Abstract
The combined use of radiation therapy (RT) and androgen deprivation for patients with localized high-risk prostate cancer is commonly accepted as the standard treatment among uro-oncologists. Preclinical studies have provided rationale for the use of this combination. Additionally, results of phase 3 studies using conventional doses of RT have supported the combined approach. Other phase 3 studies have also shown a benefit for using higher doses of RT; however, the role of androgen deprivation in this context is not clear. The optimal duration of the androgen deprivation, in both the neoadjuvant and adjuvant setting, is still under investigation. This article critically reviews the data on the use of RT combined with androgen deprivation for the treatment of high-risk prostate cancer with emphasis on the results of phase 3 trials.
prostate neoplasms; high risk; radiotherapy; hormonal therapy; androgen deprivation therapy; combined treatment
REVIEW ARTICLE
Radiation therapy and androgen deprivation in the management of high risk prostate cancer
Alan Dal Pra; Fabio L. Cury; Luis Souhami
Department of Oncology, Division of Radiation Oncology, McGill University Health Centre, Montreal, Quebec
Correspondence Correspondence address: Dr. Luis Souhami Department of Oncology Division of Radiation Oncology McGill University Health Centre 1650, Cedar Avenue Montreal, Quebec, H4X 2C5, Canada Fax: + 514 934-8220 E-mail: luis.souhami@muhc.mcgill.ca
ABSTRACT
The combined use of radiation therapy (RT) and androgen deprivation for patients with localized high-risk prostate cancer is commonly accepted as the standard treatment among uro-oncologists. Preclinical studies have provided rationale for the use of this combination. Additionally, results of phase 3 studies using conventional doses of RT have supported the combined approach. Other phase 3 studies have also shown a benefit for using higher doses of RT; however, the role of androgen deprivation in this context is not clear. The optimal duration of the androgen deprivation, in both the neoadjuvant and adjuvant setting, is still under investigation. This article critically reviews the data on the use of RT combined with androgen deprivation for the treatment of high-risk prostate cancer with emphasis on the results of phase 3 trials.
Key words: prostate neoplasms; high risk; radiotherapy; hormonal therapy, androgen deprivation therapy; combined treatment
INTRODUCTION
Despite worldwide variability in incidence rates, prostate cancer (PC) remains one of the most frequent cancers and stands among leading causes of cancer deaths (1). Although high tumor control and survival rates are observed in patients with favorable prognostic factors, 15 to 20% of tumors present unfavorable features at diagnosis responsible for the most important burden of the disease (2).
The definition of high risk prostate cancer differs according to different risk classifications, but most commonly involves PSA level above 20 ng/mL, T3-T4 disease or Gleason score (GS) higher than 7. Main treatment options for this group of patients include radiation therapy (RT) plus androgen deprivation therapy (ADT) or radical prostatectomy, in selected cases.
RT has been used for decades in the treatment of PC; however, it is known that approximately one third of all patients with clinically localized disease treated with RT alone will present tumor recurrence within 5 years post-treatment (3). If we analyze high-risk disease only, these numbers are even more significant. Therefore, attempts to improve treatment results are essential for this group of patients. Possible alternatives to improve RT results include, among others, the use of higher doses of irradiation, and the use of agents, which optimize the radiation effect.
Since the publication of a Canadian-born physician, Dr. Charles B. Huggins, showing that prostate cells depend on androgen stimulation for their growth (4), the use of androgen blockade has been widely explored in the management of PC. The pharmacological androgen ablation has some advantages over the orchiectomy including the possibility of testosterone recovery after the medication is discontinued as well as the absence of possible psychological effects related to testicles removal. Currently, different classes of drugs are used to decrease the androgen levels including luteinizing hormone-releasing hormone (LHRH) agonists, which are the most commonly used, LHRH antagonists and anti-androgens. The use of estrogens in the form of diethylstilbestrol (DES) was associated to an increased risk of thromboembolic and cardiovascular events, therefore was practically abandoned as a means of androgen suppression (5,6).
Several phase 3 studies have proved the benefit of ADT for metastatic patients (7,8). In an attempt to improve results for non-metastatic disease, the use of ADT associated with RT has also been studied for several decades (9). The purpose of this article is to critically review main randomized trials on external beam RT combined with ADT for the treatment of high risk PC.
MATERIAL AND METHODS
Data for the present review were identified by a structured MEDLINE search up to May 31, 2010. The search was carried out by combining the terms "prostate cancer", "high risk", "radiotherapy", "hormones", "androgen deprivation", "dose-escalation", "randomized trial" and "phase 3". Only publications in English were considered. All randomized trials addressing the use of a LHRH agonist with external beam RT for non-metastatic, high risk localized PC (experimental arm) in comparison to RT alone (standard arm) were included and reviewed. A search for studies including external beam radiation therapy plus hormonal therapy versus hormonal therapy alone was also performed. Relevant abstracts from meetings were also considered for the analysis. Other options of hormonal suppression such as surgical castration (10) and oral estrogen therapy (9) were not included in this review because of their irreversible castration pattern and known toxicity profile, respectively. The single study using bicalutamide as the ADT method was also excluded from this analysis given that it was not a trial exclusively designed to compare RT versus RT and ADT but rather a study comparing bicalutamide alone versus the same plus a curative treatment (11,12). In order to assess data to support the use of ADT with higher doses of RT, randomized trials on dose-escalation were also reviewed (13-17).
For the purpose of this review we adopted the Genito Urinary Radiation Oncologists of Canada definition of risk stratification (18), as follows: low risk (< T2a, PSA < 10 ng/mL and GS < 7), intermediate risk (T2b-T2c, or PSA 10-20 ng/dL or GS 7) and high risk (> T3a, PSA > 20 ng/mL or GS > 7).
BIOLOGICAL BASIS FOR THE COMBINED TREATMENT
Several experimental studies have outlined the potential benefits from the combination of ADT with RT. A dose-response study by Zietman et al. (19,20) demonstrated that androgen deprivation in the form of orchiectomy reduces the dose of RT necessary to control 50% of the tumor (TCD50). In that study, nude mice bearing Shionogi adenocarcinoma allograft were treated with RT with or without orchiectomy at different time sequences. They reported that the combination of RT and ADT led to a better tumor control, and that timing of ADT plays an important role on the effectiveness of this combined therapy. Orchiectomy 12 days prior to RT (neoadjuvant) produced a significantly greater decline in the TCD50 than if performed during or after RT.
In another study, Kaminski et al. (21) also reported as well an increased overall tumor-cell killing in animal models and, in addition, a longer doubling time in the surviving PC cells after the neoadjuvant treatment. In their experiment, rats bearing Dunning rat PC cell lines were treated with RT and temporary ADT (orchiectomy followed by testosterone replacement) at different time sequences. Temporary ADT for 14 days before RT resulted in a statistically significant lengthening of tumor growth compared with RT given during the 14 days of ADT or when RT was given before the 14 days of ADT. This study hypothesizes a protracted effect on tumor growth after neoadjuvant ADT even after the androgen level is restored.
