Abstracts
The article presents an overview of the mechanism of chromium stress in plants. Chromium is known to be a toxic metal that can cause severe damage to plants and animals. Chromium-induced oxidative stress involves induction of lipid peroxidation in plants that causes severe damage to cell membranes. Oxidative stress induced by chromium initiates the degradation of photosynthetic pigments causing decline in growth. High chromium concentration can disturb the chloroplast ultrastructure thereby disturbing the photosynthetic process. Like copper and iron, chromium is also a redox metal and its redox behaviour exceeds that of other metals like Co, Fe, Zn, Ni, etc. The redox behaviour can thus be attributed to the direct involvement of chromium in inducing oxidative stress in plants. Chromium can affect antioxidant metabolism in plants. Antioxidant enzymes like SOD, CAT, POX and GR are found to be susceptible to chromium resulting in a decline in their catalytic activities. This decline in antioxidant efficiency is an important factor in generating oxidative stress in plants under chromium stress. However, both metallothioneins and organic acids are important in plants as components of tolerance mechanisms and are also involved in detoxification of this toxic metal.
antioxidants; chromium; fenton reactions; metallothioneins; oxidative stress
Este artigo apresenta uma revisão sobre o mecanimso de estresse de cromo em plantas. O cromo é um metal tóxico que pode causar severos danos a plantas e animais. Estresse oxidativo induzido por cromo envolve peroxidação de lipídeos em plantas, o que causa danos severos às membranas celulares. O estresse oxidativo induzido pelo cromo inicia a degradação de pigmentos fotossintéticos, levando à diminuição do crescimento. Sua alta concentração pode causar distúrbios à ultra-estrutura dos cloroplastos e, conseqüentemente, afetar o processo fotossintético. Assim como o cobre e o ferro, o cromo é também um metal redox, e esse comportamento excede o de outros metais como Co, Fe, Zn, Ni, etc. O comportamento redox pode, então, ser atribuído ao direto envolvimento do cromo em induzir estresse oxidativo em plantas. O cromo pode afetar o metabolismo antioxidante em plantas. Enzimas antioxidantes como SOD, CAT, POX e GR são suscetíveis ao cromo, resultando em declínio das suas atividades catalíticas. Esse declínio na eficiência antioxidante é um importante fator na geração do estresse oxidativo em plantas sob estresse por cromo. No entanto, metalotioneínas e ácidos orgânicos são importantes componentes em plantas como mecanismos de tolerância e estão envolvidos na destoxificação desse metal tóxico.
antioxidantes; cromo; estresse oxidativo; metalotioneínas; reações de Fenton
TOXIC METALS IN PLANTS
Chromium stress in plants
Estresse de cromo em plantas
S.K. PandaI, II, * * Corresponding author: drskp_au@yahoo.com ; S. ChoudhuryII
IResearch Institute for Bioresources, Okayama University, Kurashiki 7100 046, Japan
IIPlant Biochemistry Laboratory, School of Life Sciences, Assam (Central) University, Silchar 788 011, India
ABSTRACT
The article presents an overview of the mechanism of chromium stress in plants. Chromium is known to be a toxic metal that can cause severe damage to plants and animals. Chromium-induced oxidative stress involves induction of lipid peroxidation in plants that causes severe damage to cell membranes. Oxidative stress induced by chromium initiates the degradation of photosynthetic pigments causing decline in growth. High chromium concentration can disturb the chloroplast ultrastructure thereby disturbing the photosynthetic process. Like copper and iron, chromium is also a redox metal and its redox behaviour exceeds that of other metals like Co, Fe, Zn, Ni, etc. The redox behaviour can thus be attributed to the direct involvement of chromium in inducing oxidative stress in plants. Chromium can affect antioxidant metabolism in plants. Antioxidant enzymes like SOD, CAT, POX and GR are found to be susceptible to chromium resulting in a decline in their catalytic activities. This decline in antioxidant efficiency is an important factor in generating oxidative stress in plants under chromium stress. However, both metallothioneins and organic acids are important in plants as components of tolerance mechanisms and are also involved in detoxification of this toxic metal.
Key words: antioxidants, chromium, fenton reactions, metallothioneins, oxidative stress.
RESUMO
Este artigo apresenta uma revisão sobre o mecanimso de estresse de cromo em plantas. O cromo é um metal tóxico que pode causar severos danos a plantas e animais. Estresse oxidativo induzido por cromo envolve peroxidação de lipídeos em plantas, o que causa danos severos às membranas celulares. O estresse oxidativo induzido pelo cromo inicia a degradação de pigmentos fotossintéticos, levando à diminuição do crescimento. Sua alta concentração pode causar distúrbios à ultra-estrutura dos cloroplastos e, conseqüentemente, afetar o processo fotossintético. Assim como o cobre e o ferro, o cromo é também um metal redox, e esse comportamento excede o de outros metais como Co, Fe, Zn, Ni, etc. O comportamento redox pode, então, ser atribuído ao direto envolvimento do cromo em induzir estresse oxidativo em plantas. O cromo pode afetar o metabolismo antioxidante em plantas. Enzimas antioxidantes como SOD, CAT, POX e GR são suscetíveis ao cromo, resultando em declínio das suas atividades catalíticas. Esse declínio na eficiência antioxidante é um importante fator na geração do estresse oxidativo em plantas sob estresse por cromo. No entanto, metalotioneínas e ácidos orgânicos são importantes componentes em plantas como mecanismos de tolerância e estão envolvidos na destoxificação desse metal tóxico.
