Abstract
Antioxidants production is amongst the physiological responses of plants to protect their tissues from oxidative damages caused by chilling and freezing stresses. Indeed, cold tolerance of plants is related to production of reactive oxygen species (ROS) scavengers to combat oxidative stress. In this study, two citrus cultivars including Citrus sinensis 'Siavaraz' and Citrus limon 'Lisbon' grown at the north of Iran were examined to compare antioxidants changes including total flavonoid, total phenol and total antioxidant capacities (TAC) in the flavedo tissue of the fruits under various low temperature treatments of control (15 ºC), 3, 0, -3 and -6 ºC. The results indicated that total flavonoid content and TAC in Citrus limon 'Lisbon' was significantly higher than in Citrus sinensis 'Siavaraz'. During the treatments, both cultivars showed a significant increase in the flavonoid content. Meanwhile, total phenol content significantly increased from 15 to -6 ºC in Citrus limon 'Lisbon' and from 15 to 0 ºC in Citrus sinensis 'Siavaraz'. These results suggest that the biosynthesis of phenolics and flavonoids in flavedo of citrus fruit in response to low temperature might be correlated with environmental adaptation rather than antioxidant response.
Antioxidant capacity; citrus; low temperature treatment
RESEARCH ARTICLE
Bioactive compounds and antioxidant capacities in the flavedo tissue of two citrus cultivars under low temperature
Mansour Afshar Mohammadian* * Corresponding author: email: afshar@guilan.ac.ir ; Zeinab Mobrami; Reza Hasan Sajedi
Department of Biology, Faculty of Sciences, University of Guilan, Iran
ABSTRACT
Antioxidants production is amongst the physiological responses of plants to protect their tissues from oxidative damages caused by chilling and freezing stresses. Indeed, cold tolerance of plants is related to production of reactive oxygen species (ROS) scavengers to combat oxidative stress. In this study, two citrus cultivars including Citrus sinensis 'Siavaraz' and Citrus limon 'Lisbon' grown at the north of Iran were examined to compare antioxidants changes including total flavonoid, total phenol and total antioxidant capacities (TAC) in the flavedo tissue of the fruits under various low temperature treatments of control (15 ºC), 3, 0, -3 and -6 ºC. The results indicated that total flavonoid content and TAC in Citrus limon 'Lisbon' was significantly higher than in Citrus sinensis 'Siavaraz'. During the treatments, both cultivars showed a significant increase in the flavonoid content. Meanwhile, total phenol content significantly increased from 15 to -6 ºC in Citrus limon 'Lisbon' and from 15 to 0 ºC in Citrus sinensis 'Siavaraz'. These results suggest that the biosynthesis of phenolics and flavonoids in flavedo of citrus fruit in response to low temperature might be correlated with environmental adaptation rather than antioxidant response.
Key words: Antioxidant capacity, citrus, low temperature treatment
INTRODUCTION
Reactive oxygen species including hydroxyl free radical (OH), hydrogen peroxide (H2O2) and singlet oxygen (O-2) are general metabolites of plants, and a common consequence of low temperatures is the increased production of these ROS (Pastori et al., 2000). Plant antioxidant apparatus consist of nonenzymatic and enzymatic scavenging systems. Nonenzymatic scavenging system includes ascorbic acid, carotenoids, phenolic compounds and vitamin E. Environmental stress such as chilling induce oxidative damage is related to the imbalance between ROS production and scavenging which leads to the accumulation of ROS and resulting in lipid peroxidation of cell membranes (Hodgson and Raison, 1991).
