Abstract
PURPOSE: We describe the critical steps of the laparoscopic radical prostatectomy (LRP) technique and discuss how they impact upon the pertinent issues regarding prostate cancer surgery: blood loss, potency and continence. RESULTS: A major advantage of LRP is the reduced operative blood loss. The precise placement of the dorsal vein complex stitch associated with the tamponading effect of the CO2 pneumoperitoneum significantly decrease venous bleeding, which is the main source of blood loss during radical prostatectomy. At the Cleveland Clinic, the average blood loss of our first 100 patients was 322.5 ml, resulting in low transfusion rates. The continuous venous bleeding narrowed pelvic surgical field and poor visibility can adversely impact on nerve preservation during open radical prostatectomy. Laparoscopy, with its enhanced and magnified vision in a relatively bloodless field allows for excellent identification and handling of the neurovascular bundles. During open retropubic radical prostatectomy, the pubic bone may impair visibility and access to the urethral stump, and the surgeon must tie the knots relying on tactile sensation alone. Consequently, open prostatectomy is associated with a prolonged catheterization period of 2 - 3 weeks. Comparatively, during laparoscopic radical prostatectomy all sutures are meticulously placed and each is tied under complete visual control, resulting in a precise mucosa-to-mucosa approximation. CONCLUSION: The laparoscopic approach may represent a reliable less invasive alternative to the conventional open approach. Despite the encouraging preliminary anatomical and functional outcomes, prospective randomized comparative trials are required to critically evaluate the role of laparoscopy for this sophisticated and delicate operation.
prostate; carcinoma; prostatic neoplasms; prostatectomy; laparoscopy
CLINICAL UROLOGY
Pertinent issues related to laparoscopic radical prostatectomy
Sidney C. Abreu; Inderbir S. Gill
Section of Laparoscopic and Minimally Invasive Surgery, Glickman Urological Institute, Cleveland Clinic Foundation, Cleveland, Ohio, USA
Correspondence Correspondence to Dr. Sidney C. Abreu Section of Laparoscopy and Endourology, São Carlos Hospital Av. Pontes Vieira, 2551 Fortaleza, CE, 60135-420, Brazil Fax: + 55 85 227-6393 E-mail: sidneyabreu@hotmail.com
ABSTRACT
PURPOSE: We describe the critical steps of the laparoscopic radical prostatectomy (LRP) technique and discuss how they impact upon the pertinent issues regarding prostate cancer surgery: blood loss, potency and continence.
RESULTS: A major advantage of LRP is the reduced operative blood loss. The precise placement of the dorsal vein complex stitch associated with the tamponading effect of the CO2 pneumoperitoneum significantly decrease venous bleeding, which is the main source of blood loss during radical prostatectomy. At the Cleveland Clinic, the average blood loss of our first 100 patients was 322.5 ml, resulting in low transfusion rates.
The continuous venous bleeding narrowed pelvic surgical field and poor visibility can adversely impact on nerve preservation during open radical prostatectomy. Laparoscopy, with its enhanced and magnified vision in a relatively bloodless field allows for excellent identification and handling of the neurovascular bundles.
During open retropubic radical prostatectomy, the pubic bone may impair visibility and access to the urethral stump, and the surgeon must tie the knots relying on tactile sensation alone. Consequently, open prostatectomy is associated with a prolonged catheterization period of 2 3 weeks. Comparatively, during laparoscopic radical prostatectomy all sutures are meticulously placed and each is tied under complete visual control, resulting in a precise mucosa-to-mucosa approximation.
CONCLUSION: The laparoscopic approach may represent a reliable less invasive alternative to the conventional open approach. Despite the encouraging preliminary anatomical and functional outcomes, prospective randomized comparative trials are required to critically evaluate the role of laparoscopy for this sophisticated and delicate operation.
Key words: prostate; carcinoma; prostatic neoplasms; prostatectomy; laparoscopy
INTRODUCTION
Once considered an unpopular operation with significant morbidity, radical retropubic prostatectomy has evolved into a refined, anatomically precise operation (1). In view of the already satisfactory oncological and functional outcomes achieved with the traditional open retropubic approach and encouraged by the initial inroads of laparoscopy in renal oncology, efforts have been recently focused towards developing a laparoscopic approach to radical prostatectomy, aiming to decrease the morbidity related to the open operation. However, the advantages of laparoscopic renal surgery, wherein avoidance of a flank incision markedly reduces morbidity, are not so evident for laparoscopic radical prostatectomy (LRP). In fact, the avoidance of a low midline incision used for the open retropubic approach likely will not have the same implications as regards reduction of morbidity (2). Due to the enhanced visualization and magnification during LRP, and the tamponading effect of the CO2 pneumoperitoneum, the laparoscopic approach has the potential to favorably impact upon the functional sequelae related to this intricate operation. The decreased blood loss, adequate preservation of the neurovascular bundles and superior watertight urethrovesical anastomosis may ultimately further improve potency and continence rates (3,4).
