ABSTRACT
Purpose:
Testicular germ cells tumor (TGCT) are associated with a high cure rate and are treated with platinum-based chemotherapy. However, a group of testicular cancer patients may have a very unfavorable evolution and insensitivity to the main therapeutic agent chemotherapy (CT) cisplatin. The aim of this study was to evaluate the risk of recurrence and overall survival related to the expression of nuclear factor kappa-B (NF-κB), transglutaminase 2 (TG2) and excision repair cross-complementation group 1 (ERCC1) in patients with TGCT treated with platinum combinations.
Patients and Methods:
A retrospective study was performed with TGCT patients treated with platinum-based chemotherapy. Immunohistochemical analysis was performed and the expression was correlated with clinical and laboratory data.
Results:
Fifty patients were included, the mean age was 28.4 years (18 to 45), and 76% were non-seminoma. All patients were treated with standard cisplatin, etoposide and bleomycin or cisplatin, and etoposide. Patient’s analyzed immunodetection for NF-κB, TG2, and ERCC1 were positive in 76%, 54% and 42%, respectively. Multivariate analysis identified that positive expressions to ERCC1 and NF-κB are independent risk factors for higher recurrence TGCT after chemotherapy (RR 2.96 and 3.16, respectively). Patients with positive expression of ERCC1 presented a poor overall survival rate for 10-year follow (p=0.001).
Conclusions:
The expression of ERCC1 and NF-κB give a worse prognosis for relapse, and only ERCC1 had an influence on the overall survival of TGCT patients treated with platinum-based chemotherapy. These may represent markers that predict poor clinical outcome and response to cisplatin.
Keywords:
transglutaminase 2 [Supplementary Concept]; Testicular Neoplasms; Cisplatin
INTRODUCTION
Testicular tumors account for 1% of all cancers in men. It is most frequent in men 15–35 years old and thus involves always a dramatic diagnosis (11. Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672-84.). The very majority are testicular germ cell tumors (TGCT), where 50% are seminomas, 40% non-seminomas and the others are mixed tumors (11. Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672-84., 22. Goldberg H, Klaassen Z, Chandrasekar T, Fleshner N, Hamilton RJ, Jewett MAS. Germ Cell Testicular Tumors-Contemporary Diagnosis, Staging and Management of Localized and Advanced disease. Urology. 2019;125:8-19.). Even with the advent of new drugs in chemotherapy, cisplatin remains the treatment regimen with most curative potential for testicular cancer (33. Vasey PA. Resistance to chemotherapy in advanced ovarian cancer: mechanisms and current strategies. Br J Cancer. 2003;89(Suppl 3):S23-8.). Cisplatin cytotoxic activity results of interactions with DNA and the inability to repair DNA strand can lead to tumor cell apoptosis (33. Vasey PA. Resistance to chemotherapy in advanced ovarian cancer: mechanisms and current strategies. Br J Cancer. 2003;89(Suppl 3):S23-8.
4. Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291-5.-55. Chovanec M, Abu Zaid M, Hanna N, El-Kouri N, Einhorn LH, Albany C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann Oncol. 2017;28:2670-9.). In fact, adducts between platinum and DNA inhibit cellular processes, such as replication, transcription, translation and DNA repair (33. Vasey PA. Resistance to chemotherapy in advanced ovarian cancer: mechanisms and current strategies. Br J Cancer. 2003;89(Suppl 3):S23-8.). The decrease in cellular respiration can produce reactive oxygen species, resulting in lipid peroxidation (44. Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291-5.). Furthermore, cisplatin binding to the mitochondrial DNA leads to decreased ATP and thus the decrease in ATPase activity and modification of the calcium content (44. Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291-5.).
However, a group of testicular cancer patients may have a very unfavorable evolution and insensitivity to the main therapeutic agent chemotherapy (CT), cisplatin. Around 20-30% of the cases relapse and a second line of CT is necessary (44. Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291-5.). Several mechanisms of cisplatin resistance have been proposed. Studies have linked the expression of excision repair cross-complementation group 1 (ERCC1) gene to chemoresistance as well as to poor survival in many types of cancer such as non-small-cell lung cancer, ovarian and gastric tumors (66. Kang CH, Jang BG, Kim DW, Chung DH, Kim YT, Jheon S, et al. Differences in the expression profiles of excision repair crosscomplementation group 1, x-ray repair crosscomplementation group 1, and betaIII-tubulin between primary non-small cell lung cancer and metastatic lymph nodes and the significance in mid-term survival. J Thorac Oncol. 2009;4:1307-12.
7. Friboulet L, Olaussen KA, Pignon JP, Shepherd FA, Tsao MS, Graziano S, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013;368:1101-10.
