Abstract
We conducted a systematic review to compare the effectiveness and safety of exercise versus no exercise for patients with asymptomatic aortic aneurysm. We followed the guidelines set out in the Cochrane systematic review handbook. We searched Medline, Embase, CENTRAL, LILACS, PeDRO, CINAHL, clinicaltrials.gov, ICTRP, and OpenGrey using the MeSH terms “aortic aneurysm” and “exercise”. 1189 references were identified. Five clinical trials were included. No exercise-related deaths or aortic ruptures occurred in these trials. Exercise did not reduce the aneurysm expansion rate at 12 weeks to 12 months (mean difference [MD], −0.05; 95% confidence interval [CI], −0.13 to 0.03). Six weeks of preoperative exercise reduced severe renal and cardiac complications (risk ratio, 0.54; 95% CI, 0.31–0.93) and the length of intensive care unit stay (MD, −1.00; 95% CI, −1.26 to −0.74). Preoperative and postoperative forward walking reduced the length of hospital stay (MD, −0.69; 95% CI, −1.24 to −0.14). The evidence was graded as ‘very low’ level.
Keywords:
aortic aneurysm; abdominal aortic aneurysm; exercise; postoperative complications
Resumo
Foi realizada revisão sistemática para comparar a efetividade e a segurança de exercícios versus não exercícios em pacientes assintomáticos com aneurisma de aorta. Usamos os termos MeSH aortic aneurysm e exercise para as bases MEDLINE, Embase, CENTRAL, LILACS, PeDRO, CINAHL, clinicaltrials.gov, International Clinical Trials Registry Platform (ICTRP) e OpenGrey. Foram obtidas 1.189 referências. Cinco ensaios clínicos foram incluídos. Não houve morte ou rotura associada ao exercício. Além disso, este não reduziu a velocidade de crescimento do aneurisma em 12 semanas a 12 meses [diferença de médias (DM) −0,05; intervalo de confiança de 95% (IC95%) −0,13 a 0,03]. Seis semanas de exercícios pré-operatórios reduziram complicações clínicas renais e cardíacas (razão de risco 0,54; IC95% 0,31–0,93) e a permanência em unidade de terapia intensiva (DM −1,00; IC95% −1,26 a −0,74). Caminhadas nos períodos pré e pós-operatório reduziram a permanência hospitalar. A evidência foi classificada como de muito baixa qualidade.
Palavras-chave:
aneurismas de aorta; aneurismas de aorta abdominal; exercícios; complicações pós-operatórias
INTRODUCTION
An aortic aneurysm is a permanent localized aortic dilatation that is at least 50% larger than the normal diameter.11 Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13(3):452-8. http://dx.doi.org/10.1067/mva.1991.26737. PMid:1999868.
http://dx.doi.org/10.1067/mva.1991.26737...
,22 Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2011;41(Suppl 1):S1-58. http://dx.doi.org/10.1016/j.ejvs.2010.09.011. PMid:21215940.
http://dx.doi.org/10.1016/j.ejvs.2010.09...
The estimated prevalence is 4.0% to 8.9% in men and 1.3% to 2.2% in women aged ≥55 years.33 Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø Study. Am J Epidemiol. 2001;154(3):236-44. http://dx.doi.org/10.1093/aje/154.3.236. PMid:11479188.
http://dx.doi.org/10.1093/aje/154.3.236...
,44 Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82(8):1066-70. http://dx.doi.org/10.1002/bjs.1800820821. PMid:7648155.
http://dx.doi.org/10.1002/bjs.1800820821...
Approximately 80% of aortic aneurysms are located in the abdominal aorta.55 Aggarwal S, Qamar A, Sharma V, Sharma A. Abdominal aortic aneurysm: a comprehensive review. Exp Clin Cardiol. 2011;16(1):11-5. PMid:21523201. They usually have an asymptomatic natural history and so diagnosis is made after thorough investigation.66 Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;(2):CD002945. PMid:17443519. The most feared complication of aortic aneurysms is rupture, which leads to death in up to 90% of patients.77 Lederle FA. The natural history of abdominal aortic aneurysm. Acta Chir Belg. 2009;109(1):7-12. http://dx.doi.org/10.1080/00015458.2009.11680364. PMid:19341189.
http://dx.doi.org/10.1080/00015458.2009....
The risk of rupture increases as the aneurysm diameter increases.22 Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2011;41(Suppl 1):S1-58. http://dx.doi.org/10.1016/j.ejvs.2010.09.011. PMid:21215940.
http://dx.doi.org/10.1016/j.ejvs.2010.09...
,88 Mell M, White JJ, Hill BB, Hastie T, Dalman RL, Stanford Abdominal Aortic Aneurysm Specialized Center of Clinically Oriented Research Investigators. No increased mortality with early aortic aneurysm disease. J Vasc Surg. 2012;56(5):1246-5. http://dx.doi.org/10.1016/j.jvs.2012.04.023. PMid:22832264.
http://dx.doi.org/10.1016/j.jvs.2012.04....
,99 Reed WW, Hallett JW Jr, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med. 1997;157(18):2064-8. http://dx.doi.org/10.1001/archinte.1997.00440390050007. PMid:9382661.
http://dx.doi.org/10.1001/archinte.1997....
Surgery is recommended when an aneurysm reaches 50 mm in women or 55 mm in men,1010 The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352(9141):1649-55. http://dx.doi.org/10.1016/S0140-6736(98)10137-X. PMid:9853436.
http://dx.doi.org/10.1016/S0140-6736(98)...
because at this point the risks of surveillance outweigh the risks of surgery. No clinical interventions have been found to be effective for reducing the growth rate or risk of rupture before an aneurysm reaches these diameters.1010 The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352(9141):1649-55. http://dx.doi.org/10.1016/S0140-6736(98)10137-X. PMid:9853436.
http://dx.doi.org/10.1016/S0140-6736(98)...
,1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
In one study, 87.7% of the patients diagnosed had aneurysms with diameters of <3.5 cm.1212 Newman AB, Arnold AM, Burke GL, O’Leary DH, Manolio TA. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the cardiovascular health study. Ann Intern Med. 2001;134(3):182-90. http://dx.doi.org/10.7326/0003-4819-134-3-200102060-00008. PMid:11177330.
http://dx.doi.org/10.7326/0003-4819-134-...
Therefore, despite the tremendous effort that has been expended on surgical research, there is not enough information to recommend non-pharmacologic clinical treatment for most patients, other than smoking cessation and controlling blood pressure. Even among patients with small aneurysms, the most frequent cause of death is myocardial infarction and stroke, not aneurysm rupture.1010 The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352(9141):1649-55. http://dx.doi.org/10.1016/S0140-6736(98)10137-X. PMid:9853436.
http://dx.doi.org/10.1016/S0140-6736(98)...
,1212 Newman AB, Arnold AM, Burke GL, O’Leary DH, Manolio TA. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the cardiovascular health study. Ann Intern Med. 2001;134(3):182-90. http://dx.doi.org/10.7326/0003-4819-134-3-200102060-00008. PMid:11177330.
http://dx.doi.org/10.7326/0003-4819-134-...
Moreover, when patients do undergo surgery, 41% of deaths are also related to cardiovascular events.1313 Brady AR, Fowkes FG, Greenhalgh RM, Powell JT, Ruckley CV, Thompson SG. Risk factors for postoperative death following elective surgical repair of abdominal aortic aneurysm: results from the UK Small Aneurysm Trial. Br J Surg. 2000;87(6):742-9. http://dx.doi.org/10.1046/j.1365-2168.2000.01410.x. PMid:10848851.
http://dx.doi.org/10.1046/j.1365-2168.20...