In this article, we describe the critical steps of laparoscopic radical prostatectomy and discuss how they impact upon the important issues regarding prostate cancer surgery: blood loss, positive surgical margins, and postoperative potency and continence rates.
DORSAL VEIN COMPLEX LIGATION AND TRANSECTION - IMPACT OVER BLOOD LOSS
Similar to the open counterpart, the dorsal vein complex is suture ligated in order to decrease blood loss upon it transection. After the Foley catheter is replaced by an 18F metallic urethral sound, a 2 - 0 vicryl stitch with a CT-1 needle is employed to ligate the dorsal vein. This stitch is placed in a back-hand manner from the right to the left side, distal to the apex of the prostate, between the dorsal vein complex and the urethra (Figure-1). In order to avoid inadvertent transgression of the urethra by the suture, the assistant pushes down the metallic sound, displacing the urethra posteriorly. We routinely place two stitches across the dorsal vein complex in an attempt to achieve a safe ligation. Also, we anchor the dorsal vein stitch to the pubic periosteum, aiming to achieve a retropubic urethropexy suspension in order to possibly enhance continence outcomes. A back-bleeding stitch is placed across the anterior surface of the base of the prostate. Following bladder neck transection, the prostate base is grasped and tractioned cephalad, which places the urethra and the dorsal vein complex on stretch. Subsequently, an electrosurgical J-hook or the harmonic scalpel is used to slowly divide the dorsal vein complex at the apex of the prostate achieving meticulous hemostasis. Occasionally the dorsal vein stitch may become loosened, leading to venous hemorrhage. However due to the tamponading effect of the CO2 pneumoperitoneum this hemorrhage is usually not intense, and can be controlled by placing another stitch around the transected dorsal vein.
High operative blood loss and transfusion is a common problem of open prostate surgery. A major advantage of LRP is the reduced operative blood loss. The precise placement of the dorsal vein complex stitch under laparoscopic visualization associated with the tamponading effect of the 15 mmHg pressure CO2 pneumoperitoneum significantly decrease venous bleeding, which is the main source of blood loss during radical prostatectomy. Conversely, during retropubic open surgery, a right angle clamp is blindly passed underneath the dorsal vein complex in order to control it with a tie. This maneuver is mainly based on the palpation of the Foley catheter to identify the anterior surface of the urethra and may lead to clamp misplacement and significant bleeding.
In a review of 1228 LRP at six European centers, average blood loss was 488 ml with a transfusion rate of 3.5% (5). The French team of Guillonneau et al. also reported a mean intraoperative blood loss of 354 ml with a transfusion rate of 5.7% (20 patients) in a series of 350 LRP (6). At the Cleveland Clinic, the average blood loss of our first 100 patients was 322.5 ml, resulting in low transfusion rates (2%) (7). This is in contrast to open prostatectomy series with reported blood losses between 500 ml to 1000 ml.
BLADDER NECK TRANSECTION AND APICAL DISSECTION IMPACT OVER POSITIVE SURGICAL MARGINS
The precise anatomic location of the junction between the prostate and the bladder neck is not well defined under laparoscopic visualization. A combination of maneuvers can be performed to overcome the absence of precise landmarks for identifying the bladder neck: Close laparoscopic visualization usually identifies the area where the prevesical fat ends, signifying the prostatovesical junction. Gentle blunt palpation with the elbow of the J-hook eletrocautery also aids in defining this junctional area. Repeated in and out movements of the metallic urethral dilator, with its curved tip pointing anteriorly, provide another indication where the prostate ends and where the bladder begins. At the presumed prostato-vesical junction, a horizontal incision is created using J-hook eletrocautery (Figure-2). The anterior bladder neck is divided in the midline, and the tip of the urethral dilator is delivered through the cystotomy into the space of Retzius. We do not make a major effort to spare the bladder neck in an attempt to minimize positive surgical margin at this location. However, a carefully dissected bladder neck can avoid the extra step of bladder neck reconstruction. Due to the precise mucosa-to-mucosa laparoscopic urethrovesical anastomosis, bladder neck evertion is not necessary.