The effect of hypoxia on PC has been extensively studied in the recent years. It is known that PC low oxygen levels are associated with treatment failures and poor prognosis (22). Prostate tumors often have an erratic and inefficient pattern of vascularization, which leads to intermittent or chronic hypoxia (23). An inadequate tissue oxygenation is the prime trigger of angiogenesis by increasing several angiogenic factors including Vascular Endothelial Growth Factor (VEGF) and its receptors (24). Androgen deprivation has shown to down regulate VEGF expression causing apoptosis of endothelial cells and consequently decreased vascularization. Thus, ADT may play a role in an at least transient "normalization" of the tumor vascularization not only by reducing immature, leaky tumor vessels but also by the death of peri-vascular cells causing decreased interstitial pressure (25-27). In this area, measurements of vascular efficacy like microvascular density (28,29) and vascular morphology (30) have been shown to be promising predictors of clinical outcome. As result of ADT, decreased vascular resistance has been demonstrated using Doppler ultrasound (31,32). Milosevic et al. (33) studying 237 PC patients reported significant heterogeneity in prostate oxygenation, with a range of median pO2 from 0 to 75 mm Hg. In addition, they were the first authors to clinically prove that ADT increased PC oxygenation. Thus, despite being far from a complete understanding, effects on tissue vascularization and hypoxia seem to contribute importantly for the additive effect seen with the combined treatment.
The combination of ADT plus RT leads to an increased apoptotic cell-killing and improves both tumor vasculature and tissue oxygenation and this potential synergistic effect may explain the difference in outcomes from the surgical series.
Systemically, ADT may prevent the dissemination of micro-metastasis due to inhibition of DNA synthesis and cell proliferation as well as the increased apoptotic ratio (34). There is also some evidence of tumoricidal immune system response triggered by androgen suppression (35). Despite many preclinical trials providing theoretical basis for the ADT prescription, several mechanisms still lack further elucidation.
PHASE 3 TRIALS OF RADIATION THERAPY WITH OR WITHOUT ANDROGEN DEPRIVATION THERAPY
Most studies did not define treatment options according to risk categories, clustering patients together. In these situations, we calculated, whenever possible, the proportion of high risk group disease based on patient's characteristics information (clinical stage, PSA level and Gleason score) contained within the text of the article. For the purpose of this review, we have divided the trials into two groups: those starting hormonal treatment (HT) before RT (neoadjuvant trials) and trials testing the use of HT after RT (adjuvant trials). From these studies, emphasis will be given towards the group of patients with high risk features for treatment failure.
Neoadjuvant Trials
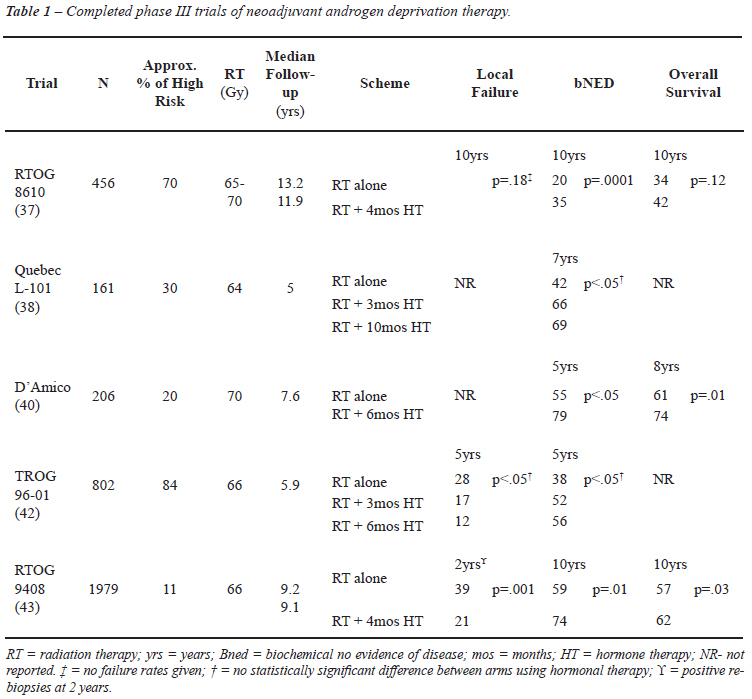
Five randomized trials directly compared the use of ADT before and during RT to RT alone (Table-1). Apart from being conducted in different eras, these trials differed in several other aspects including patient selection, HT scheduling and duration, RT delivery, and definition of end-points. These issues are discussed in detail below.
RTOG 8610
This study (36,37) was the first trial testing the hypothesis that short-term neoadjuvant ADT combined with RT could improve treatment outcomes in patients with locally advanced disease. From 1987 to 1991, 456 patients with bulky T2-T4 disease (> 5 x 5 cm of palpable tumor) were randomized to receive ADT with goserelin and flutamide for 2 months before and 2 months concomitantly with RT or to receive RT alone. The majority of patients included had high risk disease. Despite being conducted before the PSA era, for those 29% of the patients who had PSA measurement available, the median level pre-treatment was 26.3 ng/mL. After central pathological review, 66% of the patients were considered to have a Gleason score > 7. The 10-year overall survival (OS) was not statistically different between the combined treatment and the control group (42.6% vs. 33.8%, respectively, p = 0.12) (37). However, deaths resulting from PC at 8 and 10 years were significantly decreased with the short course of HT, 23% vs. 33% (p = 0.05) and 23% vs. 36% (p = 0.01), respectively. Of note, this positive outcome was based on a centrally histopathological review and not on the original institutional GS on which the randomization was based.
QUEBEC L-101
The Quebec L-101 study (38) randomly allocated 161 patients with clinical stage T2-T3 to RT alone, or to 3 months of neoadjuvant treatment prior to RT, or to ADT 3 months before, 2 months during, and 5 months after RT. Thirty percent of the patients had T3 tumors, 25% GS 7 or more, and median PSA values ranged from 9 to 12 ng/mL, thus the high risk population was only a minor part of this study. At a median follow-up of 5 years, the actuarial 7 year biochemical control was statistically better for both groups receiving ADT when compared to RT alone. However, there was no significant difference between both arms receiving ADT (p = 0.6).
D'Amico Study
D'Amico et al. (39,40) compared 6 months of total ADT (2 months before, during and after RT) to RT alone. Two hundred and six patients with clinical stage T1b-2bN0M0 and at least 1 unfavorable prognostic factor were enrolled to the study. Unfavorable prognostic factors were a PSA >10 ng/mL (maximum, 40 ng/mL), GS 7-10, evidence of extracapsular extension, or seminal vesicle invasion by endorectal magnetic resonance imaging. The majority of patients (79%) were classified as intermediate risk and the remaining as high risk PC. Those receiving RT with ADT had a statistically significant improved 8-year OS (74% vs. 61% %, p = 0.01). In a post randomization analysis of the study, the authors evaluated the benefit of ADT by risk group (41). Both patients in the intermediate and high risk groups had an improvement in overall survival, although for the latter group the difference was only marginally significant (p = 0.06). This result might be related to the small number of high risk patients, around 44, included in this trial.
TROG 9601
The Trans-Tasman Radiation Oncology Group (TROG) 9601 study (42) included about 83% of high risk patients. From 818 patients included, 38% had PSA > 20 ng/mL, 40% had T3-4 disease and 17% had GS > 8. Patients were randomized to RT alone, 3 months of total ADT and RT with neoadjuvant ADT starting 2 months before RT or 6 months of total ADT with neoadjuvant ADT starting 5 months before RT. In both arms using ADT, there were improvements in biochemical and local control. No statistically significant improvement in overall survival between treatment arms was shown; however, a trend of increasing benefit with increasing GS and PSA levels was observed. Six months of ADT significantly reduced the probability of cancer related deaths (HR = 0.56, 95% CI, 0.32 to 0.98, p = 0.04).
RTOG 9408
RTOG 9408 (43) has focused on whether a short course of ADT (same schema used in the RTOG 8610) improves OS in localized PC (T1b-T2b, PSA < 20 ng/mL and no involved nodes). Intermediate risk patients represented the majority of patients accrued (54%) followed by low risk (35%) and a minority of high risk patients (11%).