Therefore, an aortic aneurysm is a risk factor for death and has a risk of mortality 50% higher than that in persons with no aortic pathology.1212 Newman AB, Arnold AM, Burke GL, O’Leary DH, Manolio TA. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the cardiovascular health study. Ann Intern Med. 2001;134(3):182-90. http://dx.doi.org/10.7326/0003-4819-134-3-200102060-00008. PMid:11177330.
http://dx.doi.org/10.7326/0003-4819-134-...
Exercise and smoking cessation help to reduce mortality and improve quality of life.1414 Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-59. http://dx.doi.org/10.1249/MSS.0b013e318213fefb. PMid:21694556.
http://dx.doi.org/10.1249/MSS.0b013e3182...
,1515 Almeida OP, Khan KM, Hankey GJ, Yeap BB, Golledge J, Flicker L. 150 minutes of vigorous physical activity per week predicts survival and successful ageing: a population-based 11-year longitudinal study of 12 201 older Australian men. Br J Sports Med. 2014;48(3):220-5. http://dx.doi.org/10.1136/bjsports-2013-092814. PMid:24002240.
http://dx.doi.org/10.1136/bjsports-2013-...
Exercise is a subgroup of physical activity defined as planned, structured, and repetitive activities performed with the objective of improving or maintaining physical fitness.1616 Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-31. PMid:3920711. Despite the importance of exercise, there is no consensus regarding exercise recommendations for patients with aortic aneurysms,1717 Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2-77.e2. http://dx.doi.org/10.1016/j.jvs.2017.10.044. PMid:29268916.
http://dx.doi.org/10.1016/j.jvs.2017.10....
,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
because of the fear of aneurysm rupture and doubts about the effectiveness of exercise. Hence, a systematic review of the literature is crucial to describe the risks and benefits of exercise for patients with aortic aneurysms.
The study was performed to assess the effectiveness and safety of exercise for asymptomatic patients with an aortic aneurysm.
MATERIALS AND METHODS
This review was conducted in the Post-graduate Program in Evidence-based Healthcare at the Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil. It followed the recommendations contained in the Cochrane Handbook for Systematic Reviews of Interventions,1919 Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. USA: The Cochrane Collaboration; 2011. and reporting of the results complies with the PRISMA Statement for quality in publication.2020 Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-41. http://dx.doi.org/10.1016/j.ijsu.2010.02.007. PMid:20171303.
http://dx.doi.org/10.1016/j.ijsu.2010.02...
The review protocol was registered on the PROSPERO database.2121 Oliveira R, Nakajima E, Riera R, Baptista-Silva J. Physical exercises for aortic aneurysm: a systematic review. United Kingdom: NIHR; 2016. PROSPERO 2016 CRD42016041468. [cited 2019 aug 15]. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42016041468
https://www.crd.york.ac.uk/prospero/disp...
The review was also approved by the institutional ethics committee (CAAE number: 57716016.0.0000.5505).
Randomized and quasi-randomized clinical trials were considered for inclusion. Due to the nature of the intervention, crossover studies were not considered for this review.
The inclusion criteria were sedentary patients (those performing only daily activities during the last year), adults (≥18 years of age), and the presence of an aortic aneurysm confirmed by a diagnostic imaging examination.
The exclusion criteria were rapid growth of aneurysms (0.5 cm within 6 months or 1.0 cm within 1 year), saccular aneurysms, complicated aneurysms (such as symptomatic, completely thrombosed, or ruptured aneurysms), and inflammatory and infectious aneurysms. High intensity interval training exercises were excluded.
Any exercise was considered (individual or in groups, assisted or self-managed, aerobic, stretching or strengthening; any intensity, frequency, and duration; and alone or combined with any other intervention), as long as the same co-intervention was also performed in the comparison group. This group of participants was designated the exercise group. For the purposes of the present study, exercise was defined as a subgroup of physical activity that is planned, structured, and repetitive and aims to improve or maintain one or more components of physical fitness.1616 Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-31. PMid:3920711. The comparators considered in this review were patients receiving no intervention and patients on a waiting list. If a study compared different types of exercises (e.g., strength exercises versus resistance exercises), we considered performing a comparison of exercises versus advice for exercising or a different type of exercise used. These patients were designated the no exercise group.
Primary outcomes
-
All-cause mortality in the short-term (up to 30 days after beginning exercise) and long-term (from 30 days to ≥1 year after starting exercise);
-
Number of participants presenting with aneurysmal rupture;
-
Aneurysm growth rate (change, in millimeters (mm), in the aneurysm diameter from baseline to the end of the study).
Secondary outcomes
-
Quality of life, measured by any validated tool;
-
Number of participants referred for aneurysm surgery;
-
Number of participants presenting with at least one severe short-term (up to 24 hours after surgery), intermediate-term (from 24 hours to 30 days after surgery), or long-term (>30 days after surgery) complication. A severe complication was defined as myocardial infarction, prolonged inotropic support, new-onset arrhythmia, unstable angina, postoperative pneumonia, unexplained re-intubation, or renal insufficiency (requirement for dialysis or a >20% reduction in creatinine clearance);
-
Hospital stay related to aneurysm surgery (in days);
-
Intensive care unit stay after aneurysm surgery (in days);
-
Forced expiratory volume in 1 second as measured with a spirometer.
Any outcome not mentioned in the protocol was described as a non-proposed outcome in the results.
The following electronic databases were searched and updated: Literatura Latino Americana em Ciências da Saúde e do Caribe (LILACS) (via the Biblioteca Virtual em Saúde [BVS], from 1966 to 13 December 2018), Medline (via PubMed, from inception to 13 December 2018), Cochrane Central Register of Controlled Trials (CENTRAL) (via Wiley Cochrane Library, December 2018 Edition), Embase (via Elsevier, from 1974 to 13 December 2018), PEDro (via BVS, from inception to November 2018), and Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO, from inception to 7 November 2018). Additional searches were conducted on the trial registry databases ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP) search portal, and the gray literature (http://www.opengrey.eu/) (from inception to 13 December 2018). A manual search was also performed of the reference lists of all studies included and relevant systematic reviews.
There were no search limits for data, status, or language of publication. The search strategy for Medline is shown in Table 1.
Two reviewers independently screened the titles and abstracts for selection and inclusion using Rayyan software.2222 Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. http://dx.doi.org/10.1186/s13643-016-0384-4. PMid:27919275.
http://dx.doi.org/10.1186/s13643-016-038...
They also extracted data and assessed the methodological quality of the studies included as described in the PROSPERO registry database.2121 Oliveira R, Nakajima E, Riera R, Baptista-Silva J. Physical exercises for aortic aneurysm: a systematic review. United Kingdom: NIHR; 2016. PROSPERO 2016 CRD42016041468. [cited 2019 aug 15]. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42016041468
https://www.crd.york.ac.uk/prospero/disp...
A third reviewer resolved any disagreements at each stage.
The strategies for data synthesis, meta-analysis, effect size, subgroup, and sensitivity analysis are also described in the PROSPERO database.2121 Oliveira R, Nakajima E, Riera R, Baptista-Silva J. Physical exercises for aortic aneurysm: a systematic review. United Kingdom: NIHR; 2016. PROSPERO 2016 CRD42016041468. [cited 2019 aug 15]. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42016041468
https://www.crd.york.ac.uk/prospero/disp...
RevMan 5.3 software2323 Manager R. (RevMan) Version 5.3 [software]. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. was used to measure the effect size and perform a meta-analysis when possible. A funnel plot was also planned as part of the protocol.