Meticulous apical dissection is probably the most challenging step of LRP. During this step technical shortcomings can lead to incontinence (compromised urethral stump length), impotency (damage to the neurovascular bundle), and positive surgical margins (iatrogenic entry into the prostate apex) (8). After the dorsal venous complex is divided, the anterior urethral wall is identified with the aid of the metallic urethral sound. The neurovascular bundles, located posterolateral to the urethra, are mobilized laterally from the prostato-urethral junction using the fine gently curved tip of the harmonic scalpel without activation. At this point, cold Endoshears is used to transect the anterior urethral wall close to the concave notch of the prostate, which assures preservation of an excellent urethral stump. The tip of the intraurethral metallic sound is delivered through the urethral opening. The posterior urethral wall and the rectourethralis muscles are divided, completely detaching the prostate. At this point, care must be taken to avoid inadvertent entry into the rectum.
Concerns about bladder neck and apical positive surgical margins after laparoscopic radical prostatectomy have been raised (9). Laparoscopy is likely to be comparable to open surgery as regards positive surgical margins. In our experience, a significant decline on the positive surgical margin rate occurred from our first 50 cases to our third 50 cases (7). Technique refinements, especially during the apical dissection, were inherently related to this reduction of our positive surgical margins rate. In addition to this, we believe that a reasonable number of positive surgical margins present in our series may be "false-positive", related to the laparoscopic manipulation of the prostate with traumatic instruments. In fact, the laparoscopic Allis forceps used to tautly retract the prostate cephalad and laterally during neurovascular bundle dissection has a propensity to routinely remove divots from the gland. In an attempt to overcome this, we are now using an additional clamp through a 5 mm port placed at the supra-pubic region to anteriorly retract the seminal vesicles and vas deferens and maneuver the prostate, thus potentially avoiding these presumed "false positive" surgical margins.
Although the available data are sparse, it is likely that LRP will emerge as a sound oncologic alternative. Recently, Guillonneau et al. reported an oncological mid-term evaluation of 1000 patients that underwent LRP (10). In this study, the incidence of positive surgical margins for the stages pT2a, pT2b was 6.9%, 18.6%, which is comparable to the series of Lepor et al. who reported 19.9% positive margin rate in 1000 open retropubic radical prostatectomies (11). Similarly Katz et al. reported an 18.9% incidence of positive surgical margins in 169 pT2 cases (12). These authors highlight that a constant decrease was noted in the overall incidence of positive margins over time, and that avoiding bladder neck preservation eliminated positive bladder neck margins. Moreover, the overall incidence of positive lateral surgical margins in pT2 cases treated with a nerve sparing procedure was 8.4%. Rassweiller et al. reported a 16% incidence of positive margins in 180 patients, almost half of who had pT3 disease (13).
NEUROVASCULAR BUNDLE DISSECTION IMPACT OVER POSTOPERATIVE POTENCY
The optimal laparoscopic technique of nerve sparing continues to evolve. We currently employ a combined antegrade-retrograde technique. A few technical maneuvers in this regard include: 1) Upon opening the endopelvic fascia, while releasing the levator muscles from the apex and lateral aspect of the prostate, no thermal or electrical energy is used near the NVB. A laparoscopic kittner or "cold" cut scissors is used to complete the dissection; 2) Following dorsal vein ligation, the lateral fascia over the prostate is bilaterally incised superficially with "cold" Endoshears in order to release the tethering of the NVB; 3) Aiming to minimize eletrocautery trauma to the NVB near the tip of the seminal vesicles, the vesicular artery is secured with hemostatic locking clips (Weck Systems, Triangle Park, NC).
Initially, the lateral pedicles of the prostate are controlled with one or two 10 mm Hemolock clips (Weck Systems, Triangle Park, NC) (Figure-3). The postero-lateral edge of the prostate base is identified. Once this anatomical landmark is encountered, the magnified laparoscopic vision allows the surgeon to identify the neurovascular bundle. Staying approximately 2 mm away from the prostate, the harmonic scalpel is employed to develop a plane between the gland and the NVB. The harmonic scalpel is preferred because of its limited spread of thermal energy (1 2 mm). Furthermore, no electric energy (monopolar or bipolar) is involved, which could potentially disrupt nerve integrity and conduction.
