Abstract
OBJECTIVE: This study evaluated the efficacy of NitrAdineTM-based disinfecting cleaning tablets for complete denture, in terms of denture biofilm removal and antimicrobial action. MATERIAL AND METHODS: Forty complete denture wearers (14 men and 26 women) with a mean age of 62.3±9.0 years were randomly assigned to two groups and were instructed to clean their dentures according to two methods: brushing (control) - 3 times a day with denture brush and tap water following meals; brushing and immersion (Experimental) - brushing the denture 3 times a day with denture brush and tap water following meals and immersion of the denture in NitrAdineTM-based denture tablets (Medical InterporousTM). Each method was used for 21 days. Denture biofilm was disclosed by a 1% neutral red solution and quantified by means of digital photos taken from the internal surface before and after the use of the product. Microbiological assessment was conducted to quantify Candida sp. RESULTS: An independent t-test revealed a significant lower biofilm percentage for the experimental group (4.7, 95% CI 2.4 to 7.9) in comparison with the control group (mean 37.5, 95% CI 28.2 to 48.1) (t38=7.996, p<0.001). A significant reduction of yeast colony forming units could be found after treatment with Medical InterporousTM denture tablets as compared to the control group (Mann-Whitney test, Z=1.90; p<0.05). CONCLUSION: The present findings suggest that NitrAdineTM-based disinfecting cleaning tablets are efficient in removal of denture biofilm. In addition, a clear antimicrobial action was demonstrated. Therefore, they should be recommended as a routine denture maintenance method for the prevention of the development of microbial biofilm induced denture stomatitis.
Complete denture; Denture cleansers; Biofilms; Hygiene
ORIGINAL ARTICLE
Clinical and antimicrobial efficacy of NitrAdineTM-based disinfecting cleaning tablets in complete denture wearers
Cláudia Helena Silva-LovatoI; Bart de WeverII; Els AdriaensIII; Helena de Freitas Oliveira ParanhosIV; Evandro WatanabeV; Maria Xavier PisaniVI; Rafael Freitas de SouzaI; Isabel Yoko ItoVII
IDDS, MSc, PhD, Professor, Department of Dental Materials and Prosthodontics, Ribeirão Preto Dental School, University of São Paulo, Ribeirão Preto, SP, Brazil
IIPhD, MSI Laboratories AG, Vaduz, Liechtenstein
IIIPhD, Adriaens Consulting, Aalter, Belgium
IVDDS, MSc, PhD, Full Professor, Department of Dental Materials and Prosthodontics, Ribeirão Preto Dental School, University of São Paulo, Ribeirão Preto, SP, Brazil
VDDS, MSc, PhD, Graduate student, Department of Clinical Analyses, Toxicological and Bromatological, Pharmaceutical Sciences School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil
VIDDS, MSc, PhD, Graduate student, Department of Dental Materials and Prosthodontics, Ribeirão Preto Dental School, University of São Paulo, Ribeirão Preto, SP, Brazil
VIIDDS, MSc, PhD, Full Professor, Department of Clinical Analyses, Toxicological and Bromatological, Pharmaceutical Sciences School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil
Corresponding address Corresponding address: Profa. Dra. Cláudia Helena Lovato da Silva Faculdade de Odontologia de Ribeirão Preto - USP - Departamento de Materiais Dentários e Prótese Av. do Café s/n, 14040-904 Ribeirão Preto, SP - Brasil e-mail: chl@forp.usp.br
ABSTRACT
OBJECTIVE: This study evaluated the efficacy of NitrAdineTM-based disinfecting cleaning tablets for complete denture, in terms of denture biofilm removal and antimicrobial action.
MATERIAL AND METHODS: Forty complete denture wearers (14 men and 26 women) with a mean age of 62.3±9.0 years were randomly assigned to two groups and were instructed to clean their dentures according to two methods: brushing (control) - 3 times a day with denture brush and tap water following meals; brushing and immersion (Experimental) - brushing the denture 3 times a day with denture brush and tap water following meals and immersion of the denture in NitrAdineTM-based denture tablets (Medical InterporousTM). Each method was used for 21 days. Denture biofilm was disclosed by a 1% neutral red solution and quantified by means of digital photos taken from the internal surface before and after the use of the product. Microbiological assessment was conducted to quantify Candida sp.