The addition of hormonal treatment to RT improved the 10 year overall survival from 57% to 62% (p = 0.03). However, on a hypothesis-generating subset analysis by risk category, no statistically significant improvement in OS or disease specific survival was found with the addition of 4 months of HT for the high risk patients. The small number of high risk patients included in this study and the short duration of the HT may explain the lack of benefit for these patients.
In these previous trials, some differences in the RT scheme are important to highlight. They differed significantly in terms of clinical target volume (CTV). In RTOG studies 8610 and 9408 (37,43), RT was typically delivered electively to the whole pelvis to a dose of 44-46 Gy with the prostatic target volume boosted to a total dose of 65 to 70 Gy. In the TROG 9601 (42) and D'Amico et al. studies (40), pelvic lymph nodes were clearly not included in the CTV. In the TROG 9601 study, RT was delivered to the prostate and seminal vesicles to a total dose of 66 Gy, while in the D'Amico et al. trial, 45 Gy was given to the prostate and seminal vesicles followed by 22 Gy to the prostate volume alone.
Also, different criteria for biochemical failure post-RT have been used, making direct comparison between series very difficult, if not inappropriate. The only trials using the recently adopted "PSA nadir + 2 ng/mL" Phoenix criteria (44) were the TROG 9601 and RTOG 9408.
In summary, the use of neoadjuvant HT in combination with RT has consistently shown improvements in different clinical outcomes. Two randomized trials have unequivocally shown overall survival benefit for the neoadjuvant treatment; however, they only included a minor portion of high risk patients, different end-points were used and treatments varied significantly among studies making an objective interpretation of the results rather difficult. From the available data, it appears that short-term hormonal therapy, when combining with RT, is inadequate therapy for high risk PC patients.
Adjuvant Trials
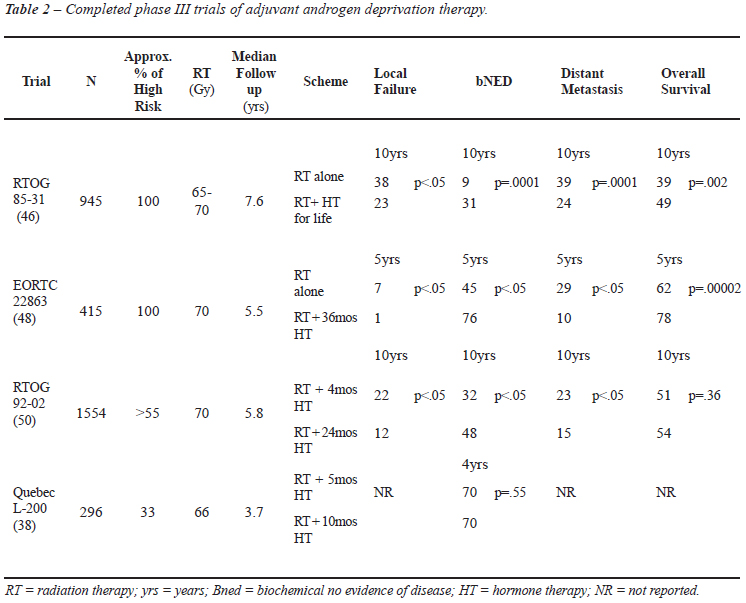
Four randomized trials compared adjuvant HT post-RT to no further treatment (38,45-50). Table-2 depicts in detail these studies. One trial using bicalutamide as the HT means (11) was not included in this analysis. Similarly to the previously described studies in the neoadjuvant setting, trials with adjuvant HT also differ in several aspects.
EORTC 22863
In the European Organization for Research and Treatment of Cancer (EORTC) 22863 study, 415 patients with T1-2 World Health Organization (WHO) histopathological grade 3 (9%) or T3-4 any histopathological grade, N0-1 (91%) were randomized to RT alone versus RT plus concurrent and adjuvant ADT. Patients received HT on the first day of RT, for 36 months. Ciproterone, an antiandrogen agent, was given for one month prior to ADT. Given the eligibility criteria, it is fair to assume that most patients were in the high risk category. This was the first study to show a survival benefit with the combined treatment for locally advanced PC. At a median follow-up of 66 months, in patients receiving ADT for 36 months the 5-year survival rate was 78% compared to 62% in the RT group (p = 0.0002) [hazard ratio of 0.51 (95% CI 0.36-0.73)].
RTOG 9202
RTOG 9202 randomized 1554 patients to receive 24 months of ADT or no further treatment after 2 months of neoadjuvant HT and 2 months of HT given concomitantly with RT. Patients with T2c-T4 disease, N0-NX, and PSA < 150 ng/mL were included. Although the use of 24 month HT after neoadjuvant and concurrent HT showed significant improvement in biochemical control, disease-specific and disease-free survival rates, there was no significantly improved 10-year OS compared to neoadjuvant and concurrent HT for the whole group.
An unmistakable analysis of results based on risk categories is obviously not possible to be performed given the lack of risk stratification in the study. However, we estimated that, at least, 55% of the patients had high risk disease considering that 55% had T3-T4 disease and 33% had PSA levels higher than 30 ng/mL at presentation. In a subgroup analysis, patients with a GS > 8 (30%) had a significant improvement in OS at 10 years (31% vs. 45%, p = 0.0061). Nevertheless, it is important to keep in mind the potential limitations of a subgroup analysis.
The lack of survival benefit for all patients included in the RTOG 9202 study may be due to the large number of patients with GS < 7 (70%) and/or the shorter duration of HT compared to the EORTC 22863 trial.
RTOG 8531
RTOG 8531 randomized 945 patients with clinical stage T3 (57%), extracapsular or seminal vesicle involvement post-operatively (15%) or patients with nodal disease (28%) to RT alone versus RT and adjuvant ADT, starting in the last week of RT, and given indefinitely or until evidence of disease progression. Although the exact percentage of high risk patients cannot be determined, the vast majority of the patients had high risk features (they had to have T3 disease or T1-T2 with nodal metastasis). The long-term results of the RTOG 8531 study (46) confirmed the significant improvement in disease-specific survival and overall survival for patients receiving the combined treatment. Additionally, subgroup analysis of node-positive patients (n = 173) has shown statistically significant improvement in 5-year overall survival and cause specific survival to be replaced by estimated progression-free survival (51). Of note, although ADT was supposed to be taken for life, its median duration was only 2.2 years (52).
QUEBEC L-200
Quebec- L200 study (38) compared neoadjuvant and concomitant ADT (total of 5 months) to neoadjuvant, concomitant and a short course of adjuvant ADT (total of 10 months). Two hundred ninety six patients with T2-T3 disease were eligible. Fifteen percent were T3, 30% had GS > 7 and 24% had PSA level higher than 20 ng/mL. Thus, at least one third of these patients are likely to be in the high risk category. At a median follow-up of 3.7 year, the 4 year biochemical control was 70% and there was no statistical difference between the 2 groups (p = 0.54). Overall survival data was not presented. Unfortunately an analysis based on risk stratification was not carried out by the authors.
All these adjuvant trials were not importantly different in terms of RT dose prescription. In the RTOG 8531, RTOG 9202, and EORTC 22863 all patients were planned to receive pelvic irradiation to a total dose of 44 to 46 Gy followed by a boost of 20 to 25 Gy to the prostate, thus achieving a total dose of 70 Gy. A lower total dose of 64 Gy was delivered in the Quebec-L200 study using field sizes of 8 x 8 cm to 10 x 10 cm.
The mortality for all causes was significantly reduced in two trials, EORTC 22863 and RTOG 8531 (Table-2). RTOG 8531, EORTC 22863 and RTOG 9202 have shown significant reduction in biochemical, local and distant failure rates with the use of HT and RT. These trials were not specifically designed for the high risk population; however subgroup analysis in some of these studies showed that the adjuvant benefit was evident for these patients.