Anatomic studies have demonstrated the precise location of the neurovascular bundle, which is familiar to most urologists. However, the continuous venous bleeding narrowed pelvic surgical field and poor visibility can adversely impact on nerve preservation during open radical prostatectomy. Laparoscopy, with its enhanced and magnified vision in a relatively bloodless field allows for excellent identification and handling of the neurovascular bundles (14). Therefore, the potential exits to match the best potency rates obtained with open surgery. Guillonneau et al. reviewed 73 of their patients who had either bilateral (46 patients) or unilateral (27 patients) nerve-sparing LRP. A remarkable 74% spontaneous erection rate was reported in the bilateral nerve sparing group and 51% in the unilateral group with a follow-up ranging from 2 to 12 months (15). More recently, Katz et al. followed-up 143 patients that underwent LRP which were potent preoperatively. Of these patients, 100, 80, 48 and 26 responded to a sexual function questionnaire at 1, 3, 6 and 12 months after surgery, respectively. Of the unilateral and bilateral nerve-sparing groups 50% and 87.5% reported spontaneous erections at 12 months after surgery, respectively (16). These authors found the overall rate of patients who had erections preoperatively and maintained erections after surgery (53.8%) to be comparable to the results for open surgery.
URETHRO-VESICAL ANASTOMOSIS IMPACT OVER POSTOPERATIVE CONTINENCE
During open retropubic radical prostatectomy, the pubic bone may impair visibility and access to the urethral stump, and the surgeon must tie 4 to 8 knots in a blind field, relying on tactile sensation alone. Consequently, open prostatectomy is associated with a prolonged catheterization period of 2 3 weeks. Comparatively, during laparoscopic radical prostatectomy all sutures are meticulously placed and each is tied under complete visual control, resulting in a precise mucosa-to-mucosa approximation (17).
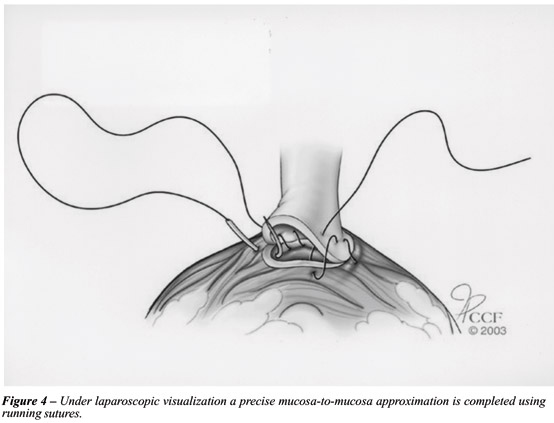
We employ a continuous running suture in an attempt to decrease postoperative urinary leak (Figure-4). A double-armed stitch is prepared by tying two 2 - 0 sutures on a UR-6 needle, one dyed Monocryl and the other undyed Caprosyn, each 10 inches in length (18). Both needles are initially passed outside in at 6 o'clock on the posterior bladder neck, thus placing the knot outside the bladder and anchoring the stitch at this position. The first stitch is run up in a clockwise direction from 6 o'clock to 9 o'clock. The second stitch is run up in a counter-clockwise direction from 6 o'clock to 3 o'clock. At this point, both stitches are placed on traction. Due to the low friction characteristics of the Monocryl and Caprosyn, the sutures glide smoothly under traction, thus tautly anchoring the entire posterior half of the bladder neck to the urethral stump. Upon creation of this posterior plate, a 22F urethral Foley catheter is easily advanced into the bladder. Anastomosis is completed by running both stitches to the 12 o'clock where they are tied together. Our preliminary results with this technique indicate a low rate of urinary leak on postoperative day 3 cystogram, allowing catheter removal at that time in the majority of the patients. Moreover, only one intracorporeal knot is tied.
Adequate preservation of a long urethral stump and an intact urinary sphincter mechanism with the laparoscopic technique may potentially result in improved postoperative continence rates. Guillonneau et al. reported on their first 133 patients with at least a one year follow-up and found 85.5% were totally continent (no protection needed during day or night). Five patients (3.8%) were classified as severely incontinent (15). Nadu et al. have reported continence rates greater then 93% in a median follow-up of 7 months (range from 1 to 15). In this particular study, the authors reported that only 15.1% of the patients had anastomotic leak on postoperative day 2 to 4 cystography (19). Despite using a single circular running stitch technique, no anastomotic stricture, pelvic abscess or urinoma were noticed in this series. At the Cleveland Clinic, we have not documented any case of anastomotic stricture in an overall experience that exceeds 300 cases of LRP. (unpublished data).