RESULTS: An independent t-test revealed a significant lower biofilm percentage for the experimental group (4.7, 95% CI 2.4 to 7.9) in comparison with the control group (mean 37.5, 95% CI 28.2 to 48.1) (t38=7.996, p<0.001). A significant reduction of yeast colony forming units could be found after treatment with Medical InterporousTM denture tablets as compared to the control group (Mann-Whitney test, Z=1.90; p<0.05).
CONCLUSION: The present findings suggest that NitrAdineTM-based disinfecting cleaning tablets are efficient in removal of denture biofilm. In addition, a clear antimicrobial action was demonstrated. Therefore, they should be recommended as a routine denture maintenance method for the prevention of the development of microbial biofilm induced denture stomatitis.
Key words: Complete denture. Denture cleansers. Biofilms. Hygiene.
INTRODUCTION
Denture-induced stomatitis (atrophic chronic candidiasis) is an inflammatory lesion of the denture bearing mucosa that affects approximately 50% of patients wearing maxillary complete dentures35, and has a multifactor etiology. Factors of particular significance are trauma caused by the denture itself, infection by Candida, poor denture hygiene, continuous denture wear, and dietary and systemic factors, including suppressed immuno-competence34. Especially elderly people wearing dentures or patients with metabolic diseases, such as diabetics, organ transplant patients, cancer patients or patients on chronic antibiotic treatment have an increased risk for developing oral candidiasis13.
The presence of denture biofilm is an important etiologic factor for this disease. Biofilm found on dentures consists of a complex mixture of fungi and bacteria and desquamated epithelial cells8. This biofilm act as a protective reservoir for oral microorganisms, and Candida albicans and other species of yeast found in biofilm have been reported as important agents for the installation, maintenance and exacerbation of denture stomatitis. Scanning electron microscopic evaluation of dentures retrieved from patients suffering from denture stomatitis demonstrates that Candida biofilm not only colonizes the surface, but also penetrate the cracks and imperfections of the denture material26.
In addition to Candida sp., also other pathogenic and opportunistic microorganisms have been isolated from patient's dentures which include Staphylococcus sp., Streptococcus sp. and Pseudomonas sp.8. Moreover, the colonization of oral surfaces, including denture-seating surfaces, could serve as a reservoir for disseminated infections, such as gastrointestinal infections17. Even respiratory pathogens that are uncommon in the oral flora have been isolated from dentures, including Escherichia coli, Klebsiella pneumoniae, MRSA or methicillin-resistant Staphylococcus aureus and Enterobacteria32. The presence of these bacteria increases the risk of aspiration pneumonia and/or aspiration pneumonitis, in particular when swallowing disorders are observed (e.g.: in patients with neurological disorder including peripheral or central neuromotor disability, such as cerebral palsy, stroke, Parkinson's disease, Alzheimer's disease or Down Syndrome)28. For the above described reasons, adequate removal of pathogenic biofilm present on dentures should be recommended as a routine practice.
The efficacy of several denture cleansers has been clinically evaluated in a number of approaches, and commercially available denture cleansing products are generally not very efficient in denture biofilm control. In addition, some of these cleansers are either corrosive or change the colour or hardness of the polymer after repeated use27,33. The ideal denture maintenance product must be effective in the removal of organic and inorganic deposits, have antibacterial and antifungal biofilm removal properties, be non-toxic to humans, compatible with the denture materials including metals, easy to handle and also have a low cost18. The aim of this study was to evaluate the clinical efficacy of a novel disinfecting cleaning tablet for complete denture cleansing, Medical InterporousTM (MSI Laboratories AG, Vaduz, Liechtenstein), in terms of denture biofilm removal and antimicrobial action in denture wearers.
MATERIAL AND METHODS
This study was conducted with 40 complete denture wearers (14 men and 26 women) with a mean age of 62.3±9.0 years (range: 45 to 70 years). Dentures were inserted at least 1 year (mean 5.5±4.8 years) prior to the study and were made from heat-polymerized acrylic resin. All participants presented adequate general health conditions. This research study was approved by the institutional Research Ethics Committee (process number 2007.1.207.58.4). Patients were informed of the nature of the investigation, and written informed consent was obtained prior to enrolment.