Phenolic compounds act as free radical terminators (Shahidi et al., 1992) and mostly include flavonoids, phenolic acids, stilbenes, coumarins and tannins (Liu, 2004). Yet the role that flavonoids have in the response of stress is not well understood. In many cases, these compounds may increase antioxidant activity as part of a general stress response (Winkel-Shirley, 2002). The mechanisms of action of flavonoids are through scavenging or chelating process (Cook and Samman, 1996; Kessler et al., 2003). Nutritional studies are now focused on examining food for their protective and health-promoting potential. Epidemiological studies have shown that fruit and vegetables are especially rich in natural antioxidants and these compounds can reduce risk of chronic diseases, such as cancer, Alzheimer's disease and diabetes (Temple, 2000; Willett, 2002; Liu, 2003). Phytochemicals, such as alkaloids, phenolics, carotenoids, and various nitrogenous compounds, in fruit and vegetables, are reported for various bioactivities, e.g. antioxidant, anti-fungal, antibacterial and antiviral activities (Dillard & German, 2000). Citrus fruits and derived products have beneficial impacts on the human health. Hence, citrus fruits have attracted much attention because of its nutritional and antioxidant properties (Del Caro et al., 2004; Dhuique-Mayer et al., 2005; Wu et al., 2007).
There is a considerable positive association between consumption of citrus fruit or juice with reduced risk of several diseases (Joshipura et al., 2001). Citrus fruit extracts have health benefits that mainly is attributed to the presence of bioactive compounds, such as vitamin C (Knekt et al., 2004), phenolics (e.g. flavanone glycosides, hydroxycinnamic acids) (Rossa et al., 2000), and carotenoids (Craig, 1997) are also found to have activities, such as anti-inflammatory, anti-tumor, anti-fungal and blood clot inhibition activities (Olson, 1988; Middleton and Kandaswami, 1994; Yehoshua et al., 1995). So far, studies mainly focused on bioactive compounds and total antioxidant capacities (TAC) of citrus (Gorinstein et al., 2004a; Yoo et al., 2004) and juice (Gardner et al., 2000; Gorinstein et al., 2004b).
There has been no report comparing the nutritional and health-promoting values of citrus fruit under chilling stress. The flavedo tissue of the citrus fruit (the outer pigmented layer of the fruit peel) is the first protective layer of the fruit facing various environmental stresses. The aim of this study was to compare the content of the bioactive compounds and the antioxidant capacity in the flavedo tissue of two citrus cultivars including Citrus sinensis 'Siavaraz' and Citrus limon 'Lisbon' under various cold treatments including control (15), 3, 0, -3 and -6 ºC.
MATERIALS AND METHODS
Plant materials and treatments: Commercially matured fruits of two citrus cultivars including Citrus sinensis 'Siavaraz' and Citrus limon 'Lisbon' were harvested from the garden of Citrus Institute located in Tonekabon city (Latitude: 36° 48' 31''N, Longitude: 50° 52' 55'' E), Iran, in December 2009. The maturity of citrus fruit was determined according to skin color. Freshly harvested fruits of the same cultivar were remained for 10 h at 3, 0, -3 and -6 ºC. The temperature of 15 ºC was used as control in order to compare the ongoing changes in antioxidant compounds of the fruits under cold treatments. Flavedo tissues of citrus fruit of each cultivar were manually separated. They were frozen in liquid nitrogen and then stored at -20˚C prior to analysis.
Extraction: The phenolic compounds were extracted according to the method reported by Singleton and Rossi (1965) with slight modifications. The frozen fruit in liquid nitrogen was ground into fine powder in a mortar and 0.25 g of ground sample was accurately weighed in a screw-capped tube.
The phytochemicals were extracted with 5 mL of 80 % methanol/water and vortexed for 1 min. The samples centrifuged at 1500 × g for 10 min to remove the solid fraction. The supernatant was used for determination of total phenolics, total flavonoids and TAC.
Determination of total phenolic content: Total phenolic contents of the fruit extracts were measured using a modified colorimetric Folin-Ciocalteu method (Singleton and Rossi, 1965) with further slight modifications. Fruit extracts (0.5 mL) were placed in a test tube. Folin-Ciocalteu reagent (2.5 mL) was added to the solution and allowed to react for 3 min. The reaction was neutralized with 2 mL of sodium carbonate (7.5 %). Absorbance at 765 nm was read after 30 min. Gallic acid was used as standard and data were expressed as mg Gallic acid equivalents (GA)/ g FW.
