Abstract
Background
Aortic cross-clamping and balloon occlusion of the aorta could lead to damage to the aorta wall.
Objective
The aim of this study was to investigate changes to the aorta wall related to the method used to interrupt flow (clamping or balloon) in the different techniques available for aortic surgery.
Methods
Experiments were performed on 40 female pigs, weighing 25-30kg, which were randomly allocated to 4 study groups: S (n=10), no intervention (sham group); C (n=10), midline transperitoneal laparotomy for infrarenal abdominal aortic access with 60 min of cross-clamping; L (n=10), laparoscopic infrarenal abdominal aortic surgery with 60 min of cross-clamping; EV (n=10), remote proximal aortic control with transfemoral arterial insertion of aortic occlusion balloon catheter, inflated to provide continued aortic occlusion for 60min. After euthanasia, the aortas were removed and cross-sectioned to obtain histological specimens for light microscopic and morphometric analyses. The remaining longitudinal segments were stretched to rupture and mechanical parameters were determined.
Results
We observed a reduction in the yield point of the abdominal aorta, decrease in stiffness and in failure load in the aortic cross-clamping groups (C and L) compared with the EV group.
Conclusions
Aortic cross-clamping during open or laparoscopic surgery can affect the mechanical properties of the aorta leading to decrease in resistance of the aorta wall, without structural changes in aorta wall histology.
Keywords:
abdominal aorta; tensile strength; mechanical stress; vascular closure devices
Resumo
Contexto
O clampeamento aórtico e a oclusão da aorta com balão poderiam levar a lesões na parede aórtica.
Objetivo
O objetivo deste estudo foi verificar as alterações da parede aórtica relacionadas ao método de interrupção de fluxo (cample ou balão) em diferentes técnicas disponíveis para cirurgia de aorta.
Métodos
Os experimentos foram realizados em 40 porcos fêmeas pesando de 25-30 kg, alocados para quatro grupos: S (n = 10), nenhuma intervenção (sham); C (n = 10), laparotomia mediana transperitoneal para acesso à aorta abdominal infrarrenal com tempo de clampeamento de 60 minutos; L (n = 10), cirurgia laparoscópica da aorta abdominal infrarrenal com tempo de clampeamento de 60 minutos; EV (n = 10), controle aórtico proximal com inserção de cateter-balão para oclusão aórtica por acesso femoral, inflado a fim de promover oclusão aórtica contínua por 60 minutos. Após a eutanásia, as aortas foram removidas e seccionadas para obtenção de espécimes histológicos destinados a análises morfométricas e por microscopia de luz. Os fragmentos longitudinais restantes foram estirados até a ruptura, e determinaram-se padrões mecânicos.
Resultados
Observou-se redução do limite de proporcionalidade da aorta abdominal, diminuição da rigidez e da carga de ruptura nos grupos submetidos a campleamento aórtico (C e L) em comparação ao grupo EV.
Conclusões
O campleamento aórtico durante cirurgia aberta ou laparoscópica pode afetar as propriedades mecânicas da aorta, ocasionando redução de resistência da parede aórtica sem desencadear alterações na estrutura histológica da parede aórtica.
Palavras-chave:
aorta abdominal; resistência à tração; estresse mecânico; dispositivos de oclusão vascular
INTRODUCTION
Choosing the most appropriate surgical approach for repair of an infrarenal abdominal aortic aneurysm (AAA) involves analysis of surgical risk, comorbidities, morphology of the AAA, patient life expectancy, experience of the surgical team with each technique, and the scientific evidence for each technique. Strategies currently available for treatment of this condition disease include conventional open surgery, videolaparoscopic surgery, and endovascular treatment.11 Coscas R, Maumias T, Capdevila C, Javerliat I, Goëau-Brissonnière O, Coggia M. Mini-invasive treatment of abdominal aneurysms: current roles of endovascular, laparoscopic and open techniques. Ann Vasc Surg. 2014;28(1):123-31. http://dx.doi.org/10.1016/j.avsg.2013.05.007. PMid:24200131.
http://dx.doi.org/10.1016/j.avsg.2013.05...
2 Pascarella L, Aboul Hosn M. Minimally invasive management of severe aortoiliac occlusive disease. J Laparoendosc Adv Surg Tech A. 2018;28(5):562-8. http://dx.doi.org/10.1089/lap.2017.0675. PMid:29346011.
http://dx.doi.org/10.1089/lap.2017.0675...
3 Ahmed N, Gollop ND, Ellis J, Khan OA. How does elective laparoscopic aortic aneurysm repair compare to endovascular aneurysm repair? Interact Cardiovasc Thorac Surg. 2014;18(6):814-20. http://dx.doi.org/10.1093/icvts/ivu031. PMid:24578481.
http://dx.doi.org/10.1093/icvts/ivu031...
-44 Robertson L, Nandhra S. Laparoscopic surgey for elective abdominal aortic aneurysm repair. Cochrane Database Syst Rev. 2017;5:CD012302. Despite the initial enthusiasm for laparoscopic aortic surgery, the technique has not been widely adopted in vascular surgery because of the challenges inherent to the procedure and the long learning curve, so its use has remained restricted to a few specialized centers.22 Pascarella L, Aboul Hosn M. Minimally invasive management of severe aortoiliac occlusive disease. J Laparoendosc Adv Surg Tech A. 2018;28(5):562-8. http://dx.doi.org/10.1089/lap.2017.0675. PMid:29346011.
http://dx.doi.org/10.1089/lap.2017.0675...
,33 Ahmed N, Gollop ND, Ellis J, Khan OA. How does elective laparoscopic aortic aneurysm repair compare to endovascular aneurysm repair? Interact Cardiovasc Thorac Surg. 2014;18(6):814-20. http://dx.doi.org/10.1093/icvts/ivu031. PMid:24578481.
http://dx.doi.org/10.1093/icvts/ivu031...
There is evidence to suggest that elective laparoscopic surgery to repair an AAA has comparable invasivity to endovascular repair (EVAR), with the advantages of a lower conversion rate and similar morbidity and mortality, while offering a minimally invasive option for treatment of patients with anatomy that is unsuitable for EVAR.11 Coscas R, Maumias T, Capdevila C, Javerliat I, Goëau-Brissonnière O, Coggia M. Mini-invasive treatment of abdominal aneurysms: current roles of endovascular, laparoscopic and open techniques. Ann Vasc Surg. 2014;28(1):123-31. http://dx.doi.org/10.1016/j.avsg.2013.05.007. PMid:24200131.
http://dx.doi.org/10.1016/j.avsg.2013.05...
,33 Ahmed N, Gollop ND, Ellis J, Khan OA. How does elective laparoscopic aortic aneurysm repair compare to endovascular aneurysm repair? Interact Cardiovasc Thorac Surg. 2014;18(6):814-20. http://dx.doi.org/10.1093/icvts/ivu031. PMid:24578481.
http://dx.doi.org/10.1093/icvts/ivu031...
,44 Robertson L, Nandhra S. Laparoscopic surgey for elective abdominal aortic aneurysm repair. Cochrane Database Syst Rev. 2017;5:CD012302.