Exclusion criteria were: time of denture use less than one year and absence of biofilm on internal surface of upper dentures during the first examination. This assessment was conducted by the Additive Index of Ambjørnsen, et al.1 (1982). Only subjects wearing upper complete dentures with scores of "1" or greater were selected. In other words, a zero in one or more areas of the Additive Index precluded inclusion.
Volunteers were instructed to clean their dentures according to two methods:
1) Control: brushing 3 times a day using a denture brush (Denture - Condor S.A., São Bento do Sul, SC, Brazil) and tap water following meals (breakfast, lunch and dinner);
2) Experimental: brushing the denture using the denture brush and tap water following meals (breakfast, lunch and dinner), and treating the denture with Medical InterporousTM Denture tablets, by immersing the denture into 150 mL of lukewarm water and then adding one tablet. The denture was then allowed to soak in the solution for at least 15 min once a day for 21 days. After treatment, the denture was removed from the solution and rinsed vigorously under running water before replacing back in the mouth.
The 40 patients were randomly distributed to each group and received verbal information and practical demonstration of both control and experimental techniques. After participants had received their denture maintenance instructions and hygiene materials, disclosure of the internal surface of maxillary denture of each participating denture wearer was assessed using a 1% neutral red solution24. The disclosed dentures were then brushed until complete removal of disclosed biofilm (biofilm free). For evaluation of the efficacy in the removal of biofilm, after 21 days of the use of both denture maintenance methods (control and experimental), the internal surfaces of upper dentures were disclosed again by 1% neutral red solution. The surfaces were then photographed (digital camera Canon EOS Digital Rebel EF-S 18-55; and flash: Canon MR-14 EX, Canon Inc., Tokyo, Japan) with standard lens-object distance and exposure time. The camera was fixed on a stand (CS-4 Copy Stand, Testrite Inst. Co., Inc., Newark, NJ, USA). After quantification, biofilm was eliminated by brushing with the denture brush and liquid soap (JOB Química, Produtos para Limpeza Ltda., Monte Alto, SP, Brazil).
Photographs were transferred to a computer and the total surface area and the areas corresponding to the stained region were measured using image processing software (Image Tool 2.02, UTHSC, San Antonio, Texas). Biofilm percentage was calculated using the relation between biofilm area multiplied by 100, and total surface area of the denture's internal base.
For the microbiological analysis, at the end of the 21 days evaluation period, the biofilm was collected by brushing in saline solution20. Serial dilutions were prepared in PBS (10-4) and cultivated in CHROMagarTMCandida (CHROMagar, Paris, France) culture medium (CM) under laminar flow, in duplicate at 37°C for 48 h. The counting of the number of colony-forming units (cfu) of yeast-like fungi was performed and the identification was carried out on the basis of the macroscopic morphology and use of code of colors (Candida albicans - green; Candida dubliniensis - green; Candida glabrata - purpura; Candida tropicalis - blue; Candida parapsilosis - white). The identity of the isolated yeasts was confirmed by the tests of tube formation of germination (GT), chlamydoconidia and tests of fermentation and assimilation.
The influence of the treatments on the denture biofilm percentage was assessed with an independent sample t-test. The homogeneity of the variances was tested with a Levene's test and normality of the residuals was tested with a Shapiro-Wilk test (SW). A significance level of P<0.05 was used. The data were transformed to their square root to meet the assumptions. In the tables, the back-transformed mean values together with their 95% confidence interval are shown.
The analysis of the antimicrobial action was performed after processing the original data (cfu) in Log 10. Candida sp. counts were not normally distributed and were compared by the Mann-Whitney test.
RESULTS
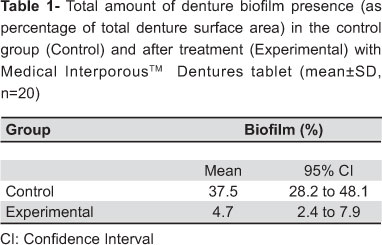
Table 1 shows the biofilm percentage in the internal surface of the upper complete denture after the use of the Control method and after the use of the Medical InterporousTM denture tablets (Experimental). The percentage was obtained from the measurement of the total and disclosed area with biofilm.