Palavras-chave: antioxidantes, cromo, estresse oxidativo, metalotioneínas, reações de Fenton.
INTRODUCTION
Chromium (Cr) is the seventh most abundant metal in the earth's crust (Katz and Salem, 1994) and an important environmental contaminant released into the atmosphere due to its huge industrial use (Nriagu and Nieboer, 1988). In nature, Cr exists in two different stable oxidation states; trivalent (CrIII) and hexavalent (CrVI) chromium. Both CrIII and CrVI differ in terms of mobility, bioavailability and toxicity. CrVI is found to be more toxic than CrIII (Panda and Patra, 1997). CrVI forms chromate and dichromate and is highly soluble in water. CrIII on the other hand is less soluble in water and is required in trace amounts as an inorganic nutrient for animals. Both chromate and dichromate are considered to be negatively charged and there is a limited chance of it being adsorbed by organic materials. CrVI is thus considered to be more mobile than that of CrIII. Cr is extensively used in both the trivalent and hexavalent forms in industries like steel, leather, textile, etc (Dixit et al., 2002). The hexavalent form of Cr is a biologically toxic oxidation state and to date there is no evidence indicating any potential biological role in plants (Von Burg and Liu, 1993). Both oxidized forms, however, have the capacity to form complexes with other species (NRC, 1980; Bartlett, 1991).
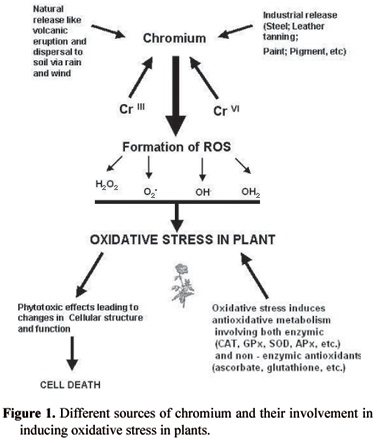
In line with other heavy metals like As, Cd, Co, Cu, Ni, Sn and Zn, Cr is a broadline heavy metal, and is phytotoxic either at all concentrations or above certain threshold levels (Nieboer and Richardson, 1980). Cr phytotoxicity can result in inhibition of seed germination, degrade pigment status, nutrient balance, antioxidant enzymes and induce oxidative stress in plants (Poschenrieder et al., 1991; Barcelo and Poschenrieder, 1997; Panda and Patra, 1997, 1998, 2000; Panda et al., 2003; Panda, 2003). Beside these effects, Cr can alter chloroplast and membrane ultrastructure in plants (Bassi et al., 1990; Choudhury and Panda, 2004). The phytotoxicity of both CrIII and CrVI has been studied in many higher and lower plants. CrIII is thought essential for animals in trace amounts, can be toxic and induces oxidative stress (Panda and Patra, 2000). CrIII is toxic to plants even at low concentration and reported to causes severe oxidative damage to plant cells. It can affect growth, water balance, pigment content and initiate lipid peroxidation causing oxidative damage to plants (Bonet et al., 1991; Poschenrieder et al., 1991; Barcelo and Poschenrieder, 1997; Panda and Patra, 2000). CrVI on the other hand is more phytotoxic than CrIII (Han et al., 2004) and retards growth, reduces the number of palisade and spongy parenchyma cells in leaves, and increases the number of vacuoles and electron dense material along the walls of xylem and phloem (Han et al., 2004). As a consequence of a wide range of abiotic stresses including heavy metals, toxic reactive oxygen species (ROS) like H2O2, O2-, OH-, etc are produced. Heavy metals usually form ROS either directly or through involvement in a redox reaction. Cr was thought to be a non-redox metal that could not participate in Fenton reactions, however, other studies have shown that Cr can indeed participate in Fenton reactions, proving its redox character (Shi and Dalal, 1989). Cr reactivity can be considered from its interaction with glutathione, NADH and H2O2, forming OH- radicals in cell-free systems (Shi and Dalal, 1989; Aiyar et al., 1991). Production of H2O2, OH- and O2- under Cr stress has been demonstrated in many plants, generating oxidative stress leading to damage of DNA, proteins and pigments as well as initiating lipid peroxidation (Bagchi et al., 2000; Panda and Patra, 2000; Panda et al., 2003; Panda, 2003; Choudhury and Panda, 2004). The source of chromium and subsequent imposition of oxidative stress is summarized in figure 1.
Cr effects in relation to phytotoxicity have been investigated by many workers on important crop plants and also in lower plants like mosses (Turner and Rust, 1971; McGrath, 1982; Vazquez et al., 1987; Bishnoi et al., 1993; Panda and Patra, 1998; Panda and Patra, 2000; Dixit et al., 2002; Panda et al., 2002 a,b; Panda, 2003; Panda and Khan, 2003; Panda et al., 2003; Choudhury and Panda, 2004; Panda and Choudhury, 2004; Han et al., 2004), and it has been clearly demonstrated that CrVI is more phytotoxic than CrIII (Panda and Patra, 2000; Han et al., 2004). Soil and aquatic ecosystems in the vicinity of Cr-releasing sources are adversely affected by it, making arable land unproductive and unfertile. Unlike other heavy metals such as Cd, Al, Pb, Cu, Zn, etc Cr detoxification via mycorhizal fungi or other mechanisms as well as its phytoaccumulation and phytoremediation has been studied very little. Mycorhizas can alleviate Cr toxicity and supports greater plant growth in Cr-rich soils (Davis Jr et al., 2001). It has been demonstrated in sorghum that transcription rates of MTs are specifically high under Cr stress and the H2O2 generated as a result can act as a signal, inducing MT-mRNA transcription (Shanker et al., 2004). Cr stress, especially CrVI, cannot induce phytochelatins (PCs) suggesting a role for MTs in Cr detoxification and tolerance in plants (Shanker et al., 2004).