Conventional and laparoscopic surgery both involve use of hemostatic clamps (atraumatic clamps) to control blood flow and reflux. However, despite their “atraumatic” label, these clamps cause acute injury to the artery wall. The degree of injury appears to be dependent on the pressure applied and the duration of clamping, and ranges from distortion of the intima to complete breakdown of the tunica media of the vessel, with weakening of the artery wall, intimal hyperplasia, and restenosis.55 Slayback JB, Bowen WW, Hinshaw DB. Intimal injury from arterial clamps. Am J Surg. 1976;132(2):183-7. http://dx.doi.org/10.1016/0002-9610(76)90045-3. PMid:952348.
http://dx.doi.org/10.1016/0002-9610(76)9...
Margovsky et al.66 Margovsky AI, Lord RSA, Meek AC, Bobryshev YV. Artery wall damage and platelet uptake from so-called atraumatic arterial clamps: an experimental study. Cardiovasc Surg. 1997;5(1):42-7. http://dx.doi.org/10.1016/S0967-2109(96)00064-6. PMid:9158122.
http://dx.doi.org/10.1016/S0967-2109(96)...
observed formation of cavities in the tunica media, a change known as cystic necrosis of the media that is found in degenerative processes involving the aorta, such as aortic dissections, degenerative aneurysms, and aging.77 Borges LF, Gutierrez PS, Marana HR, Taboga SR. Picrosirius polarization staining method as an efficient histopathological tool for collagenolysis detection in vesical prolapse lesions. Micron. 2007;38(6):580-3. http://dx.doi.org/10.1016/j.micron.2006.10.005. PMid:17126553.
http://dx.doi.org/10.1016/j.micron.2006....
,88 Jaldin RG, Castardelli É, Perobelli JE, et al. Morphologic and biomechanical changes of thoracic and abdominal aorta in a rat model of cigarette smoke exposure. Ann Vasc Surg. 2013;27(6):791-800. http://dx.doi.org/10.1016/j.avsg.2013.03.002. PMid:23880458.
http://dx.doi.org/10.1016/j.avsg.2013.03...
Loh et al.99 Loh CS, Al-Jafari MS, Croton R. Acute rupture of the abdominal aorta from cross-clamp injury. Eur J Vasc Surg. 1990;4(6):647-8. http://dx.doi.org/10.1016/S0950-821X(05)80824-2. PMid:2279578.
http://dx.doi.org/10.1016/S0950-821X(05)...
described acute rupture of the abdominal aorta provoked by clamping, documenting localized ischemic parietal injury that weakened the structural integrity of the aorta. Nevertheless, it has been observed that, although clamping caused morphological changes,1010 Chen HY, Navia JA, Shafique S, Kassab GS. Fluid-structure interaction in aortic cross-clampig:implications for vessel injury. J Biomech. 2010;43(2):221-7. http://dx.doi.org/10.1016/j.jbiomech.2009.08.042. PMid:19883917.
http://dx.doi.org/10.1016/j.jbiomech.200...
there were no significant changes to the mechanical properties of the artery wall over the long term.1111 Dobrin PB, McGurrin JF, McNulty JA. Chronic histologic changes after vascular clamping are not associated with altered vascular mechanics. Ann Vasc Surg. 1992;6(2):153-9. http://dx.doi.org/10.1007/BF02042737. PMid:1599834.
http://dx.doi.org/10.1007/BF02042737...
However, there is a lack of studies that correlate the acute changes caused by clamping of the aorta with mechanical changes to its walls.
While the aorta is not clamped during routine endovascular treatment, there is temporary occlusion of the aortic flow by devices for deployment of stent-grafts and by inflation of the balloon used for positioning the device after release.1212 Thompson MM, Nasim A, Sayers RD, et al. Oxygen free radical and cytokine generation during endovascular and conventional aneurysm repair. Eur J Vasc Endovasc Surg. 1996;12(1):70-5. http://dx.doi.org/10.1016/S1078-5884(96)80278-4. PMid:8696901.
http://dx.doi.org/10.1016/S1078-5884(96)...
,1313 Malina M, Veith F, Ivancev K, Sonesson B. Balloon occlusion of the aorta during endovascular repair of ruptured abdominal aortic aneurysm. J Endovasc Ther. 2005;12(5):556-9. http://dx.doi.org/10.1583/05-1587.1. PMid:16212455.
http://dx.doi.org/10.1583/05-1587.1...
The negative effects on the mechanical properties of the aorta wall of oversizing stent-grafts has already been studied.1414 Sincos IR, Aun R, Silva ES, et al. Impact of stent-graft oversizing on the thoracic aorta: experimental study in a porcine model. J Endovasc Ther. 2011;18(4):576-84. http://dx.doi.org/10.1583/11-3470.1. PMid:21861750.
http://dx.doi.org/10.1583/11-3470.1...
There are also clinical scenarios in which prolonged balloon inflation is needed in the absence of the effects of contact between an endoprosthesis and the artery wall – for example in resuscitative endovascular balloon occlusion of the aorta (REBOA), used for treatment of a ruptured aorta or for intraoperative complications.1313 Malina M, Veith F, Ivancev K, Sonesson B. Balloon occlusion of the aorta during endovascular repair of ruptured abdominal aortic aneurysm. J Endovasc Ther. 2005;12(5):556-9. http://dx.doi.org/10.1583/05-1587.1. PMid:16212455.
http://dx.doi.org/10.1583/05-1587.1...
,1515 Matsuda H, Tanaka Y, Hino Y, et al. Transbrachial arterial insertion of aortic occlusion balloon catheter in patients with shock from ruptured abdominal aortic aneurysm. J Vasc Surg. 2003;38(6):1293-6. http://dx.doi.org/10.1016/S0741-5214(03)00774-2. PMid:14681630.
http://dx.doi.org/10.1016/S0741-5214(03)...
Injuries caused by intraluminal inflation of the balloon range from endothelial damage (abrasion and dissection) to necrosis of the tunica media, chiefly interfering in the function of components of the extracellular matrix.1616 Batchelor WB, Robinson R, Strauss BH. The extracellular matrix in balloon arterial injury: a novel target for restenosis prevention. Prog Cardiovasc Dis. 1998;41(1):35-49. http://dx.doi.org/10.1016/S0033-0620(98)80021-2. PMid:9717858.
http://dx.doi.org/10.1016/S0033-0620(98)...
Keris et al.1717 Keris V, Ozolanta I, Enina G, Kasyanovs V, Aide H, Bricis R. Biomechanical and structure assessment of transluminal angioplasty. Med Eng Phys. 1998;20(5):339-46. http://dx.doi.org/10.1016/S1350-4533(98)00032-0. PMid:9773687.
http://dx.doi.org/10.1016/S1350-4533(98)...
observed that arterial segments subjected to balloon angioplasty had reduced tangential elastic modulus in the circumferential direction, which could predispose to increases in the diameter of the vessel when subjected to normal blood pressure.
Although it appears that less invasive methods are beneficial in terms of the systemic repercussions of conventional surgical trauma, studies are needed that can shed light on the body’s pathophysiologic responses after open surgery, endovascular repair, or videolaparoscopic surgery on the aorta.33 Ahmed N, Gollop ND, Ellis J, Khan OA. How does elective laparoscopic aortic aneurysm repair compare to endovascular aneurysm repair? Interact Cardiovasc Thorac Surg. 2014;18(6):814-20. http://dx.doi.org/10.1093/icvts/ivu031. PMid:24578481.
http://dx.doi.org/10.1093/icvts/ivu031...