The data were transformed to their square root prior to the analysis to obtain homogeneity of the variances (F1,38=1.797, P=0.188) and normality of the residuals (SW40=0.946, p=0.056). An independent t-test revealed a significant lower biofilm percentage for the experimental group [4.7, 95% confidence interval (CI) 2.4 to 7.9] in comparison with the control group (mean 37.5, 95% CI 28.2 to 48.1) (t38=7.996, p<0.001).
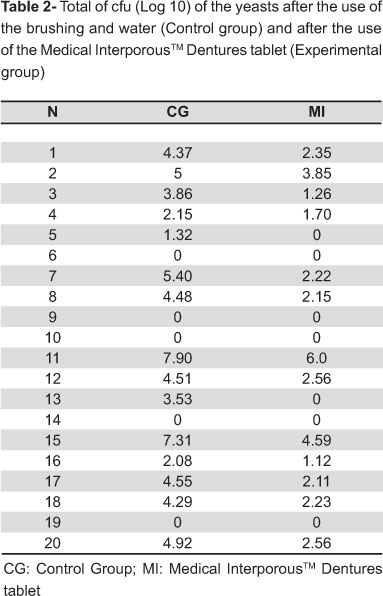
Table 2 presents the total cfu counts (in Log 10) of yeast obtained for the control group (CG) and for the experimental group (MI). A significant reduction of yeast cfu could be found after treatment with Medical InterporousTM denture tablets as compared to the control group. The data of Table 2 were analyzed by the Mann-Whitney test, which showed significant difference between the groups.
DISCUSSION
The study of oral hygiene in elderly denture wearers is becoming increasingly important because of the increasing numbers of elderly people in the world. Today, approximately 600 million people are aged over 60 years, and this number is estimated to double by 2025, 80% of which live in developing countries. In Brazil, by the year 2050, over 100 million people will be old-aged25. Denture stomatitis is a common clinical pathology observed in elderly populations. Microbial biofilm on the fitting surface of the denture is one of the principal causes of denture stomatitis10. The correlation between poor denture cleanliness and denture stomatitis is statistically significant according to Kulak-Ozkan, et al.13 (2002). Removal of the denture biofilm by means of good denture hygiene is important in the prevention of the denture stomatitis, in aesthetic aspects and in the maintenance of a healthy mucosa18. Efficient biofilm control by mechanical or chemical denture cleansing has produced significant resolution of this oral disease18. However, the incidence of denture stomatitis is still significant affecting 11-67% of denture wearers and recent studies indicate that 1 in every 3 removable denture wearers in the United States suffers from denture stomatitis4.
A variety of chemical denture cleansing products are commercially available. These type of denture cleansers can be divided into five groups: alkaline peroxides, alkaline hypochlorite, diluted organic and inorganic acids, disinfectants and enzymes16,18. While chemical biofilm control is a useful and appropriate procedure, such treatment should always be accompanied by mechanical control of biofilm in the fitting surface of the denture24, and brushing the fitting surface of the prosthesis should be indicated as an essential technique of hygiene, at least for patients with motors disabilities. Because of this evidence, the association of both methods was used in the present study in the experimental group (brushing and immersion in Medical InterporousTM for 15 min)22,23.
Dentures are made of poly-methyl methacrylate (PMMA) and all of them become quickly colonized by various microorganisms once they are being placed in the mouth. These microorganisms, including bacteria, fungi and viruses, initially adhere to the surface of the denture material and subsequently penetrate into the dentures via a complex of pores and tracks formed by the release of gases during the construction polymerization process2,14. The dentures in this study were at least 1 year old once it is necessary for patients to be really adapted to the daily use of the denture, which ensures the formation of biofilm. Moreover, with the continuous use, the resin looses gradually its polished surface, which also contributes to the buildup of biofilm and penetration of microorganisms in the porous of the acrylic resin. So, the effectiveness of the product in biofilm removal, as well as its antimicrobial action can be evaluated. In another study, Jeganathan, et al.11 (1996) suggested that the increasing occurrence of stomatitis in some of the analyzed denture wearing patients may be related to the age of the denture: the denture may become traumatic because of old age and the surface may present porosities that make it more difficult to keep clean. This may partially explain the varying number of cfu of Candida albicans in the experimental group in this present study.