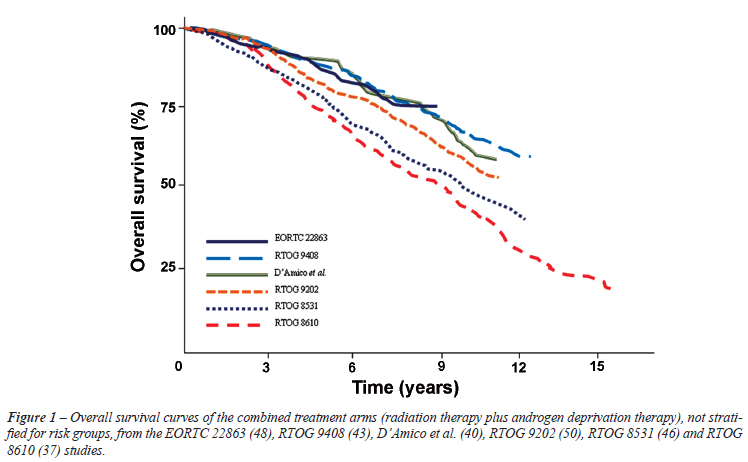
All studies taken together, overall survival rates from neoadjuvant and adjuvant trials reflect an important heterogeneity in terms of patients' selection, timing and duration of HT (Figure-1).
Optimal Duration of Hormonal Therapy
With regards to the neoadjuvant treatment, the optimal duration of the HT and the timing of RT are uncertain. In most studies, RT begins 2 to 3 months after institution of ADT. In a multicenter Canadian trial (53,54), 378 men with low-risk (n = 98), intermediate-risk (n = 163), or high-risk (n = 117) localized disease were randomized to conventional dose RT (66 Gy) with either 3 or 8 months of neoadjuvant ADT. At a median follow-up of 6.6 years, disease-free survival, OS, and patterns of failure were similar in both groups. However, 8 months of neoadjuvant ADT was associated with a significant prolongation of 7-year disease-free survival rate for men with high-risk disease (59% versus 33%; p = 0.01). An update of the Canadian study (55) shows that the biochemical response to neoadjuvant ADT before RT, and not duration of HT, appears to be the most critical determinant of benefit in the setting of the combined therapy. Men achieving a PSA < 0.1 ng/mL before RT seem to have a significantly higher biochemical control compared to those whose PSA pre-RT is > 0.1 ng/mL (55.3% vs. 49.4%, p = 0.014).
A recent secondary analysis of the RTOG 9413 study (56), which randomized patients to short course neoadjuvant and concomitant HT plus RT versus short course adjuvant HT shows that those who reached a PSA level < 0.3 ng/mL at completion of HT had an improved cancer-specific survival when compared to patients with a PSA > 0.3 ng/mL, regardless of the sequencing of the HT (F. Cury, personal communication, April, 2010). This study included more than 70% of patients with high risk features. De Crevoisier et al. (2) also reported similar findings to the predictive value of the PSA decline prior to RT. For high risk patients, a PSA < 0.2 ng/mL after 3 months of neoadjuvant ADT predicted higher rates of biochemical and clinical control. These findings are in agreement with the Canadian trial and if this provocative finding proves to be real, the HT duration may be individually tailored to the PSA nadir, avoiding unnecessary hormone-related toxicities and costs.
The optimal duration of adjuvant HT after RT is also under investigation. A recent study by the EORTC (57) compared, on a non-inferiority design, RT plus 36 months of ADT versus the same RT plus 6 months of ADT for high risk patients. Good performance patients (970 evaluable) with T1c-T2b stage, pathological nodal stage N1-N2, and no clinical evidence of metastatic spread (M0) or with clinical tumor stages T2c-T4, clinical nodal stages N0-N2, and no clinical evidence of metastatic spread were enrolled. After a median follow-up of 6.4 years, the 5-year overall mortality and prostate-specific mortality for short-term vs. long-term suppression was 19.0% vs. 15.2% and 4.7% vs. 3.2%, respectively. Despite the relatively short follow-up period, these results are important and appear to confirm the importance of long-term HT for high risk patients.
A challenging, hypothesis-generating secondary analysis of the RTOG 8531 study (52) reported that prolonged HT with LHRH agonist for more than 5 years might be associated with improved outcomes in patients with locally advanced localized PC, as compared to a shorter duration. D'Amico et al. (58), in a non-randomized fashion, compared short versus long-term HT from a pooled analysis of patients enrolled in three prospective randomized trials and treated either with 36 or 6 months of androgen suppression and pelvic RT. They concluded that the longer use of hormonal therapy was not associated with increased survival.
Quebec PCS IV (Principal investigator: Dr. A. Nabid) is a recently completed study that randomized over 600 patients with high risk disease to 18 versus 36 months of HT, both arms receiving RT. The forthcoming results of the Quebec trial together with those of the ongoing RADAR study (59) that compares 6 to 18 months of HT with will hopefully shed further light on this intriguing and important scheduling question.
HORMONAL THERAPY ALONE FOR HIGH RISK DISEASE
After the positive results from EORTC 22836 with the use of long-term adjuvant HT in combination with RT, some investigators questioned the real value of RT in these patients and hypothesized that perhaps the major benefits achieved was in fact related to the use of HT alone. To determine whether long-term HT alone would be as effective as the combination of HT and RT, 3 randomized trials addressing this issue have been completed and are described below.
In 1996, the Scandinavian Prostate Cancer Group and the Swedish Association for Urological Oncology started a phase III trial exploring the role of local RT in addition to HT in patients with high-risk disease (60). They randomized patients to ADT alone or RT plus ADT. The HT consisted of 3 months of total androgen blockade followed by flutamide until progression or death. After a median follow-up time of 7.6 years, prostate specific mortality at 10 years, the primary endpoint of the study, was 24% in the ADT alone group versus 12% in the combined treatment group (95% CI 4.9-19.1%, p < 0.0001). The overall mortality at 10 years was 39.4% in the ADT alone group and 30% in the combined treatment group (0.8-18.8%, p = 0.004). The biochemical control at 10 year was also significantly higher in the combined group (74.7% vs. 25.9%, p < 0.0001).
Two other studies recently reported in abstract form have as well tested the impact of ADT alone in the treatment of high risk disease (61,62). In the Canadian PR.3 trial (61), the primary endpoint was OS. High risk patients were randomized to lifelong ADT (bilateral orchiectomy or LHRH agonist) with or without RT. After a median follow-up of 6 years, the risk of death was significantly reduced in the combined treatment group (hazard ratio [HR] 0.77, 95% CI 0.61-0.98, p = 0.033). The prostate cancer specific mortality at 10 years was 15% with the combined treatment and 23% with ADT alone (p < 0.05). In a French study (62), instead of lifelong ADT, 3 years was used in both groups (plus or minus RT). With a median follow-up of 5.6 years, the 5-year progression-free survival (biochemical or clinical progression) was 61% for the combined treatment group vs. 8.5% for ADT alone (p < 0.001). Clinical progression, including loco-regional and systemic disease, was significantly reduced when RT was added to hormonal deprivation. The effect in overall survival was not reported likely due to the short follow-up.
In summary, the current body of evidence from 3 randomized trials clearly demonstrated the significant benefits of RT when combined with HT in the treatment of high risk patients. The use of ADT alone, regardless of treatment duration or method, has been shown to confer inferior OS, biochemical control and clinical progression free survival when compared to the combination and should not be recommended as a primary therapy for patients with high risk disease. Considering that the RT doses used in these studies were lower than current standards, it is even possible that a greater benefit in disease control may be seen when modern RT is delivered to such patients.