The objective of this study was to conduct comparative assessments of the structural and biomechanical changes to the aorta wall provoked by methods used to temporarily interrupt flow through the aorta, depending on the surgical access used to approach the aorta.
MATERIALS AND METHODS
A prospective, randomized, experimental study was conducted. The study complies with the Guide for Care and Use of Laboratory Animals and was approved by the institution’s Animal Experimentation Ethics Committee (protocol 899-2011). Female pigs, Large White - Landrace cross, weighing 25 to 30 kg were used. After an adaptation period of 5 to 10 days, the animals were allocated at random by simple lots to one of three experimental groups, with 10 animals in each: Group C (open surgery), Group L (videolaparoscopy), or Group EV (endovascular surgery). An additional group, Group S (Sham), was made up of aorta specimens from 10 animals with the same origin and weight range that were removed by the study soon after slaughter at the abattoir used by the farm that reared them. This group was used as the standard of normality for biomechanical and histological parameters.
Anesthesia procedures
The animals were kept in preoperative fasting for 8 hours. Premedication comprised a combination of 0.1 mg/kg of acepromazine 1%, 8 mg/kg of ketamine, 0.5 mg/kg of xylazine, and 0.5 mg/kg of morphine, via intramuscular injection. Fifteen minutes after premedication, the central vein of the ear was cannulated and used for anesthesia induction by administration of 2 mg/kg of ketamine and 2 mg/kg of diazepam. The animal was then placed in the prone position on the operating table for oral endotracheal intubation. Anesthesia was maintained with isoflurane at 5-10 mL/kg/min. Mechanical ventilation was provided with a tidal volume of 12-15 mL/kg of oxygen, at a rate of 10 to 12 respiratory movements/min, to maintain expiratory carbon dioxide pressure in the range of 35 to 45 mmHg. Baseline hydration was maintained with Ringer’s lactate solution infused at 5 mL/kg/h with an intravenous infusion pump and complemented with infusion of saline 0.9% according to hemodynamic requirements identified by a veterinary anesthetist throughout the procedure. Intraoperative monitoring comprised pulse oximetry with a sensor placed on the animal’s tongue, a rectal thermometer for body temperature, and invasive blood pressure monitoring via a carotid access, with arterial catheterization using an 11 cm 6F introducer.
Surgical procedures
Group C: Animals were positioned on the operating table in horizontal dorsal decubitus. After antisepsis and draping of the surgical field, a midline laparotomy was performed with transperitoneal exposure of the aorta. The infrarenal aorta was exposed from the point it crosses the left renal vein and the origin of the renal arteries and the aortic bifurcation were identified. The infrarenal aorta was then clamped with Debakey atraumatic forceps to interrupt flow through the aorta for 60 minutes.
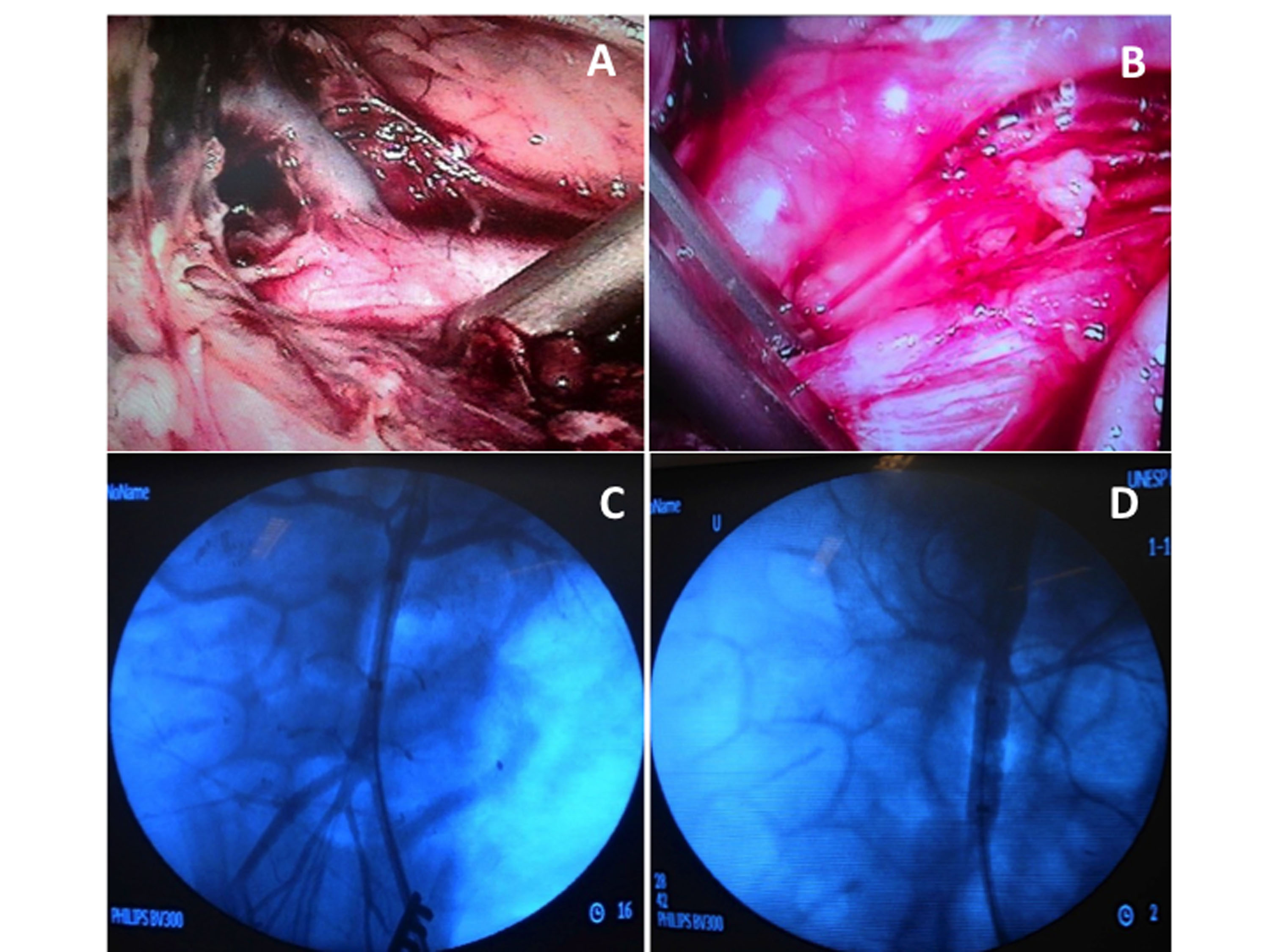
Group L: Animals were placed on the operating table in right lateral decubitus. Antiseptic solution was applied and the surgical field was draped. The pneumoperitoneum procedure was initiated via a percutaneous puncture with a Veress needle. After pneumoperitoneum was established with CO2 at a pressure of 16 mmHg, an 11 mm trocar was positioned lateral to the umbilical scar to introduce a 30° optical lens. After reestablishing pressure at 12 mmHg, two further 11 mm trocars were positioned lateral to the midline, above and below the line of the umbilicus. Another three 11 mm trocars were placed along the left side of the abdominal wall, using the costal margin, the midaxillary line, the large dorsal muscle, and the iliac crest as references. Exposure of the aorta began by medial elongation of the left colon, the left kidney, and the splenic flexure, using laparoscopic graspers, scissors, and harmonic scalpel (Ultracision®, Johnson & Johnson®). After completing dissection of the abdominal aorta, a laparoscopic aortic clamp (Storz®) was applied immediately below the left renal artery to interrupt flow through the aorta for 60 minutes (Figure 1).