The overall objective of immersing a denture in a cleaning maintenance solution is to obtain a clean but also decontaminated prosthesis through the destruction of microorganisms by means of the chemical actions including the effervescency properties of the cleanser30. It has been shown previously that 24 h accumulations can be removed moderately effectively after 15 min of immersion and completely with overnight soaking29. Coenye, et al.3 (2008) affirmed that the contact time with NitrAdineTM (MSI Laboratories AG, Vaduz, Liechtenstein) needs to be sufficiently long (i.e., at least 10-15 min) in order to obtain meaningful reductions, and this may be attributed to the time needed for the disinfectant to penetrate in to the biofilm. In the present study, the percentage of biofilm in the experimental group has diminished substantially (from 37.5% to 4.7%) in comparison to the control group after 21 days of use. Although the use of NitrAdineTM is indicated daily for 2 weeks, a period of 21 days was used in this study as a parameter of comparison with others studies found in literature19,23. Panzeri, et al.19 (2009), while evaluating the mechanical method, found significant differences between the control and experimental groups, and brushing with experimental dentifrice presented greater effectiveness in removing the biofilm.
Hence, regular overnight soaking in this product may increase the antimicrobial potential even further, clean the denture completely and leave polished and shiny surfaces. According to Shay, et al.29 (2000) in addition to the usual instructions in oral hygiene that should be given to patients wearing prosthesis, the use of disclosing solutions to identify biofilm accumulations was demonstrated. Since mature biofilm is much more resistant to removal than the 24 h biofilm29, the quantification of the biofilm can be regarded as a good outcome measurement for denture hygiene. The use of neutral red solution is justified by its high affinity to oral biofilms and the ease of removal31. In addition, photography1 combined with quantitative analysis21 was employed to standardize an objective evaluation method. A limitation of most biofilm measurement techniques, including the present method is the two dimensional nature of the recording. The use of a disclosing method appears to present no disadvantage when compared with the biofilm weight15. Furthermore, biofilm staining is the most commonly used technique for denture biofilm quantification18, and hence, provides a more efficient method if data need to be compared with previous studies. The analysis used in this study was based on biofilm quantification on the internal surface of the maxillary complete denture, in accordance with the studies by Paranhos, et al.24 (2007), Keng and Lim12 (1996), Sheen, et al.30 (2000). This surface has greater potential for build-up of pathogenic micro-organisms and the consequent development of denture stomatitis. As suggested by Paranhos, et al.24 (2007), we did not measure the external surface, since it accumulates much less biofilm.
The ideal denture cleanser should be efficient in removal of organic and inorganic depositions, non toxic, low cost, ease to use and reduce the amount of biofilm, stain and food of the dentures surface. We observed a significant difference between the control group and the experimental group in reduction in Candida sp however with some variability. This could be related to the variability in the number of cfu of Candida sp, between the periods of study and the subjects, as previously described by with Gornitsky, et al.9 (2002).
Medical InterporousTM denture tablets contain NitrAdineTM, a disinfecting formula that demonstrated high in vitro biofilm removal activity against a variety of micro-organisms, namely Candida albicans, Pseudomonas aeruginosa, Staphylococcus aureus, including the MRSA type, and viruses5,7. The efficacy of these tablets in reducing the biofilm percentage may also be due to the presence of sodium lauryl sulfate (SLS) in its formula, Moore, et al.16 (1984) reported that in a group of six cleansers alkaline peroxide cleansers, only two of them had a superior performance against yeasts, maybe because of the presence of SLS. SLS is a detergent used to solubilize protein in a variety of biochemical analysis techniques.
Coenye, et al.3 (2008), while testing the modified Robbins device to study the in vitro biofilm removal efficacy of NitrAdineTM found that the number of C. albicans biofilm cells on various materials was reduced by 3-4 log units after a single 15 min treatment, while reductions of c. 3 log units were obtained for MRSA and Pseudomonas aerouginosa. This study showed the efficacy of NitrAdineTM and the optimal conditions for the application of this disinfectant.
Dills, et al.6 (1988) showed that brushing combined with soaking treatment removed significantly more organisms than brushing alone: the level of microorganisms recovered from the prosthesis were significantly lower, which confirms the results from our current study. In the same study, Dills, et al.6 (1988) observed that the denture cleansers tested were not selective in its antimicrobial action, and that denture cleanser soak treatment displayed a broad spectrum activity in eliminating gram negative anaerobic rods, gram positive facultative cocci, and gram negative anaerobic cocci. These results support the need to use a denture disinfectant in addition to brushing the prosthesis for proper denture hygiene.
