multivariate analysis of the data. As advantages of the morphotyping found during this work, we can mention quickness in the identification of the morphotypes; no need for very long and specific training and simple and low-cost materials used to carry out activities.
Acknowledgments
The authors thank the Foundation for the Support of Research and Innovation in the State of Santa Catarina (Process: 6.309/2011-6/FAPESC) and to the National Council for Scientific and Technological Development (Process: 563251/2010-7/CNPq) for the support of the research. Dilmar Baretta thanks the Productivity Research Scholarship (Process: 305939/2018-1) granted by CNPq. Danielle Cristina Ortiz thanks FAPESC (notice 05/2015) for the grant of a master's degree scholarship, and the Graduate Program in Forestry Engineering of UDESC Lages.
References
- ALEF, K. & NANNIPIERI, P. 1995. Methods in applied soil microbiology and biochemistry. London, p.576.
- ALMEIDA, H. S., TROMBETA, H. W., WELTER, P. D. 2017. Organismos edáficos em sistema de integração lavoura pecuária. J. Agron. Sci. 6:23-40.
- ANTONIOLLI, Z., REDIN, M., SOUZA, E.L. & POCOJESKI, E. 2013. Metais pesados, agrotóxicos e combustíveis: efeito na população de colêmbolos no solo. Cienc. Rural 43(6):992-998.
- BARETTA, D., PIRES, J.C.P., SEGAT, J.C., GEREMIA, E.V., OLIVEIRA FILHO, L.C.I. & ALVES, M.V. 2011. Fauna edáfica e qualidade do solo. In Tópicos Especiais em Ciência do Solo. (O. Klauberg Filho., A.L. Mafra., L.C. Gatiboni eds). Viçosa: Sociedade Brasileira de Ciência do Solo, v.7, p.141-192.
- BARETTA, D., BARTZ, M .L. C., FACHINI, I., ANSELMI, R., ZORTÉA, T., BARETTA, C. R. D. M. 2014. Soil fauna and its relation with environmental variables in soil management systems. Rev. Ciênc. Agron 45(5): 871-879.
- BARTZ, M.L.C., PASINI, A. & BROWN, G.G. 2013. Earthworms as soil quality indicators in Brazilian no-tillage systems. Appl. Soil Ecol. 69:39-48.
- BELLINGER, P.F., CHRISTIANSEN, K.A. & JANSSENS, F. 2018. Collembola of the World. http://www.collembola.org (último acesso em: 05/11/2018).
» http://www.collembola.org - BELLINI, B.C. 2016. Colêmbolos: uma riqueza microscópica no Semiárido. In Conhecendo os artrópodes do Semiárido 1 ed. (F. Bravo & A.R. Calor eds). Métis Produção Editorial, São Paulo, p.43-53.
- BERUDE, M., GALOTE, J.K. B., PINTO, P.H. & AMARAL, A.A. 2015. A mesofauna do solo e sua importância como bioindicadora. Encicl. Biosf. 11:14-28.
- CASSAGNE, N., GERS, C. & GAUQUELIN, T. 2003. Relationships between Collembola, soil chemistry and humus types in forest stands. Biol. Fertil. Soils 37: 355-361.
- CASSAGNE, N., BAL-SERIN, M.C., GERS, C & GAUQUELIN, T. 2004. Changes in humus properties and collembolan communities following the replanting of beech forests with spruce. Pedobiologia 48: 267-276.
- CLAESSEN, M.E.C. 1997. Manual de métodos de análise de solo. 2 ed. Embrapa Solos, Rio de Janeiro.
- GARCÍA-PRÉCHAC, F., ERNST, O., SIRI-PRIETO, G., TERRA, J.A. 2004. No-till into crop-pasture rotations in Uruguay. Soil Till Res 77(1):1-13.
- LIMA e SILVA, C., BRENNAN, N., BROUWER, J.M., COMMANDEUR, D., VERWEIJ, R.A. & VAN GESTEL, C.A.A. 2017. Comparative toxicity of imidacloprid and thiacloprid to different species of soil invertebrates. Ecotoxicology 26:555-564.
- LOSS, A., COSTA, E.M., PEREIRA, M.G. & BEUTLER, S.J. 2014. Agregação, matéria orgânica leve e carbono mineralizável em agregados do solo. Rev. Fac. Agron. 113(1):1-8.
- MACHADO, D.L., PEREIRA, M.G., CORREIA, M.E. F., DINIZ, A.R. & MENEZES, C.E.G. 2015. Fauna edáfica na dinâmica sucessional da Mata Atlântica em Floresta Estacional Semidecidual no Rio Paraíba do Sul, RJ. Cienc. Florest. 25:91-106.