8. Urun Y, Leow JJ, Fay AP, Albiges L, Choueiri TK, Bellmunt J. ERCC1 as a prognostic factor for survival in patients with advanced urothelial cancer treated with platinum based chemotherapy: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;120:120-6.-99. Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983-91.). In TGCT cancer cell lines it has been reported an association of the cisplatin non-sensitivity with high levels of ERCC1, suggesting that this marker could be a potential mediator of response to cisplatin and a prognostic factor (1010. Mendoza J, Martínez J, Hernández C, Pérez-Montiel D, Castro C, Fabián-Morales E, et al. Association between ERCC1 and XPA expression and polymorphisms and the response to cisplatin in testicular germ cell tumours. Br J Cancer. 2013;109:68-75.). Likewise, the overexpression of ERCC1 and XPF in TGCT was previously described during the progression of seminoma to non-seminoma (1111. Köberle B, Brenner W, Albers A, Usanova S, Thüroff JW, Kaina B. ERCC1 and XPF expression in human testicular germ cell tumors. Oncol Rep. 2010;23:223-7.). In addition, the transcription factor nuclear factor kappa-B (NF-κB) has been described to mediate cisplatin resistance. NF-κB is involved in many cellular functions, including the regulation of apoptosis and platinum-based chemotherapy resistance (1212. Koga F, Yoshida S, Tatokoro M, Kawakami S, Fujii Y, Kumagai J, et al. ErbB2 and NFκB overexpression as predictors of chemoradiation resistance and putative targets to overcome resistance in muscle-invasive bladder cancer. PLoS One. 2011;6:e27616.). Other studies demonstrate its role in tumorigenesis, CT resistance and a worse prognosis in bladder and head and neck cancer (1212. Koga F, Yoshida S, Tatokoro M, Kawakami S, Fujii Y, Kumagai J, et al. ErbB2 and NFκB overexpression as predictors of chemoradiation resistance and putative targets to overcome resistance in muscle-invasive bladder cancer. PLoS One. 2011;6:e27616.
13. Li Z, Yang Z, Lapidus RG, Liu X, Cullen KJ, Dan HC. IKK phosphorylation of NF-κB at serine 536 contributes to acquired cisplatin resistance in head and neck squamous cell cancer. Am J Cancer Res. 2015;5:3098-110.-1414. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309-24.). Another marker is transglutaminase 2 (TG2), a trans-peptidase with a wide distribution in various tissues that plays an important role in malignancy progression by suppressing apoptosis (1515. Tabolacci C, De Martino A, Mischiati C, Feriotto G, Beninati S. The Role of Tissue Transglutaminase in Cancer Cell Initiation, Survival and Progression. Med Sci (Basel). 2019;7.). It is overexpressed in several neoplasms such as breast, ovaries, pancreas, and colon (1616. Huang L, Xu AM, Liu W. Transglutaminase 2 in cancer. Am J Cancer Res. 2015;5:2756-76.). TG2 is considered a prognostic marker in various cancers, due to its participation in promoting malignant cell mobility, invasion, metastasis, and chemoresistance, especially by platinum (1717. Cao L, Petrusca DN, Satpathy M, Nakshatri H, Petrache I, Matei D. Tissue transglutaminase protects epithelial ovarian cancer cells from cisplatin-induced apoptosis by promoting cell survival signaling. Carcinogenesis. 2008;29:1893-900.). Further mechanisms can be involved in platinum resistance such as decreased tumor blood flow, reduced platinum uptake, increased efflux, decreased binding, DNA repair, alteration of antiapoptotic factors and effects of various signaling pathways, among others (1818. Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12-31.).
A previous study showed that high expression of ERCC1 was associated with non-sensitivity to cisplatin-based CT in patients with non-seminomas TGCT (1010. Mendoza J, Martínez J, Hernández C, Pérez-Montiel D, Castro C, Fabián-Morales E, et al. Association between ERCC1 and XPA expression and polymorphisms and the response to cisplatin in testicular germ cell tumours. Br J Cancer. 2013;109:68-75.), but little is known about other mechanisms involved in platinum resistance in testicular cancer. Therefore, the identification of other molecular markers to platinum-resistance is essential to a better treatment selection, avoiding unnecessary toxicity associated with platinum-based CT. In this study, we assessed the correlation of NF-κB, TG2 and ERCC1 expression with clinical outcomes in patients with TGCT treated with standard platinum combinations.