This review describes the physiological and molecular aspects of Cr stress in plants. It focuses on the phytotoxicity of Cr generating oxidative stress via ROS and also on the subsequent antioxidant metabolism and the redox behaviour of Cr. Detailed accounts on how mycorhizas and MTs are involved in Cr detoxification and tolerance have been included in this review.
Chromium toxicity in plants
Heavy metals like Zn, Fe, Cu, Mn are essential for plant growth and important constituents of many enzymes of metabolic importance. Other metals like Pb, Cd, As, Se, Cr and Al are biologically non-essential and toxic above certain threshold levels. Cr is toxic to plants and does not play any role in plant metabolism (Dixit et al., 2002). Accumulation of Cr by plants can reduce growth, induce chlorosis in young leaves, reduce pigment content, alter enzymatic function, damage root cells and cause ultrastructural modifications of the chloroplast and cell membrane (McGrath, 1985; Panda and Patra, 1997, 1998; Panda and Dash, 1999; Panda and Patra, 2000; Panda, 2003; Panda et al., 2002, 2003; Choudhury and Panda, 2004; Hu et al., 2004). Cr toxicity can reduce seed germination and radicle growth in plants (Atta Aly et al., 1991; Corradi et al., 1993; Liu et al., 1993; Nayari et al., 1997; Panda et al., 2002). Growth inhibition in plants can be due to inhibition of cell division by inducing chromosomal aberrations (Liu et al., 1993). However, in many plants an increase in DNA content has been observed under Cr and the amount of DNA increased with the increase in concentration of Cr (Bishnoi et al., 1993; Zeid, 2001). During seed germination, hydrolysis of proteins and starch takes place, providing amino acids and sugars. Under Cr treatment, a decrease in both a and b - amylase has been reported, which is one of the important factors for germination inhibition in many plants in view of the impaired supply of sugar to developing embryo axes (Zied, 2001). At very low concentrations of Cr, however, an increase in a - amylase activity has been reported (Zeid, 2001).
Phytotoxic effects of Cr on plant growth have been thoroughly studied in many plant species like mosses, rice, pea, wheat, etc in relation to oxidative stress. Cr exposure at the micromolar range can lead to severe phytotoxic symptoms in plant cells. Both CrIII and CrVI can reduce chlorophyll content and thereby inhibit growth (Panda and Patra, 2000; Panda et al., 2003). It can cause ultrastructural changes in the chloroplast leading to inhibition of photosynthesis. Such alterations in the chloroplast have been observed in the case of plants like Lemna minor, Pistia sp., Taxithelium nepalense (Bassi et al., 1990; Choudhury and Panda, 2004). Cr can affect roots of plants causing wilting and plasmolysis in root cells (Bassi et al., 1990; McGrath, 1995). A series of studies with Cr in the moss T. nepalense showed changes in chloroplast membrane structure accompanied by changes in thylakoid arrangement. Moreover, at high concentrations (1 mM) complete distortion of the chloroplastidic membrane was observed together with severe disarrangement of thylakoids indicating that Cr in its hexavalent form can cause severe phytotoxic effects (Choudhury and Panda, 2004). Cr can also inhibit the Hill reaction, affecting both the dark and light reaction (Krupa and Baszynski, 1995; Zied, 2001). Aquatic plants like Vallisneria spiralis can accumulate significant amounts of Cr in their tissues with decreases in the biomass of the plant (Sen et al., 1987; Gupta et al., 1994; Vajpayee et al, 1999, 2000, 2001). In aquatic plants uptake and bioaccumulation of Cr can influence many physiological and biochemical processes and in many plant species photosynthetic pigments are affected by Cr (Panda and Patra, 2000; Vajpayee et al., 2001; Panda, 2003). Decrease in total chlorophyll, chlorophyll a and b, and carotenoids have been well documented under Cr stress in plants (McGrath, 1982; Panda and Patra, 1998, 2000; Tripati and Smith, 2000; Panda, 2003; Panda and Khan, 2003; Panda et al., 2003; Choudhury and Panda, 2004; Panda and Choudhury, 2004). Cr possesses the capacity to degrade d-aminolevulinic acid dehydratase, an important enzyme involved in chlorophyll biosynthesis, thereby affecting d - aminolevulinic acid (ALA) utilization (Vajpayee et al., 2000). Cr, mostly in its hexavalent form can replace Mg ions from the active sites of many enzymes and deplete chlorophyll content (Vajpayee et al., 2000). Like other heavy metals Cr can induce degradation of carotenoids in plants (Baszynski et al., 1981; Rai et al., 1992). In Vallisneria spiralis and other aquatic plants, however, an increase in carotenoids content was seen under Cr treatment (Ralph and Burchett, 1998; Tripati and Smith, 2000; Vajpayee et al., 2001). The increase in carotenoids content may act as an antioxidant to scavenge ROS generated as a result of Cr toxicity.