Determination of total flavonoid content: The total flavonoid content of the samples was measured using a colorimetric method (Zhishen et al., 1999; Dewanto et al., 2002). The methanolic extract (250 µL) was mixed respectively with 1.25 mL DI water and 75 µL of 5% NaNO2 solution, then allowed to mix for 6 min. After addition of 150 µL of 10% AlCl3 solution and mixing for 5 min, the reaction was initiated by adding 0.5 mL of 1 M NaOH and the total volume was made up to 2.5 mL with DI water. Sample absorbance was read at 510 nm using a UV/vis spectrophotometer. (+)- catechin standard was used as standard and total flavonoid content was expressed as µg (+)-catechin equivalents (CA)/g FW.
Free radical scavenging activity determination: Total antioxidant capacity (TAC) was determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity (DRSC) assays (Blois, 1958) with modifications. 0.2 mL of powder extract dissolved in methanol (0.05 mg/ml) were mixed with 0.6 mL of 0.1 mM DPPH reagent in methanol was freshly prepared and kept in the dark prior to analysis. After 40 min absorbance was measured at 593 nm using a UV/vis spectrophotometer (Shimadzu model UV-2450). The experiments were repeated for three times.
Statistical analysis: A completely randomized design with three replicates was used for the experiments. Statistical comparisons of the mean values were performed by analysis of variance (ANOVA), followed by Duncan's multiple-range test (P < 0.05), using SAS 8.3 software (SAS Ins. Inc., Cary, USA).
RESULTS AND DISCUSSION
Flavonoid and total phenol content: Antioxidants are compounds that can delay or inhibit the oxidation of lipids or other molecules by inhibiting the initiation or propagation of oxidizing chain reactions (Velioglu et al., 1998). The antioxidant activity of phenolic compounds is mainly due to their redox properties, which can play an important role in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen or decaying peroxides (Rice-Evans et al., 1997). However, the role of phenolic compounds in plant abiotic stresses particularly low temperature stress, has received much less attention.
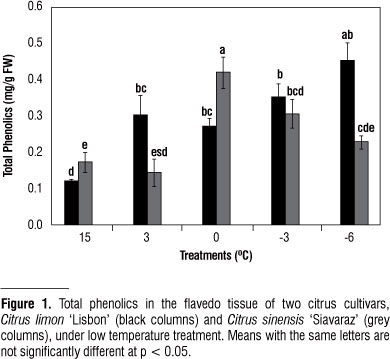
Since only a few of the entire spectra of compounds could be resolved by the HPLC analysis in this study, the spectrophotometric patterns were used to estimate the total amount of phenolics and flavonoids present in the samples. Initial total phenolic was 0.123 mg/g FW for Citrus limon 'Lisbon' and 0.173 mg/g FW for Citrus sinensis 'Siavaraz' (Table 1). This is in agreement with less total phenolics content of Citrus limon 'Lisbon' than orange (Li et al., 2006). Folin-Ciocalteu reagent is nonspecific for phenolic compounds since it measures sample reducing capacity through electronic transfer-based antioxidant capacity and, thus it can be reduced by many non-phenolic compounds such as vitamin C, sugars and organic acids (Huang et al., 2005, Li et al., 2006). The amount of total phenolics was relatively depended on the flavonoid content in Citrus limon 'Lisbon' (R2 = 0.547; p<0.01) (data not shown).
In this study a significant increase in total phenol was found in Citrus limon 'Lisbon' and Citrus sinensis 'Siavaraz' during low temperature treatments from 15 to -6 ºC (Fig. 1). Rapisarda et al. (1999) reported that during sweet orange cold storage (6±1 ºC), there was an initial increase and subsequent decrease in total phenolics in both 'T. Messina' and 'Ovale' reflecting the trend of vitamin C content. Also, Pennycooke et al. (2004) found that cold acclimation induced accumulation of total phenolics and increased antioxidant capacity.