PATIENTS AND METHODS
Study design and data collection
A retrospective study was performed to evaluate tumor markers of cisplatin resistance in patients with testicular cancer receiving chemotherapy treatment. Eligible patients included male individuals (aged 18 years or above) with the confirmed diagnosis of testicular germ cell tumors. Seventy-six (76) cases of patients diagnosed with testicular cancer were evaluated in the Oncology Department-Hospital São Lucas/PUCRS in the period 2001 to 2011. Twenty-six 26 patients were excluded from the study due to the following reasons: lack of adherence to treatment or follow-up, incomplete data and loss of paraffin blocks. Histological indicative of TGCT was required to confirm the diagnosis. Data collection was retrospectively done through medical chart analysis of the cases treated. Patient’s characteristics and tumor markers alpha-fetoprotein (AFP), beta-hCG and lactate dehydrogenase (LDH) were collected. The measurement of the tumor markers was made usually after the first-month post orchiectomy. The cut-off points and patient’s stratification risk were evaluated according to the International Germ Cell Consensus Classification (IGCCCG) (1919. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.). This study was approved by the Institutional Ethics Committee of PUCRS (CEP number 0804398).
End-points
The endpoints were the relapse/recurrence rate and overall survival (OS). Recurrence was defined when the progression of disease in computer tomography was confirmed. OS was calculated from the time of diagnosis to the date of death. The follow-up time for recurrence and OS was of 120 months for any cause of death.
Immunohistochemistry
To determine the expression of ERCC1, NF-κB and TG2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, EUA), in the germ cell tumors we performed immunohistochemistry assay ((99. Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983-91., 1313. Li Z, Yang Z, Lapidus RG, Liu X, Cullen KJ, Dan HC. IKK phosphorylation of NF-κB at serine 536 contributes to acquired cisplatin resistance in head and neck squamous cell cancer. Am J Cancer Res. 2015;5:3098-110., 2020. Park MJ, Baek HW, Rhee YY, Lee C, Park JW, Kim HW, et al. Transglutaminase 2 expression and its prognostic significance in clear cell renal cell carcinoma. J Pathol Transl Med. 2015;49:37-43.). The tumors were excised after surgery and fixed in buffered neutral formalin, sectioned, processed to paraffin wax and mounted onto a microscope slide. For the immunostaining study, sections were deparaffinized and rehydrated. The sections were submitted to antigenic retrieval being incubated with TRIS-EDTA, pH 9.0 for 30 minutes in a water bath at 98°C. For detection of the antibodies, it was used the REVEAL Biotin-Free Detection System (Spring Bioscience). The incubation of the primary antibodies (anti-NF-κB p65, clone C22B4, anti-TG2 clone CUB7402, anti-ERCC1, clone 8F1) was performed overnight at 4°C. Chromogenic detection (DAB) was used and the slides were counterstained with hematoxylin. The slides were mounted with glass coverslips using Canada Balsam and viewed with a microscope equipped with a camera. Images were captured in 400x amplification. For control experiments, primary antibody was omitted and evaluated for specificity or background staining levels. ERCC1, NF-κB and TG2 expression was considered as positive or negative by a pathologist blinded to the clinical outcome of each patient. The staining intensity was graded on a scale of 0 to 3. The percentage of positive nuclei was calculated for each specimen, and a proportion score was assigned (0 if 0%, 0.1 if 1 to 9%, 0.5 if 10 to 49% and 1.0 if 50% or more), as previously described (2121. Reed E. Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev. 1998;24:331-44.). This proportion score was multiplied by the staining intensity of nuclei to obtain a final semi-quantitative H score. The median value of all the H scores was a priori chosen as a cut off point for separating positive tumors from negative tumors.
Statistical analysis
Quantitative data were described as mean±standard deviation (SD). Categorical data were presented by counts and percentages. Fisher’s exact test or Pearson Chi-Square were used in categorical data. To obtain estimates of the association between ERCC1, NF-κB and TG2 markers with the occurrence of relapse we used a negative binomial regression model that provided relative risk estimates and their 95% confidence intervals. The relative risk was adjusted by age, stage, AFP, beta-hCG, and LDH histology. The differences in OS between categories of interest were analyzed using the log-rank test, and the hazard ratios (HRs) of the adjusted ERCC1, NF-κB and TG2 were calculated using the Cox model. The survival curves were constructed using the Kaplan-Meier method and significant between-group differences were assessed by the log-rank test. The significance level was set at α=0.05. Data were analyzed with the aid of the program SPSS version 22 and Sigma-Plot version 11.
RESULTS
In this study, we assessed the correlation of NF-κB, TG2 and ERCC1 expression to clinical outcomes in 50 patients with TGCT treated with standard platinum combinations. The characteristics of the patients studied are presented in Table-1. Median age (range) of the group analyzed was 28.0 (18 to 45) years, 18 (36%) patients were clinical stage I, 10 (20%) were clinical stage II and 21 were stage III (42%), 12 cases (24%) were of seminoma and 38 cases (76%) of non-seminoma. Patient’s stratification risk for non-seminoma was 16% poor prognosis, 38% intermediate risk and 46% good prognosis and, 58% good prognosis and, 42% intermediate to the seminoma cases.