The GRADE approach was used to evaluate the quality of the body of evidence.2424 GRADEpro GDT. GRADEpro Guideline Development Tool [software]. Hamilton: McMaster University; 2015. Each decision to downgrade the quality of studies was justified (Tables 2, 3, 4, 5, and 6). A summary-of-findings table was created using GRADEpro GDT considering the primary outcomes and the main comparisons (exercise vs. no exercise at 7- to 12-week surveillance and at 3 years; exercise vs. no exercise before surgery; exercise vs. no exercise after surgery; and exercise vs. no exercise before and after surgery).2424 GRADEpro GDT. GRADEpro Guideline Development Tool [software]. Hamilton: McMaster University; 2015. The outcomes were death, aortic rupture, aneurysm growth rate, number of patients with at least one cardiovascular complication, and number of patients referred for surgery.
GRADEpro-GDT judgment of the quality of evidence: GRADE question: Should exercise be indicated for patients with aortic aneurysms at surveillance?
GRADEpro-GDT judgment of the quality of the evidence: GRADE question: Should exercise be indicated for patients with aortic aneurysms at surveillance?
GRADEpro-GDT judgment of the quality of the evidence: GRADE question: Should exercise be indicated for patients with aortic aneurysms before surgery?
GRADEpro-GDT judgment of the quality of the evidence: GRADE question: Should exercise be indicated for patients with aortic aneurysms after surgery?
GRADEpro-GDT judgment of the quality of the evidence: GRADE question: Should exercise be indicated for patients with aortic aneurysms before and after surgery?
RESULTS
The search strategy returned 1189 references (Figure 1). From these, 8 references from 5 clinical trials involving a total of 387 participants were included (212 participants in exercise groups and 175 in no exercise groups).1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
,2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
29 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
-3030 Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239. PMid:28028979.
http://dx.doi.org/10.17219/acem/62239...
Three studies were conducted during the surveillance period.1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
,2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
One study was conducted in the preoperative period (preoperative study).2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
Finally, one study was performed in both the preoperative and postoperative periods (preoperative and postoperative study).3030 Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239. PMid:28028979.
http://dx.doi.org/10.17219/acem/62239...
The clinical trial authors also published three other articles based on the same studies: two2525 Lima RM, Vainshelboim B, Ganatra R, Dalman R, Chan K, Myers J. Exercise training improves ventilatory efficiency in patients with a small abdominal aortic aneurysm: A randomized controlled study. J Cardiopulm Rehabil Prev. 2018;38(4):239-45. http://dx.doi.org/10.1097/HCR.0000000000000270. PMid:28727673.
http://dx.doi.org/10.1097/HCR.0000000000...
,2626 Myers JN, White JJ, Narasimhan B, Dalman RL. Effects of exercise training in patients with abdominal aortic aneurysm: preliminary results from a randomized trial. J Cardiopulm Rehabil Prev. 2010;30(6):374-83. http://dx.doi.org/10.1097/HCR.0b013e3181ebf2db. PMid:20724934.
http://dx.doi.org/10.1097/HCR.0b013e3181...
from Myers et al.1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
and one2727 Barakat HM, Shahin Y, Barnes R, et al. Supervised exercise program improves aerobic fitness in patients awaiting abdominal aortic aneurysm repair. Ann Vasc Surg. 2014;28(1):74-9. http://dx.doi.org/10.1016/j.avsg.2013.09.001. PMid:24332259.
http://dx.doi.org/10.1016/j.avsg.2013.09...
from Barakat et al.2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
PRISMA flow chart for the review. This figure shows the PRISMA flow chart illustrating the processing of searching for, selecting, excluding, and including studies. There were three references from the same studies: two2525 Lima RM, Vainshelboim B, Ganatra R, Dalman R, Chan K, Myers J. Exercise training improves ventilatory efficiency in patients with a small abdominal aortic aneurysm: A randomized controlled study. J Cardiopulm Rehabil Prev. 2018;38(4):239-45. http://dx.doi.org/10.1097/HCR.0000000000000270. PMid:28727673.
http://dx.doi.org/10.1097/HCR.0000000000... ,2626 Myers JN, White JJ, Narasimhan B, Dalman RL. Effects of exercise training in patients with abdominal aortic aneurysm: preliminary results from a randomized trial. J Cardiopulm Rehabil Prev. 2010;30(6):374-83. http://dx.doi.org/10.1097/HCR.0b013e3181ebf2db. PMid:20724934.
http://dx.doi.org/10.1097/HCR.0b013e3181... from Myers et al.1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182... and one2727 Barakat HM, Shahin Y, Barnes R, et al. Supervised exercise program improves aerobic fitness in patients awaiting abdominal aortic aneurysm repair. Ann Vasc Surg. 2014;28(1):74-9. http://dx.doi.org/10.1016/j.avsg.2013.09.001. PMid:24332259.
http://dx.doi.org/10.1016/j.avsg.2013.09... from Barakat et al.,2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000... resulting in eight references from five original studies.
Table 7 describes the studies and their characteristics, including the reasons for inclusion or exclusion.
Risk of bias in included studies
The Cochrane risk of bias table was used as follows:
Random sequence generation (selection bias): Kothmann et al.,2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
Barakat et al.,2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
and Wnuk et al.3030 Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239. PMid:28028979.
http://dx.doi.org/10.17219/acem/62239...
described their randomization methods and were classified as “low risk.” The remaining studies did not describe their randomization methods and were classified as “unclear risk.”1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
Allocation concealment (performance bias and detection bias): all studies were judged to have an “unclear risk” of bias because the allocation method was not described.1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
,2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
29 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
-3030 Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239. PMid:28028979.
http://dx.doi.org/10.17219/acem/62239...
Blinding of personnel and participants (performance bias): Due to the nature of the intervention, it was presumably impossible to blind participants and personnel. Therefore, all studies were classified as “high risk” for this domain.
Blinding of outcome assessment (detection bias): Myers et al.1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
described blinding of the outcome assessment, but the assessors who performed the blinding were not described. Tew et al.1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
described their study as an open study. Thus, these two studies were judged as “unclear risk.” Kothmann et al.2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
and Wnuk et al.3030 Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239. PMid:28028979.
http://dx.doi.org/10.17219/acem/62239...
responded by email regarding the blinding of the outcome assessment and were judged as “low risk.” Barakat et al.2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
described blinding of the outcome assessment and was also judged as “low risk.”
Incomplete outcome data (attrition bias): Kothmann et al.,2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
Tew et al.,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
and Wnuk et al.3030 Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239. PMid:28028979.
http://dx.doi.org/10.17219/acem/62239...
described >20.00% to 27.41% of losses to follow-up and the reasons for these losses. How this could impact the results was not clear; therefore, they were graded as “unclear risk.” Myers et al.1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
was graded as “high risk” because the reasons for the 54% loss to follow-up were uncertain. Barakat et al.2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
described no loss to follow-up for the proposed outcomes, and their study was judged “low risk.”
Selective reporting (reporting bias): All studies described every proposed outcome and were therefore considered to have a “low risk” of bias.
Other potential sources of bias: Myers et al.1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
described a baseline imbalance between the groups with respect to body mass index (p = 0.002) and the prevalence of diabetes (30% in the exercise group vs. 12% in the usual care group [p = 0.01]). To what extent these imbalances could affect the results remained unclear. Tew et al.1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
and Kothmann et al.2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
did not describe the balance between the intervention and control groups because their study included no p values. These studies were classified as “unclear risk.” Barakat et al.2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
and Wnuk et al.3030 Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239. PMid:28028979.
http://dx.doi.org/10.17219/acem/62239...
reported no baseline imbalances. Barakat et al.2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
used two interventions with different prognoses (endovascular and open surgery), but the number of interventions was balanced between the groups. Therefore, their study was judged “low risk.” There was no other source of bias detected in the study by Wnuk et al.3030 Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239. PMid:28028979.
http://dx.doi.org/10.17219/acem/62239...