Increase of phenolic compounds in tissues during chilling treatments may be partially due to chilling adaptation as defense mechanisms for scavenging ROS and also to mediate these stresses (Christie et al., 1994). Consequent decrease in freezing temperatures was also probably due to polymerization and oxidation of phenolic compounds. It is assumed that freezing temperatures with disruption of cell membranes may trigger the release of oxidative and hydrolytic enzymes that would destroy the antioxidants. Probably, deactivating these enzymes avoid the loss of phenolics and, therefore, lead to the increase of total phenolics content in Citrus limon 'Lisbon'. According to the reports, the decrease in total phenolics and increase in lignin contents in Mangosteen fruit stored at low temperature (6 ºC), suggest that phenolics are also incorporated into lignin (Bunsiri et al., 2003).
The results of the current research indicated that initial total flavonoid content was 11.1 µg/g FW and 20.2 mg/g FW in Citrus sinensis 'Siavaraz' and Citrus limon 'Lisbon', respectively (Table 1). There was significant increase in the content of total flavonoid in the flavedo of both citrus cultivars during low temperature treatments from 0 to -6 ºC. At the same time, total flavonoid in Citrus limon 'Lisbon' was significantly higher than that of Citrus sinensis 'Siavaraz' during low temperature treatments (Fig. 2).
Increase of flavonoids during low temperature treatment indicates that flavonoids may act as part of antioxidant system for ROS scavenging. Previous studies also demonstrated that cold storage at 6±1 ºC increased flavanones, anthocyanins, HCA in blood oranges (Rapisarda et al., 1999).
Antioxidant activity: There was no significant change in the antioxidant activity of flavedo tissue of both cultivars during low temperature treatments from 15 to -6 ºC. However, there was a significant reduction in the antioxidant activity in the flavedo of Citrus sinensis 'Siavaraz' at -6 ºC compared with control samples at 15 ºC (Fig. 3). Rapisarda et al. (1999) also reported that antioxidant activity increased during 6±1 ºC cold storage of blood oranges caused mainly by phenolic accumulation. Additionally, initial TAC in the flavedo of Citrus limon 'Lisbon' was significantly higher than in Citrus sinensis 'Siavaraz'. This is in agreement with the results found by Li et al. (2006) in Yen Ben Citrus limon 'Lisbon' compared with orange.
Relationship between total phenolic, total flavonoids and TAC: According to the results of the current study, good correlation could not be found between total phenolic content and the TAC (data not shown). This is in contrary with finding of Abeysinghe et al. (2007), Anagnostopoulou et al. (2006) and Rapisarda et al. (1999) but in agreement with the results of Li et al. (2006). However, the relationship between the antioxidant activity and phenolic compounds depend on several factors such as chemical structure of individual component, synergistic interaction among them and specific conditions applied in different assay (Huang et al., 2005). It seems that the antioxidant activity of Citrus limon 'Lisbon' and orange not to be the property of a single phytochemical compound but is widely distributed between vitamin C and phenolics constituents. According to Kähkönen et al. (2001), ascorbic acid could exert a synergistic effect with phenolic components. Also, due to differences in chemical structure of phenolic compounds may be the total phenolic content is high but the total antioxidant activity is low (Li et al., 2006).
There was a positive linear relationship between total flavonoid content and the total antioxidant activity of both cultivars (Fig. 4). The high flavonoid content resulted in a high antioxidant activity. Antioxidant activity correlated strongly with total flavonoid content in Citrus limon 'Lisbon' (Fig. 4A) (R2 = 0.973; p<0.05) and Citrus sinensis 'Siavaraz' (Fig. 4B) (R2 = 0.903; p<0.01). Cakir et al. (2003) also reported that antioxidant activity was increased in experimented plants which were rich in flavonoids content.