ANDROGEN DEPRIVATION THERAPY FOR HIGH RISK PROSTATE CANCER IN THE DOSE ESCALATION CONTEXT
Trials previously described, comparing RT to RT plus ADT, used RT doses which are now considered suboptimal local therapy, particularly in terms of PSA control. Several randomized trials (13-17,63) indicate that RT dose-escalation provides better biochemical control rates than conventional RT doses (< 74 Gy). However, the role of hormonal treatment in this context is poorly explored. The only trials in which ADT was allowed were the Medical Research Council (MRC) (16) and a Dutch trial (14).
The MRC study (16) randomized 843 patients to receive a standard dose (64 Gy) or escalated-dose RT (74 Gy) and included neoadjuvant and concomitant ADT for 3 to 6 months to all patients. The 5-year biochemical control for the entire cohort was signi?cantly improved in the high-dose arm (71% vs. 60%, p = 0.0007). Of the whole group, 362 patients (43%) were in the high-risk group. The 5-year biochemical control for high-risk PC treated with high-dose versus low-dose were 57% versus 43%, respectively (HR = 0.6, 95% CI = 0.44-0.81).
The Dutch trial (14) randomized 664 patients with localized PC to receive either 68 Gy or 78 Gy. Of those, 362 patients (55%) were high risk. Of these, 125 patients (34%) received 6 or 36 months of HT. Of the whole group, the 6-year biochemical failure rate was improved from 51% in the low-dose arm to 63% in high-dose arm (p = 0.04). In the subgroup analysis, the difference between both arms was not statistically signi?cant in the high-risk group (49% vs. 40%, p = 0.15). Considering that in the high-dose arm 11% received a dose lower than the protocol dose of 78 Gy, the authors divided patients in two non-overlapping dose-groups: patients who received < 73 Gy and those who received > 73 Gy (64). Of the high-risk group, 125 patients (35%) received HT. The 6-year actuarial failure rate in high-risk patients who received HT and escalated-dose RT (> 73 Gy) was lower than in patients who were treated with HT and conventional-dose RT (< 73 Gy), although not statistically significant (66% vs. 50%, respectively, p = 0.07).
Whether dose-escalation would increase even further the magnitude of the HT benefit or, on the other hand, obviate the need of ADT remains an unanswered question. Results of randomized trials on the real benefit of HT in combination with high dose RT will be forthcoming from the on-going studies.
TOXICITY
Androgen deprivation has been associated with numerous side-effects including sexual dysfunction, gynecomastia, bone mineral loss, anemia, fatigue, muscular pain, hot-flashes, metabolic complications and potentially increased cardiovascular events (65-68). The latter is a controversial issue receiving increasing attention in the decision-making process.
Some retrospective studies with large cohorts have reported increased risks of both cardiovascular events and incident diabetes with the use of ADT (69,70). Tsai et al. (71) using CaPSURE database demonstrated increased risk of cardiovascular events for patients receiving ADT in the prostatectomy context, but interestingly not for patients who underwent RT. A recent study from Ontario with more than 19,000 users of ADT found an increased risk of diabetes but not an excess risk of myocardial infarction or sudden cardiac death (72). A combined analysis of 3 randomized trials published by D'Amico et al. (73) showed that in men over 65 years the use of short term ADT had not changed the overall rate of cardiac events; however the time to develop fatal myocardial infarction was decreased. This study is limited by the small number of events, 51 myocardial infarctions. Another single institution study has recently presented data on increased all-cause mortality for patients receiving neoadjuvant ADT and who had pre-existing heart failure or a history of myocardial infarction (74). This is likely the subgroup of patients that requires specific counseling when ADT is being considered.
Reanalyzes of the RTOG randomized trials 9202 (75), 8610 (37), 8531 (75) and 9408 (35) as well as the EORTC 22961 (59) have not shown any significant difference in cardiovascular mortality between experimental and control arms. However, these studies might be underpowered to detect difference for this end-point, contributing for the current uncertainty on the issue.
Undoubtedly, there is a need to clarify whether a direct causal relationship between ADT and cardiovascular disease exists, what are the mechanisms involved and whether the risk is maintained after cessation of the ADT. Despite all conflicting data, patients should be advised for early screening and detection of insulin resistance, diabetes, hyperlipidemia and hypertension and oriented towards maintenance of a healthy diet and regular physical activity.
CONCLUSION
For patients with high risk disease, there is level 1 evidence supporting the combination of RT and long-term HT. Results of randomized trials suggest that the addition of ADT improves major outcomes including overall survival; therefore, for those high risk patients who are candidates to RT, the combined approach is currently considered the standard of care. The optimal duration of the HT in this population is yet to be determined. Taking all results together, it is justifiable to use HT for at least 24 months, starting 2-3 months before RT. The differences in survival favoring the combined approach range from 5 to 16%. Even though statistically significant, these differences are modest and one has to consider the impact the combined therapy may have on quality of life and the costs to the patient and to the health system. One way to measure the magnitude of the benefit is by calculating the number of patients needed to treat (NNT) in order to benefit one single patient. Considering overall survival as the endpoint, NNTs calculated from the studies included in this analysis ranged from 8 to 20. In the context of oncology in general, these numbers can be considered adequate and are indeed superior to many already accepted standard cancer treatments.
Notably, randomized trials included patients with diverse risk groups treated with older RT modalities, a variety of HT scheduling and duration and, importantly, suboptimal RT doses. The use of HT with higher doses of RT has to be properly assessed.
In conclusion, all these issues, including increasing evidence of cardiovascular toxicity related to the long-term ADT, have to be prospectively evaluated. The results of ongoing randomized trials addressing these topics will hopefully clarify most of these uncertainties.
CONFLICT OF INTEREST
Dr. Dal Pra is an Astra Zeneca Genito-Urinary Fellow.
Accepted after revision: September 27, 2010
EDITORIAL COMMENT
This is a comprehensive review of a clinically important controversy about high risk prostate cancer where, to rephrase the question asked by Willet F Whitmore, cure is certainly necessary, but may not be possible. The review is well written and generally well balanced, and the authors should be congratulated on critically exploring the controversies about combination treatment for high risk prostate cancer.
The authors discuss the possible biological basis for the use of combined radiotherapy (RT) and androgen deprivation therapy (ADT). However, this discussion is incomplete without reference to the enigmatic phenomenon that neoadjuvant ADT prior to radical prostatectomy (RP) is not more effective than RP alone. Several studies have shown that neoadjuvant ADT prior to RP reduces the rate of positive surgical margins, but does not lead to greater long-term disease-free or overall survival after RP.
Possible explanations are that RP is equally effective in eradicating cancer in the prostate, whether neoadjuvant ADT has been given or not, whereas ADT is not effective in eradicating microscopic systemic disease present at surgery, so that eventual disease progression occurs regardless of whether neoadjuvant ADT was given before RP or not.
Since the effect of neoadjuvant ADT on systemic disease present at the time of treatment must be the same for RP and RT, this must mean that RT is not as effective as RP in eradicating cancer from the prostate, but that the effect of RT on cancer cells in the prostate and pelvis is enhanced by ADT, which is responsible for the improved long-term outcome. It remains an intriguing enigma why neoadjuvant ADT should prove beneficial when followed by RT, but not RP.
Although survival rates without evidence of biochemical, local or metastatic recurrence are important for the purpose of evaluating treatment efficacy, from the individual patient's point of view, it is only overall survival that is important. In prostate cancer, because of its generally indolent nature, this means evaluating 10-15-years overall survival.