The protocols of CT administered to the patients studied were in agreement with the currently first-line treatment pattern for TGCT (1515. Tabolacci C, De Martino A, Mischiati C, Feriotto G, Beninati S. The Role of Tissue Transglutaminase in Cancer Cell Initiation, Survival and Progression. Med Sci (Basel). 2019;7.). Patients received intravenously: BEP (cisplatin 20mg/m2 on days 1 to 5, etoposide 100mg/m2 on days 1 to 5 and bleomycin 30UI, on days 2, 9 and 16) or EP (cisplatin 20mg/m2, days 1 to 5 plus etoposide 100mg/m2 days 1 to 5), every 21 days, intravenously (2222. Culine S, Kerbrat P, Kramar A, Théodore C, Chevreau C, Geoffrois L, et al. Refining the optimal chemotherapy regimen for good-risk metastatic nonseminomatous germ-cell tumors: a randomized trial of the Genito-Urinary Group of the French Federation of Cancer Centers (GETUG T93BP). Ann Oncol. 2007;18:917-24.). In this study, 36 (72%) patients received BEP (x3), 14 (28%) received EP (x4), and 4 (8%) patients used radiotherapy after CT (Table-1). The assessment of tumor markers was made after the orchiectomy. The AFP, beta--hCG, and LDH were elevated, on the 30h day post orchiectomy, in 68%, 70% and 56% of cases, respectively (Table-1). Immunodetection for ERCC1, NF-κB, and TG2 markers was positive in 42%, 76% and 46% of the patient’s samples analyzed, respectively, and there were no statistically significant differences between seminoma and non-seminoma (Figures 1 A, B and C, Table-2).
Immunohistochemical analysis of ERCC1, NF-κB, and TG2 in patients with testicular cancer (n=50).
Representative images of H&E and immunostaining for the tumor markers (x400): A) H&E of non-seminoma testicular Tumor. Arrows indicate immunopositivity for: B) ERCC1; C) NF-κB; D) TG2.
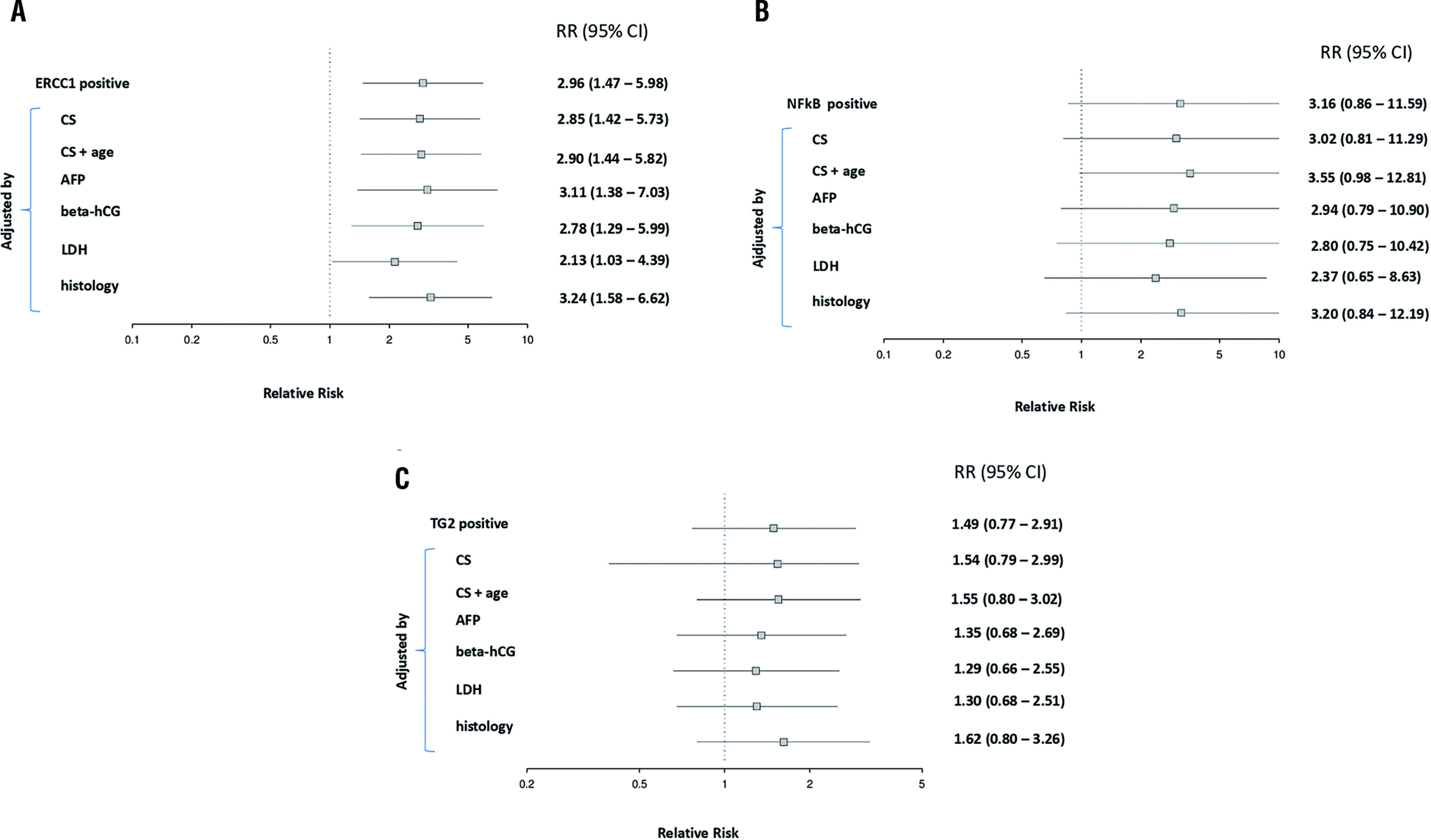
The relative risk (RR) of ERCC1, NF-κB, and TG2 for testicular cancer relapse after completion of chemotherapy are depicted in Figure-2. It is presented also the adjusted RR for possible confounding factors in the outcome. The data on ERCC1 expression was significantly associated with a higher risk of relapse. When we adjusted stratified