Lipid peroxidation, which is considered an indication of oxidative stress in plants, can be induced via free radicals or ROS that are generated as a result of heavy metal toxicity in plants. Lipid peroxidation can degrade biological membranes making them susceptible to oxidative damage (Panda, 2002). Under Cr, lipid peroxidation can be initiated resulting in oxidative stress. ROS that are common consequences of most biotic and abiotic stresses are also formed as a result Cr toxicity (Dixit et al., 2002; Panda et al., 2003; Choudhury and Panda, 2004). In many crop plants like rice, wheat, pea and also in lower plants significant increases in ROS production can be observed with concomitant increases in lipid peroxidation (Panda and Patra, 1997, 1998; Behra et al., 1999; Panda and Patra, 2000; Dixit et al., 2001; Panda and Patra, 2002; Panda, 2003; Panda et al., 2003; Choudhury and Panda, 2004). High production of H2O2 and O2- radicals were reported in many plant species exposed to Cr and the metal has been implicated in the generation of oxidative stress (Roy et al., 1992; Dixit et al., 2001; Panda, 2003; Panda and Khan, 2003; Panda et al., 2003; Choudhury and Panda, 2004).
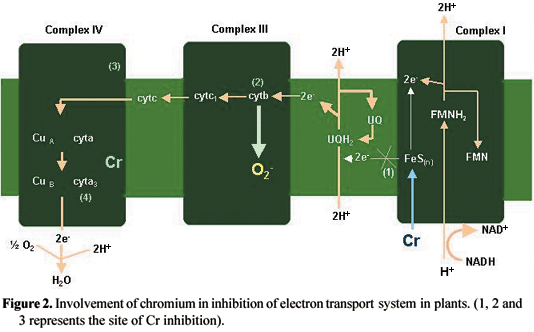
Cr can degrade proteins. Degradation of protein in plants can result in the inhibition of nitrate reductase (NR) activity (Solomonson and Barber, 1990; Vajpayee et al., 1999; 2000; Panda and Choudhury, 2004). The correlation between NR activity and proteins has been well documented in plants (Rai et al., 1992). The amino acid cysteine is an important component of phytochelatin (Vajpayee et al., 2001). A decline in cysteine may result in the degradation of sulphate-reducing enzymes leading to toxic effects (Vajpayee et al., 2001). Cr, at both toxic and mild concentrations, can inhibit uncoupled electron transport (Dixit et al., 2001), indicating the electron transport chain to be a common site of Cr binding in plants. Inhibition of electron transport by Cr may be a consequence of the redox change in the Cu and Fe carriers, where Cr may be transferred by cytochrome in the mitochondria to reduce it or the reduced heme group of cytochrome may act as a site for Cr binding, blocking electron transport (Dixit et al., 2001). The severe inhibition of cytochrome oxidase activity may be due to the binding of Cr to complex IV where Cr may also bind to cytochrome a3 (Dixit et al., 2001). Another alternative mechanism is the generation of O2- radicals in the mitochondria (Scandalios, 1993; Vranova et al., 2002). In pea plants, the treatment of Cr at different concentrations showed that O2- is generated in the cytochrome b region (complex III) of root mitochondria. The O2- generation at this specific site was high under Cr (Dixit et al., 2001). The Cr-induced inhibition of electron transport is represented in figure 2.
Fenton type and redox reactions of chromium
The oxidation-reduction or redox reactions occur in both soil and aquatic environments. The Fenton-type reactions involve transition metals and hydrogen peroxide or hydroperoxide leading to the formation oxidizing species (Goldstein and Czaspki, 1990). The redox reactions that occur in the soil are important in altering mobility and phytotoxicity. The involvement of chromium in the Fenton reaction is not very well understood or studied (Strile et al., 2003). Beside the wellknown redox metals like Cu and Fe, Cr also appears to participate in Redox or Fenton reactions (Shi and Dalal, 1991). The oxidation state of Cr is important because the common triplet oxidation state (CrIII) is not toxic as compared to the hexavalent form (Carter, 1995). Mn in both the trivalent and tetravalent state oxidizes CrIII to CrVI, while FeS and organic matter in the soil can reduce CrVI to the more stable and less phytotoxic CrIII. CrIII and H2O2 can cause breakage of the DNA strand at pH 6 to 8, but not at pH 4, and it is believed that CrIII and CrII enters the Fenton reaction (Strile et al., 2003). During radical oxidation of CrIII, CrIV and CrV intermediates are thought to be involved (Strile et al., 2003). Beside CrIV and CrV, other species like thiyl (GS-) and hydroxyl radicals (OH-) are considered to be toxic and carcinogenic (Alcedo and Wetterhahn, 1990). The ESR studies at pH 5.8 and 7.1 for CrIII / H2O2 showed that the lower oxidation states of Cr can effectively participate in generating oxidizing species without generating CrVI (Strile et al., 2003). At higher pH (8.9), the oxidation of CrIII is rapid. Although the concentration CrVI reaches a steady state, the production of oxidizing species like CrIV and CrV proceeds at a slow measurable rate (Strile et al., 2003). This result indicates that both CrIV and CrV are catalytically active and possess the capability to generate ROS such as the hydroxyl radical (OH-) (Strile et al., 2003). The catalytic activity of CrIII is much higher in a Fenton reaction system compared to other metals like Co II, Cd II, Zn II, Mn II and Fe III but lower than Cu II (Strile et al., 2003).