Site of interruption of flow through the aorta for minimally invasive techniques. (A) Laparoscopic dissection and identification of the left renal artery; (B) Placement of the laparoscopic hemostatic forceps; (C) Aortography to identify emergence of the renal arteries; (D) Aortography with the balloon inflated.
Group EV: Animals were positioned on the operating table in horizontal dorsal decubitus. After antisepsis of the groin, the surgical field was draped. A transverse inguinotomy was performed and the left common femoral artery was accessed. Under direct view, the common femoral artery was punctured with a 21G single-wall needle and a rigid metal “J” tip guidewire inserted, enabling arterial catheterization with an 11 cm 6F introducer using the Seldinger technique. A 260 cm 0.035” Roadrunner hydrophilic guidewire (Cook Medical®) was advanced to the infrarenal aorta under radiographic guidance (Phillips®, BV 300 C-arm, United States), then a 5F Pig Tail angiographic catheter (Cook Medical®) was inserted to conduct aortography with injection of 20 mL of Optiray® nonionic contrast, delineating the renal arteries by road mapping. Aortography was performed before and after inflation of the balloon, with a total contrast volume of 40 mL. After removal of the introducer and provoking hemostasis by manual compression, the angiographic catheter was substituted for a complacent balloon catheter with a diameter of 32 mm for aortic occlusion (Coda Balloon Catheter®, Cook Medical®, USA). The angiographic catheter was then inserted via the carotid access. After positioning the balloon just below the origin of the renal arteries, it was inflated until flow was entirely interrupted, using 15 mL of contrast solution, with angiographic control, and maintained inflated for 60 minutes (Figure 1).
Experimental protocol
All of the surgical procedures were conducted by the same team, following the same experimental sequence. Prior to aortic clamping, sodium heparin was administrated intravenously at a dosage of 100 UI/kg to animals in all groups. In all groups, the duration of interruption of aortic blood flow was 60 minutes. After this period, animals were euthanized by anesthetic overdose and median laparotomy was performed to access the aorta and remove specimens for study. Segments of abdominal aorta approximately 5 mm in length were taken from the site of clamping/ballooning from five animals per group, including the point at which the clamp/balloon had been positioned, and fixed in buffered formalin 10% for histological study at a later date. From the other five animals in each group, samples of the aorta measuring approximately 3 cm (1 suprarenal cm and 2 infrarenal cm, to include the point where flow had been interrupted) were taken for use in the tensile strength tests.
Histology
Segments of aorta preserved in formol were processed in a Leica TP102 tissue processor and set in paraffin blocks in a Leica EG 1160. Posteriorly, serial cross-sections of approximately 5 µm were cut in a Leica RM 2155 microtome, mounted on glass slides and stained with hematoxylin and eosin (H & E) and Picrosirius Red, for collagen, and Verhoeff’s stain, for analysis of elastic fibers in the aorta wall. General changes to the vascular wall were recorded, such as loss of the lamellar architecture, reduction of smooth muscle cells, mononuclear cellular infiltrate, disorganization of collagen fibers, intensity of Picrosirius staining, and reduction or fragmentation of elastic fibers.
Biomechanical tests
The segments of abdominal aorta, including the portions immediately above and immediately below the point at which flow had been interrupted, were subjected to destructive uniaxial tensile testing, using a method employed previously88 Jaldin RG, Castardelli É, Perobelli JE, et al. Morphologic and biomechanical changes of thoracic and abdominal aorta in a rat model of cigarette smoke exposure. Ann Vasc Surg. 2013;27(6):791-800. http://dx.doi.org/10.1016/j.avsg.2013.03.002. PMid:23880458.
http://dx.doi.org/10.1016/j.avsg.2013.03...
,1818 Bertanha M, Moroz A, Jaldin RG, et al. Morphofunctional characterization of decellularized vena cava as tissue engineering scaffolds. Exp Cell Res. 2014;326(1):103-11. http://dx.doi.org/10.1016/j.yexcr.2014.05.023. PMid:24929113.
http://dx.doi.org/10.1016/j.yexcr.2014.0...
19 Cerqueira NF, Yoshida WB, Müller SS, Sequeira JL, Rodrigues AC, Padovani CR. Morphological and biomechanical study of abdominal aorta of rats submitted to experimental chronic alcoholism. Acta Cir Bras. 2005;20(3):213-8. http://dx.doi.org/10.1590/S0102-86502005000300004. PMid:16033179.
http://dx.doi.org/10.1590/S0102-86502005...
-2020 Yoshida WB, Müller SS, Carvalho I, Fabris VE, Naresse LE, Maffei FHA. Tensile strengthand histological changes of abdominal aorta of malnourished rats. Cardiovasc Surg. 1995;3(4):437-9. http://dx.doi.org/10.1016/0967-2109(95)94165-S. PMid:7583001.
http://dx.doi.org/10.1016/0967-2109(95)9...
for evaluation of mechanical properties. The ends of each segment were fixed using the machine’s clamps, which are smooth, non-cutting, metal plates, enabling the aortic segment to be stretched longitudinally. The traction velocity adopted was 30 mm/min. The apparatus employed was an EMIC® Universal Mechanical Test Machine, model DL 10.000 (Equipments and Testing Systems, Ltd., Curitiba, PR, Brazil), which is a system with precision of ± 0.018+F/3700 KN, as tested according to the Brazilian Association for Technical Standards (ABNT) NBR6156 and NBR6674 specifications. The machine operates in conjunction with a microcomputer with the Windows 98® operating system installed, running Mtest 1.00 software. At the end of the test, the program provides values for the mechanical properties chosen by the user and a load vs. elongation graph. These diagrams can be used to derive the following parameters (Figure 2): Yield point (N): maximum load value at which the material still has the capacity to return to its original length if the load is removed; graphically, this corresponds to the maximum tension value at which the linear function of the load-elongation curve still obeys Hooke’s Law, calculated using the Johnson method; Coefficient of stiffness (N/mm): force (N) divided by elongation (mm) at the elastic limit; which, since it is a constant, linear, numeric relationship, represents a material’s deformation capacity as the load is applied; Maximum load – force at failure (N): the greatest load withstood by the material before rupture, i.e., the limit of resistance.
Mathematical model used for tensile testing of the aorta. (A) Segment of aorta including the clamped portion, fixed in the machine’s jaws; (B) Universal Mechanical Test Machine in the Biomechanical Testing Laboratory at the Surgical Techniques and Experimental Surgery Laboratory and the uniaxial force vector applied to the sample; (C) Load vs. elongation diagram; (D) Elastic and plastic phases on load vs. elongation diagram; (E) Elastic limit calculated by the Johnson method; (F) Example of a graph from the test.
Statistical analysis
The sample size of 10 animals per experimental group was calculated with the help of the institution’s Research Support Office on the basis of previous experimental studies of aortic surgery using porcine models,2121 Suk P, Cundrle I Jr, Hruda J, et al. Porcine model of ruptured abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2012;43(6):698-704. http://dx.doi.org/10.1016/j.ejvs.2012.02.020. PMid:22421373.
http://dx.doi.org/10.1016/j.ejvs.2012.02...