for factors such as age, clinical stage, alpha-fetoprotein, beta-hCG, lactate dehydrogenase, and histology non-seminoma, there was persistent the risk of recurrence (Figure-2A). Interestingly, we showed, for the first time, that the risk for relapse is around three times as high in the group NF-κB positive when compared to NF-κB negative, and this difference remains even after the adjustment of a potential factor of influence, with the exception of the tumor maker LDH (Figure-2B). No differences were observed with TG2 marker (Figure-2C). Interestingly, when we evaluated the impact of ERCC1 positive plus NF-kB positive versus ERCC1 positive plus NF-kB negative, there was a significant increase in the risk of recurrence for the markers combined positiveness (71.4% vs. 29.4%; p=0.001).
A) Relative risk for relapse to ERCC1 positive cases; stratified mode adjustments for factors such as age, clinical stage (CS), alpha-fetoprotein (AFP), beta-hCG, lactate dehydrogenase (LDH) and histology non-seminoma. B) The relative risk for relapse to NF-KB positive cases; stratified mode adjustments for factors such as age, clinical stage (CS), alphafetoprotein (AFP), beta-hCG, lactate dehydrogenase (LDH) and histology non-seminoma. C) The relative risk for relapse to TG2 positive cases; stratified mode adjustments for factors such as age, clinical stage (CS), alpha-fetoprotein (AFP), beta-hCG, lactate dehydrogenase (LDH) and histology non-seminoma. Amounts right corresponds to p and ranges (n=50).
The evaluation of overall survival among patients with ERCC1-negative tumor, 1, 3, 5 and 10-year overall survival rate were 100%, 96%, 89% and 62%, compared to 100%, 85%, 57% and 9% for patients with positive expression of ERCC1 (p=0.001) (Figure-3A). The levels of NF-κB and TG2 protein expression had no significant influence on overall survival (Figures 3B and C).
Kaplan-Meier estimate of overall survival probability according to ERCC1, p <0.001. A) NF-κB, p=0.084; B) and TG2, p=0.066; C). The differences in OS between categories of interest were analyzed using the log-rank test, and the significance level was set at α=0.05.