CONCLUSION
We believe that radical prostatectomy is the gold standard for the definitive treatment of localized prostate cancer in the appropriate patient. The laparoscopic approach may represent a reliable less invasive alternative to the conventional open approach. Despite the encouraging preliminary anatomical and functional outcomes above discussed, prospective randomized comparative trials are required to critically evaluate the role of laparoscopy for this sophisticated and delicate operation.
Received: September 9, 2003
Accepted: October 14, 2003
- 1. Walsh PC, Donker PJ: Impotency following radical prostatectomy: insight into etiology and prevention. J Urol. 1982; 128: 492-7.
- 2. Andriole GL: Laparoscopic radical prostatectomy: CON. Urology. 2001; 58: 507-8.
- 3. Steinberg A, Gill I: Laparoscopic radical prostatectomy. Contemp Urol. 2002; 14: 3449.
- 4. Gill I, Zippe C: Laparoscopic radical prostatectomy: technique. Urol Clin North Am. 2001; 28: 423-36.
- 5. Susler T, Guillenneau B, Vallancien G, Gaston R., Piechaud T, Turk I, et al.: Complications and initial experience with 1228 laparoscopic radical prostatectomy at 6 European centers. J Urol. 2001; 165: (Abst# 605).
- 6. Guillonneau B, Cathelineau X, Doublet JD, Vallancien G: Laparoscopic radical prostatectomy: the lessons learned. J Endourol. 2001; 15: 441-5.
- 7. Farouk A, Gill I, Kaouk J, Matin S, Meraney A, Abreu SC, et al.: 150 laparoscopic radical prostatectomy: learning curve in the United the States. J Endourol. 2002; 16: A33.
- 8. Steinberg AP, Abreu SC, Gill, IS: Laparoscopic Radical Prostatectomy. In: Smith, A. (ed.), Smith's Textbook of Endourology, New York, Quality Medical Publishing (In Press 2004).
- 9. Walsh PC: Minimally invasive treatment of prostate cancer. J Endourol. 2001; 15: 447-8.
- 10. Guillonneau B, El Fettouh H, Baumert HC: Laparoscopic radical prostatectomy: Oncological miditerm evaluation about 1000 patients at Montsouris Institute. J Urol. 2002; 169: 1261-6.
- 11. Lepor H, Ferrandino M, Nieder AM: The intraoperative and postoperative complications following radical retropubic prostatectomy in a consecutive series of 1000 cases. J Urol. 2001; 165: 1347A.
- 12. Katz R, Salomon L, Hoznek A, Taille A, Antiphon P, Abbou CC: Surgical margins in laparoscopic radical prostatectomy: the impact of apical dissection, bladder neck remodling and nerve preservation. J Urol. 2003; 169: 2049-52.
- 13. Rassweiler J, Sentker L, Seemann O, Hatzinger M, Rumpelt HJ: Laparoscopic radical prostatectomy with the Heilbronn technique: an analysis of the first 180 cases. J Urol. 2001; 166: 2101-8.
- 14. Kaouk HJ, Desai MM, Abreu SC, Papay F, Gill IS: Robotic assisted laparoscopic sural nerve grafting during radical prostatectomy: initial experience. J Urol. 2003; 170: 909-12.
- 15. Guillonneau B, Cathelineau X, Doublet JD: Prospective assessment of functional results after laparoscopic radical prostatectomy. J Urol. 2001; 165: 614A.
- 16. Katz R, Salomon L, Hoznek A: Patient reported sexual function following laparoscopic radical prostatectomy. J Urol. 2002; 168: 2078-82.
- 17. Hoznek A, Salomon L, Rabii R, Slama MB, Cicco A, Antiphon P, et al.: Vesicourethral anastomosis during laparoscopic radical prostatectomy: the running suture method. J Endourol. 2000; 14: 749-53.
- 18. Von Vethoven RF, Ahlering TE, Peltier A, Skarecky DW, Clayman RV: Technique for laparoscopic running urethrovesical anastomosis: the single knot method. Urology. 2003; 61: 699-702.
- 19. Nadu A, Salomon L, Hoznek A, Olsson LE, Saint F, Taile A, et al.: Early removal of the catheter after laparoscopic radical prostatectomy. J Urol. 2001; 166: 1662-4.
Correspondence to
Publication Dates
-
Publication in this collection
07 May 2004 -
Date of issue
Dec 2003
History
-
Received
09 Sept 2003 -
Accepted
14 Oct 2003