Especially in view of the long-term adverse effects of ADT and its possible contribution to non-cancer causes of mortality, it is important to critically evaluate the effect of ADT on overall survival. The importance of "significant" p-values is highly overrated, because statistical significance is often determined more by the size of the study population than by the real size of the difference between treatment effects. It is equally (or perhaps more) important to consider the clinical significance of outcome differences.
Looking at the 5 randomized trials of RT without or with neoadjuvant ADT (Table-1 in the paper), two trials did not report on overall survival. The 10-year overall survival was 34% vs. 42% in RTOG86-10, and 57% vs. 62% in RTOG9408, with high-risk cancer being present in 70% and 84% of study patients, respectively. The 8-year overall survival was 61% vs. 74% in the D'Amico et al. study, in which 20% of patients had high risk cancer. This shows that treatment differences were, at best, rather modest. In the two studies with predominantly high risk cancer, the real difference between treatment arms was 8 and 5 percentage points, respectively, and in the study with predominantly intermediate risk cancer, the difference was 13 percentage points.
Looking at the 4 randomized trials of RT alone compared with adjuvant ADT and RT (Table-2), one study did not report overall survival, and in one the 10-year overall survival was 51% vs. 54% (not statistically significant). The 10-year overall survival was 39% vs. 49% in study RTOG85-31, and the 5-year overall survival was 62% vs. 78% in study EORTC 22863 (both had 100% of patients with high risk cancer). Once again, the treatment differences are rather modest, especially when the financial costs and quality-of-life (QoL) consequences of long-term ADT are considered. This raises some doubt as to the real magnitude of the clinical benefit of ADT combined with RT, and whether the cost-benefit ratio justifies routine recommendation of combined treatment.
Alexander et al. (1) showed that the biochemical response to neoadjuvant ADT before RT, and not the duration of ADT, appears to be the most critical determinant of benefit in the setting of combined therapy. De Crevoisier et al. presented similar findings (2). In their study, in patients who received ADT plus RT, the median PSA prior to ADT was 18.2 ng/mL, and after 3 months of ADT it was 1.3 ng/mL. This shows a quite rapid and dramatic overall PSA response after just 3 months of ADT, before the initiation of RT. The authors found an "undetectable" PSA (< 0.2 ng/mL) in 12% of patients, and in this group the 10-year prostate cancer specific survival rate was 100%.
It is generally accepted that an undetectable PSA (< 0.1 ng/mL) 3 months after RP indicates that all cancer cells have been eradicated. The findings in the paper by De Crevoisier et al. suggest that in some patients 3 months of ADT may be "as effective" as RP in eradicating prostate cancer. This raises the question whether RT is necessary in patients with PSA < 0.2 ng/mL after 3 months of ADT.
The important message of this review should be that RT alone is not sufficient as intended curative therapy for high risk prostate cancer, therefore neoadjuvant and/or adjuvant ADT (probably long-term or lifelong) is required to improve the results. An important, unanswered question concerns the role of RP for high risk cancer, combined with adjuvant RT and/or ADT for disease recurrence after surgery, and the cost-benefit ratio and QoL outcomes of RP compared with RT. There is a deplorable lack of prospective, randomized studies comparing RP vs. RT in the management of localized or high-risk prostate cancer. Until such studies have been completed with adequate follow-up, the controversy about the optimal treatment of high risk prostate cancer will continue.
In the Abstract the authors state that the combined use of RT and ADT for patients with localized high risk prostate cancer is commonly accepted as the standard treatment among uro-oncologists. They moderate this statement by pointing out that there are negative and inconclusive studies with regard to overall survival, and that the financial and QoL issues have not been adequately evaluated. Moreover, the role of RP rather than RT as primary treatment modality for high risk cancer, combined with ADT prior to or after disease recurrence, remains undefined, and prospective, randomized studies are urgently needed to address this issue.
REFERENCES
Dr. C. F. Heyns
Department of Urology
Univ. of Stellenbosch and Tygerberg Hospital
Tygerberg, South Africa
E-mail: cfh2@sun.ac.za
EDITORIAL COMMENT
This well organized review article revealed that there are evidences that the combination of radiation treatment (RT) and hormone treatment (HT) results in increased biochemical disease-free survival, increased local control, reduced incidence of distant metastases, and reduced cause-specific mortality in patients with high risk disease. A lot of randomized controlled trials reported the benefit of adding HT to RT. Interestingly, the effect of hypoxia on RT has been studied by many researchers, and it is important point in multimodal treatment of prostate cancer.
There are several limitations of the present studies, most of the studies are retrospective, and have relatively short follow-up. Also, although most patients were treated uniformly, there was some variation in terms of definition of high risk disease and duration of HT used. As the authors mentioned in the manuscript, we need to study about optimal HT schedule and duration prospectively.
REFERENCES
Dr. Taek Won Kang
Department of Urology
Chonnam National University Med. Sch.
Gwangju, Republic of Korea
E-mail: sydad@hanmail.net
REPLY BY THE AUTHORS
We appreciate and thank Drs. Heyns and Kang for their thoughtful comments on our article (1) aimed at studying high-risk prostate cancer outcomes following radiation therapy (RT) and androgen deprivation therapy (ADT).
We fully agree with Dr. Heyns that the lack of long-term benefit on the use of neo-adjuvant hormonal therapy prior to radical prostatectomy (RP) remains an intriguing phenomenon. However, one has to keep in mind that some of the randomized surgical trials were underpowered and involved a broad range of patients, including men with low- and intermediate-risk prostate cancer. Moreover, neoadjuvant hormonal therapy has been shown to lead to morphological pathological changes that may result in underdetection of positive margins and capsular involvement by prostatic adenocarcinoma. Bazinet and colleagues (2) found more extensive intracapsular, capsular and extracapsular tumor involvement and higher rate of positive margins when cytokeratin immunohistochemistry was used as compared to hematoxylin-eosin staining. Thus, the improved pathological effect observed in the neoadjuvant surgical trials may be an artifact due to an underestimation of residual tumor and underreporting of positive margins. Finally, direct comparisons of neoadjuvant ADT in surgical and RT series is rather difficult, if not inappropriate, considering important differences in patient selection.
Individualized treatments for high-risk disease with possible omission of RT according to biochemical response to ADT would certainly be an interesting approach if we had no convincing level 1 evidence from three randomized trials addressing hormonal therapy (HT) alone versus HT plus RT (3-5). Regardless of HT duration or method, HT alone confers significant lower rates of biochemical control, overall survival and clinical progression-free survival when compared to the combined treatment. Solberg et al. (6), from the Scandinavian Prostate Cancer Group, has recently shown improved local control through prostate gland biopsies in the combined treatment (78% vs. 34%, p < 0.0001). In addition, the delivery of higher RT doses using modern RT techniques might even potentiate further these outcomes.
REFERENCES
The Authors
- 1. Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G: The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 2008; 15: 3866-71.
- 2. Greene KL, Cowan JE, Cooperberg MR, Meng MV, DuChane J, Carroll PR, et al.: Who is the average patient presenting with prostate cancer? Urology. 2005; 66(5 suppl): 76-82.
- 3. Kuban DA, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA, et al.: Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys. 2003; 57: 915-28.
- 4. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002; 168: 9-12.
- 5. de Voogt HJ, Pavone-Macaluso M, Smith PH, de Pauw M, Suciu S: Lessons from phase III-trials on the hormonal treatment of prostatic cancer. I: Results of EORTC-trials 30761 and 30762. Prog Clin Biol Res. 1988; 260: 111-7.
- 6. [No authors listed]: Management of advanced cancer of prostate and bladder. Proceedings of a symposium on the tenth anniversary of EORTC, Leeds, England, October 3rd, 1986 and the Fifth Course in Urological Oncology, Erice, Sicily, November 28-December 4th, 1986. Prog Clin Biol Res. 1988; 260: 1-658.