; therefore, the study was also judged “low risk.”
The authors were contacted, and Kothmann et al.2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
replied that it is “totally inappropriate to conduct significance tests on baseline values,” citing Senn.4040 Senn S. Testing for baseline balance in clinical trials. Stat Med. 1994;13(17):1715-26. http://dx.doi.org/10.1002/sim.4780131703. PMid:7997705.
http://dx.doi.org/10.1002/sim.4780131703...
Additionally, these authors did not provide a significance test for the baseline values. This systematic review follows the Cochrane Handbook for Systematic Reviews of Interventions, which recommends inclusion of imbalances between groups in the domain “other source of bias.”1919 Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. USA: The Cochrane Collaboration; 2011. The reasons for each judgment are presented in Table 8.
Effects of interventions
The following comparisons were analyzed:
Comparison 1: Exercise for patients with small aneurysms during surveillance.1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
,2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
In total, 106 subjects in the exercise group underwent a 7-week2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
to 36-month1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
supervised exercise program, and 92 were included in no exercise groups.
Proposed outcomes:
-
No mortality was reported;
-
No patients developed aneurysm rupture;
-
The aneurysm growth rate did not change in the pooled studies from the 12-week to 12-month follow-up (mean difference [MD], −0.05; 95% confidence interval [CI], −0.13 to 0.03).1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182... ,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07... Additionally, there was a statistical tendency to reach significance at the 95% CI; -
Despite the fact that Tew et al.1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07... described quality of life, no data were depicted. The study reported a non-significant change in eight evaluated domains; -
There was a tendency for the number of patients referred for surgery to reduce, but it was not statistically significant (risk ratio [RR], 0.31; 95% CI, 0.09–1.11) (Figure 2);1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182... ,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07... ,2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...Figure 2
Number of patients referred for surgery at any time during surveillance. This figure shows patients referred for surgery at any time during the surveillance period. They were described at 12 weeks (1.2.1) and at 12 months (1.2.2). M-H, Mantel–Haenszel; Random, random-effects model; CI, confidence interval; Events, number of patients referred for surgery; Total, total number of patients; Total (95% CI), effect size at 95% confidence interval.
-
Cardiovascular adverse events were not different between the intervention and control groups (RR, 1.57; 95% CI, 0.07–35.46).1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182... ,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07... ,2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20... In the study by Kothmann et al.,2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20... one patient in the intervention group had a severe adverse cardiac event after seven sessions.
Non-proposed outcomes:
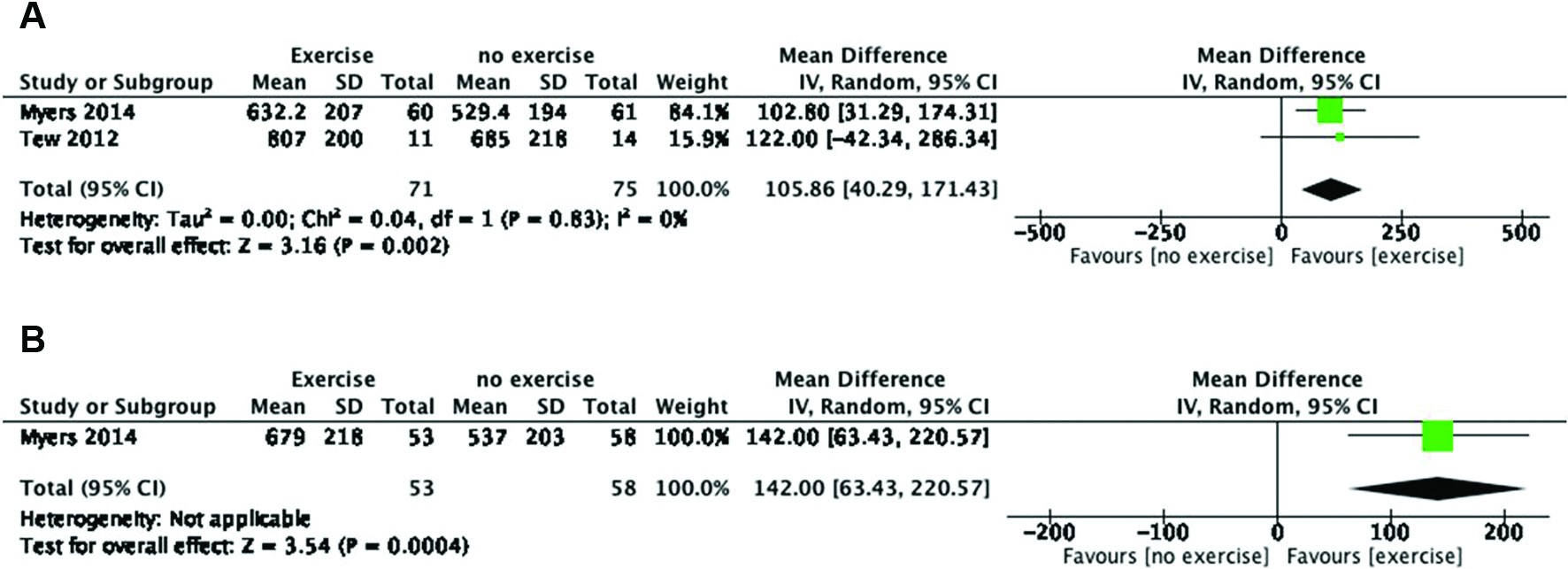
There was an improvement in the exercise time at 12 weeks (MD, 105.86; 95% CI, 40.29–171.43) (Figure 3A).1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
This result was even stronger in the 12-month clinical trial (MD, 142.00; 95% CI, 63.43–220.57) (Figure 3B).1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
Exercise time during surveillance. (A) Exercise time during surveillance at 12 weeks (exercise vs. no exercise). (B) Exercise time during surveillance at 12 months (exercise vs. no exercise). IV, inverse variance; Random, random-effects model; CI, confidence interval; Total, total number of patients; Total (95% CI), effect size at 95% confidence interval.
The change in anaerobic threshold improved after at least 7 weeks of exercise (MD, 1.55; 95% CI, 0.27–2.82) (Figure 4A).1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
,2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
Although the maximal rate of oxygen consumption during incremental exercise (VO2 peak) improved, the difference did not attain statistical significance (MD, 1.15; 95% CI, −0.09 to 2.38) (Figure 4B).1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
,1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
,2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
Change in anaerobic threshold and peak VO2 during surveillance. (A) Change in total anaerobic threshold values at 7 and 12 weeks during surveillance (exercise vs. no exercise). (B) Peak VO2 during surveillance at 7 and 12 weeks (exercise vs. no exercise). IV, inverse variance; Random, random-effects model; CI, confidence interval; Total, total number of patients; Total (95% CI), effect size at 95% confidence interval.
Comparison 2: Exercise in the preoperative period2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
One study assessed this comparison.2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
The study included 62 patients in the exercise group and 62 in the no exercise group for 6 weeks. Data were assessed in the interquartile range, which was transformed to standard deviation by dividing the interquartile range by 1.35, as described in the seventh chapter of the Cochrane handbook.1919 Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. USA: The Cochrane Collaboration; 2011.