DISCUSSION
Platinum-based chemotherapy remains the first line of treatment of TGCT for more than 30 years (11. Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672-84.). This study aimed to assess new molecular markers involved in cisplatin resistance, and their correlation with tumors relapse in patients with testicular tumors. In this study, the majority of patients studied were young males with TGCT non-seminoma and had a poor or intermediate prognosis classification. One of the main problems related to the recurrence of TGCT is platinum resistance and the mechanisms associated with cisplatin resistance involve many different cellular processes ((1818. Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12-31., 2323. Sonnenburg D, Spinella MJ, Albany C. Epigenetic Targeting of Platinum Resistant Testicular Cancer. Curr Cancer Drug Targets. 2016;16:789-95.). Previous studies have demonstrated that platinum damage can be repaired by the nucleotide excision repair system, especially by ERCC1 (2121. Reed E. Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev. 1998;24:331-44.). Our results showed that the expression of ERCC1 is associated with increased risk for TGCT relapse after treatment with platinum-based chemotherapy. When multivariate analysis was performed, none of the confounding factors in the outcome was able to change this point. These results show, in an important manner, that ERCC1 overexpression may predict that the curative chemotherapy has a relative risk of 2.96 to failure. In effect, predictive and prognostic values of ERCC1 expression have been studied in many solid tumors. It has been reported in some studies in ovarian, head and neck, and particularly in lung cancer that this marker could predict the response to chemotherapy (77. Friboulet L, Olaussen KA, Pignon JP, Shepherd FA, Tsao MS, Graziano S, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013;368:1101-10., 99. Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983-91., 1010. Mendoza J, Martínez J, Hernández C, Pérez-Montiel D, Castro C, Fabián-Morales E, et al. Association between ERCC1 and XPA expression and polymorphisms and the response to cisplatin in testicular germ cell tumours. Br J Cancer. 2013;109:68-75., 2424. Polat G, Yılmaz U, Anar C, Kömürcüoğlu B, Aydoğdu Z. Is there relationship between excision repair cross-complementation 1 expression level and response to treatment and prognosis in an advanced stage lung cancer treated with cisplatin-based chemotherapy? Indian J Cancer. 2015;52:277-80., 2525. Jun HJ, Ahn MJ, Kim HS, Yi SY, Han J, Lee SK, et al. ERCC1 expression as a predictive marker of squamous cell carcinoma of the head and neck treated with cisplatin-based concurrent chemoradiation. Br J Cancer. 2008;99:167-72.). Corroborating to our results, Mendoza et al. (1010. Mendoza J, Martínez J, Hernández C, Pérez-Montiel D, Castro C, Fabián-Morales E, et al. Association between ERCC1 and XPA expression and polymorphisms and the response to cisplatin in testicular germ cell tumours. Br J Cancer. 2013;109:68-75.) demonstrated that high levels of ERCC1 were associated with non-cisplatin sensitivity, suggesting that ERCC1 could be used as a potential indicator of the response to cisplatin and prognosis in non-sensitive TGCTs. Furthermore, the study of Olaussen et al. (99. Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983-91.) demonstrated that the benefit of adjuvant chemotherapy with cisplatin was lost when there was a high expression of ERCC1 in the small-cell lung cancer tumor. In patients with squamous cell carcinoma, the expression of ERCC1 predicts a lower response to chemotherapy treatment (2525. Jun HJ, Ahn MJ, Kim HS, Yi SY, Han J, Lee SK, et al. ERCC1 expression as a predictive marker of squamous cell carcinoma of the head and neck treated with cisplatin-based concurrent chemoradiation. Br J Cancer. 2008;99:167-72.). Interestingly, overexpression of ERCC1 gene seems to be associated with a reduction in the therapeutic efficacy of cisplatin, and the clinical response varies with polymorphisms ERCC1 (66. Kang CH, Jang BG, Kim DW, Chung DH, Kim YT, Jheon S, et al. Differences in the expression profiles of excision repair crosscomplementation group 1, x-ray repair crosscomplementation group 1, and betaIII-tubulin between primary non-small cell lung cancer and metastatic lymph nodes and the significance in mid-term survival. J Thorac Oncol. 2009;4:1307-12.). The mechanism by which ERCC1 contributes to cisplatin resistance involves a nucleoside excision repair, which removes platinum-DNA adducts and repairs the DNA double-strand breaks, and other reports mention an inherent biologic characteristic of the tumor (2424. Polat G, Yılmaz U, Anar C, Kömürcüoğlu B, Aydoğdu Z. Is there relationship between excision repair cross-complementation 1 expression level and response to treatment and prognosis in an advanced stage lung cancer treated with cisplatin-based chemotherapy? Indian J Cancer. 2015;52:277-80., 2525. Jun HJ, Ahn MJ, Kim HS, Yi SY, Han J, Lee SK, et al. ERCC1 expression as a predictive marker of squamous cell carcinoma of the head and neck treated with cisplatin-based concurrent chemoradiation. Br J Cancer. 2008;99:167-72.). Our results suggest that the evaluation of ERCC1 expression may contribute as a more accurate predictor of patient’s selection who are at increased risk of recurrence following standard treatment with cisplatin.