- 7. [No authors listed]: Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet. 2000; 355: 1491-8.
- 8. [No authors listed]: Maximum androgen blockade in advanced prostate cancer: an overview of 22 randomised trials with 3283 deaths in 5710 patients. Prostate Cancer Trialists' Collaborative Group. Lancet. 1995 Jul 29; 346: 265-9.
- 9. Zagars GK, Johnson DE, von Eschenbach AC, Hussey DH: Adjuvant estrogen following radiation therapy for stage C adenocarcinoma of the prostate: long-term results of a prospective randomized study. Int J Radiat Oncol Biol Phys. 1988; 14: 1085-91.
- 10. Granfors T, Modig H, Damber JE, Tomic R: Long-term followup of a randomized study of locally advanced prostate cancer treated with combined orchiectomy and external radiotherapy versus radiotherapy alone. J Urol. 2006; 176: 544-7.
- 11. See WA, Tyrrell CJ; CASODEX Early Prostate Cancer Trialists' Group: The addition of bicalutamide 150 mg to radiotherapy significantly improves overall survival in men with locally advanced prostate cancer. J Cancer Res Clin Oncol. 2006; 132(suppl 1): S7-16.
- 12. Tyrrell CJ, Payne H, See WA, McLeod DG, Wirth MP, Iversen P, et al.: Bicalutamide ('Casodex') 150 mg as adjuvant to radiotherapy in patients with localised or locally advanced prostate cancer: results from the randomised Early Prostate Cancer Programme. Radiother Oncol. 2005; 76: 4-10.
- 13. Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al.: Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008; 70: 67-74.
- 14. Peeters ST, Heemsbergen WD, Koper PC, van Putten WL, Slot A, Dielwart MF, et al.: Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006; 24: 1990-6.
- 15. Sathya JR, Davis IR, Julian JA, Guo Q, Daya D, Dayes IS, et al.: Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005; 23: 1192-9.
- 16. Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, et al.: Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007; 8: 475-87.
- 17. Zietman AL, DeSilvio ML, Slater JD, Rossi CJ Jr, Miller DW, Adams JA, et al.: Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005; 294: 1233-9. Erratum in: JAMA. 2008; 299: 899-900.
- 18. Lukka H, Warde P, Pickles T, Morton G, Brundage M, Souhami L, et al.: Controversies in prostate cancer radiotherapy: consensus development. Can J Urol. 2001; 8: 1314-22.
- 19. Zietman AL, Prince EA, Nakfoor BM, Park JJ: Androgen deprivation and radiation therapy: sequencing studies using the Shionogi in vivo tumor system. Int J Radiat Oncol Biol Phys. 1997; 38: 1067-70.
- 20. Zietman AL, Prince EA, Nakfoor BM, Shipley WU: Neoadjuvant androgen suppression with radiation in the management of locally advanced adenocarcinoma of the prostate: experimental and clinical results. Urology. 1997; 49(3A Suppl): 74-83.
- 21. Kaminski JM, Hanlon AL, Joon DL, Meistrich M, Hachem P, Pollack A: Effect of sequencing of androgen deprivation and radiotherapy on prostate cancer growth. Int J Radiat Oncol Biol Phys. 2003; 57: 24-8.
- 22. Stewart GD, Ross JA, McLaren DB, Parker CC, Habib FK, Riddick AC: The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int. 2010; 105: 8-13.
- 23. Jain RK: Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005; 307: 58-62.
- 24. Folkman J: Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995; 333: 1757-63.
- 25. Fukumura D, Jain RK: Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007; 74: 72-84.
- 26. Joseph IB, Isaacs JT: Potentiation of the antiangiogenic ability of linomide by androgen ablation involves down-regulation of vascular endothelial growth factor in human androgen-responsive prostatic cancers. Cancer Res. 1997; 57: 1054-7.
- 27. Joseph IB, Nelson JB, Denmeade SR, Isaacs JT: Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin Cancer Res. 1997; 3: 2507-11.
- 28. Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J: Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993; 143: 401-9.
- 29. Borre M, Offersen BV, Nerstrøm B, Overgaard J: Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer. 1998; 78: 940-4.
- 30. Mucci LA, Powolny A, Giovannucci E, Liao Z, Kenfield SA, Shen R, et al.: Prospective study of prostate tumor angiogenesis and cancer-specific mortality in the health professionals follow-up study. J Clin Oncol. 2009; 27: 5627-33.
- 31. Okihara K, Watanabe H, Kojima M: Kinetic study of tumor blood flow in prostatic cancer using power Doppler imaging. Ultrasound Med Biol. 1999; 25: 89-94.
- 32. Huang SF, Chang RF, Moon WK, Lee YH, Chen DR, Suri JS: Analysis of tumor vascularity using three-dimensional power Doppler ultrasound images. IEEE Trans Med Imaging. 2008; 27: 320-30.
- 33. Milosevic M, Chung P, Parker C, Bristow R, Toi A, Panzarella T, et al.: Androgen withdrawal in patients reduces prostate cancer hypoxia: implications for disease progression and radiation response. Cancer Res. 2007; 67: 6022-5.
- 34. Isaacs JT, Lundmo PI, Berges R, Martikainen P, Kyprianou N, English HF: Androgen regulation of programmed death of normal and malignant prostatic cells. J Androl. 1992; 13: 457-64.
- 35. Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, et al.: Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004; 173: 6098-108.
- 36. Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P, et al.: Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001; 50: 1243-52.
- 37. Hirsch FR, Hansen HH, Hansen M, Osterlind K, Vindeløv LL, Dombernowsky P, et al.: The superiority of combination chemotherapy including etopside based on in vivo cell cycle analysis in the treatment of extensive small-cell lung cancer: a randomized trial of 288 consecutive patients. J Clin Oncol. 1987; 5: 585-91.
- 38. Laverdiere J, Nabid A, De Bedoya LD, Ebacher A, Fortin A, Wang CS, et al.: The efficacy and sequencing of a short course of androgen suppression on freedom from biochemical failure when administered with radiation therapy for T2-T3 prostate cancer. J Urol. 2004; 171: 1137-40.
- 39. D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW: 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004; 292: 821-7.
- 40. D'Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW: Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008; 299: 289-95.
- 41. Nguyen PL, Chen MH, Beard CJ, Suh WW, Renshaw AA, Loffredo M, et al.: Radiation with or without 6 months of androgen suppression therapy in intermediate- and high-risk clinically localized prostate cancer: a postrandomization analysis by risk group. Int J Radiat Oncol Biol Phys. 2010; 77: 1046-52.
- 42. Denham JW, Steigler A, Lamb DS, Joseph D, Mameghan H, Turner S, et al.: Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005; 6: 841-50.
- 43. McGowan D, Hunt D, Jones C, Amin M, Leibenhaut M, Husian S, et al.: Short-term Endocrine Therapy Prior to and during Radiation Therapy Improves Overall Survival in Patients with T1b-T2b Adenocarcinoma of the Prostate and PSA = 20: Initial Results of RTOG 94-08. Int J Radiat Oncol Biol Phys. 2010; 77: 1. Abstract: # LB 1.
- 44. Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al.: Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006; 65: 965-74.
- 45. Pilepich MV, Caplan R, Byhardt RW, Lawton CA, Gallagher MJ, Mesic JB, et al.: Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol. 1997; 15: 1013-21.
- 46. Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, wr al.: Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005; 61: 1285-90.
- 47. Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al.: Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997; 337: 295-300.
- 48. Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al.: Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002; 360: 103-6.
- 49. Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V, et al.: Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003; 21: 3972-8. Erratum in: J Clin Oncol. 2004; 22: 386.