22 Martín-Cancho MF, Sánchez-Margallo FM, Soria F, et al. Physiological responses to different ischemic periods during laparoscopic infrarenal aortic cross-clamping: evaluation in an experimental animal model. Ann Vasc Surg. 2009;23(4):506-18. http://dx.doi.org/10.1016/j.avsg.2008.12.002. PMid:19375889.
http://dx.doi.org/10.1016/j.avsg.2008.12...
23 Martin-Cancho MF, Crisostomo V, Soria F, et al. Physiologic responses to infrarenal aortic cross-clamping during laparoscopic or conventional vascular surgery in experimental animal model: comparative study. Anesthesiol Res Pract. 2008;581948:1-8. http://dx.doi.org/10.1155/2008/581948. PMid:21197458.
http://dx.doi.org/10.1155/2008/581948...
-2424 Alric P, Ryckwaert F, Branchereau P, Marty-Ane C, Mary H, Colson P. A porcine model of systemic and renal haemodynamic responses to infrarenal aortic cross-clamping. Eur J Vasc Endovasc Surg. 2003;25(1):72-8. http://dx.doi.org/10.1053/ejvs.2002.1789. PMid:12525815.
http://dx.doi.org/10.1053/ejvs.2002.1789...
and was adopted as the reference for constituting the groups. First, normality of the data was tested, showing that they were symmetrical. Therefore, analysis of variance (ANOVA), followed by the Tukey test was used for multiple comparisons to test whether there were differences between the C, L, EV, and S groups.
RESULTS
Biomechanical tests
The EV group exhibited the greatest resistance to load, with higher stiffness coefficient (p < 0.05), maximum load (p < 0.05), and yield point (p < 0.05), than groups C and L. The aorta samples from the EV group had similar mechanical behavior to the S group in the tensile tests (Figures 3, 4, and 5).
Absolute comparison between groups for the parameter maximum load, where the difference in maximum load was statistically significant (p < 0.05) between groups EV and L and between EV and C.
Absolute comparison between groups for the parameter elasticity, where the difference in elastic limit was statistically significant (p < 0.05) between groups EV and L and between EV and C.
Absolute comparison between groups for the parameter stiffness coefficient, where the difference in stiffness coefficient was statistically significant (p < 0.05) between groups EV and L and between EV and C.
Histological analysis of the aorta specimens
The histology of the aorta specimens was preserved in all of the cases analyzed. No changes were observed in cellular structure, collagen fibers, or elastic fibers in the samples assessed, irrespective of study group (Figure 6).
Histological sections of abdominal aorta. (A, B, C). The lamellar arrangement is preserved, characterized by the parallel pattern of the fibers of the tunica media. Number and nuclei of smooth muscle cells are preserved. Absence of mononuclear inflammatory infiltrate (hematoxylin and eosin, 200x); (D, E, F) Collagen fibers with normal organization and staining (Picrosirius, 200x); (G, H, I) Sinuous elastic fibers present throughout the vascular segment, without fragmentation and in normal quantities (Verhoeff, 200x).
DISCUSSION
The main findings of this study consist of identification of changes provoked by the hemostatic surgical forceps at the clamping site, which is unavoidable during aortic surgery. Whenever a vessel is manipulated, there is a possibility of plaque rupture, intimal injury, and formation of thrombi during and after placement of the hemostatic clamps. Even after endovascular surgery, 1 hour after arterial ballooning for angioplasty, artery wall changes have already occurred, such as: endothelial denudation, deposition of platelets, mural thrombi, and endothelial tears involving the internal elastic lamina.2525 Gutierrez PS, Reis MM, Higuchi ML, Aiello VD, Stolf NAG, Lopes EA. Distribution of hyaluronan and dermatan/chondroitin sulfate proteoglycans in human aortic dissection. Connect Tissue Res. 1998;37(3-4):151-61. http://dx.doi.org/10.3109/03008209809002435. PMid:9862217.
http://dx.doi.org/10.3109/03008209809002...
The balloon used during EVAR is complacent, but it is kept inflated above the animal’s blood pressure for a long time, and could therefore be a source of artery wall injury. When it undergoes balloon angioplasty, it is subjected to radial tensions that exceed its physiological range and so damage could occur, in particular to collagen fibers.2626 Taghizadeh N, Vonk JM, Boezen HM. Lifetime smoking history and cause-specific mortality in a cohort study with 43 years of follow-up. PLoS One. 2016;11(4):e0153310. http://dx.doi.org/10.1371/journal.pone.0153310. PMid:27055053.
http://dx.doi.org/10.1371/journal.pone.0...
The balloon’s complacency causes the area of contact between the balloon and the aorta wall to be greater than its nominal surface, because it accommodates to the smaller diameter of the pig aorta. Experiments have demonstrated that this injury will induce thickening of the wall of the vessel and will be determined by the stress at the surface of the vessel wall that is in contact with the balloon.2727 Steele PM, Chesebro JH, Stanson AW, et al. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ Res. 1985;57(1):105-12. http://dx.doi.org/10.1161/01.RES.57.1.105. PMid:3159504.
http://dx.doi.org/10.1161/01.RES.57.1.10...
Consigny et al.2828 Consigny PM, Tulenko TN, Nicosia RF. Immediate and long-term effects of angioplasty-balloon dilation on normal rabbit iliac artery. Arteriosclerosis. 1986;6(3):265-76. http://dx.doi.org/10.1161/01.ATV.6.3.265. PMid:2939818.
http://dx.doi.org/10.1161/01.ATV.6.3.265...
observed an immediate increase in arterial diameter, endothelial denudation, injuries to smooth muscle cells, reduced arterial thickness, and increased elastic modulus soon after arterial ballooning. In the present study, the aortic segment in contact with the balloon could have been a point of localized ischemic changes, caused by compression of the vasa vasorum, by a lack of contact between the aorta and circulating blood, and by reperfusion.
The hypothesis of acute changes to the aorta wall after prolonged ballooning is not without foundation, even using a complacent balloon, since it is inflated at high pressure and for a long time. However, the mechanical behavior of the aortas in which flow was interrupted by balloon inflation was similar to those from the normal controls. These results may suggest that inflation of the complacent balloon inside the aorta, even for a prolonged period, does not provoke structural changes in the wall, which is fundamental to the durability of endovascular techniques. Increased aortic diameter due to a possible injury and weakening of the wall at the site of ballooning (the proximal anchorage for endoprostheses) could lead to degeneration of the neck and consequent type I endoleaks and stent migration.2929 Savlovskis J, Krievins D, de Vries J-PPM, et al. Artic neck enlargement after endovascular aneurysm repair using balloon-expandable versus self-expanding endografts. J Vasc Surg. 2015;62(3):541-9. http://dx.doi.org/10.1016/j.jvs.2015.04.393. PMid:26213274.
http://dx.doi.org/10.1016/j.jvs.2015.04....
Additionally, endovascular hemostasis is increasingly achieved during aortic emergencies using an intraluminal balloon and the REBOA technique,3030 Ribeiro MAF Jr, Feng CYD, Nguyen ATM, et al. The complications associated with Ressuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). World J Emerg Surg. 2018;13(1):20-5. http://dx.doi.org/10.1186/s13017-018-0181-6. PMid:29774048.
http://dx.doi.org/10.1186/s13017-018-018...
so it is important to accumulate data showing that this technique does not provoke persistent mechanical changes to the aorta wall.