Chromium-induced oxidative stress
One of the common responses to a wide range of biotic and abiotic stresses is the generation of reactive oxygen species or ROS. ROS are produced in cells as an intermediate product during the reduction of O2 to H2O. Heavy metals are known to initiate ROS production which is implicated as a stress response (Dietz et al., 1999). Cr is a toxic heavy metal that can generate ROS like H2O2, O2-, OH- which cause oxidative damage to plants (Panda and Patra, 1997, 2000, 2002; Dixit et al., 2002; Panda, 2003; Panda and Khan, 2003; Panda et al., 2003; Choudhury and Panda, 2004).
The nature and extent of ROS production by a particular metal depends mainly on its redox or non-redox character. Redox metals like Cu and Fe can undergo Fenton reactions generating toxic ROS species. The participation of Cr in such redox reactions is, however, not clearly understood (Strile et al., 2003), but one study has demonstrated its participation in Redox or Fenton reactions just as Cu or Fe (Shi and Dalal, 1989). Plants exposed to toxic concentrations Cr can suffer from oxidative stress leading to disruption of its cellular functions and structure. Exposure to Cr at different concentrations can initiate the process of lipid peroxidation in plants. Lipid peroxidation is considered to be an indication of oxidative damage by which the integrity and functionality of the membrane is lost. In wheat, Cr exposed to 1, 10 and 100 mM concentrations initiated the process of lipid peroxidation and the amount of malonyldialdehyde (MDA) increased with the increase in concentration and duration of Cr exposure (Panda et al., 2003). Increase in MDA content has been also reported in mosses exposed to Cr. In moss species like Taxithelium nepalense and Polytrichum commune exposure of Cr initiated the process of lipid peroxidation via free radical generation (Choudhury and Panda, 2004; Panda and Choudhury, 2004).
Antioxidant responses in plants to chromium-induced oxidative stress
In most of the aerobic organisms, there is a need to effectively eliminate the toxic oxygen species (ROS) generated as a result of environmental stresses. Plants have developed a complex antioxidant system by which they scavenge the ROS thereby protecting the cell from oxidative attack. ROS are produced as intermediate products in a number of metabolic reactions in various cellular organelles, generated by oxidative stress in plants (figure 3). Heavy metals are known to generate these toxic ROS such as H2O2, O2-, OH-, OH2, etc. which degrade important cellular components by inducing oxidative stress (Dietz et al., 1999; Panda, 2002). In order to control the level of ROS and protect the cells from oxidative injury, plants have developed a complex antioxidant defense system to scavenge the ROS and these antioxidant systems include various enzymes and non-enzymes, which may also play a significant role in ROS signaling in plants (Vranova et al., 2002). The antioxidant response of plants to metal-induced oxidative stress is variable and depends on the type of plant and the metal involved. However, unlike other heavy metals like Cd, Zn and Fe, information on the antioxidant metabolism for Cr stress is scarce.
The antioxidant enzymes catalase (CAT), guaiacol peroxidase (GPx), glutathione reductase (GR), ascorbate peroxidase (APx) and superoxide dismutase (SOD) have been thoroughly studied for plants like rice, wheat, pea and even in lower plants like mosses. CAT is an important heme-containing enzyme that catalyses the dismutation of H2O2 to H2O and oxygen and is localized in the peroxisomes. CAT is an indispensable enzyme required for ROS detoxification in plants. Activity of CAT in response to Cr has been studied in many crop plants like rice, wheat, green gram and even in lower plants like mosses (Panda and Patra, 1998; Panda and Dash, 1999; Panda and Patra, 2000, 2002; Panda, 2003; Panda et al., 2002, 2003; Panda and Choudhury, 2004; Choudhury and Panda, 2004). In rice, Cr can either induce CAT activity or suppress it. Treatment of developing wheat seedlings to different concentrations of Cr showed varied responses. In most of the studies conducted, a gradual decrease in CAT activity was observed in plants while in mosses both alleviation and decline was observed (Panda and Patra, 1998, 2000, 2002; Panda, 2003; Panda et al., 2002, 2003; Panda and Choudhury, 2004; Choudhury and Panda, 2004). In wheat response to Cr was also variable. Exposure of Cr to developing wheat seedlings decreased the CAT activity after 7 d and 9 d of Cr treatment at 0.01, 0.1 and 1 mM (Panda and Patra, 2000). Moreover, at much higher concentrations (10 and 100 mM) severe inhibition in CAT activity was observed after 2 d and 4 d of Cr treatment (Panda et al., 2003). A similar inhibition of CAT was reported when Cr was supplied in addition to nitrogen nutrition in wheat (Panda et al., 2002).