- 50. Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, et al.: Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008; 26: 2497-504.
- 51. Lawton CA, Winter K, Grignon D, Pilepich MV: Androgen suppression plus radiation versus radiation alone for patients with stage D1/pathologic node-positive adenocarcinoma of the prostate: updated results based on national prospective randomized trial Radiation Therapy Oncology Group 85-31. J Clin Oncol. 2005; 23: 800-7. Erratum in: J Clin Oncol. 2005; 23: 8921.
- 52. Souhami L, Bae K, Pilepich M, Sandler H: Impact of the duration of adjuvant hormonal therapy in patients with locally advanced prostate cancer treated with radiotherapy: a secondary analysis of RTOG 85-31. J Clin Oncol. 2009; 27: 2137-43.
- 53. Crook J, Ludgate C, Malone S, Lim J, Perry G, Eapen L, et al.: Report of a multicenter Canadian phase III randomized trial of 3 months vs. 8 months neoadjuvant androgen deprivation before standard-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004; 60: 15-23.
- 54. Crook J, Ludgate C, Malone S, Perry G, Eapen L, Bowen J, et al.: Final report of multicenter Canadian Phase III randomized trial of 3 versus 8 months of neoadjuvant androgen deprivation therapy before conventional-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009; 73: 327-33.
- 55. Alexander A, Crook J, Jones S, Malone S, Bowen J, Truong P, et al.: Is biochemical response more important than duration of neoadjuvant hormone therapy before radiotherapy for clinically localized prostate cancer? An analysis of the 3- versus 8-month randomized trial. Int J Radiat Oncol Biol Phys. 2010; 76: 23-30.
- 56. Roach M 3rd, DeSilvio M, Lawton C, Uhl V, Machtay M, Seider MJ, et al.: Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003 ;21: 1904-11.
- 57. Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, et al.: Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009; 360: 2516-27.
- 58. D'Amico AV, Denham JW, Bolla M, Collette L, Lamb DS, Tai KH, et al.: Short- vs long-term androgen suppression plus external beam radiation therapy and survival in men of advanced age with node-negative high-risk adenocarcinoma of the prostate. Cancer. 2007; 109: 2004-10.
- 59. Haworth A, Kearvell R, Greer PB, Hooton B, Denham JW, Lamb D, et al.: Assuring high quality treatment delivery in clinical trials - Results from the Trans-Tasman Radiation Oncology Group (TROG) study 03.04 "RADAR" set-up accuracy study. Radiother Oncol. 2009; 90: 299-306.
- 60. Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al.: Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009; 373: 301-8. Erratum in: Lancet. 2009; 373: 1174.
- 61. Warde PR, Mason MD, Sydes MR, Gospodarowicz MK, GP S, Kirkbride P, et al.: Intergroup randomized phase III study of androgen deprivation therapy (ADT) plus radiation therapy (RT) in locally advanced prostate cancer (CaP) (NCIC-CTG, SWOG, MRC-UK, INT: T94-0110; NCT00002633). J Clin Oncol. 2010; 28(suppl): Abstract # CRA4504
- 62. Mottet N, Peneau M, Mazeron J, Molinie V, Richaud P: Impact of radiotherapy (RT) combined with androgen deprivation (ADT) versus ADT alone for local control in clinically locally advanced prostate cancer. J Clin Oncol. 2010; 28(suppl): Abstract # 4505
- 63. Zietman AL: Correction: Inaccurate analysis and results in a study of radiation therapy in adenocarcinoma of the prostate. JAMA. 2008; 299: 898-9.
- 64. Al-Mamgani A, van Putten WL, Heemsbergen WD, van Leenders GJ, Slot A, Dielwart MF, et al.: Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008; 72: 980-8.
- 65. Sharifi N, Gulley JL, Dahut WL: Androgen deprivation therapy for prostate cancer. JAMA. 2005; 294: 238-44.
- 66. Alibhai SM, Gogov S, Allibhai Z: Long-term side effects of androgen deprivation therapy in men with non-metastatic prostate cancer: a systematic literature review. Crit Rev Oncol Hematol. 2006; 60: 201-15.
- 67. Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM: Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005; 90: 6410-7.
- 68. Kim SO, Kang TW, Kwon D, Park K, Ryu SB: Risk factors for bone loss with prostate cancer in Korean men not receiving androgen deprivation therapy. Int Braz J Urol. 2009; 35: 183-8; discussion 189.
- 69. Keating NL, O'Malley AJ, Smith MR: Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006; 24: 4448-56.
- 70. Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS, et al.: Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007; 110: 1493-500.
- 71. Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR: Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007; 99: 1516-24.
- 72. Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, et al.: Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009; 27: 3452-8.
- 73. D'Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, Lamb DS, et al.: Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007; 25: 2420-5.
- 74. Nanda A, Chen MH, Braccioforte MH, Moran BJ, D'Amico AV: Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009; 302: 866-73.
- 75. Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, et al.: Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009; 27: 92-9.
- 1. Alexander A, Crook J, Jones S, Malone S, Bowen J, Truong P, et al.: Is biochemical response more important than duration of neoadjuvant hormone therapy before radiotherapy for clinically localized prostate cancer? An analysis of the 3- versus 8-month randomized trial. Int J Radiat Oncol Biol Phys. 2010; 76: 23-30.
- 2. de Crevoisier R, Slimane K, Messai T, Wibault P, Eschwege F, Bossi A, et al.: Early PSA decrease is an independent predictive factor of clinical failure and specific survival in patients with localized prostate cancer treated by radiotherapy with or without androgen deprivation therapy. Ann Oncol. 2010; 21: 808-14.
- 1. Nilsson S, Norlén BJ, Widmark A: A systematic overview of radiation therapy effects in prostate cancer. Acta Oncol. 2004; 43: 316-81.
- 2. Stewart GD, Ross JA, McLaren DB, Parker CC, Habib FK, Riddick AC: The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int. 2010; 105: 8-13.
- 1. Dal Pra A, Cury FL, Souhami L: Radiation therapy and androgen deprivation in the management of high risk prostate cancer. Int Braz J Urol. 2011: 37 (in press).
- 2. Bazinet M, Zheng W, Begin LR, Aprikian AG, Karakiewicz PI, Elhilali MM: Morphologic changes induced by neoadjuvant ablation may result in underdetection of positive surgical margins and capsular involvement by prostatic adenocarcinoma. Urology. 1997; 49: 721-5.
- 3. Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al.: Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009; 373: 301-8. Erratum in: Lancet. 2009; 373: 301-8.
- 4. Warde PR, Mason MD, Sydes MR, Gospodarowicz MK, Swanson GP, Kirkbride P, et al.: Intergroup randomized phase III study of androgen deprivation therapy (ADT) plus radiation therapy (RT) in locally advanced pros¬tate cancer (CaP) (NCIC-CTG, SWOG, MRC-UK, 15INT: T94-0110; NCT00002633). J Clin Oncol. 2010; 28(suppl): Abstract # CRA4504.
- 5. Mottet N, Peneau M, Mazeron J, Molinie V, Richaud P: Impact of radiotherapy (RT) combined with androgen deprivation (ADT) versus ADT alone for local control in clinically locally advanced prostate cancer. J Clin Oncol. 2010; 28(suppl): Abstract # 4505.
- 6. Solberg A, Haugen OA, Viset T, Bergh A, Tasdemir I, Ahlgren G, et al: Residual Prostate Cancer in Patients Treated With Endocrine Therapy With or Without Radical Radiotherapy: A Side Study of the SPCG-7 Randomized Trial. Int J Radiat Oncol Biol Phys. 2011; 80: 55-61.
Publication Dates
-
Publication in this collection
30 May 2011 -
Date of issue
Apr 2011