Proposed outcomes:
-
There was no difference in 30-day mortality between the groups (RR, 1.00; 95% CI, 0.93–1.08) (Figure 5);
Figure 5
Thirty-day mortality after surgery in patients in the preoperative study. This figure compares 30-day mortality after surgery in patients in the exercise and no exercise groups during the preoperative period. EVAR, endovascular aneurysm repair. Comparison 2 involves the subset treated with EVAR (2.5.1) and the subset treated with open surgery (2.5.2). M-H: Mantel–Haenszel; Random, random-effects model; CI, confidence interval; Events, number of deaths up to 30 days after surgery; Total, total number of patients; Total (95% CI), effect size at 95% confidence interval.
-
No participants developed an aneurysm rupture;
-
Quality of life was not measured;
-
Overall, postoperative complications were reduced in the exercise group (RR, 0.54; 95% CI, 0.31–0.93). In a subgroup analysis, cardiac complications (RR, 0.36; 95% CI, 0.14–0.93), and renal complications (RR, 0.31; 95% CI, 0.11–0.89) had the most important benefit. Despite a tendency to reduce pulmonary complications, this was not statistically significant (RR, 0.54; 95% CI, 0.23–1.26). When analyzed by surgical subgroups, renal complications were lower in open aneurysm surgery (RR, 0.54; 95% CI, 0.34–0.87) than in endovascular repair (RR, 1.00; 95% CI, 0.07–15.04). The same trend occurred in cardiac complications: open aneurysm repair (RR, 0.36; 95% CI, 0.13–1.04) versus an endovascular approach (RR, 0.33; 95% CI, 0.04–2.97). Pulmonary complications were not significantly reduced in endovascular repair (RR, 0.11; 95% CI, 0.01–1.95) or open repair (RR, 0.78; 95% CI, 0.32–1.88);
-
Hospital stay was not reduced in endovascular repair (MD, −1.00; 95% CI, −4.22 to 2.22) or open aneurysm repair groups (MD, 0.00; 95% CI, −0.55 to 0.55);
-
There was a detectable reduction in the critical care stay in the exercise group (MD, −1.00; 95% CI, −1.26 to −0.74).
Eleven of 62 patients who were referred for exercise (17.7%) did not attend the scheduled exercise sessions. There were no losses to follow-up after initiating the study.
Non-proposed outcomes:
Bleeding was described clinically or as a need for transfusion of more than four bags and was not affected by inclusion in either the preoperative exercise or no exercise study groups (RR, 0.57; 95% CI, 0.18–1.85).2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
There was an improvement in anaerobic threshold (MD, 1.80; 95% CI, 0.68–2.92) and VO2 peak oxygen consumption (MD, 1.60; 95% CI, 0.40–2.80).2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
Comparison 3: Exercise in the preoperative and postoperative periods3030 Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239. PMid:28028979.
http://dx.doi.org/10.17219/acem/62239...
One of the studies included assessed 22 patients who performed backward walking, 22 who performed forwarding walking, and 21 in a control group during the preoperative and postoperative periods. After contact, the author reported 18 drop-outs: 7 in the backward walking group (due to myocardial infarction in 3 patients, respiratory failure in 3, and refusal to exercise after surgery in 1), 6 in the forward walking group (myocardial infarction in 2 patients, respiratory failure in 2, and exclusion due to blood coagulation dysfunction in 2), and 5 patients in the control group (all due to myocardial infarction). A per-protocol analysis was conducted (including 15 patients in the backward walking group, 16 in the forwarding walking group, and 16 in the control group). All patients were men, and the results for proposed outcomes were as follows:
-
No mortality was reported;
-
Quality of life was not measured;
-
The number of participants presenting with at least one severe complication was not reported;
-
The hospital stay was detectably reduced in the forward walking group compared with the control group (MD, −0.69; 95% CI, −1.24 to −0.14). No difference was observed between the backward walking group and control group (MD, −0.06; 95% CI, −0.53 to 0.41);
-
Length of intensive care unit stay after aneurysm surgery (in days) was not assessed;
-
During forward walking, the forced expiratory volume in 1 second was not different between the intervention group and control group (RR, 0.27; 95% CI, −0.12 to 0.66).
The proposed subgroup analysis was not performed because of limited data available.
Using GRADEpro-GDT,2424 GRADEpro GDT. GRADEpro Guideline Development Tool [software]. Hamilton: McMaster University; 2015. we judged the quality of the evidence as “very low” for all outcomes. Quality ratings were downgraded due to methodological limitations (impossibility of blinding personnel and participants, attrition bias) and imprecision (single study for some outcomes and low numbers of participants) (Tables 2, 3, 4 and 5).
DISCUSSION
This review revealed no differences in mortality rates between patients with and without exercise during surveillance, preoperative, or postoperative periods. Additionally, no aneurysm ruptures were detected in any intervention groups (total of 209 patients). These clinical trials did not identify reduction in aneurysmal expansion rates or referrals for surgery (Figure 2). However, 6 weeks of preoperative exercise was an effective intervention for reducing cardiac and renal complication rates after surgical interventions and also the length of critical care stay.2828 Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609. PMid:26756766.
http://dx.doi.org/10.1097/SLA.0000000000...
Indeed, a forward walking program started before and continued after surgery reduced the hospital stay.3030 Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239. PMid:28028979.
http://dx.doi.org/10.17219/acem/62239...
A retrospective cohort with a longer follow-up showed that exercise is an effective intervention to reduce aneurysmal expansion and aortic aneurysm repair rates.3232 Nakayama A, Morita H, Nagayama M, et al. Cardiac rehabilitation protects against the expansion of abdominal aortic aneurysm. J Am Heart Assoc. 2018;7(5):e007959. http://dx.doi.org/10.1161/JAHA.117.007959. PMid:29487112.
http://dx.doi.org/10.1161/JAHA.117.00795...
This result is similar to that in an animal model study.4141 Nakahashi TK, Hoshina K, Tsao PS, et al. Flow loading induces macrophage antioxidative gene expression in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2002;22(12):2017-22. http://dx.doi.org/10.1161/01.ATV.0000042082.38014.EA. PMid:12482828.
http://dx.doi.org/10.1161/01.ATV.0000042...
Because these clinical trials had short-term follow-up and low numbers of patients, the effect direction may yet change with the addition of new studies.
Patients with aortic aneurysms have a life expectancy lower than that of individuals of the same age in the same population,1717 Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2-77.e2. http://dx.doi.org/10.1016/j.jvs.2017.10.044. PMid:29268916.
http://dx.doi.org/10.1016/j.jvs.2017.10....
and it has been recognized the exercise decreases mortality in patients with stable coronary heart disease.4242 Stewart RAH, Held C, Hadziosmanovic N, et al. Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol. 2017;70(14):1689-700. http://dx.doi.org/10.1016/j.jacc.2017.08.017. PMid:28958324.
http://dx.doi.org/10.1016/j.jacc.2017.08...
Although exercise did not reduce the mortality rates in this review, some mortality can be attributed to patients’ associated clinical risk factors.1717 Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2-77.e2. http://dx.doi.org/10.1016/j.jvs.2017.10.044. PMid:29268916.
http://dx.doi.org/10.1016/j.jvs.2017.10....