Analyses showed that the mechanical parameters of specimens from groups C and L were inferior to those of specimens from the EV group, whereas the biomechanical variables for the S group were similar to those observed for the EV group, revealing a reduction in the resistance of the aorta wall after use of hemostatic clamps. The distensibility of the aorta is dependent on components in the tunica media – collagen, elastin, and proteoglycanes – and all of these can affect its resistance to traction,88 Jaldin RG, Castardelli É, Perobelli JE, et al. Morphologic and biomechanical changes of thoracic and abdominal aorta in a rat model of cigarette smoke exposure. Ann Vasc Surg. 2013;27(6):791-800. http://dx.doi.org/10.1016/j.avsg.2013.03.002. PMid:23880458.
http://dx.doi.org/10.1016/j.avsg.2013.03...
since the elastic capacity of cardiovascular tissues is directly related to their biomechanical behavior.1818 Bertanha M, Moroz A, Jaldin RG, et al. Morphofunctional characterization of decellularized vena cava as tissue engineering scaffolds. Exp Cell Res. 2014;326(1):103-11. http://dx.doi.org/10.1016/j.yexcr.2014.05.023. PMid:24929113.
http://dx.doi.org/10.1016/j.yexcr.2014.0...
In the final analysis, tests of tensile resistance reflect the stiffness and the elasticity of the aorta, i.e., they analyze the capacity of collagen and elastin to enable the aorta to distend, which is a fundamental element in its function.88 Jaldin RG, Castardelli É, Perobelli JE, et al. Morphologic and biomechanical changes of thoracic and abdominal aorta in a rat model of cigarette smoke exposure. Ann Vasc Surg. 2013;27(6):791-800. http://dx.doi.org/10.1016/j.avsg.2013.03.002. PMid:23880458.
http://dx.doi.org/10.1016/j.avsg.2013.03...
Maximum load, yield point, and stiffness coefficient are the parameters most related to these biomechanical properties. It is possible that these factors contributed to the changes observed in the C and L groups during the biomechanical tests, since the mechanical tension in the artery wall is dependent on the load applied and the deformed vascular geometry.3131 Polindara C, Waffenschmidt T, Menzel A. Simulation of balloon angioplasty in residually stressed blood vessels-Application of a gradient-enhanced fibre damage model. J Biomech. 2016;49(12):2341-8. http://dx.doi.org/10.1016/j.jbiomech.2016.01.037. PMid:26924658.
http://dx.doi.org/10.1016/j.jbiomech.201...
32 Zubilewicz T, Wronski J, Bourriez A, et al. Injury in vascular surgery--the intimal hyperplastic response. Med Sci Monit. 2001;7(2):316-24. PMid:11257743.-3333 Barone GW, Cornely JM, Flagagan TL, Kron IL. Assessing clamp related vascular injuries by measurement of associated vascular dysfunction. Surgery. 1989;105(4):465-71. PMid:2648627.
Since the biomechanical parameters of the artery wall are to a great extent due to its collagen and elastic fibers, stains specific to these components of the wall were included, but even so, no marked and significant changes were observed in these slides under light microscopy. Borges et al.77 Borges LF, Gutierrez PS, Marana HR, Taboga SR. Picrosirius polarization staining method as an efficient histopathological tool for collagenolysis detection in vesical prolapse lesions. Micron. 2007;38(6):580-3. http://dx.doi.org/10.1016/j.micron.2006.10.005. PMid:17126553.
http://dx.doi.org/10.1016/j.micron.2006....
demonstrated that Picrosirius Red analyzed under polarized light, together with conventional light microscopy would be the best method for evaluating the structure of collagen, since it allows the arrangement and grouping of collagen fibers to be studied, because of its normal birefringence. This stain can be used to view the morphology of intact collagen bundles and fragmented bundles and collagenolysis can also be detected. It is possible that using polarized light would have shown some type of rearrangement of the three-dimensional structure of the collagen fibers that was not detected with the conventional techniques employed.
The histological analyses were not sufficiently sensitive to detect acute structural changes in the components of the aorta wall, but functional or ultrastructural changes may have occurred. Studies with ultramicroscopy, with histochemistry for other components of the media2525 Gutierrez PS, Reis MM, Higuchi ML, Aiello VD, Stolf NAG, Lopes EA. Distribution of hyaluronan and dermatan/chondroitin sulfate proteoglycans in human aortic dissection. Connect Tissue Res. 1998;37(3-4):151-61. http://dx.doi.org/10.3109/03008209809002435. PMid:9862217.
http://dx.doi.org/10.3109/03008209809002...
and with immunohistochemistry for elastases could possibly have shown some type of change that would have confirmed the biomechanical changes observed. There are studies that suggest that ultrastructural damage to the artery wall is provoked by clamping after surgery, even in the absence of histological damage identifiable under light microscopy.3434 Sassani SG, Kakisis J, Tsangaris S, Sokolis DP. Layer-dependent wall properties of abdominal aortic aneurysms: Experimental study and material characterization. J Mech Behav Biomed Mater. 2015;49:141-61. http://dx.doi.org/10.1016/j.jmbbm.2015.04.027. PMid:26011656.
http://dx.doi.org/10.1016/j.jmbbm.2015.0...
,3535 Taghizadeh H, Tafazzoli-Shadpour M. Characterization of mechanical properties of lamellar structure of the aortic wall: effect of aging. J Mech Behav Biomed Mater. 2017;65:20-8. http://dx.doi.org/10.1016/j.jmbbm.2016.08.011. PMid:27544616.
http://dx.doi.org/10.1016/j.jmbbm.2016.0...
Hemostatic forceps have grooves that exert significant local pressure on the artery wall, which invariably results in trauma to the vessel.2424 Alric P, Ryckwaert F, Branchereau P, Marty-Ane C, Mary H, Colson P. A porcine model of systemic and renal haemodynamic responses to infrarenal aortic cross-clamping. Eur J Vasc Endovasc Surg. 2003;25(1):72-8. http://dx.doi.org/10.1053/ejvs.2002.1789. PMid:12525815.
http://dx.doi.org/10.1053/ejvs.2002.1789...
The pressure exerted by the clamp on the aorta wall, in addition to local transitory ischemia followed by reperfusion, could have provoked changes to the vascular structure and biomechanical parameters. Although the acute changes to the aorta wall caused by clamping have been documented in many studies, apparently they do not result in permanent weakening of the vessel.3636 Babin-Ebell J, Gimpel-Henning K, Sievers H-H, Scharfschwerdt M. Influence of clamp duration and pressure on endothelial damage in aortic cross-clamping. Interact Cardiovasc Thorac Surg. 2010;10(2):168-71. http://dx.doi.org/10.1510/icvts.2009.220996. PMid:19934161.
http://dx.doi.org/10.1510/icvts.2009.220...
Dobrin et al.3737 Dobrin PB, McGurrin JF, McNulty JA. Chronic histologic changes after vascular clamping are not associated with altered vascular mechanics. Ann Vasc Surg. 1992;6(2):153-9. http://dx.doi.org/10.1007/BF02042737. PMid:1599834.
http://dx.doi.org/10.1007/BF02042737...
described persistent injuries to the clamped area for up to 6 months afterwards, but they were not associated with chronic mechanical changes to the aorta. These findings could explain the safety of these techniques that have been used for decades.