Since most elderly people do not know how to keep dentures clean, knowledge of the efficacy of different denture maintenance protocols is of importance to improve the quality of life of these dentures wearing patients24 and also the durability of the denture itself will be prolonged. This study suggests that NitrAdineTM-based disinfecting cleaning tablets are efficient in removal of denture biofilm. In addition a clear antimicrobial action was demonstrated. So, the Medical InterporousTM product can be useful clinically, especially for institutionalized patients, frail elderly patients in nursing homes, patients undergoing cancer treatment, and HIV infected patients, and seem to be effective against methicillinresistant Staphylococcus aureus (MRSA), which can cause a type of staph infection that is hard to treat because it cannot be killed by many common antibiotics3.
Moreover, the "tablet form" application is very easy-to-use and a consumer friendly method, which is another benefit for the elderly denture wearer. Additional studies are currently ongoing to address the physicochemical properties of the Medical InterporousTM as well as its mechanism of action in the prevention of denture stomatitis symptoms.
CONCLUSION
Microbial denture biofilm counts were substantially higher in the control method (denture brushing with tap water), when compared with the treatment based on denture brushing and the immersion in Medical InterporousTM denture tablets. Therefore, these tablets should be recommended as a routine denture maintenance method for the prevention of the development of microbial biofilm induced denture stomatitis.
ACKNOWLEDGEMENTS
We would like to thank Prof. Tom Coenye and Prof. Bart Vande Vannet for critical review of the paper content.
Received: March 16, 2009
Modification: March 24, 2010
Accepted: May 21, 2010
- 1- Ambjørnsen E, Valderhaug J, Norheim PW, Fløystrand F. Assessment of an additive index for plaque accumulation on complete maxillary dentures. Acta Odontol Scand. 1982;40:203-8.
- 2- Chau VB, Saunders TR, Pimsler M, Elfring DR. In-depth disinfection of acrylic resins. J Prosthet Dent. 1995;74:309-3.
- 3- Coenye T, De Prijck K, De Wever B, Nelis HJ. Use of the modified Robbins device to study the in vitro biofilm removal efficacy of NitrAdineTM, a novel disinfecting formula for the mantainance of oral medical devices. J Appl Microbiol. 2008;105:733-40.
- 4- Cunha-Cruz J. One in 3 removable denture users in the United States has denture stomatitis. J Evid Based Dent Pract. 2006;6:197-8.
- 5- De Prijck K, Nelis H, Coenye T. Efficacy of silver-releasing rubber for the prevention of Pseudomonas aeruginosa biofilm formation in water. Biofouling. 2007;23:405-11.
- 6- Dills SS, Olshan A, Goldner S, Brogdon C. Comparison of the antimicrobial capability of an abrasive paste and chemical-soak denture cleansers. J Prosthet Dent. 1988;60:467-70.
- 7- Glass RT, Bullard JW, Conrad RS, Blewett EL. Evaluation of the sanitization effectiveness of a denture-cleaning product on dentures contaminated with known microbial flora. An in vitro study. Quintessence Int. 2004;35:194-9.
- 8- Glass RT, Bullard JW, Hadley CS, Mix EW, Conrad RS. Partial spectrum of microorganisms found in dentures and possible disease implications. J Am Osteopath Assoc. 2001;101:92-4.
- 9- Gornitsky M, Paradisl I, Landaverde G, Malo AM, Velly AM. A clinical and microbiological evaluation of denture cleansers for geriatric patients in long-term care institutions. J Can Dent Assoc. 2002;68:39-45.
- 10- Hutchins DW, Parker WA. A clinical evaluation of the ability of denture cleaning solutions to remove dental plaque from prosthetic devices. NY State Dent J. 1973;39:363-7.
- 11- Jeganathan S, Thean HP, Thong KT, Chan YC, Singh M. A clinically viable index for quantifying denture plaque. Quintessence Int. 1996;27:569-73.
- 12- Keng SB, Lim M. Denture plaque distribution and the effectiveness of a perborate-containing denture cleanser. Quintessence Int. 1996;27:341-5.
- 13- Kulak-Ozkan Y, Kazazoglu E, Arikan A. Oral hygiene habits, denture cleanliness, presence of yeasts and stomatitis in elderly people. J Oral Rehabil. 2002;29:300-4.