Exercise could also be advocated to improve patients’ quality of life, but data are insufficient to assess this outcome.1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
With respect to safety concerns, in all studies exercise did not increase the risks of rupture, death, or severe cardiovascular adverse effects. Additionally, there are presumably large numbers of patients with undiagnosed small abdominal aortic aneurysms in exercise programs and rupture rates are low. Indeed, cardiorespiratory fitness is a marker of mortality,4343 Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women. JAMA. 2009;301(19):2024-35. http://dx.doi.org/10.1001/jama.2009.681. PMid:19454641.
http://dx.doi.org/10.1001/jama.2009.681...
and improved fitness can be a valuable intervention to prevent at least serious complications whenever surgery is necessary. Good fitness levels are considered important to reduce hospital stay with no reduction in surgical mortality rates.3333 Hayashi K, Hirashiki A, Kodama A, et al. Impact of preoperative regular physical activity on postoperative course after open abdominal aortic aneurysm surgery. Heart Vessels. 2016;31(4):578-83. http://dx.doi.org/10.1007/s00380-015-0644-6. PMid:25666952.
http://dx.doi.org/10.1007/s00380-015-064...
These facts still do not constitute evidence to support recommending exercise to patients with small aneurysms at surveillance, since few patients have been evaluated.
A recent review included five studies and conducted a descriptive analysis.4444 Pouwels S, Willigendael EM, van Sambeek MR, Nienhuijs SW, Cuypers PW, Teijink JA. Beneficial effects of pre-operative exercise therapy in patients with an abdominal aortic aneurysm: a systematic review. Eur J Vasc Endovasc Surg. 2015;49(1):66-76. http://dx.doi.org/10.1016/j.ejvs.2014.10.008. PMid:25457300.
http://dx.doi.org/10.1016/j.ejvs.2014.10...
We decided that two of those studies could not be appropriately included in the systematic review without increasing clinical heterogeneity. One study had no control group,2727 Barakat HM, Shahin Y, Barnes R, et al. Supervised exercise program improves aerobic fitness in patients awaiting abdominal aortic aneurysm repair. Ann Vasc Surg. 2014;28(1):74-9. http://dx.doi.org/10.1016/j.avsg.2013.09.001. PMid:24332259.
http://dx.doi.org/10.1016/j.avsg.2013.09...
and the other evaluated respiratory physiotherapy, not exercise.3131 Dronkers J, Veldman A, Hoberg E, van der Waal C, van Meeteren N. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: a randomized controlled pilot study. Clin Rehabil. 2008;22(2):134-42. http://dx.doi.org/10.1177/0269215507081574. PMid:18057088.
http://dx.doi.org/10.1177/02692155070815...
Another systematic review has problems related to selection since it included the same study three times in the meta-analysis and described surrogate outcomes.4545 Kato M, Kubo A, Green FN, Takagi H. Meta-analysis of randomized controlled trials on safety and efficacy of exercise training in patients with abdominal aortic aneurysm. J Vasc Surg. 2019;69(3):933-43. http://dx.doi.org/10.1016/j.jvs.2018.07.069. PMid:30578072.
http://dx.doi.org/10.1016/j.jvs.2018.07....
The overall quality of the evidence of this review was graded “very low” because of the use of a rigorous methodology to reduce the risk of bias for clinical trials. A comprehensive and sensitive literature search was carried out, and at least two authors collected, extracted, and assessed the quality of data from studies. Additionally, a validated study was used to determine the risk of bias of the studies included.4646 Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. USA: The Cochrane Collaboration; 2011. Finally, the GRADE approach was used to grade the final quality of the body of the evidence.2424 GRADEpro GDT. GRADEpro Guideline Development Tool [software]. Hamilton: McMaster University; 2015.
There was heterogeneity in the amount, duration, and type of exercise among the studies included, possibly leading to different fitness levels. This heterogeneity could also lead to variation in individuals’ physiologic responses. Furthermore, the rate of loss to follow-up was high during the interventions in the clinical trials; however, this was sometimes impossible to avoid (e.g., some patients were withdrawn due to acute myocardial infarction and respiratory failure). A per-protocol analysis was thus chosen for analytic purposes. These concerns led us to conclude that the optimal duration and intensity of exercise remain undetermined. Indeed, aortic aneurysms larger than 70 mm have a lower prevalence but the worst prognosis.4747 Conway KP, Byrne J, Townsend M, Lane IF. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? J Vasc Surg. 2001;33(4):752-7. http://dx.doi.org/10.1067/mva.2001.112800. PMid:11296328.
http://dx.doi.org/10.1067/mva.2001.11280...
Thus, the evidence is not valid for this subgroup of patients.
One limitation of this review is that most of the studies included were performed in a well-controlled environment, which does not represent everyday life. Indeed, one patient in the intervention group in the study by Kothmann et al.2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
had ventricular fibrillation and was successfully resuscitated. Aneurysms are more prevalent in men, and no study included a sufficient number of women to arrive at any conclusions for this subgroup, despite the fact that it has worse prognosis.4848 Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007;115(22):2865-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.671859. PMid:17548742.
http://dx.doi.org/10.1161/CIRCULATIONAHA...
Whether the effect of intervention is limited to the duration of exercise or can be extended even when a patient becomes sedentary later in life remains unknown. In two clinical trials, no patients were referred for surgery during surveillance, because of the short follow-up period.1818 Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012. PMid:22846453.
http://dx.doi.org/10.1016/j.apmr.2012.07...
,2929 Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x. PMid:19086999.
http://dx.doi.org/10.1111/j.1365-2044.20...
Additionally, the causes of aneurysm growth are unclear,4949 Brewster DC, Cronenwett JL, Hallett JW Jr, et al. Guidelines for the treatment of abdominal aortic aneurysms: report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37(5):1106-17. http://dx.doi.org/10.1067/mva.2003.363. PMid:12756363.
http://dx.doi.org/10.1067/mva.2003.363...
and some rapidly expanding aneurysms reach the threshold for surgery before the expected time.5050 Scott RA, Tisi PV, Ashton HA, Allen DR. Abdominal aortic aneurysm rupture rates: a 7-year follow-up of the entire abdominal aortic aneurysm population detected by screening. J Vasc Surg. 1998;28(1):124-8. http://dx.doi.org/10.1016/S0741-5214(98)70207-1. PMid:9685138.
http://dx.doi.org/10.1016/S0741-5214(98)...
This may indicate the presence of a subgroup of patients with increased exercise-related risks. Thus, to ensure safety, it is essential to set intervals for conducting ultrasound surveillance during exercise periods for patients with both small and large aneurysms.
Aneurysm diameter was imbalanced between intervention and control groups. Because aneurysm growth rate is directly dependent on original aneurysm diameter,1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
related outcomes (e.g., aneurysm growth rate and rupture) could also be influenced.
Two-thirds of patients in the study by Myers et al.1111 Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8. PMid:23793234.
http://dx.doi.org/10.1249/MSS.0b013e3182...
were not able to achieve the amount and intensity of exercise required for inclusion. Other types, durations, and intensities of exercise might be of value for these patients. Additionally, all studies only evaluated patients with abdominal aortic aneurysms.
There is a glaring need to perform more pragmatic clinical trials with longer follow-ups to achieve a sufficient number of patients to reduce uncertainty. Prospective studies with women are also necessary.
CONCLUSION
The results of this systematic review and meta-analysis showed that there is very low quality evidence that exercise was effective and safe for patients with asymptomatic aortic aneurysms. Exercise did not impact aneurysm expansion rates. Six weeks of preoperative exercise decreased renal and cardiovascular surgical complications and reduced intensive care unit stays. Preoperative and postoperative forward walking reduced hospital stays. These outcomes need more studies to confirm the potential use of exercise for aortic aneurysm patients, since the quality of the evidence was judged as very low quality for all the outcomes studied.