The data presented herein showed, for the first time, the expression of NF-κB in TGCT. The detection of high levels of NF-κB in patients with testicular cancer supports the hypothesis of a higher risk of recurrence after the treatment with cisplatin. Although there were no differences in the risk of recurrence for positive expression of NF-κB alone, we observed a significant increase in the risk of recurrence when we evaluated combined positive expression of ERCC1 plus NF-κB. The mechanism of resistance could be explained since cisplatin significantly increases NF-κB DNA binding activity, and NF-κB may antagonize apoptosis induced by cisplatin ((2626. Kim JK, Kim KD, Lee E, Lim JS, Cho HJ, Yoon HK, et al. Up-regulation of Bfl-1/A1 via NF-kappaB activation in cisplatin-resistant human bladder cancer cell line. Cancer Lett. 2004;212:61-70., 2727. Eichholtz-Wirth H, Sagan D. IkappaB/NF-kappaB mediated cisplatin resistance in HeLa cells after low-dose gammairradiation is associated with altered SODD expression. Apoptosis. 2000;5:255-63.). It has been described that NF-κB inhibitors augment platinum activity against some cancer cell lines and tumor xenograft models ((2828. Upadhyay AK, Singh S, Chhipa RR, Vijayakumar MV, Ajay AK, Bhat MK. Methyl-beta-cyclodextrin enhances the susceptibility of human breast cancer cells to carboplatin and 5-fluorouracil: involvement of Akt, NF-kappaB and Bcl 2. Toxicol Appl Pharmacol. 2006;216:177-85., 2929. Mohammad RM, Banerjee S, Li Y, Aboukameel A, Kucuk O, Sarkar FH. Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in BxPC-3 pancreatic tumor xenografts. Cancer. 2006;106:1260-8.). On the other hand, NF-κB activation increased cisplatin efficacy in some cell lines, and enhanced efficacy of higher cisplatin concentrations (3030. Shehata M, Shehata M, Shehata F, Pater A. Apoptosis effects of Xrel3 c-Rel/Nuclear Factor-kappa B homolog in human cervical cancer cells. Cell Biol Int. 2005;29:429-40.). For the other marker studied, there was no significant association between TG2 with the recurrence of TGCT.
It is worth to mention that due to its retrospective design, the number of clinical samples, and survival bias could potentially threaten the conclusions of this study. A further prospective cohort of patients should be performed in order to confirm the results achieved herein. Another limitation of this study is the inherent weaknesses of immunohistochemical staining, such as its semi-quantitative nature and interobserver variation. It is worth to consider that in this study the markers were analyzed only after surgery, which could limit the assessment.
In conclusion, we demonstrated that ERCC1 and NF-κB expression confer a worse prognosis for recurrence in patients with TGCT treated with standard platinum-based chemotherapy. The identification of resistance markers in TCGT patients who are potentially non-sensitive to cisplatin chemotherapy can improve their quality of life by avoiding the adverse effects caused by this agent.
ACKNOWLEDGMENTS
This work was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) scholarships and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil) - Finance Code 001, PUCRS (Pontifícia Universidade Católica do Rio Grande do Sul). The authors thank Dr. Wagner M, for his technical advice with the statistical analysis, Dr. Gaiger AM for immunohistochemical analysis, and Bittencourt B for the help in data collection.
REFERENCES
-
1Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672-84.
-
2Goldberg H, Klaassen Z, Chandrasekar T, Fleshner N, Hamilton RJ, Jewett MAS. Germ Cell Testicular Tumors-Contemporary Diagnosis, Staging and Management of Localized and Advanced disease. Urology. 2019;125:8-19.
-
3Vasey PA. Resistance to chemotherapy in advanced ovarian cancer: mechanisms and current strategies. Br J Cancer. 2003;89(Suppl 3):S23-8.
-
4Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291-5.
-
5Chovanec M, Abu Zaid M, Hanna N, El-Kouri N, Einhorn LH, Albany C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann Oncol. 2017;28:2670-9.
-
6Kang CH, Jang BG, Kim DW, Chung DH, Kim YT, Jheon S, et al. Differences in the expression profiles of excision repair crosscomplementation group 1, x-ray repair crosscomplementation group 1, and betaIII-tubulin between primary non-small cell lung cancer and metastatic lymph nodes and the significance in mid-term survival. J Thorac Oncol. 2009;4:1307-12.
-
7Friboulet L, Olaussen KA, Pignon JP, Shepherd FA, Tsao MS, Graziano S, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013;368:1101-10.
-
8Urun Y, Leow JJ, Fay AP, Albiges L, Choueiri TK, Bellmunt J. ERCC1 as a prognostic factor for survival in patients with advanced urothelial cancer treated with platinum based chemotherapy: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;120:120-6.
-
9Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983-91.
-
10Mendoza J, Martínez J, Hernández C, Pérez-Montiel D, Castro C, Fabián-Morales E, et al. Association between ERCC1 and XPA expression and polymorphisms and the response to cisplatin in testicular germ cell tumours. Br J Cancer. 2013;109:68-75.