- 14- Lin JJ, Cameron SM, Runyan DA, Craft DW. Disinfection of denture base acrylic resin. J Prosthet Dent. 1999;81:202-6.
- 15- McCracken GI, Preshaw PM, Steen IN, Swan M, deJager M, Heasman PA. Measuring plaque in clinical trials: index or weight? J Clin Periodontol. 2006;33:172-6.
- 16- Moore TC, Smith DE, Kenny GE. Sanitization of dentures by several denture hygiene methods. J Prosthet Dent. 1984;52:158-63.
- 17- Nikawa H, Hamada T, Yamamoto T. Denture plaque - past and recent concerns. J Dent. 1998;26:299-304.
- 18- Nikawa H, Hamada T, Yamashiro H, Kumagai H. A review of in vitro and in vivo methods to evaluate the efficacy of denture cleansers. Int J Prosthodont. 1999;12:153-9.
- 19- Panzeri H, Lara EHG, Paranhos HF, Silva-Lovato CH, Souza RF, Souza Gugelmin MC, et al. In vitro and clinical evaluation of specific dentifrices for complete denture hygiene. Gerodontology. 2009;26:26-33.
- 20- Paranhos HF, Panzeri H, Lara EH, Candido RC, Ito IY. Capacity of denture plaque/biofilm removal and antimicrobial action of a new denture paste. Braz Dent J. 2000;11:97-104.
- 21- Paranhos HF, Silva-Lovato CH. Comparative study of methods for the quantification of biofilm on complete dentures. Braz Oral Res. 2004;18:215-23.
- 22- Paranhos HF, Silva-Lovato CH, Souza RF, Cruz PC, Freitas KM, Peracini A. Effects of mechanical and chemical method on denture biofilm accumulation. J Oral Rehabil. 2007;34:606-12.
- 23- Paranhos HF, Silva-Lovato CH, Souza RF, Cruz PC, Freitas-Pontes KM, Watanabe E, et al. Effects of three methods for cleaning dentures on biofilm formed in vitro on acrylic resin. J Prosthodont. 2009;18:427-31.
- 24- Paranhos HF, Silva-Lovato CH, Venezian GC, Macedo LD, Souza RF. Distribution of biofilm on internal and external surfaces of upper complete dentures: the effect of hygiene instruction. Gerodontology. 2007;24:162-8.
- 25- Petersen PE, Yamamoto T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2005;33:81-92.
- 26- Ramage G, Tomsett K, Wickes BL, López-Ribot JL, Redding SW. Denture stomatitis, a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:53-9.
- 27- Rodrigues Garcia RC, Joane Augusto S Jr, Rached RN, Del Bel Cury AA. Effect of denture cleansers on the surface roughness and hardness of a microwave-cured acrylic resin and dental alloys. J Prosthodont. 2004;13:173-8.
- 28- Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk of nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8:54-6.
- 29- Shay K. Restorative considerations in the dental treatment of the older patient. Gen Dent. 2000;48:550-4.
- 30- Sheen SR, Harisson A. Assessment of plaque prevention on dentures using an experimental cleanser. J Prosthet Dent. 2000;84:594-601.
- 31- Silva-Lovato CH, Paranhos Hde F, Ito Y. Biofilm disclosing agents in complete denture: clinical and antimicrobial evaluation. Pesqui Odontol Bras. 2002;16:270-5.
- 32- Sumi Y, Miura H, Sunakawa M, Michiwaki Y, Sakagami N. Colonization of denture plaque by respiratory pathogens in dependent elderly. Gerondontology. 2002;19:25-9.
- 33- Webb BC, Thomas CJ, Whittle T. A 2-year study of Candida-associated denture stomatitis treatment in aged care subjects. Gerondontology. 2005;22:168-76.
- 34- Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: a review. Part 2. Oral diseases caused by Candida species. Aust Dent J. 1998;43:160-6.
- 35- Wilson J. The aetiology, diagnosis and management of denture stomatitis. Br Dent J. 1998;185:380-4.
Publication Dates
-
Publication in this collection
14 Jan 2011 -
Date of issue
Dec 2010
History
-
Received
16 Mar 2009 -
Accepted
21 Mar 2010 -
Reviewed
24 Mar 2010