Mechanism of chromium detoxification: the possible role of metallothionines and organic acids
Heavy metals like Cr, Cd, Zn, Fe, Al, Pb, As are highly reactive and toxic to living cells. Some heavy metals, particularly Cu, Zn and Fe are essential micronutrients involved in various physiological processes but become toxic above certain threshold concentrations. Plants have developed complex mechanisms by which they control the uptake and accumulation of heavy metals (Cobbett and Goldsbrough, 2002). These mechanisms involve chelation and sequestering of metals ions by a particular class of metal binding ligands denominated phytochelatins (PCs) and metallothioneins (MTs) (Cobbett, 2000; Cobbett and Goldsbrough, 2002). MTs have a possible role in Cr detoxification in plants and it has been reported for sorghum that MT-like proteins are expressed under Cr stress (Shanker et al., 2004). MTs are the product of mRNA translation and are characterized as low molecular weight cysteine-rich metal-binding protein (Kagi, 1991). The role of MTs or even PCs in Cr detoxification in plants has not been thoroughly studied compared to that other heavy metals like Cd, Hg, Cu, etc. and consequently there is very little information about the involvement of these metal-binding ligands in Cr detoxification in plants. A study of CrVI effects on the MT3 gene expression using Cr tolerant and susceptible varieties revealed a high intensity band matching the gene of interest in the tolerant variety compared to the susceptible one (Shanker et al., 2004). This suggests that under Cr stress there could be high transcription rates of MTs, particularly in the tolerant variety (Shanker et al., 2004). The role of PCs in regulating metal toxicity has been reported in plants. It was suggested that the production of ROS and H2O2 as a result of Cr exposure might have triggered signals to induce MT mRNA transcription (Shanker et al., 2004). Thus, MTs may have a very important role in Cr detoxification in plants, possibly by binding Cr ions and making them non-toxic. However, the role of MTs in Cr detoxification in plants is not well understood nor thoroughly studied, so their role in this respect still remains a challenge for the future.
The root contains organic acids that bind to metals from highly insoluble forms in the soil and acids like citric acid and malic acids can act as essential ligands for metals (Rauser, 1999). The role of citric acid in regulating Al III and Ni II detoxification in plants has been clearly demonstrated (Yang et al., 2003). The root exudates are very important agents that form complexes with trace metals and affect their redox behaviour (Hale and Griffin, 1974; Caltado et al., 1988). Root exudates containing organic acids can form complexes with Cr compounds, making them available for plant uptake (Bartlett and James, 1988). Studies on the role of organic acids in Cr toxicity in Lycopersicon esculentum showed that in the presence of organic acids like carboxylic acid and amino acids, Cr uptake in roots is enhanced (Srivastava et al., 1999). However, of these types of organic acids, amino acids have been found to be less effective in mobilizing chromium (Srivastava et al. 1999). Organic acids like citric acid, aspartic acid and oxalic acid can convert inorganic Cr to organically bound Cr, making it soluble for a longer period of time and thereby available to plants (James and Bartlett, 1983). Whether organic acids can play significant role in Cr detoxification is still not completely understood.
CONCLUSION
The understanding of the basic mechanism involved in chromium uptake, transport, accumulation and detoxification in plants together with its physiological effects is necessary for the phytoremediation of the chromium-polluted environments using molecular and genetic techniques. These approaches may include the identification of hyperaccumulators that can provide efficient phytoremediation of chromium-polluted soils, the study of biochemical and molecular responses of these plants to chromium, and the identification of genes that express PCs or MTs involved in the detoxification of the metal within the plant. These technologies will prove useful in environmental cleanup procedures and subsequent restoration of soil fertility.
- Aiyar J, Buerkovitis HJ, Floyd RA, Borges K (1991) Reaction of chromium (VI) with glutathione or with hydrogen peroxide: Identification of reactive intermediates and their role in chromium (VI) û induced DNA damage. Environ. Health. Persp. 92:53û62.
- Alcedo JA, Wetterhahn R (1990) Chromium toxicity and carcinogenesis. In: Richter GW, Solez K, Aisen P, Cohen G (eds), International review of experimental pathology, pp. 85-108. Academic Press, New York.
- Atta Aly MA, Shehata NG, Kobbia TM (1991) Effect of cobalt on tomato plant growth and mineral content. Ann. Agril. Sci. (Cairo) 36:617-624.
- Bagchi C, Joshi SS, Bagchi M, Balmoori J, Benner EJ, Kuszynski CA (2000) Cadmium û and Chromium û induced oxidative stress, DNA damage, and apoptotic cell death in cultured human chronic myelogenous leukemic K562 cell, promyelocytic leukemic HL û 60 cells, and normal human peripheral blood mononuclear cells. J. Biol. Mol. Toxicol. 14:3û41.
- Barcelo J, Poschenrieder C (1997) Chromium in plants. In: Carati S, Tottarelli F, Seqmi P (eds), Chromium environmental issue, pp.101û129. Francotangati Press, Milan.
- Barlett RJ (1991) Chromium cycling in soil and water: Links, gaps and methods. Environ. Health. Persp. 92:14û24.
- Barlett RJ, James BR (1988) Mobility and bioavailability of chromium in soils. In: Nriagu JO, Nieboer E (eds), Chromium in natural and human environment, pp. 267û304. John Wiley and Sons Inc., New York.
- Baszynski T, Krol M, Wolinka D (1981) Effect of chromate on photosynthetic apparatus of Lemna minor L. In: Akoynoglou G (ed) Photosynthesis II. Electron transport and photophosphorylation, pp.245û246. Balbon International Science Services.
- Bassi M, Corradi MG, Realini M (1990) Effects of chromium (VI) on two freshwater plants, Lemna minor and Pistia stratiotes 1. Morphological observations. Cytobios 62:27û38.
- Bishnoi NR, Anita D, Gupta VK, Shawaney SK (1993) Effect of chromium on seed germination seedling growth and yield of peas. Agric. Ecol. Ecosyt. 47:47û57.
- Bonet A, Poschenrieder C, Barcelo J (1991) Chromium III ion interaction in Fe deficient and Fe sufficient bean plants. I. Growth and Nutrient content. J. Plant Nutr. 14:403û414.