- MARTINS, C., NATAL-DA-LUZ, T., SOUSA, J.P., GONÇALVES, M.J., SALGUEIRO, L. & CANHOTO, C. 2013. Effects of essential oils from Eucalyptus globulus leaves on soil organisms involved in leaf degradation. Plos One 8(4):1-7.
- OLIVEIRA FILHO, L.C.I. & BARETTA, D. 2016. Por que devemos nos importar com os colêmbolos edáficos? Sci. Agrar. 17(2):21-40.
- OLIVEIRA FILHO, L.C.I., KLAUBERG FILHO, O., BARETTA, D., TANAKA, C.A.S. & SOUSA. J.P. 2016. Collembola community structure as a tool to assess land use effects on soil quality. Rev. Bras. Cienc. Solo 40:1-18.
- REIS, F., CARVALHO, F., SILVA, P.M., MENDES, S., SANTOS, S.A.P. & SOUSA, J.P. 2016. The use of a functional approach as surrogate of Collembola species richness in European perennial crops and forests. Ecol. Indic. 61:676-682.
- RIEFF, G.G., NATAL-DA-LUZ, T., SOUSA, J.P. & SÁ, E.L.S. 2014. Diversity of springtails and mites of a native forest in southern Brazil: relationship with the indices of temperature and precipitation in the native environment. Int. J. Emerg. Technol. Adv. Eng. 4(9):684-692.
- RIEFF, G.G., NATAL-DA-LUZ, T., SOUSA, J.P., WALLAU, M.O., HAHN, L., SACCOL DE SÁ, E.L. 2016. Collembolans and mites communities as a tool for assessing soil quality: effect of eucalyptus plantations on soil mesofauna biodiversity. Curr. Sci. 110(4):713-719,
- SABAIS, A.C.W., SCHEU, S. & EISENHAUER, N. 2011. Plant species richness drives the density and diversity of Collembola in temperate grassland. Acta Oecol. 37: 195-202.
- SANTOS, M.A.B., OLIVEIRA FILHO, L.C.I., POMPEO, P.N., ORTIZ, D.C., MAFRA, Á.L., KLAUBERG FILHO, O., BARETTA, D. 2018. Morphological diversity of springtails in land use systems. Rev. Bras. Cienc. Solo 42:e0170277.
- TEDESCO, M.J., GIANELLO, C., BISSANI, C.A., BOHNEN, H. & VOLKWEISS, S.J. 1995. Análise de solo, plantas e outros materiais. 2 ed. Universidade Federal do Rio Grande do Sul, Porto Alegre, p.174.
- TÓTOLA, M.R. & CHAER, G.M. 2002. Microrganismos e processos microbiológicos como indicadores da qualidade do solo. In Tópicos em Ciência do Solo (V.H. Alvarez, C.E.G.R Schaefer, N.F. Barros, J.W.V. Melo, L.M. Costa eds) Sociedade Brasileira de Ciência do Solo, Viçosa. 2:195-276.
- TSIAFOULI, M. A., THÉBAULT, E., SGARDELIS, S.P., RUITER, P.C., VAN DE PUTTEN, W.H., BIRKHOFER, K., HEMERIK, L., VRIES, F.T., BARDGETT, R.D., BRADY, M.V., BJORNLUND, L., JØRGENSEN, H.B., CHRISTENSEN, S., HERTEFELDT. T.D., HOTES, S., GERA HOL, W., FROUZ, J., LIIRI, M., MORTIMER, S.R., SETÄLÄ, H., TZANOPOULOS, J., UTESENY, K., PIŽL, V., STARY, J., WOLTERS, V. & HEDLUND, K. 2015. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change. Biol. 21(2):973-985.
- VANCE, E.D., BROOKS, P.C. & JENKINSON, D.S. 1987. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703-707.
- VILELA, l., MARTHA JUNIOR, G.B., MACEDO, M. C.M., MARCHÃO, R.L., GUIMARÃES JÚNIOR, R., PULROLNIK, K., MACIEL. 2011. Sistemas de integração lavoura-pecuária na região do Cerrado. Pesq. agropec. bras 46(10):1127-1138.
- ZHANG, B., CHANG, L.; NI, Z., CALLAHAM, M. A., SUN, X. & WU, D. 2014. Effects of land use changes on winter-active Collembola in Sanjiang Plain of China. Appl. Soil Ecol. 83:51-58.
- ZORTÉA, T., BARETTA, D., SEGAT, J.C., MACCARI, A.P. & BARETTA, C.R.M. 2015. Comportamento de fuga de colêmbolos expostos a solos contaminados com cipermetrina. Sci. Agrar. 16(4):49-58.
Publication Dates
-
Publication in this collection
26 Aug 2019 -
Date of issue
2019
History
-
Received
17 Dec 2018 -
Reviewed
09 May 2019 -
Accepted
30 May 2019