CONCLUSION
The results of this study indicated that low temperatures stimulated phenolics and flavonoids biosynthesis in Citrus limon 'Lisbon', whereas in Citrus sinensis 'Siavaraz' total phenolic content decreased significantly during freezing temperatures which could be attributed to freezing-related degradation. Surprisingly, in the present experimental conditions, total phenolics content did not correlate with radical scavenging activity and the antioxidant activity negatively correlated with flavonoid content. These results suggest that the biosynthesis of phenolics and flavonoids in flavedo of citrus fruit in response to low temperature treatments might be correlated with environmental adaptation. The current study also demonstrated that the methanolic extracts from flavedo of Citrus limon 'Lisbon' possessed higher total flavonoid content and antioxidant activity based on the DPPH assay. Therefore, citrus flavedo tissue which usually discarded as waste can be used as a rich source of phenolic antioxidants.
Future studies should focus on the modern medicals and chemical techniques to evaluate and modify the structures of certain purified plant ingredients into better agents with high efficiency and chilling stress tolerance. In regard to the present unusual findings on the antioxidant system, it is becoming clearer that the mechanisms of chilling stress tolerance in citrus fruits are complex and may act in concert with other biochemical and physiological mechanisms to maintain normal physiological functions under chilling conditions.
Acknowledgments: This work was supported by the fund of the University of Guilan, Iran.
Received: 04 April 2011
Accepted: 13 October 2011
- Abeysinghe DC, Xian Li, Chong De Sun, Wang Shu Zhang, Chun Hua Zhou, Kun Song Chen (2007) Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 104:1338-1344.
- Anagnostopoulou MA, Panagiotis Kefalas, Vassilios P Papageorgio, Andreana N Assimopooulou Dimitrios Boskou (2006) Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem . 94:19-25.
- Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature. 26: 1199- 1200.
- Bunsiri A, Ketsa S, Paull RE (2003a) Phenolic metabolisms and lignin synthesis in damaged pericarp of mangosteen fruit after impact. Postharvest Biol Technol . 29:61-71
- Cakir A, Mavi AYA, Duru ME, Harmandar M, Kazaz C (2003) Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium L by activity-guided fractionation. J Ethonopharmacol. 87:73-83.
- Christie PJ, Alfenito MR, Walbot V (1994) Impact of low temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta. 194: 541-549.
- Cook NC, Samman S (1996) Flavonoids- chemistry, metabolism, cardioprotective effects, and dietary sources. Nutr Biochem. 7:66- 76.
- Craig WJ (1997) Phytochemicals: guardians of our health. J Amer Diet Assoc. 97:199-204.
- Del Caro A, Piga A, Vacca V, Agabbio M (2004) Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chem. 84:99-105.
- Dewanto V, Wu XZ, Adom KK, Liu, RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agricult Food Chem. 50:3010-3014.
- Dhuique-Mayer C, Caris-Veyrat C, Ollitrault P, Curk F, Amiot MJ (2005) Varietal and interspecific influence on micronutrient contents in citrus from the Mediterranean area. J Agricult Food Chem. 53:2140-2145.
- Dillard CJ, German JB (2000) Phytochemicals: nutraceuticals and human health. J Sci Food Agricult. 80:1744-1756.
- Gardner PT, White TAC, McPhail DB, Duthie GG (2000) The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 68:471-474.
- Gorinstein S, Cvikrova M, Machackova I, Haruenkit R, Park YS, Jung ST, et al (2004a) Characterization of antioxidant compounds in Jaffa sweeties and white grapefruits. Food Chem. 84:503-510.
- Gorinstein S, Leontowicz H, Leontowicz M, Krzeminski R, Gralak M, Martin Belloso O, et al (2004b) Fresh Israeli jaffa blond (Shamouti) orange and Israeli jaffa red star ruby (Sunrise) grapefruit juices affect plasma lipid metabolism and antioxidant capacity in rats fed added cholesterol. J Agricult Food Chem. 52:4853-4859.
- Hodgson RAJ, Raison, JK (1991) Superoxide production by thylakoids during chilling and its implication in the susceptibility of plants to chilling-induced photoinhibition. Planta. 183:222-228.