CONCLUSIONS
Use of clamping during open or laparoscopic surgery causes acute mechanical changes to the aorta suggestive of reduced resistance, even without apparent morphological changes. Prolonged inflation of the intraluminal balloon did not change the mechanical properties of the wall, denoting maintenance of its structural integrity.
-
How to cite: Prata MP, Jaldin RG, Lourenção PLTA, et al. Acute aortic wall injury caused by aortic cross-clamping: morphological and biomechanical study of the aorta in a swine model of three aortic surgery approaches. J Vasc Bras. 2020;19:e20190025. https://doi.org/10.1590/1677-5449.190025
-
Financial support: Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (process no. 2012/50159-3, 2014/13616-2).
-
The study was carried out at Departamento de Cirurgia e Ortopedia, Faculdade de Medicina de Botucatu, Universidade Estadual Paulista (UNESP), São Paulo, SP, Brazil.
REFERÊNCIAS
-
1Coscas R, Maumias T, Capdevila C, Javerliat I, Goëau-Brissonnière O, Coggia M. Mini-invasive treatment of abdominal aneurysms: current roles of endovascular, laparoscopic and open techniques. Ann Vasc Surg. 2014;28(1):123-31. http://dx.doi.org/10.1016/j.avsg.2013.05.007 PMid:24200131.
» http://dx.doi.org/10.1016/j.avsg.2013.05.007 -
2Pascarella L, Aboul Hosn M. Minimally invasive management of severe aortoiliac occlusive disease. J Laparoendosc Adv Surg Tech A. 2018;28(5):562-8. http://dx.doi.org/10.1089/lap.2017.0675 PMid:29346011.
» http://dx.doi.org/10.1089/lap.2017.0675 -
3Ahmed N, Gollop ND, Ellis J, Khan OA. How does elective laparoscopic aortic aneurysm repair compare to endovascular aneurysm repair? Interact Cardiovasc Thorac Surg. 2014;18(6):814-20. http://dx.doi.org/10.1093/icvts/ivu031 PMid:24578481.
» http://dx.doi.org/10.1093/icvts/ivu031 -
4Robertson L, Nandhra S. Laparoscopic surgey for elective abdominal aortic aneurysm repair. Cochrane Database Syst Rev. 2017;5:CD012302.
-
5Slayback JB, Bowen WW, Hinshaw DB. Intimal injury from arterial clamps. Am J Surg. 1976;132(2):183-7. http://dx.doi.org/10.1016/0002-9610(76)90045-3 PMid:952348.
» http://dx.doi.org/10.1016/0002-9610(76)90045-3 -
6Margovsky AI, Lord RSA, Meek AC, Bobryshev YV. Artery wall damage and platelet uptake from so-called atraumatic arterial clamps: an experimental study. Cardiovasc Surg. 1997;5(1):42-7. http://dx.doi.org/10.1016/S0967-2109(96)00064-6 PMid:9158122.
» http://dx.doi.org/10.1016/S0967-2109(96)00064-6 -
7Borges LF, Gutierrez PS, Marana HR, Taboga SR. Picrosirius polarization staining method as an efficient histopathological tool for collagenolysis detection in vesical prolapse lesions. Micron. 2007;38(6):580-3. http://dx.doi.org/10.1016/j.micron.2006.10.005 PMid:17126553.
» http://dx.doi.org/10.1016/j.micron.2006.10.005 -
8Jaldin RG, Castardelli É, Perobelli JE, et al. Morphologic and biomechanical changes of thoracic and abdominal aorta in a rat model of cigarette smoke exposure. Ann Vasc Surg. 2013;27(6):791-800. http://dx.doi.org/10.1016/j.avsg.2013.03.002 PMid:23880458.
» http://dx.doi.org/10.1016/j.avsg.2013.03.002 -
9Loh CS, Al-Jafari MS, Croton R. Acute rupture of the abdominal aorta from cross-clamp injury. Eur J Vasc Surg. 1990;4(6):647-8. http://dx.doi.org/10.1016/S0950-821X(05)80824-2 PMid:2279578.
» http://dx.doi.org/10.1016/S0950-821X(05)80824-2 -
10Chen HY, Navia JA, Shafique S, Kassab GS. Fluid-structure interaction in aortic cross-clampig:implications for vessel injury. J Biomech. 2010;43(2):221-7. http://dx.doi.org/10.1016/j.jbiomech.2009.08.042 PMid:19883917.
» http://dx.doi.org/10.1016/j.jbiomech.2009.08.042 -
11Dobrin PB, McGurrin JF, McNulty JA. Chronic histologic changes after vascular clamping are not associated with altered vascular mechanics. Ann Vasc Surg. 1992;6(2):153-9. http://dx.doi.org/10.1007/BF02042737 PMid:1599834.
» http://dx.doi.org/10.1007/BF02042737 -
12Thompson MM, Nasim A, Sayers RD, et al. Oxygen free radical and cytokine generation during endovascular and conventional aneurysm repair. Eur J Vasc Endovasc Surg. 1996;12(1):70-5. http://dx.doi.org/10.1016/S1078-5884(96)80278-4 PMid:8696901.
» http://dx.doi.org/10.1016/S1078-5884(96)80278-4 -
13Malina M, Veith F, Ivancev K, Sonesson B. Balloon occlusion of the aorta during endovascular repair of ruptured abdominal aortic aneurysm. J Endovasc Ther. 2005;12(5):556-9. http://dx.doi.org/10.1583/05-1587.1 PMid:16212455.
» http://dx.doi.org/10.1583/05-1587.1 -
14Sincos IR, Aun R, Silva ES, et al. Impact of stent-graft oversizing on the thoracic aorta: experimental study in a porcine model. J Endovasc Ther. 2011;18(4):576-84. http://dx.doi.org/10.1583/11-3470.1 PMid:21861750.
» http://dx.doi.org/10.1583/11-3470.1 -
15Matsuda H, Tanaka Y, Hino Y, et al. Transbrachial arterial insertion of aortic occlusion balloon catheter in patients with shock from ruptured abdominal aortic aneurysm. J Vasc Surg. 2003;38(6):1293-6. http://dx.doi.org/10.1016/S0741-5214(03)00774-2 PMid:14681630.
» http://dx.doi.org/10.1016/S0741-5214(03)00774-2 -
16Batchelor WB, Robinson R, Strauss BH. The extracellular matrix in balloon arterial injury: a novel target for restenosis prevention. Prog Cardiovasc Dis. 1998;41(1):35-49. http://dx.doi.org/10.1016/S0033-0620(98)80021-2 PMid:9717858.
» http://dx.doi.org/10.1016/S0033-0620(98)80021-2 -
17Keris V, Ozolanta I, Enina G, Kasyanovs V, Aide H, Bricis R. Biomechanical and structure assessment of transluminal angioplasty. Med Eng Phys. 1998;20(5):339-46. http://dx.doi.org/10.1016/S1350-4533(98)00032-0 PMid:9773687.