- Caltado DA, McFadden KM, Thomas R, Garland Wilddung RE (1988) Organic constituent and complexation of Nickel (II), Cadmium (II) and Plutonium (VI) in soybean xylem exudates. Plant Physiol. 86:734û739.
- Carter D E (1995) Oxidation reduction of metal ions. Environ. Health Persp. 103:17û19.
- Choudhury S, Panda SK (2004) Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under chromiun and lead phytotoxicity. Water Air Soil Pollut. (Submitted).
- Corradi MG, Bianchi A, Albasini A (1993) Chromium toxicity in Salvia sclarea: 1. Effects of hexavalent chromium on seed germination and seedling development. Environ. Exp. Bot. 33:405û413.
- Cobbett C, Goldsbrough P (2001) Phytochelatins and metallothioneins: Role in heavy metal detoxification and homeostasis. Ann. Rev. Plant. Biol. 53:59û82.
- Cobbett C (2000) Phytochelatins and their role in heavy metal detoxification. Plant. Physiol. 123:463û469.
- Davis Jr FT, Puryear JD, Newton RJ, Egilla JN, Grossi JAS (2001) Mycorhizal fungi enhances accumulation and tolerance of chromium in sunflower (Halianthus annuus). J. Plant Physiol. 158:777-786.
- Dixit V, Pandey V, Shyam R (2002) Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv: Azad) root mitochondria. Plant Cell Env. 25:687-693.
- Dietz KJ, Baier M, Kramer U (1999) Free radicals and reactive oxygen species as mediator of heavy metal toxicity in plants. In: Prasad MNV, Hagemeyer J (eds), Heavy metal stress in plants: From molecules to ecosystem, pp.73û79. Springer û Verlag, Berlin.
- Goldstein S, Czaspki G (1990) Transition metals and oxygen radicals. In: Richter G, Solez K, Aisen P, Cohen G (eds), International review of experimental pathology, pp.133-164. Academic Press, New York.
- Gupta M, Sinha S, Chandra P (1994) Uptake and toxicity of metal in Scripus lacustris L. J. Environ. Sci. Health 29:2185-2202.
- Hale MG, Griffin GJ (1974) Effect of injury on exudation from immature and mature plant fruits. Plant Physiol. Abstracts. 13.
- Han FX, Sridhar BBM, Monts DL, Su Y (2004) Phytoavailability and toxicity of trivalent and hexavalent chromium to Brassica juncea New Phytol. 162:489.
- James BR, Barlett RJ (1983) Behavior of chromium in soils. VI. Interactions between oxidation û reduction and organic complexation. J. Environ. Qual. 12:173-176.
- Kagi JHR (1991) Overview of metallothionein. Methods Enzymol. 205:613-626.
- Katz SA, Salem H (1994) The biological and environmental chemistry of chromium. Verlagsgesellschaft mbH, Weinheim, Pappelallae 3, Postfach.
- Krupa Z, Baszynski T (1995) Some aspect of heavy metal toxicity towards photosynthetic apparatus û direct and indirect effect of light and dark reactions. Acta. Physiol. Plant. 17:177-190.
- Liu DH, Jaing WS, Li MX (1993) Effect of chromium on root growth and cell division of Allium cepa Israel J. Plant Sci. 42:235-243.
- Mc Grath SP (1982) The uptake and translocation of tin and hexavalent chromium and effects on the growth of oat in flowering nutrient solution and in soil. New Phytol. 92:381-390.
- Mc Grath SP (1995) Chromium and Nickel. In: Alloway BJ (eds) Heavy metals in soil, pp.139-155. Chapman and Hall, London, UK.
- Nayari HF, Szalai T, Kadar I, Castho P (1997) Germination characteristics of pea seeds originating from a field trial treated with different level of harmful elements. Acta Agron. Hung. 45:147-154.
- Nieboer E, Richardson DHS (1980) The replace of the nondescript term << heavy metals >> by a biologically and chemically significant classification of metal ions. Environ. Pollut. Ser. B1:3-26.
- Nriagu JO, Neiboer E (1988) Chromium in the natural and human environments. Wiley, New York.
- NRC [National Research Council] (1980) Committee on biological effect of atmospheric pollutants chromium, Natl. Acad. Sci., Washington DC, 155.
- Panda SK, Parta HK (1997) Physiology of chromium toxicity in plants û A Review. Plant Physiol. Biochem. 24(1):10-17.
- Panda SK, Patra HK (1998) Alteration of nitrate reductase activity by chromium ions in excised wheat leaves. Ind. J. Agric. Biochem. 2(2) 56-57.
- Panda SK, Dash M (1999) Regulation of senescence by Cr (VI) ions in excised wheat leaves. J. Nat. Bot. Soc. 53:35-37.
- Panda SK, Patra HK (2000) Does Cr(III) produces oxidative damage in excised wheat leaves. J. Plant Biol. 27(2):105-110.
- Panda SK, Mahapatra S, Patra HK (2002) Chromium toxicity and water stress simulation effects in intact senescing leaves of greengram (Vigna radiata L.var Wilckzeck K851), In: Panda SK (ed), Advances in stress physiology of plants, pp.129-136. Scientific Publishers, India.
- Panda SK, Khan MH (2003) Antioxidant efficiency in rice (Oryza sativa L.) leaves under heavy metal toxicity. J. Plant Biol. 30:23-29.
- Panda SK (2003) Heavy metal phytotoxicity induces oxidative stress in Taxithelium sp. Curr. Sci. 84:631-633.