PERSPECTIVE
Patients with aortic aneurysms are faced with a dilemma: although exercising could increase the risk of aneurysm rupture, a sedentary lifestyle increases the risk of death, mainly due to coronary artery disease. The prevalence of aortic aneurysms is high in older patients,33 Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø Study. Am J Epidemiol. 2001;154(3):236-44. http://dx.doi.org/10.1093/aje/154.3.236. PMid:11479188.
http://dx.doi.org/10.1093/aje/154.3.236...
but most patients have small abdominal aortic aneurysms with higher mortality rates compared with patients of the same age, depending on the clinical condition.1212 Newman AB, Arnold AM, Burke GL, O’Leary DH, Manolio TA. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the cardiovascular health study. Ann Intern Med. 2001;134(3):182-90. http://dx.doi.org/10.7326/0003-4819-134-3-200102060-00008. PMid:11177330.
http://dx.doi.org/10.7326/0003-4819-134-...
Therefore, this issue is relevant for patients, exercise professionals, and stakeholders involved in creation of new treatment interventions. To our knowledge, no other systematic review has addressed this issue with the same level of quality. This review revealed no deaths or aneurysm ruptures related to exercise. Additionally, although the clinical trials showed no reduction in aneurysm growth rates, a retrospective cohort with longer follow-up showed reductions in aneurysm growth rate and in the number of patients referred for surgery. This evidence demonstrates reductions in cardiac and renal complication rates, hospital stays, and intensive care unit stays. The present review identifies a new patient population in whom the benefits of exercise should be studied. While the general population experiences increased quality and quantity of life from exercising, patients with aneurysms might benefit from exercise as a treatment option.
ACKNOWLEDGMENTS
We thank Paulo Minalli, physical educator and content specialist, for helping us to understand contexts, definitions, and outcomes. We also thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
-
How to cite: Oliveira RÁ, Nakajima E, Vasconcelos VT, Riera R, Baptista-Silva JCC. Effectiveness and safety of structured exercise vs. no exercise for asymptomatic aortic aneurysm: systematic review and meta-analysis. J Vasc Bras. 2020;19:e20190086. https://doi.org/10.1590/1677-5449.190086
-
Financial support: None.
-
The study was carried out at Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil.
REFERENCES
-
1Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13(3):452-8. http://dx.doi.org/10.1067/mva.1991.26737 PMid:1999868.
» http://dx.doi.org/10.1067/mva.1991.26737 -
2Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2011;41(Suppl 1):S1-58. http://dx.doi.org/10.1016/j.ejvs.2010.09.011 PMid:21215940.
» http://dx.doi.org/10.1016/j.ejvs.2010.09.011 -
3Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø Study. Am J Epidemiol. 2001;154(3):236-44. http://dx.doi.org/10.1093/aje/154.3.236 PMid:11479188.
» http://dx.doi.org/10.1093/aje/154.3.236 -
4Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82(8):1066-70. http://dx.doi.org/10.1002/bjs.1800820821 PMid:7648155.
» http://dx.doi.org/10.1002/bjs.1800820821 -
5Aggarwal S, Qamar A, Sharma V, Sharma A. Abdominal aortic aneurysm: a comprehensive review. Exp Clin Cardiol. 2011;16(1):11-5. PMid:21523201.
-
6Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;(2):CD002945. PMid:17443519.
-
7Lederle FA. The natural history of abdominal aortic aneurysm. Acta Chir Belg. 2009;109(1):7-12. http://dx.doi.org/10.1080/00015458.2009.11680364 PMid:19341189.
» http://dx.doi.org/10.1080/00015458.2009.11680364 -
8Mell M, White JJ, Hill BB, Hastie T, Dalman RL, Stanford Abdominal Aortic Aneurysm Specialized Center of Clinically Oriented Research Investigators. No increased mortality with early aortic aneurysm disease. J Vasc Surg. 2012;56(5):1246-5. http://dx.doi.org/10.1016/j.jvs.2012.04.023 PMid:22832264.
» http://dx.doi.org/10.1016/j.jvs.2012.04.023 -
9Reed WW, Hallett JW Jr, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med. 1997;157(18):2064-8. http://dx.doi.org/10.1001/archinte.1997.00440390050007 PMid:9382661.
» http://dx.doi.org/10.1001/archinte.1997.00440390050007 -
10The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352(9141):1649-55. http://dx.doi.org/10.1016/S0140-6736(98)10137-X PMid:9853436.
» http://dx.doi.org/10.1016/S0140-6736(98)10137-X -
11Myers J, Mcelrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2-9. http://dx.doi.org/10.1249/MSS.0b013e3182a088b8 PMid:23793234.
» http://dx.doi.org/10.1249/MSS.0b013e3182a088b8 -
12Newman AB, Arnold AM, Burke GL, O’Leary DH, Manolio TA. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the cardiovascular health study. Ann Intern Med. 2001;134(3):182-90. http://dx.doi.org/10.7326/0003-4819-134-3-200102060-00008 PMid:11177330.
» http://dx.doi.org/10.7326/0003-4819-134-3-200102060-00008 -
13Brady AR, Fowkes FG, Greenhalgh RM, Powell JT, Ruckley CV, Thompson SG. Risk factors for postoperative death following elective surgical repair of abdominal aortic aneurysm: results from the UK Small Aneurysm Trial. Br J Surg. 2000;87(6):742-9. http://dx.doi.org/10.1046/j.1365-2168.2000.01410.x PMid:10848851.
» http://dx.doi.org/10.1046/j.1365-2168.2000.01410.x -
14Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-59. http://dx.doi.org/10.1249/MSS.0b013e318213fefb PMid:21694556.
» http://dx.doi.org/10.1249/MSS.0b013e318213fefb -
15Almeida OP, Khan KM, Hankey GJ, Yeap BB, Golledge J, Flicker L. 150 minutes of vigorous physical activity per week predicts survival and successful ageing: a population-based 11-year longitudinal study of 12 201 older Australian men. Br J Sports Med. 2014;48(3):220-5. http://dx.doi.org/10.1136/bjsports-2013-092814 PMid:24002240.
» http://dx.doi.org/10.1136/bjsports-2013-092814 -
16Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-31. PMid:3920711.
-
17Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2-77.e2. http://dx.doi.org/10.1016/j.jvs.2017.10.044 PMid:29268916.
» http://dx.doi.org/10.1016/j.jvs.2017.10.044 -
18Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93(12):2148-53. http://dx.doi.org/10.1016/j.apmr.2012.07.012 PMid:22846453.
» http://dx.doi.org/10.1016/j.apmr.2012.07.012 -
19Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. USA: The Cochrane Collaboration; 2011.
-
20Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-41. http://dx.doi.org/10.1016/j.ijsu.2010.02.007 PMid:20171303.
» http://dx.doi.org/10.1016/j.ijsu.2010.02.007 -
21Oliveira R, Nakajima E, Riera R, Baptista-Silva J. Physical exercises for aortic aneurysm: a systematic review. United Kingdom: NIHR; 2016. PROSPERO 2016 CRD42016041468. [cited 2019 aug 15]. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42016041468
» https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42016041468 -
22Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. http://dx.doi.org/10.1186/s13643-016-0384-4 PMid:27919275.
» http://dx.doi.org/10.1186/s13643-016-0384-4 -
23Manager R. (RevMan) Version 5.3 [software]. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
-
24GRADEpro GDT. GRADEpro Guideline Development Tool [software]. Hamilton: McMaster University; 2015.