» http://dx.doi.org/10.1016/S1350-4533(98)00032-0 -
18Bertanha M, Moroz A, Jaldin RG, et al. Morphofunctional characterization of decellularized vena cava as tissue engineering scaffolds. Exp Cell Res. 2014;326(1):103-11. http://dx.doi.org/10.1016/j.yexcr.2014.05.023 PMid:24929113.
» http://dx.doi.org/10.1016/j.yexcr.2014.05.023 -
19Cerqueira NF, Yoshida WB, Müller SS, Sequeira JL, Rodrigues AC, Padovani CR. Morphological and biomechanical study of abdominal aorta of rats submitted to experimental chronic alcoholism. Acta Cir Bras. 2005;20(3):213-8. http://dx.doi.org/10.1590/S0102-86502005000300004 PMid:16033179.
» http://dx.doi.org/10.1590/S0102-86502005000300004 -
20Yoshida WB, Müller SS, Carvalho I, Fabris VE, Naresse LE, Maffei FHA. Tensile strengthand histological changes of abdominal aorta of malnourished rats. Cardiovasc Surg. 1995;3(4):437-9. http://dx.doi.org/10.1016/0967-2109(95)94165-S PMid:7583001.
» http://dx.doi.org/10.1016/0967-2109(95)94165-S -
21Suk P, Cundrle I Jr, Hruda J, et al. Porcine model of ruptured abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2012;43(6):698-704. http://dx.doi.org/10.1016/j.ejvs.2012.02.020 PMid:22421373.
» http://dx.doi.org/10.1016/j.ejvs.2012.02.020 -
22Martín-Cancho MF, Sánchez-Margallo FM, Soria F, et al. Physiological responses to different ischemic periods during laparoscopic infrarenal aortic cross-clamping: evaluation in an experimental animal model. Ann Vasc Surg. 2009;23(4):506-18. http://dx.doi.org/10.1016/j.avsg.2008.12.002 PMid:19375889.
» http://dx.doi.org/10.1016/j.avsg.2008.12.002 -
23Martin-Cancho MF, Crisostomo V, Soria F, et al. Physiologic responses to infrarenal aortic cross-clamping during laparoscopic or conventional vascular surgery in experimental animal model: comparative study. Anesthesiol Res Pract. 2008;581948:1-8. http://dx.doi.org/10.1155/2008/581948 PMid:21197458.
» http://dx.doi.org/10.1155/2008/581948 -
24Alric P, Ryckwaert F, Branchereau P, Marty-Ane C, Mary H, Colson P. A porcine model of systemic and renal haemodynamic responses to infrarenal aortic cross-clamping. Eur J Vasc Endovasc Surg. 2003;25(1):72-8. http://dx.doi.org/10.1053/ejvs.2002.1789 PMid:12525815.
» http://dx.doi.org/10.1053/ejvs.2002.1789 -
25Gutierrez PS, Reis MM, Higuchi ML, Aiello VD, Stolf NAG, Lopes EA. Distribution of hyaluronan and dermatan/chondroitin sulfate proteoglycans in human aortic dissection. Connect Tissue Res. 1998;37(3-4):151-61. http://dx.doi.org/10.3109/03008209809002435 PMid:9862217.
» http://dx.doi.org/10.3109/03008209809002435 -
26Taghizadeh N, Vonk JM, Boezen HM. Lifetime smoking history and cause-specific mortality in a cohort study with 43 years of follow-up. PLoS One. 2016;11(4):e0153310. http://dx.doi.org/10.1371/journal.pone.0153310 PMid:27055053.
» http://dx.doi.org/10.1371/journal.pone.0153310 -
27Steele PM, Chesebro JH, Stanson AW, et al. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ Res. 1985;57(1):105-12. http://dx.doi.org/10.1161/01.RES.57.1.105 PMid:3159504.
» http://dx.doi.org/10.1161/01.RES.57.1.105 -
28Consigny PM, Tulenko TN, Nicosia RF. Immediate and long-term effects of angioplasty-balloon dilation on normal rabbit iliac artery. Arteriosclerosis. 1986;6(3):265-76. http://dx.doi.org/10.1161/01.ATV.6.3.265 PMid:2939818.
» http://dx.doi.org/10.1161/01.ATV.6.3.265 -
29Savlovskis J, Krievins D, de Vries J-PPM, et al. Artic neck enlargement after endovascular aneurysm repair using balloon-expandable versus self-expanding endografts. J Vasc Surg. 2015;62(3):541-9. http://dx.doi.org/10.1016/j.jvs.2015.04.393 PMid:26213274.
» http://dx.doi.org/10.1016/j.jvs.2015.04.393 -
30Ribeiro MAF Jr, Feng CYD, Nguyen ATM, et al. The complications associated with Ressuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). World J Emerg Surg. 2018;13(1):20-5. http://dx.doi.org/10.1186/s13017-018-0181-6 PMid:29774048.
» http://dx.doi.org/10.1186/s13017-018-0181-6 -
31Polindara C, Waffenschmidt T, Menzel A. Simulation of balloon angioplasty in residually stressed blood vessels-Application of a gradient-enhanced fibre damage model. J Biomech. 2016;49(12):2341-8. http://dx.doi.org/10.1016/j.jbiomech.2016.01.037 PMid:26924658.
» http://dx.doi.org/10.1016/j.jbiomech.2016.01.037 -
32Zubilewicz T, Wronski J, Bourriez A, et al. Injury in vascular surgery--the intimal hyperplastic response. Med Sci Monit. 2001;7(2):316-24. PMid:11257743.
-
33Barone GW, Cornely JM, Flagagan TL, Kron IL. Assessing clamp related vascular injuries by measurement of associated vascular dysfunction. Surgery. 1989;105(4):465-71. PMid:2648627.
-
34Sassani SG, Kakisis J, Tsangaris S, Sokolis DP. Layer-dependent wall properties of abdominal aortic aneurysms: Experimental study and material characterization. J Mech Behav Biomed Mater. 2015;49:141-61. http://dx.doi.org/10.1016/j.jmbbm.2015.04.027 PMid:26011656.
» http://dx.doi.org/10.1016/j.jmbbm.2015.04.027 -
35Taghizadeh H, Tafazzoli-Shadpour M. Characterization of mechanical properties of lamellar structure of the aortic wall: effect of aging. J Mech Behav Biomed Mater. 2017;65:20-8. http://dx.doi.org/10.1016/j.jmbbm.2016.08.011 PMid:27544616.
» http://dx.doi.org/10.1016/j.jmbbm.2016.08.011 -
36Babin-Ebell J, Gimpel-Henning K, Sievers H-H, Scharfschwerdt M. Influence of clamp duration and pressure on endothelial damage in aortic cross-clamping. Interact Cardiovasc Thorac Surg. 2010;10(2):168-71. http://dx.doi.org/10.1510/icvts.2009.220996 PMid:19934161.
» http://dx.doi.org/10.1510/icvts.2009.220996 -
37Dobrin PB, McGurrin JF, McNulty JA. Chronic histologic changes after vascular clamping are not associated with altered vascular mechanics. Ann Vasc Surg. 1992;6(2):153-9. http://dx.doi.org/10.1007/BF02042737 PMid:1599834.
» http://dx.doi.org/10.1007/BF02042737
Publication Dates
-
Publication in this collection
03 Apr 2020 -
Date of issue
2020
History
-
Received
18 Mar 2019 -
Accepted
01 May 2019