- Panda SK, Chaudhury I, Khan MH (2003) Heavy metals induce lipid peroxidation and affects antioxidants in wheat leaves. Biol. Plant. 46:289-294.
- Panda SK, Choudhury S (2004) Changes in nitrate reductase (NR) activity and oxidative stress in moss Polytrichum commune subjected to chromium, copper and zinc toxicity. Braz. J. Plant Physiol. (Submitted).
- Panda SK (2002) The biology of oxidative stress in green cells: A Review. In: Panda SK (ed), Advances of stress physiology of plants, pp.1-13. Scientific Publishers, Jhodpur, India.
- Panda SK, Choudhury S (2004) Changes in nitrate reductase activity, lipid peroxidation and antioxidant system in moss Polytrichum sp subjected to hexavalent chromium treatment. Brazl. J. Plant Physiol. (Submitted, MS-01090/04).
- Poschenrieder C, Vazquez MD, Bonet A, Barcelo J (1991) Chromium III iron interaction in iron sufficient and iron deficient bean plants. II Ultrastructural aspects. J. Plant. Nutr. 14:415-428.
- Rai UN, Tripati RD, Kumar N (1992) Bioaccumulation of chromium and toxicity on growth, photosynthetic pigments, photosynthesis, in vivo nitrate reductase activity and protein in a chlorococcalean green alga Glaucocystis nostochinearum Itzigsohn. Chemosphere. 25:721-732.
- Ralph PJ, Burchett MD (1998) Photosynthetic response of Halophila ovalis to heavy metal stress. Environ. Pollut. 103:91-101.
- Roy S, Ihantola R, Hannien O (1992) Peroxidase activity in lake macrophytes and its relation to pollution tolerance. Environ. Exp. Bot. 32:457-464.
- Shi X, Dalal N S (1989) Chromium (V) and hydroxyl radical formation during the glutathione reductase û catalyzed reduction of chromium (VI). Biochem. Biophys. Res. 163:627-634.
- Solomonson LP, Barber MJ (1990) Assimilatory nitrate reductase: functional properties and regulation. Ann. Rev. Plant. Physiol and Plant Mol. Biol. 41:225-253.
- Sen AK, Mondol NG, Mondal S (1987) Studies of uptake and toxic effects of Cr (VI) on Pisita stratioites Wat. Sci. Tech. 19:119-127.
- Strile M, Kolar J, Selih VS, Kocar D, Pihlar B (2003) A comparative study of several transition metals in Fenton like reaction system at circum û neutral pH. Acta Chin. Slov. 50:619-632.
- Srivastava S, Srivastava S, Prakash S, Srivastava MM (1999) Fate of trivalent chromium in presence of organic acids. Chem. Spec. Bioavail. 10:147-150.
- Shanker AK, Djanaguiraman M, Sudhagar R, Jayaram K, Pathmanabhan G (2004) Expression of metallothioneins 3 û like protein mRNA in sorghum cultivars under chromium (VI) stress. Curr. Sci. 86:901-902.
- Scandalios JG (1993) Oxygen stress and superoxide dismutase. Plant Physiol. 101:7-12.
- Turner MA, Rust RH (1971) Effects of chromium on growth and mineral nutrition of soybean. Soil Sci. Soc. Ammer. Proc. 35:755-758.
- Tripati RD, Smith S (2000) Effect of chromium (VI) on growth and physiology of giant duckweed Spirodella polyrrhiza (L.) Schileiden. In: Yunus, M N, Singh, L, de Kok, J (eds), Environmental Stress: Indication, Mitigation and Eco û Conservation, pp.195-205. Kluwer Academic Publishers, The Northlands.
- Vajpayee P, Sharma SC, Rai UN, Tripati RD, Yunus M (1999) Bioaccumulation of chromium and toxicity to photosynthetic pigments, nitrate reductase activity and protein content of Nelumbo nucifera Gaetrn. Chemosphere 39:2159-2169.
- Vajpayee P, Tripati RD, Rai UN, Ali MB, Singh SN (2000) Chromium accumulation reduces chlorophyll biosynthesis, nitrate reductase activity and protein content of Nymphaea alba Chemosphere 41:1075-1082.
- Vajpayee P, Rai UN, Ali MB, Tripati RD, Yadav V, Sinha S, Singh SN (2001) Chromium û induced physiologic changes in Vallisneria spiralis L. and its role in phytoremedation of tannery effluents. Bull. Environ. Cont. Toxicol. 67:246-256.
- Von Burg R, Liu D (1993) Chromium and hexavalent chromium. J. Appl. Toxicol. 13:225-230.
- Vranova E, Inze D, Van Breusegem F (2002) Signal transduction during oxidative stress. J. Exp. Bot. 53:1227-1236.
- Vazquez MD, Poschenrieder C, Barcelo J (1987) Chromium VI induced structural changes in bush bean plants (Phaseolus vulgaris L.). Ann. Bot. 59:427-438.
- Yang MN, Wang J, Wang SH, Xu LL (2003) Salicylic acid induce aluminum tolerance by modulation of citrate efflux from roots of Cassia tora L. Planta. 217:168-174.
- Zeid IM (2001) Responses of Phaseolus vulgaris to chromium and cobalt treatment. Biol. Plant. 44:111-115.
Publication Dates
-
Publication in this collection
03 June 2005 -
Date of issue
Mar 2005