- Huang D, Boxin O, Prior RL (2005) The chemistry behind antioxidant capacity assay. J Agricult Food Chem. 53:1841-1856.
- Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. (2001) The effect of fruit and vegetable intake on risk for coronary heart disease. Annals Intern Med. 134:1106-1114.
- Kähkönen MP, Hopia AI, Heinonen M (2001) Berry phenolics and their antioxidant activity. J Agricult Food Chem. 49:4076-4082.
- Kessler M, Ubeaud G, Jung L (2003) Anti- and pro-oxidant activity of rutin and quercetin derivatives. J Pharm Pharmacol. 55: 131-142.
- Knekt P, Ritz J, Pereira MA, O'Reilly EJ, Augustsson K, Fraser GE, et al. (2004) Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Amer J Clinic Nutr. 80:1508-1520.
- Li BB, Smith B, Hossain MdM (2006) Extraction of phenolics from citrus peels. Separat Purif Technol 48: 182-188.
- Liu RH (2003) Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Amer J Clin Nutr. 78:517-520.
- Liu RH (2004) Potential synergy of phytochemicals in cancer prevention: Mechanism of action. Amer Soc Nutr Sci. 134:3479s-3485s.
- Middleton EJ, Kandaswami C (1994) Potential health promoting properties of citrus flavonoids. Food Technol. 18:115-120.
- Olson RED (1988) Limonene, an anticarcinogenic terpene. Nutr Rev 46: 363-365.
- Pastori G, Foyer CH, Mullineaux P (2000) Low temperature-induced change in the distribution of H2O2 and antioxidants between the bundle sheath and mesophyll cells of maize leaves. J Exp Bot. 51:107-113.
- Pennycooke JC, Vepachedu R, Stushnoff C, Jones ML (2004) Expression of an α-galactosidase gene in petunia is up-regulated during low temperature deacclimation. J Amer Soc Hort Sci. 129:491-496.
- Rapisarda P, Tomaino A, LoCascio R, Bonina F, DePasquale A, Saija A (1999) Antioxidant effectiveness as influenced by phenolic content of fresh orange juices. J Agricult Food Chem. 47:4718-4723
- Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci. 2: 152-159.
- Rossa SA, Ziskac DS, Ke Zhaod , ElSohlya MA (2000) Variance of common flavonoids by brand of grapefruit juice. Fitoterapia. 71:154-161.
- Shahidi F, Janitha PK, Wanasundara PD (1992) Phenolic antioxidants. Crit Rev Food Sci Nutr. 32:67-103.
- Singleton and Rossi (1965) Analysis of total phenols and other oxidation substrates and antioxidants by means of the Folin-Ciocalteu reagent. Meth Enzymol. 299:152-178.
- Temple NJ (2000) Antioxidants and disease: more questions than answers. Nutr Res. 20:449-459.
- Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agricult Food Chem. 46: 4113-4117.
- Willett WC (2002) Balancing life-style and genomics research for disease prevention. Science. 296:695-698.
- Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol. 5:218-23.
- Wu T, Guan Y, Ye J (2007) Determination of flavonoids and ascorbic acid in grapefruit peel and juice by capillary electrophoresis with electrochemical detection. Food Chem. 100:1573-1579.
- Yehoshua SB, Rodov V, Fang DQ, Kim JJ (1995) Preformed antifungal compounds of citrus fruit: Effect of postharvest treatments with heat and growth regulators. J Agricult Food Chem. 43:1062-1066.
- Yoo KM, Lee KW, Park JB, Lee HJ, Hwang IK (2004) Variation in major antioxidants and total antioxidant activity of Yuzu (Citrus Junos Sieb ex Tanaka) during maturation and between cultivars. J Agricult Food Chem. 52:5907-5913.
- Zhishen J, Mengcheng T, JianmingW (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64:555-559.
Publication Dates
-
Publication in this collection
20 Jan 2012 -
Date of issue
2011
History
-
Received
04 Apr 2011 -
Accepted
13 Oct 2011