-
11Köberle B, Brenner W, Albers A, Usanova S, Thüroff JW, Kaina B. ERCC1 and XPF expression in human testicular germ cell tumors. Oncol Rep. 2010;23:223-7.
-
12Koga F, Yoshida S, Tatokoro M, Kawakami S, Fujii Y, Kumagai J, et al. ErbB2 and NFκB overexpression as predictors of chemoradiation resistance and putative targets to overcome resistance in muscle-invasive bladder cancer. PLoS One. 2011;6:e27616.
-
13Li Z, Yang Z, Lapidus RG, Liu X, Cullen KJ, Dan HC. IKK phosphorylation of NF-κB at serine 536 contributes to acquired cisplatin resistance in head and neck squamous cell cancer. Am J Cancer Res. 2015;5:3098-110.
-
14Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309-24.
-
15Tabolacci C, De Martino A, Mischiati C, Feriotto G, Beninati S. The Role of Tissue Transglutaminase in Cancer Cell Initiation, Survival and Progression. Med Sci (Basel). 2019;7.
-
16Huang L, Xu AM, Liu W. Transglutaminase 2 in cancer. Am J Cancer Res. 2015;5:2756-76.
-
17Cao L, Petrusca DN, Satpathy M, Nakshatri H, Petrache I, Matei D. Tissue transglutaminase protects epithelial ovarian cancer cells from cisplatin-induced apoptosis by promoting cell survival signaling. Carcinogenesis. 2008;29:1893-900.
-
18Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12-31.
-
19International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.
-
20Park MJ, Baek HW, Rhee YY, Lee C, Park JW, Kim HW, et al. Transglutaminase 2 expression and its prognostic significance in clear cell renal cell carcinoma. J Pathol Transl Med. 2015;49:37-43.
-
21Reed E. Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev. 1998;24:331-44.
-
22Culine S, Kerbrat P, Kramar A, Théodore C, Chevreau C, Geoffrois L, et al. Refining the optimal chemotherapy regimen for good-risk metastatic nonseminomatous germ-cell tumors: a randomized trial of the Genito-Urinary Group of the French Federation of Cancer Centers (GETUG T93BP). Ann Oncol. 2007;18:917-24.
-
23Sonnenburg D, Spinella MJ, Albany C. Epigenetic Targeting of Platinum Resistant Testicular Cancer. Curr Cancer Drug Targets. 2016;16:789-95.
-
24Polat G, Yılmaz U, Anar C, Kömürcüoğlu B, Aydoğdu Z. Is there relationship between excision repair cross-complementation 1 expression level and response to treatment and prognosis in an advanced stage lung cancer treated with cisplatin-based chemotherapy? Indian J Cancer. 2015;52:277-80.
-
25Jun HJ, Ahn MJ, Kim HS, Yi SY, Han J, Lee SK, et al. ERCC1 expression as a predictive marker of squamous cell carcinoma of the head and neck treated with cisplatin-based concurrent chemoradiation. Br J Cancer. 2008;99:167-72.
-
26Kim JK, Kim KD, Lee E, Lim JS, Cho HJ, Yoon HK, et al. Up-regulation of Bfl-1/A1 via NF-kappaB activation in cisplatin-resistant human bladder cancer cell line. Cancer Lett. 2004;212:61-70.
-
27Eichholtz-Wirth H, Sagan D. IkappaB/NF-kappaB mediated cisplatin resistance in HeLa cells after low-dose gammairradiation is associated with altered SODD expression. Apoptosis. 2000;5:255-63.
-
28Upadhyay AK, Singh S, Chhipa RR, Vijayakumar MV, Ajay AK, Bhat MK. Methyl-beta-cyclodextrin enhances the susceptibility of human breast cancer cells to carboplatin and 5-fluorouracil: involvement of Akt, NF-kappaB and Bcl 2. Toxicol Appl Pharmacol. 2006;216:177-85.
-
29Mohammad RM, Banerjee S, Li Y, Aboukameel A, Kucuk O, Sarkar FH. Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in BxPC-3 pancreatic tumor xenografts. Cancer. 2006;106:1260-8.
-
30Shehata M, Shehata M, Shehata F, Pater A. Apoptosis effects of Xrel3 c-Rel/Nuclear Factor-kappa B homolog in human cervical cancer cells. Cell Biol Int. 2005;29:429-40.
Publication Dates
-
Publication in this collection
30 Mar 2020 -
Date of issue
May-Jun 2020
History
-
Received
08 Jan 2019 -
Accepted
17 Sept 2019 -
Published
30 Oct 2019