-
25Lima RM, Vainshelboim B, Ganatra R, Dalman R, Chan K, Myers J. Exercise training improves ventilatory efficiency in patients with a small abdominal aortic aneurysm: A randomized controlled study. J Cardiopulm Rehabil Prev. 2018;38(4):239-45. http://dx.doi.org/10.1097/HCR.0000000000000270 PMid:28727673.
» http://dx.doi.org/10.1097/HCR.0000000000000270 -
26Myers JN, White JJ, Narasimhan B, Dalman RL. Effects of exercise training in patients with abdominal aortic aneurysm: preliminary results from a randomized trial. J Cardiopulm Rehabil Prev. 2010;30(6):374-83. http://dx.doi.org/10.1097/HCR.0b013e3181ebf2db PMid:20724934.
» http://dx.doi.org/10.1097/HCR.0b013e3181ebf2db -
27Barakat HM, Shahin Y, Barnes R, et al. Supervised exercise program improves aerobic fitness in patients awaiting abdominal aortic aneurysm repair. Ann Vasc Surg. 2014;28(1):74-9. http://dx.doi.org/10.1016/j.avsg.2013.09.001 PMid:24332259.
» http://dx.doi.org/10.1016/j.avsg.2013.09.001 -
28Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg. 2016;264(1):47-53. http://dx.doi.org/10.1097/SLA.0000000000001609 PMid:26756766.
» http://dx.doi.org/10.1097/SLA.0000000000001609 -
29Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. Anaesthesia. 2009;64(1):9-13. http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x PMid:19086999.
» http://dx.doi.org/10.1111/j.1365-2044.2008.05690.x -
30Wnuk BR, Durmała J, Ziaja K, Kotyla P, Woźniewski M, Błaszczak E. A controlled trial of the efficacy of a training walking program in patients recovering from abdominal aortic aneurysm surgery. Adv Clin Exp Med. 2016;25(6):1241-371. http://dx.doi.org/10.17219/acem/62239 PMid:28028979.
» http://dx.doi.org/10.17219/acem/62239 -
31Dronkers J, Veldman A, Hoberg E, van der Waal C, van Meeteren N. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: a randomized controlled pilot study. Clin Rehabil. 2008;22(2):134-42. http://dx.doi.org/10.1177/0269215507081574 PMid:18057088.
» http://dx.doi.org/10.1177/0269215507081574 -
32Nakayama A, Morita H, Nagayama M, et al. Cardiac rehabilitation protects against the expansion of abdominal aortic aneurysm. J Am Heart Assoc. 2018;7(5):e007959. http://dx.doi.org/10.1161/JAHA.117.007959 PMid:29487112.
» http://dx.doi.org/10.1161/JAHA.117.007959 -
33Hayashi K, Hirashiki A, Kodama A, et al. Impact of preoperative regular physical activity on postoperative course after open abdominal aortic aneurysm surgery. Heart Vessels. 2016;31(4):578-83. http://dx.doi.org/10.1007/s00380-015-0644-6 PMid:25666952.
» http://dx.doi.org/10.1007/s00380-015-0644-6 -
34Bailey TG, Perissiou M, Windsor MT, et al. Effects of acute exercise on endothelial function in patients with abdominal aortic aneurysm. Am J Physiol Heart Circ Physiol. 2018;314(1):H19-30. http://dx.doi.org/10.1152/ajpheart.00344.2017 PMid:28939648.
» http://dx.doi.org/10.1152/ajpheart.00344.2017 -
35Weston M, Batterham AM, Tew GA, et al. Patients awaiting surgical repair for large abdominal aortic aneurysms can exercise at moderate to hard intensities with a low risk of adverse events. Front Physiol. 2016;7:684. PMid:28119627.
-
36ClinicalTrials.gov. Surviving aneurysm surgery: a pilot study on exercise training in abdominal aortic aneurysm patients. USA: U. S. National Library of Medicine; 2017. NCT01805973.
-
37ClinicalTrials.gov. The assessment of the feasibility of a home based exercise programme in the older patient following major surgery. USA: U. S. National Library of Medicine; 2017. NCT03064308.
-
38ClinicalTrials.gov. Prehabilitation for aortic repair patients - full text view. USA: U. S. National Library of Medicine; 2017. NCT02767518.
-
39Mosk A. Pre operative optimisation pathway for elderly surgical patients - OUTO. The Netherlands: Netherlands Trial Register. NTR5932.
-
40Senn S. Testing for baseline balance in clinical trials. Stat Med. 1994;13(17):1715-26. http://dx.doi.org/10.1002/sim.4780131703 PMid:7997705.
» http://dx.doi.org/10.1002/sim.4780131703 -
41Nakahashi TK, Hoshina K, Tsao PS, et al. Flow loading induces macrophage antioxidative gene expression in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2002;22(12):2017-22. http://dx.doi.org/10.1161/01.ATV.0000042082.38014.EA PMid:12482828.
» http://dx.doi.org/10.1161/01.ATV.0000042082.38014.EA -
42Stewart RAH, Held C, Hadziosmanovic N, et al. Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol. 2017;70(14):1689-700. http://dx.doi.org/10.1016/j.jacc.2017.08.017 PMid:28958324.
» http://dx.doi.org/10.1016/j.jacc.2017.08.017 -
43Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women. JAMA. 2009;301(19):2024-35. http://dx.doi.org/10.1001/jama.2009.681 PMid:19454641.
» http://dx.doi.org/10.1001/jama.2009.681 -
44Pouwels S, Willigendael EM, van Sambeek MR, Nienhuijs SW, Cuypers PW, Teijink JA. Beneficial effects of pre-operative exercise therapy in patients with an abdominal aortic aneurysm: a systematic review. Eur J Vasc Endovasc Surg. 2015;49(1):66-76. http://dx.doi.org/10.1016/j.ejvs.2014.10.008 PMid:25457300.
» http://dx.doi.org/10.1016/j.ejvs.2014.10.008 -
45Kato M, Kubo A, Green FN, Takagi H. Meta-analysis of randomized controlled trials on safety and efficacy of exercise training in patients with abdominal aortic aneurysm. J Vasc Surg. 2019;69(3):933-43. http://dx.doi.org/10.1016/j.jvs.2018.07.069 PMid:30578072.
» http://dx.doi.org/10.1016/j.jvs.2018.07.069 -
46Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. USA: The Cochrane Collaboration; 2011.
-
47Conway KP, Byrne J, Townsend M, Lane IF. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? J Vasc Surg. 2001;33(4):752-7. http://dx.doi.org/10.1067/mva.2001.112800 PMid:11296328.
» http://dx.doi.org/10.1067/mva.2001.112800 -
48Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007;115(22):2865-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.671859 PMid:17548742.
» http://dx.doi.org/10.1161/CIRCULATIONAHA.106.671859 -
49Brewster DC, Cronenwett JL, Hallett JW Jr, et al. Guidelines for the treatment of abdominal aortic aneurysms: report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37(5):1106-17. http://dx.doi.org/10.1067/mva.2003.363 PMid:12756363.
» http://dx.doi.org/10.1067/mva.2003.363 -
50Scott RA, Tisi PV, Ashton HA, Allen DR. Abdominal aortic aneurysm rupture rates: a 7-year follow-up of the entire abdominal aortic aneurysm population detected by screening. J Vasc Surg. 1998;28(1):124-8. http://dx.doi.org/10.1016/S0741-5214(98)70207-1 PMid:9685138.
» http://dx.doi.org/10.1016/S0741-5214(98)70207-1
Publication Dates
-
Publication in this collection
08 May 2020 -
Date of issue
2020
History
-
Received
15 Aug 2019 -
Accepted
01 Oct 2019